Abstract
The solute carrier (SLC) superfamily encompasses a large variety of membrane-bound transporters required to transport a diverse array of substrates over biological membranes. Physiologically, they are essential for nutrient uptake, ion transport and waste removal. However, accumulating evidence suggest that up- and/or downregulation of SLCs may play a pivotal role in the pathogenesis of human malignancy. Endogenous substrates of SLCs include oestrogen and its conjugates, the handling of which may be of importance in hormone-dependent cancers. The SLCs play a significant role in the handling of therapeutic agents including anticancer drugs. Differential SLC expression in cancers may, therefore, impact on the efficacy of treatments. However, there is also a small body of evidence to suggest the dysregulated expression of some of these transporters may be linked to cancer metastasis. This review draws on the current knowledge of the roles of SLC transporters in human cancers in order to highlight the potential significance of these solute carriers in breast cancer pathogenesis and treatment.
Graphical abstract
Keywords: Breast cancer, SLCO, SLC22, Drug transport, Chemotherapeutic drugs, Oestrogen transport
Introduction
A spectrum of mechanisms underpin the pathogenesis of breast cancer (BC), and characterizing these can not only help us understand how BCs develop and progress but also provide potential targets for treatment. Membrane transporters (both efflux and influx) significantly influence the progression of BC but less is known about their role compared with that in other tissues.
Most studies to date have focused on the role of ABC (ATP-binding cassette) transporters in BC. These have shown that upregulation of several ABC transporters (ABCB1, ABCG2 and ABCC1) in response to chemotherapeutic agents can promote development of drug resistance [1]. However, focus has increasingly shifted to other membrane transporters that contribute to BC progression, including those that mediate substrate uptake, including SLC families that are key candidates due to their ability to transport a wide range of organic anions/cations and zwitterions [2]. Substrate specificities and differences in expression profiles of these SLC transporters between normal and cancer cells may reflect differing metabolic demands and could give clues to cell behaviour, including differences in drug sensitivities, cell proliferation and efficacy of chemotherapeutics [3].
Currently, there is relatively little information on the role of SLC transporters in BC. This review aims to draw together this limited data, together with information on other cancer types, in order to build a picture of their likely significance in BC, and to guide further research to exploit their potential as prognostic indicators and therapeutic targets.
SLC transporters physiological expression and substrates
The SLC superfamily is the second largest family of membrane proteins. They are classified into 52 families containing 395 transporters which together are ubiquitously expressed throughout the body particularly within epithelial tissues. Two of these families will be the focus of this review, the SLCO family comprising organic anion–transporting peptides (OATP) and the SLC22A family comprising organic anionic (OAT), organic cationic (OCT) and organic carnitine/zwitterion (OCTN) transporters. They play an essential role in the maintenance of homeostasis through the uptake of solutes that do not freely diffuse across biological membranes, and are predominantly facultative transporters requiring an electrochemical gradient to drive substrate movement into cells or secondary-active transporters relying on ion gradients created by ion extrusion [4] [5].
SLCO transporters
Eleven OATPs have been identified in humans that transport a plethora of substrates mainly organic anions and endo- and xenobiotics as outlined in Table 1.
Table 1.
The human organic anion transporters: Gene name, corresponding transport protein, tissue distribution and main substrates [5–8]
| Gene | Protein | Tissue expression | Substrates |
|---|---|---|---|
| SLCO1A2 | OATP1A2 | Breast, brain, kidney, intestine, liver, eye, lung, testis | Bile salts |
| Organic anion and cations | |||
| Bilirubin | |||
| Steroid hormone metabolites | |||
| SLCO1B1 | OATP1B1 | Breast, liver, kidney | Bile salts |
| Organic anions | |||
| Bilirubin | |||
| Steroid hormone metabolites | |||
| Thyroid hormones | |||
| Inflammatory mediators | |||
| SLCO1B3 | OATP1B3 | Breast, liver, kidney | Bile salts |
| Organic anions | |||
| Bilirubin | |||
| Steroid hormones | |||
| Thyroid hormones | |||
| Inflammatory mediators | |||
| SLCO1C1 | OATP1C1 | Brain, testis | Thyroid hormones |
| Steroid hormones | |||
| SLCO2A1 | OATP2A1 | Ubiquitous (including breast) | Prostaglandins |
| Inflammatory mediators | |||
| SLCO2B1 | OATP2b1 | Liver, placenta, intestine, breast, eye, mammary gland, skin, heart, skeletal muscle, brain | E-3-S |
| DHEA-S | |||
| Steroid hormones | |||
| Thyroid hormones | |||
| Inflammatory mediators | |||
| SLCO3A1 | OATP3A1 |
SLCO3A1_v1: testis, heart, brain, breast, ovary, lung, spleen, thyroid gland SLCO3A1_v2: testis, brain |
E-3-S |
| Prostaglandins | |||
| Steroid hormones | |||
| Thyroid hormones | |||
| Inflammatory mediators | |||
| SLCO4A1 | OATP4A1 | Ubiquitous (including breast) | Taurocholate |
| Thyroid hormones | |||
| Prostaglandin | |||
| Bile salts | |||
| Steroid hormones | |||
| SLCO4C1 | OATP4C1 | Breast, kidney, liver | Bile salts |
| Digoxin | |||
| Thyroid hormones | |||
| Methotrexate | |||
| Steroid hormones | |||
| SLCO5A1 | OATP5A1 | Breast, foetal brain, prostate, skeletal muscle, thymus | Non-identified |
| SLCO6A1 | OATP6A1 | Testis, spleen, brain, foetal brain, placenta | Non-identified |
SLC22 transporters
The human SLC22 genes encode 23 transport proteins of which 13 have been well characterised. Within each subgroup (OCT, OAT and OCTN) the transporters share overlapping substrate specificities and are distributed throughout similar tissues as outlined in Table 2 [13].
Table 2.
The human organic anionic and cationic transporters: Gene name, corresponding transport protein, tissue distribution and main substrates [9–12]
| Gene | Protein | Tissue expression | Substrates |
|---|---|---|---|
| SLC22A1 | OCT1 | Liver, intestine, kidney, lung, skeletal muscle, brain, adipose tissue, immune cells | Organic cations |
| Oxiplatin | |||
| Cisplatin | |||
| Carboplatin | |||
| SLC22A2 | OCT2 | Kidney, small intestine, lung, placenta, thymus, brain, inner ear | Organic cations |
| Oxaliplatin | |||
| Cisplatin | |||
| SLC22A3 | OCT3 | Heart, skeletal muscle, brain, small intestine, liver, lung, kidney, bladder, breast, blood vessels, placenta | Organic cations |
| Oxaliplatin | |||
| SLC22A4 | OCTN1 | Kidney, intestine, spleen, heart, skeletal muscle, brain, breast, thymus, prostate, airways, testis, eye, immune cells, bone marrow, pancreas, placenta, lung | Zwitterions |
| Organic cations | |||
| l-Carnitine | |||
| Oxaliplatin | |||
| SLC22A5 | OCTN2 | Skeletal muscle, kidney, breast, prostate, lung, pancreas, heart, small intestine, thyroid gland, liver, placenta and spinal cord | Zwitterions |
| Organic cations | |||
| l-Carnitine | |||
| SLC22A6 | OAT1 | Kidney, placenta, brain, eyes, smooth muscle | Small xenobiotics |
| Prostaglandin | |||
| Urate | |||
| Antivirals | |||
| Methotrexate | |||
| SLC22A7 | OAT2 | Liver, kidney, lung, brain, small intestine, eye, heart | Antivirals |
| cGMP | |||
| Prostaglandin | |||
| Tetracycline | |||
| Estrone-3-sulfate | |||
| SLC22A8 | OAT3 | Liver, kidney, brain, skeletal muscle, retina, testes | |
| Small xenobiotic | |||
| Conjugated steroids | |||
| Carnitine | |||
| Prostaglandin | |||
| Vitamins | |||
| Estrone-3-sulfate | |||
| Estradiol | |||
| Methotrexate | |||
| Tetracycline | |||
| SLC22A9 | OAT7 | Liver | Oestrogen-3-sulfate |
| SLC22A10 | OAT5 | Liver | Unknown |
| SLC22A11 | OAT4 | Kidney, placenta, brain | Estrone-3-sulfate |
| Prostaglandin | |||
| Urate | |||
| Tetracycline | |||
| Methotrexate | |||
| SLC22A12 | URAT1 | Kidney, smooth muscle | Urate |
| SLC22A13 | OAT10 | Kidney, colon, small intestine, brain, heart | Urate |
| Organic anions | |||
| Nicotinate | |||
| SLC22A16 | OCT6 | Testis, bone marrow, kidney | l-Carnitine |
| Non-charged compounds | |||
| SLC22A20 | OCT6 | Olfactory mucosa, testes | Estrone-3-sulfate |
SLC transporters and cancer
SLC transporters are expressed in many cancers and can be differentially expressed between malignant and non-malignant tissues. This has led to an interest in the roles these transporters play in cancer progression.
OATPs
SLCO1A2 (OATP1A2) is present in numerous tissues and is recognised as a prostaglandin transporter. Its other substrates are steroid hormones/conjugates; estradiol-17β-glucuronide, estrone-3-sulfate (E-3-S), dehydroepiandrosterone sulfate (DHEA-S) and anticancer drugs; imatinib, methotrexate, paclitaxel, doxorubicin and docetaxel [14]. Physiologically, SLCO1A2 is expressed most highly in the brain and, to a lesser extent, in several other tissues (Table 1). In cancer, tissue distribution includes breast as well as, gliomas, colon polyps, bone, prostate, pancreatic and head and neck cancers [14–21]. Although differential expression of OATP1A2 has been found in cancer cells compared with healthy tissue, the pattern varies between cell types; in head and neck squamous cell carcinoma its expression is significantly increased compared with healthy adjacent tissue but expression appears to be lower in colonic tumours than healthy colon [16, 21]. In the prostate, SLCO1A2 may play a role in the growth of prostate cancer cells through DHEA-S uptake suggesting that inhibition of this SLCO1A2 could help to attenuate tumour cell growth [15].
SLCO1A2 protein expression has been detected in the cell membrane and cytoplasm of breast carcinoma cells but not in cells of adjacent healthy tissues. High expression was also observed in BC cell lines ZR-75-1 and T-47D [14]. A significant correlation between the expression of this transporter and the pregnane X receptor (PXR) has been demonstrated suggesting regulation of SLCO1A2 expression may be achieved through PXR [20]. Supporting this, a 10-fold higher expression has been observed in BC versus healthy adjacent breast tissue, and SLCO1A2 expression is inducible in BC cell lines upon stimulation by the PXR activator, rifampicin, resulting in E-3-S uptake and proliferation [22].
SLCO1B1 (OATP1B1) and SLCO1B3 (OATP1B3)
Physiologically, SLCO1B1 and SLCO1B3 are regarded as a largely liver-specific, localizing to the basolateral membrane of hepatocytes [5]. In cancer, they are expressed more widely and are typically overexpressed. SLCO1B1 overexpression has been demonstrated in colonic, ovarian, prostate and pancreatic cancers [16, 18, 23, 24]. SLCO1B3 is overexpressed in colonic, lung, breast, prostate, pancreatic, testicular and ovarian cancers [25–31]. SLCO1B1 expression in pancreatic cancer has been investigated as a potential target for selective toxicity to microcystin cyclopeptides [18]. SLCO1B1 is overexpressed in colon cancer and a significant relationship between SLCO1B1 expression levels and degree of differentiation in colon and liver cancer has been reported [29]. Higher mRNA levels of SLCO1B1 and SLCO1B3 in ovarian tissues and cell lines correlates with higher uptake rates of paclitaxel, suggesting a role for these transporters in the deposition of clinically relevant drugs [23].
SLCO1B3 overexpression in pancreatic adenocarcinoma compared with normal pancreas has been suggested as a potential marker of early-stage disease or as a target for drug treatments [32]. Likewise, its overexpression in colorectal cancer is suggested to confer a survival advantage by altering P53-dependent pathways [26]. A significant increase in SLCO1B3 expression has been shown to correlate with increasing Gleason score [29]. In contrast to their widespread overexpression in some cancers, several groups have shown that expression of both SLCO1B3 and SLCO1B1 is decreased in hepatocellular carcinoma at both the mRNA and protein levels [33–36]. This reduction may be attributed to de-differentiation in liver tumours and subsequent reduction in metabolic function [29].
While it has been presumed that the SLCO1B3 gene detected in cancers was identical to that seen in the healthy liver, a cancer-specific variant, OATP1B3 V1, is now known to arise due to alternative splicing. This variant differs from the wild-type (WT) protein as it lacks 28 amino acids in its N-terminus and is thought to reside predominantly in the cytoplasm rather than the plasma membrane like its WT counterpart and exhibits more restricted transport abilities of model substrates [31, 37].
There are conflicting reports of SLCO1B1 expression in BC cell lines and tissues. One study assessed SLCO1B1 expression in five paired samples of normal breast tissue and BC but found that SLCO1B1 expression was below the detection limit of qPCR [38]. Likewise, another study comparing expression in normal breast tissue and tumour samples found SLCO1B1 was not highly expressed in these tissues via qPCR, a similar finding to a study involving microarray analysis of human normal breast and cancer tissues [29, 39]. Conversely, assessment of mRNA expression in MCF10A, MCF-7, MDA435/LCC6, MDA-MB-468 and MDA-MB-231 cell lines showed expression to be highest in MDA-MD-231 cells and low, but detectable, in MDA435 and MCF10A cell lines. SLCO1B1 was also found to be expressed at the protein level with similar expression pattern, again with high expression in MDA-MB-231 cells [40].
As with SLCO1B1, there are contradictory reports of expression of SLCO1B3 in BC. In cell lines, SLCO1B3 was found to be expressed using qPCR in MDA-MB-231 and MCF10A but was not detected in the MCF-7 cell line [40]. Conversely, another study showed SLCO1B3 expression in the MCF-7 cell line via DNA microarray analysis [41]. However, in normal breast tissue samples and tumours, SLCO1B3 expression was detectable in two studies using both microarray analysis and qPCR [29, 39]. In contrast, another study examining SLCO1B3 expression in 102 breast carcinoma samples using IHC found SLCO1B3 expression in 50% of samples, and where immune reactivity was found this was inversely correlated to tumour size and was associated with a lower incidence of BC recurrence. The authors suggested that SLCO1B3 overexpression may be associated with a hormone-dependent growth mechanism and that expression of this transporter could be a potent prognostic factor in BC [28].
These conflicting reports may be due to a number of factors including; BC type, sample type and detection technique or could be due to expression of a cancer-specific variant gene, e.g. SLCO1B3 v1 found in colon and pancreatic cancers. Whether or not this is present in BC and involved in BC progression needs to be investigated.
Going forward, definitive analysis of the expression pattern of SLCO1B1 and SLCO1B3 in BC is important, since their substrate specificities suggest they may have a potential significance in different aspects of BC including cell growth (E-3-S and DHEA-S), chemotherapy (taxanes, methotrexate, rapamycin, methotrexate, paclitaxel, doxorubicin, doxertaxel, imatinib and SN-38), and also in diagnostic imaging, since it is recognised that gadolinium-based compounds such as gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) are also substrates, and these are used in magnetic resonance imaging (MRI) to assess breast tumour enhancement [8, 14, 42–44].
Little is known about the role of SLCO1C1 in cancer/BC. It is predominantly expressed in the brain and testes and has been identified as a transporter of thyroid hormones [45]. No anticancer drugs have been identified as substrates to date; however, E-3-S and estradiol-17β-glucuronide are thought to be substrates [46]. SLCO1C1 is expressed in human osteosarcomas, metastasised renal cancers and aneurysmal bone cysts, though its function in these tissues has not been established [19].
SLOC2A1 is ubiquitously expressed and was originally identified as a prostaglandin transporter due to its high affinity for this substrate. Prostaglandins are thought to promote cancer progression by affecting cell proliferation, apoptosis, angiogenesis and immune response [5, 47]. Currently, no anticancer drugs are known to be transported by SLCO2A1; however, expression is known to be variable in cancer tissues. For example, SLCO1A2 expression was decreased in colon/rectum tumours as well as stomach, ovary, lung and kidney compared with normal tissues [47]. Conversely, increased expression has been shown in other cancers including breast, liver, and bone metastases from renal cancer [19, 48, 49].
SLCO2A1 expression is documented in several cancers including BC where, overall, it appears expression may be increased. A study comparing SLCO2A1 expression using qPCR in hormone-dependent BC cell lines (MCF-7 and ZR-75-1) to a breast epithelial cell line (MCF10A) found over a 3-fold increase in expression in the BC cell lines. The authors also assessed expression in 13 matched BC and non-malignant tissue samples via qPCR, and again, expression appeared to be increased in malignant tissue. Expression of SLCO2A1 via microarray analysis has also been demonstrated in normal breast tissue and breast tumour samples [39]. The apparent increased expression of this transporter in BC may be of significance since its main substrates are prostaglandins; increased intra-tumoural prostaglandins are considered indicators of poor prognosis in BC, as prostaglandins initiate signalling pathways associated with several hallmarks of cancer (angiogenesis, anti-apoptosis, proliferation, migration, invasion, immune evasion and epithelial mesenchymal transition and support of cancer stem cell-like phenotype). Another study investigated the expression of SLCO2A1 in different BC subtypes, and increased expression was seen in normal tissue and HER2-enriched or luminal A tumours than luminal B or basal. SLCO2A1 expression was also found to be significantly decreased in triple-negative breast tumours [50].
SLCO2B1 is another ubiquitously expressed transporter (Table 1), with highest expression reported in the liver [5]. Its substrate specificity is largely pH-dependent. At pH 7.4, its main substrates are steroid hormone conjugates; however, studies in the intestine show that increasing acidity increases transporter activity, with changes both in the substrate turnover and affinity [51]. Despite this broad and variable substrate specificity, to date, no cancer drugs are known to be substrates [8]. Nevertheless, SLCO2B1 expression has been shown in several cancers with some differences between malignant and non-malignant tissues. In bone, SLCO2B1 has been detected in both benign and malignant tumours, with significantly higher mRNA levels in aneurysmal bone cysts (benign) as compared with osteosarcomas (malignant) [19]. Likewise higher expression is seen in malignant versus non-malignant breast tissue, and expression increases with tumour grade [49, 52]. In the liver and pancreas, lower mRNA expression of SLCO2B1 in liver cancer was associated with decreasing differentiation and significantly lower expression of SLCO2B1 was seen in pancreatic cancer compared with normal pancreatic tissue. Conversely, expression was higher in thyroid cancer than normal tissue and expression increased with cancer stage [29].
SLCO2B1 has been identified in normal breast tissue and has been localised to the myoepithelium surrounding the ductal epithelial cells. This differs from invasive ductal carcinoma where it was found in the membrane of cytokeratin-positive epithelial cells [53]. However, the same study showed that in three BC cell lines (MCF-7, MDA-MB-231 and T47-D), SLCO2B1 remained under the detection limit via qPCR [53]. Likewise, several other studies have found SLCO2B1 expression to be undetectable or very low in cell lines via qPCR [40, 49, 54]. Protein expression also remained very low in cell lines [40]. SLCO2B1 expression at the transcriptional level has been detected in malignant and non-malignant tissue samples, with expression being higher in non-malignant tissues [49]. Conversely, another study analysing 120 tumour and 23 normal breast tissue samples using qPCR found higher expression in malignant versus non-malignant tissues, and that expression of SLCO2B1 increased with tumour grade, though neither of these findings reached statistical significance [52]. In agreement with previous studies, Kindla et al. detected SLCO2B1 in normal and malignant tissues however no statistical difference in expression was seen between the two groups. Their analysis using immunohistochemistry (IHC) and immunofluorescence (IF) showed SLCO2B1 expression was localised to the lobules/ducts and capillary epithelium in normal tissue but localisation in malignant tissue was mainly to the cytoplasm of malignant cells and plasma membrane of non-malignant cells [38]. SLCO2B1 expression in normal breast tissue and tumours is also supported by microarray analysis [39]. Among an array of substances transported by SLCO2B1, important substrates relevant to BC include E-3-S, DHEA-S and Prostaglandin E2 [14, 53, 55–57].
SLCO3A1 (OATP3A1) is also ubiquitously expressed showing highest expression in the testes, brain and heart (Table 1). This expression is the result of two splice variants; SLCO3A1_v1 and SLCO3A1_v2. While largely similar to the v2 variant, the v1 variant lacks 18aa in its COOH-terminal end. As a result, the v1 variant is widely expressed throughout bodily tissues whereas v2 expression is limited to testes and brain (Table 1) [58]. Substrate specificity remains largely similar between both variants and includes steroid hormones and conjugates, prostaglandins, and antibiotics [14]. SLCO3A1 has been detected in several cancers including breast, lung, colon, ovary and pancreas [57]. Additionally, higher SLCO3A1 expression has been found in several cancer types compared with normal or benign tissue, including, hormone-resistant metastases versus untreated prostate cancers, in primary and metastatic liver cancer versus normal liver, in aneurysmal bone cysts versus osteosarcomas and in BC cell lines versus non-malignant breast cell lines [19, 24, 40, 48].
In normal breast SLCO3A1 localises to the plasma membrane of lactiferous duct epithelial cells, but localisation alters in BC, shifting to the cytoplasm. This is not however associated with a change in expression level, as qPCR of matched normal and BC tissues showed no significant change in expression between the two groups [38]. Expression has been detected in breast cell lines via IHC (MCF-7, HBL-100 and BT-474) and qPCR (MCF-7, MCF-10A, MDA/LCC6–435, MDA-MB-468 and MDA-MB-231). Significantly higher expression was only seen between MDA435/LCC6 cells compared with MCF10A cells [40, 54]. In agreement with the molecular expression pattern, protein expression via western blot analysis showed significantly higher levels in MCF-7 and MDA435/LCC6 cells versus MCF-10A [40]. Wleck et al. also confirmed expression in MCF-7, ZR-75-1, MCF-10A and MDA-MB-231 cells, with significantly higher expression in the normal epithelial and hormone-independent cell lines compared with the hormone-dependent cell lines [49]. In tissues, expression was detected in both normal and malignant samples, with expression significantly higher in non-malignant tissue [49].
To establish if transporters play a role in E-3-S transport and proliferation in BC, uptake of E-3-S by T47-D and MCF-7 cells was measured and resulted in increased proliferation. SLCO3A1 and SLCO4A1 were identified as candidate transporters for this uptake via RT-PCR, and inhibition studies supported the notion that an OATP may be responsible [56, 59].
SLCO4A1 (OATP4A1) shares a similar ubiquitous expression pattern to SLCO3A1; likewise, it also transports E-3-S and prostaglandins [57]. SLCO4A1 expression has been shown in cancer cell lines derived from the breast, lung, colon, prostate, ovary and pancreas [57]. As for SLCO3A1, SLCO4A1 expression appears to be increased in aneurysmal bone cysts and liver cancers, BCs and prostate cancers [19, 24, 48, 49].
Studies show SLCO4A1 expression in several breast cell lines: MCF-10A, MCF-7, MDA/LCC6–435, MDA-MB-231, MDA-MB-468, ZR-75-1 and T47-D via a combination of molecular techniques (RT-PCR and qPCR) and protein analysis (Western blot) [40, 49, 53]. Expression was also seen in normal mammary gland tissue and matched normal, malignant and non-malignant breast tissue [49, 53]. Overall, these studies suggest that SLCO4A1 expression is higher in non-malignant versus malignant breast tissue, and higher in hormone-responsive versus hormone non-responsive BC [40, 49, 53].
Little is known about the role of SLCO4C1 in normal breast and BC. It was originally classified as kidney specific, however, while highest expression is seen in kidney moderate-to-low expression is also seen in breast, liver, lung and skin [39]. In the kidney, SLCO4C1 is localised to the basolateral membrane of proximal tubule cells where it facilitates excretion of exogenous drugs and endogenous compounds including methotrexate, thyroid hormones and E-3-S [5]. It remains poorly characterised in cancers in general, though microarray analysis has detected expression in renal, ovarian, leukaemia, lung, and glioblastoma cancer cell lines [60]. SLCO4C1 has also been detected in BC cell lines and tissues via qPCR [49]; expression was found in MCF-7, ZR-75-1 and MDA-MB-231 cells, with highest expression seen in MCF-7. Conversely, no expression was detected in the MCF-10A cells. In tissue specimens, 7 of 13 paired non-malignant and malignant samples showed higher expression in the non-malignant tissue [49]. Studies in rat have shown SLCO4C1 expression in breast epithelial cells significantly increases in lactating versus non-lactating tissue [61] indicating response to hormonal drive.
Both SLCO5A1 and SLCO6A1 remain poorly characterised in terms of transporter function and substrate specificity. SLCO5A1 may be expressed in the brain, prostate, skeletal muscle and thymus according to microarray analysis; however, this requires further verification [39]. SLCO6A1 was originally identified as testis specific; however, weak expression has been detected in normal spleen, brain and placenta [5]. Expression of both transporters has been detected in several cancers; for SLCO5A1, these include breast, bone, prostate, liver and small cell lung cancer [19, 38, 48, 62]. SLCO6A1 has been detected in lung cancer cell lines and tumours of the lung, bladder, oesophagus and brain [63, 64].
In terms of functionality, a study using SLCO5A1-HEK-293 transfected cells has shown increased resistance to Satraplatin, a potential chemotherapeutic agent for breast, prostate and lung cancer treatment [62].
Unsurprisingly, there is a paucity of information on the potential role of SLCO5A1 and SLCO6A1 in normal breast tissue and BC. SLCO5A1 expression has been shown via immunohistochemistry and immunofluorescence in the membrane of normal lactiferous ducts, but expression become less membrane-bound and more cytoplasmic in BC, showing a similar expression pattern to SLCO3A1 [38]. mRNA expression has been demonstrated in cell lines (MCF-7, ZR-75-1 and MCF-10A) and in normal and malignant tissue samples. In the cell lines, SLCO5A1 expression was 3-fold higher in MCF-7 cells than MCF-10A control cells. Conversely, in the tissue specimens, 8 of 13 normal tissue samples had higher SLCO5A1 expression than malignant tissues, however, the results were quite variable [49].
OCTs and OCTNs
OCT1 is considered liver-specific, however weak expression has also been shown in other tissues such as heart, skeletal muscle, kidney and brain [65], and expression has been noted in several cancers [66]; (Table 2). Substrates include anticancer drugs such as imatinib and oxaliplatin [67]. Significant overexpression of OCT1 has been found in cell lines and samples from lymphoma patients, and this is thought to contribute to susceptibility to antineoplastic drugs [68]. Similarly, OCT1 is expressed in chronic myeloid leukaemia where it is associated with uptake of imatinib [69]. OCT1 has also been detected in healthy colonic tissues, colon cancer and polyps, where its overexpression is deemed a determinant of the anticancer effects of oxaliplatin [16, 70].
OAT2 is predominantly expressed in the kidney, however some expression is also seen in the liver, with comparatively low expression seen in many other normal tissues [71]. Notable substrates are platinum-based drugs (Table 2). OCT2 expression has been detected in glioma (SK-MG1) and colonic adenocarcinoma (Caco-2) cell lines [72] and its expression has been documented in 4 of 8 renal cancer cell lines, 4 of 6 ovarian cancer cell lines, 2 of 5 brain cancer cell lines and 1 of 8 colon cancer cell lines [73]. In tissue samples, OCT2 was present at very low levels in many tissues but absent in some cancer tissues including breast, colon, liver, lung, ovarian, prostate and thyroid [60].
OCT3 shows a different tissue specificity to both OCT1 and OCT2, and is more ubiquitously expressed, but shows stronger expression in the liver, placenta, kidney and skeletal muscle [74]. Investigations into OCT3 in cancer have mainly focused on it as a transporter of chemotherapeutic agents. Low OCT3 expression has been detected in human liver cell lines, normal healthy liver tissue and hepatocellular carcinoma (HCC), and it has been shown to be downregulated in HCC compared with healthy liver [75]. Conversely, it is highly expressed in colorectal cancer–derived cell lines compared with other OCTs and almost ten times more highly expressed in colorectal cancer cells than normal tissue. OCT3 highly expressing tissues are more sensitive to the cytotoxic effects of oxaliplatin, which has led to the suggestion that it may be used as a marker of efficacy of oxaliplatin treatment in colorectal cancer [76]. Likewise, OCT3 expression is seen in renal carcinoma cell lines and its expression has been linked to increased sensitivity to cytostatic drugs suggesting that the presence of OCT3 may be used to tailor therapies [77].
There are a few reports showing expression of OCT1–3 in breast tissues. In lactating and non-lactating mammary epithelial cells (MEC), OCT1 and OCT 3 but not OCT2 were detected, in lactating MEC, with OCT1 levels over 4-fold higher in lactating MEC versus non-lactating MEC [78]. In BC models, one study used qPCR to assess OCT1–3 expression in 9 cell lines; 4 luminal human BC (MCF-7, SK-BR-3, ZR-75-1 and BT-474) and 5 basal cancer cell lines (BT-20, MDA-MB-435S, MDA-MB-231, MDA-MB-468 and BT-549). In most of the cell lines, the levels of all three transporters were negligible; however, in MDA-MB-231, MDA-MB-468 and BT-549, their levels were relatively high [79]. Protein expression of OCT1 and OCT3 using Western blot analysis was also detected in MDA-MB-468, MDA-MB-435S and MDA-MB-232 cell lines. Using malignant breast tissue, non-malignant adjacent tissue and normal breast tissue, OCT3 showed the highest expression, followed by OCT1, with negligible OCT2 expression. Interestingly, OCT3 was lower in malignant compared with adjacent non-malignant tissue; however, this was not statistically significant [79].
OCTN1 is a transporter of zwitterions such as l-carnitine, which is required for beta-oxidation of fatty acids and ultimately energy production. This transporter shows ubiquitous expression but is more highly expressed in the kidney, trachea, and bone marrow (Table 2) [80]. To date, there is little evidence for a link between OCTN1 and cancer; however, expression has been documented in cancer cell lines including lung, colorectal, myelogenous leukaemia and HeLa [11, 80]. Expression of OCTN1 has however been linked to heightened sensitivity to mitoxantrone and doxorubicin [60]. Like the aforementioned cation transporters, OCTN1 transports oxaliplatin and its overexpression has been linked to drug accumulation and cytotoxic effects [81].
Like OCTN1, OCTN2 is a transporter of cations and is a sodium-dependent high-affinity transporter of carnitine. High OCTN2 expression is seen in various tissues including heart, placenta, skeletal muscle and kidney [82], as well as in several human cancer–derived cell lines including melanoma, lung, colorectal, chronic myeloid leukaemia and cervical carcinoma, showing a similar pattern of expression to OCTN1 [82]. The role of OCTN2 in cancer remains unclear, however one hypothesis is that its expression may be reduced in cancers bringing about a reduction in carnitine transport and in turn having knock-on effects for mitochondrial fatty acid ß-oxidation [83]. This is complementary to the Warburg effect which shows that cancer tumours favour a metabolic switch to energy production via glycolysis rather than β-oxidation regardless of the oxygen environment [84]. OCTN2 has also been identified as a transporter of both oxaliplatin and imatinib [85, 86].
OCTN2 has been localised to the luminal alveolar membrane of breast tissue [87]. It has also been identified as an oestrogen-dependent transporter associated with ER status in BC cells and tissues; its expression was confirmed in 15 BC cell lines using qPCR, with significant overexpression in ER-positive compared with ER-negative cell lines [88]. This was supported by cDNA microarray of BC cell lines and breast tumour tissue, and it was determined that OCTN2 acts as an oestrogen-activated intronic enhancer element that is crucial for carnitine homeostasis, lipid metabolism, and BC cell proliferation [88].
OCT6 is a carnitine transporter mainly expressed in the testis but also in the bone marrow, kidney and leukocytes [89]. RT-PCR has shown OCT6 expression in leukaemia cell lines and samples from leukaemia patients. Furthermore, uptake via OCT6 of doxorubicin, a commonly used treatment for acute myeloid leukaemia confers sensitivity in a leukaemia cell line [90, 91]. OCT6 is also highly expressed in liver- and colon-derived cancer tissues and cell lines, and is moderately expressed in cancer cell lines and tissues derived from prostate and uterus [91]. When SLC22A16 is highly expressed in testicular cancer there is increased sensitivity to treatment with bleomycin, a known substrate for this transporter [92].
OATs
SLC22A6-A11 and SLC22A13
Of the 11 known OAT transporters, only SLC22A6 (OAT1), A7 (OAT2) A8 (OAT3), A11 (OAT4), A10 (OAT5), A9 (OAT7) and A13 (OAT10) are known to be expressed in humans [3]. OAT1–3 and OAT10 were originally identified as kidney specific while OAT5/7 and OAT4 were found to be expressed in the liver and placenta respectively. Although initially appearing to show tissue-specific expression, several of these have now been identified in multiple tissues (Table 2) [12]. Currently, the role they play in cancer is largely unknown. Some studies have suggested that they may transport anticancer drugs, for example 5-fluorouracil, a substrate for OAT2. High OAT2 expression is thought to be a predictor of good treatment response to 5-fluorouracil in colon cancer [93]. Similarly, methotrexate is transported by OAT1, OAT2 and OAT3 [94].
Expression in BC needs to be fully established; a study of 6 cancer cell lines revealed none expressed SLC22A6, A7 and A8 at the mRNA level, while analysis of human mammary mRNA samples revealed all were expressed [60].
URAT1 (OAT4L) is a kidney-specific transporter located in the apical luminal membrane of proximal tubule cells, where its main role is in the reabsorption of urate [95]. In humans, disease mutation in the URAT1 gene leads to hereditary renal hypouricemia; however, association of URAT1 with cancer remains un-investigated [96].
SLC22A20 (OAT6) was originally identified from the Ensembl mouse genome database, it is expressed in the nasal epithelium and, to a lesser extent, the testis [97]. It Is hypothesised to be involved in odorant detection due to its expression pattern and odorant substrates [98]. Additionally, OAT6 was demonstrated to transport E-3-S in Xenopus oocyte expression assays and Chinese hamster ovary cells transfected with mOAT6 [99]. To date, few connections have been made between SLC22A20 expression and cancer. However, analysis of RNAseq data showed expression in liver, kidney and thyroid carcinoma [74]. Further analysis via qPCR also showed expression of SLC22A20 in leukaemia and lung, kidney and liver carcinoma cell lines [100].
Defining the role ole SLC transporters in breast Cancer
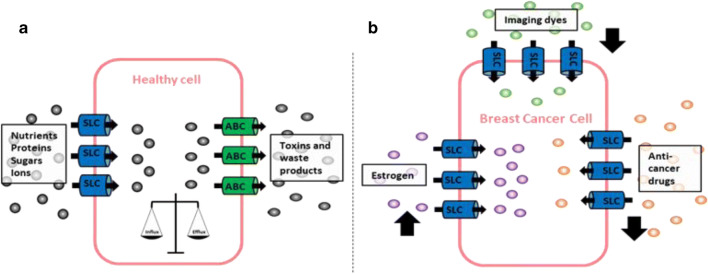
It is evident that there are differences in expression of solute carriers in different types of cancer and that this expression pattern can differ from that of healthy tissues. Major substrates for several SLC transporters include steroid hormones such as oestrogen conjugates and DHEA-S, as well as various anticancer drugs. As nearly two-thirds of newly diagnosed BCs are hormone dependent, it makes sense to look further at the role these transporters play in hormone-dependent BCs [101].
Estrogens are known promoters of BC cell proliferation. Free estrogens are lipophilic and so are able to freely diffuse across the plasma membrane. However, sulfo-conjugated steroid hormones (E-3-S) and the oestrogen precursor DHEA-S are hydrophilic and hold a net negative charge, thus are not freely diffusible. These hormones therefore require uptake transporters to enter cells. The SLCO/OATP subfamily has a potential role here, since estrogens are proven substrates for 7 of the 11 known human SLCO transporters: 1A2, 1B1, 1B3, 1C1, 2B1, 3A1 and 4A1 [102]. Although there is a decline in free estrogens in postmenopausal women the majority of hormone-dependent BCs occur in this group. This has been attributed to the biosynthesis of free estrogens from E-3-S and DHEA-S through the action of sulfatase and aromatase enzymes in BC cells that drive hormone-dependent cell proliferation [103]. In agreement with the role of estrogens as promoters of BC cell proliferation, it has been shown that oestrogen-dependent BC cell lines T47-D and MCF-7 proliferate on stimulation with E-3-S and that inhibition of E-3-S transporters results in the suppression of cell proliferation. The authors suggest several transporters may be responsible for this uptake of E-3-S and subsequent oestrogen-dependent proliferation of BC cells, but conclude that further investigation into these transporters is required [56, 59]. SLC transporters themselves may be regulated by hormonal stimulation, and this may occur through activation of PXR [20].
Current data pertaining to the clinical significance of SLC transporters in BC progression is mixed. Gene microarray results of 48,000 gene transcripts in 132 invasive breast carcinomas identified SLCO1B3 as a novel gene associated with the basal phenotype [104]. SLCO1B3 expression was associated with tumours of high histological grade, adverse survival and increased risk of early recurrence [104]. However, immunohistological analysis correlated with clinicopathological parameters in 102 breast carcinomas revealed SLCO1B3 expression is inversely correlated with tumour size and is associated with a decreased risk of recurrence [105]. In ER+ tumours, SLCO1B3 expression signified a good prognosis, and as oestrogen is a substrate for this transporters, it has been implicated in hormone-dependent growth mechanisms [105].
Thirty-one polymorphisms of the PXR, SLCO1A2, SLCO1B1 and SLCO1B3 transporters were analysed using MALDI-TOF MS and revealed that none of the 31 polymorphisms showed an association with breast cancer risk or tumour characteristics [106]. Polymorphisms were also investigated for SLCO1B1 (CYP2D6*10, A388G, T521C) in 296 hormone receptor-positive invasive breast tumours following adjuvant tamoxifen (TAM) therapy and revealed that there was a significant difference in overall survival between T521C and A388G but no difference in overall survival between CYP2D6*10 and A388G [107].
With respect to drug transport, SLCO1A2 and SLC22A16 expression has been investigated as a predictor of response to neoadjuvant chemotherapy. Immunohistochemical analysis was performed for 124 patients, pre- and post-anthracycline/taxane–based neoadjuvant chemotherapy. Analysis revealed that combined high OATP1A2/high OCT6 may be a potential predictor of pathological good/complete response to anthracycline/taxane-based chemotherapy in breast cancer, especially in triple-negative tumours [108].
Clearly, there is a great deal of information indicating the potential importance of SLCO transporters in BC development, as well as in its treatment, but more work needs to be done to develop a unifying picture of their precise roles.
Compared with the SLCO transporters, less is known about the role of SLC22 transporters in BC. There is clearly some evidence for expression of SLC22A1, SLC22A2 and SLC22A3 in breast tissue, their differential expression in normal versus malignant breast tissues, and their ability to transport certain anticancer drugs is certainly of potential significance [67]. Still, less is known about the expression and function of other SLC22 transporters. SLC22A6, SLC22A7, SLC22A8 and SLC22A11 are of potential interest because of their substrate specificities, which, like the SLCOs, include oestrogen conjugates and certain anticancer drugs [10, 13, 109]. Despite the apparent absence of SLC22A6, SLC22A7 and SLC22A8 in 6 BC cell lines, all were found in mRNA of human mammary tissue [60].
Is there a role for SLC transporters in breast cancer metastasis?
The role of SLC transporters for all metastatic cancers remains largely unclear and primarily un-investigated. It has been reported that SLCO2A1, SLCO3A1, SLCO4A1 and SLCO5A1 are all expressed at both the mRNA and protein levels in both cancerous and non-cancerous liver tissue, including expression in liver metastasis [48]. In addition, in a study of primary small cell lung cancer (SCLC), using cells and tissue from both normal lung tissue and from lung biopsies and metastases of untreated SCLC patients, it was observed that while SLCO4A1 was the most widely expressed of 11 SLC transporters investigated, SLCO5A1 was the most highly expressed of the OATPs in a cell line derived from an SCLC patient liver metastasis (DMS153). Moreover, this patient had been treated with the chemotherapeutic reagents cytoxan and methotrexate [110]. This small emerging body on patterns of expression of SLC transporters associated with metastatic cancer supports their potential to contribute to development of drug-resistant metastatic disease and implies they could be novel therapeutic targets.
Conclusion and next steps
There is enough preliminary evidence to suggest a potentially important role of solute transporters in BC. However, a comprehensive analysis of expression of all transport proteins in BC has yet to be carried out; once completed, it may be possible to paint a clearer picture of the roles of SLC family members, not only in the hormonal control of BC development but also as potential targets for hormonal and anticancer drug treatments. Functional experiments will subsequently help to establish definitive roles of SLC transporters in BC cells. Key to understanding their roles will be a better understanding of the interplay between SLC transporters, nuclear receptors, and ABC efflux transporters, which in turn will lead to the identification of targetable pathways to prevent progression to metastatic disease.
BC is a heterogeneous disease and expression profiling needs to take this into consideration. With a greater emphasis now on individualised therapies, this may be of significant importance in trying to reach that goal. In the future, genetic typing of transporter expression may even be used as a potential predictor of disease prognosis.
Abbreviations
- BC
Breast cancer
- ABC
ATP-binding cassette
- SLC
Solute carrier
- OATP
Organic anion–transporting peptide
- OAT
Organic anion transporter
- OCT
Organic cation transporter
- OCTN
Organic zwitterions/cation transporter
- E-3-S
Estrone-3-sulfate
- DHEA-S
Dehydroepiandrosterone sulfate
- IHC
Immunohistochemistry
- PXR
Pregnane X receptor
- WT
Wild type
- Gd-EOB-DTPA
Gadolinium-ethoxybenzyl diethylenetriamine pentaacetic acid
- MRI
Magnetic resonance imaging
- IF
Immunofluorescence
- IHC
Immunohistochemistry
- HCC
Hepatocellular carcinoma
Funding information
Rachel Sutherland is funded by the Women’s Cancer Detection Society, Queen Elizabeth Hospital, Gateshead, NE9 6SX, UK (grant number: BH175048).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rachel Sutherland, Email: r.sutherland2@newcastle.ac.uk.
Annette Meeson, Email: annette.meeson@newcastle.ac.uk.
Simon Lowes, Email: simon.lowes@doctors.org.uk.
References
- 1.Choi YH, Yu A-M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Current Pharmaceutical Design. 2014;20(5):793–807. doi: 10.2174/138161282005140214165212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hediger MA, Clémençon B, Burrier RE, Bruford EA. The ABCs of membrane transporters in health and disease (SLC series): Introduction. Molecular aspects of medicine. 2013;34(2–3):95–107. doi: 10.1016/j.mam.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Shu Y. Role of solute carriers in response to anticancer drugs. Molecular and cellular therapies. 2014;2:15. doi: 10.1186/2052-8426-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin L, Yee SW, Kim RB, Giacomini KM. SLC transporters as therapeutic targets: Emerging opportunities. Nature reviews Drug discovery. 2015;14(8):543–560. doi: 10.1038/nrd4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. British journal of pharmacology. 2012;165(5):1260–1287. doi: 10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagenbuch B, Stieger B. The SLCO (former SLC21) superfamily of transporters. Molecular Aspects of Medicine. 2013;34(2):396–412. doi: 10.1016/j.mam.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stieger B, Hagenbuch B. Organic anion-transporting polypeptides. Current topics in membranes. 2014;73:205–232. doi: 10.1016/B978-0-12-800223-0.00005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thakkar N, Lockhart AC, Lee W. Role of organic anion-transporting polypeptides (OATPs) in cancer therapy. The AAPS journal. 2015;17(3):535–545. doi: 10.1208/s12248-015-9740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anzai N, Kanai Y, Endou H. Organic anion transporter family: current knowledge. Journal of Pharmacological Sciences. 2006;100(5):411–426. doi: 10.1254/jphs.crj06006x. [DOI] [PubMed] [Google Scholar]
- 10.Koepsell H. The SLC22 family with transporters of organic cations, anions and zwitterions. Molecular Aspects of Medicine. 2013;34(2):413–435. doi: 10.1016/j.mam.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Nakanishi T, Tamai I. Solute carrier transporters as targets for drug delivery and pharmacological intervention for chemotherapy. Journal of Pharmaceutical Sciences. 2011;100(9):3731–3750. doi: 10.1002/jps.22576. [DOI] [PubMed] [Google Scholar]
- 12.Nigam SK, Bush KT, Martovetsky G, Ahn S-Y, Liu HC, Richard E, Bhatnagar V, Wu W. The organic anion transporter (OAT) family: A systems biology perspective. Physiological Reviews. 2015;95(1):83–123. doi: 10.1152/physrev.00025.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volk C. OCTs, OATs, and OCTNs: structure and function of the polyspecific organic ion transporters of the SLC22 family. Wiley Interdisciplinary Reviews: Membrane Transport and Signaling. 2014;3(1):1–13. [Google Scholar]
- 14.Obaidat A, Roth M, Hagenbuch B. The expression and function of organic anion transporting polypeptides in normal tissues and in cancer. Annual review of pharmacology and toxicology. 2012;52:135–151. doi: 10.1146/annurev-pharmtox-010510-100556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arakawa H, Nakanishi T, Yanagihara C, Nishimoto T, Wakayama T, Mizokami A, Namiki M, Kawai K, Tamai I. Enhanced expression of organic anion transporting polypeptides (OATPs) in androgen receptor-positive prostate cancer cells: possible role of OATP1A2 in adaptive cell growth under androgen-depleted conditions. Biochemical Pharmacology. 2012;84(8):1070–1077. doi: 10.1016/j.bcp.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 16.Ballestero MR, Monte MJ, Briz O, Jimenez F, Gonzalez-San Martin F, Marin JJ. Expression of transporters potentially involved in the targeting of cytostatic bile acid derivatives to colon cancer and polyps. Biochemical Pharmacology. 2006;72(6):729–738. doi: 10.1016/j.bcp.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Bronger H, Konig J, Kopplow K, Steiner HH, Ahmadi R, Herold-Mende C, et al. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Research. 2005;65(24):11419–11428. doi: 10.1158/0008-5472.CAN-05-1271. [DOI] [PubMed] [Google Scholar]
- 18.Kounnis V, Ioachim E, Svoboda M, Tzakos A, Sainis I, Thalhammer T, et al. Expression of organic anion-transporting polypeptides 1B3, 1B1, and 1A2 in human pancreatic cancer reveals a new class of potential therapeutic targets. OncoTargets and therapy. 2011;4:27–32. doi: 10.2147/OTT.S16706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liedauer R, Svoboda M, Wlcek K, Arrich F, Ja W, Toma C, et al. Different expression patterns of organic anion transporting polypeptides in osteosarcomas, bone metastases and aneurysmal bone cysts. Oncology reports. 2009;22(6):1485–1492. doi: 10.3892/or_00000591. [DOI] [PubMed] [Google Scholar]
- 20.Miki Y, Suzuki T, Kitada K, Yabuki N, Shibuya R, Moriya T, Ishida T, Ohuchi N, Blumberg B, Sasano H. Expression of the steroid and xenobiotic receptor and its possible target gene, organic anion transporting polypeptide-A, in human breast carcinoma. Cancer Research. 2006;66(1):535–542. doi: 10.1158/0008-5472.CAN-05-1070. [DOI] [PubMed] [Google Scholar]
- 21.Zolk O, Schnepf R, Muschler M, Fromm MF, Wendler O, Traxdorf M, Iro H, Zenk J. Transporter gene expression in human head and neck squamous cell carcinoma and associated epigenetic regulatory mechanisms. The American journal of pathology. 2013;182(1):234–243. doi: 10.1016/j.ajpath.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Meyer zu Schwabedissen HE, Tirona RG, Yip CS, Ho RH, Kim RB. Interplay between the nuclear receptor pregnane X receptor and the uptake transporter organic anion transporter polypeptide 1A2 selectively enhances estrogen effects in breast cancer. Cancer Research. 2008;68(22):9338–9347. doi: 10.1158/0008-5472.CAN-08-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svoboda M, Wlcek K, Taferner B, Hering S, Stieger B, Tong D, et al. Expression of organic anion-transporting polypeptides 1B1 and 1B3 in ovarian cancer cells: relevance for paclitaxel transport. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2011;65(6):417–26. [DOI] [PubMed]
- 24.Wright JL, Kwon EM, Ostrander EA, Montgomery RB, Lin DW, Vessella R, Stanford JL, Mostaghel EA. Expression of SLCO transport genes in castration-resistant prostate cancer and impact of genetic variation in SLCO1B3 and SLCO2B1 on prostate cancer outcomes. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(4):619–627. doi: 10.1158/1055-9965.EPI-10-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamada A, Sissung T, Price DK, Danesi R, Chau CH, Sharifi N, Venzon D, Maeda K, Nagao K, Sparreboom A, Mitsuya H, Dahut WL, Figg WD. Effect of SLCO1B3 haplotype on testosterone transport and clinical outcome in caucasian patients with androgen-independent prostatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(11):3312–3318. doi: 10.1158/1078-0432.CCR-07-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee W, Belkhiri A, Lockhart AC, Merchant N, Glaeser H, Harris EI, Washington MK, Brunt EM, Zaika A, Kim RB, el-Rifai W. Overexpression of OATP1B3 confers apoptotic resistance in colon cancer. Cancer Research. 2008;68(24):10315–10323. doi: 10.1158/0008-5472.CAN-08-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monks NR, Liu S, Xu Y, Yu H, Bendelow AS, Moscow JA. Potent cytotoxicity of the phosphatase inhibitor microcystin LR and microcystin analogues in OATP1B1- and OATP1B3-expressing HeLa cells. Molecular Cancer Therapeutics. 2007;6(2):587–598. doi: 10.1158/1535-7163.MCT-06-0500. [DOI] [PubMed] [Google Scholar]
- 28.Muto M, Onogawa T, Suzuki T, Ishida T, Rikiyama T, Katayose Y, Ohuchi N, Sasano H, Abe T, Unno M. Human liver-specific organic anion transporter-2 is a potent prognostic factor for human breast carcinoma. Cancer science. 2007;98(10):1570–1576. doi: 10.1111/j.1349-7006.2007.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pressler H, Sissung TM, Venzon D, Price DK, Figg WD. Expression of OATP family members in hormone-related cancers: potential markers of progression. PLoS One. 2011;6(5):e20372. doi: 10.1371/journal.pone.0020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svoboda M, Riha J, Wlcek K, Jaeger W, Thalhammer T. Organic anion transporting polypeptides (OATPs): regulation of expression and function. Current drug metabolism. 2011;12(2):139–153. doi: 10.2174/138920011795016863. [DOI] [PubMed] [Google Scholar]
- 31.Thakkar N, Kim K, Jang ER, Han S, Kim K, Kim D, Merchant N, Lockhart AC, Lee W. A cancer-specific variant of the SLCO1B3 gene encodes a novel human organic anion transporting polypeptide 1B3 (OATP1B3) localized mainly in the cytoplasm of colon and pancreatic cancer cells. Molecular Pharmaceutics. 2013;10(1):406–416. doi: 10.1021/mp3005353. [DOI] [PubMed] [Google Scholar]
- 32.Hays A, Apte U, Hagenbuch B. Organic anion transporting polypeptides expressed in pancreatic cancer may serve as potential diagnostic markers and therapeutic targets for early stage adenocarcinomas. Pharmaceutical Research. 2013;30(9):2260–2269. doi: 10.1007/s11095-012-0962-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui Y, Konig J, Nies AT, Pfannschmidt M, Hergt M, Franke WW, et al. Detection of the human organic anion transporters SLC21A6 (OATP2) and SLC21A8 (OATP8) in liver and hepatocellular carcinoma. Laboratory investigation; a journal of technical methods and pathology. 2003;83(4):527–538. doi: 10.1097/01.lab.0000065015.02412.48. [DOI] [PubMed] [Google Scholar]
- 34.Kinoshita M, Miyata M. Underexpression of mRNA in human hepatocellular carcinoma focusing on eight loci. Hepatology. 2002;36(2):433–438. doi: 10.1053/jhep.2002.34851. [DOI] [PubMed] [Google Scholar]
- 35.Vavricka SR, Jung D, Fried M, Grützner U, Meier PJ, Kullak-Ublick GA. The human organic anion transporting polypeptide 8 (SLCO1B3) gene is transcriptionally repressed by hepatocyte nuclear factor 3β in hepatocellular carcinoma. Journal of Hepatology. 2004;40(2):212–218. doi: 10.1016/j.jhep.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Zollner G, Wagner M, Fickert P, Silbert D, Fuchsbichler A, Zatloukal K, Denk H, Trauner M. Hepatobiliary transporter expression in human hepatocellular carcinoma. Liver international : official journal of the International Association for the Study of the Liver. 2005;25(2):367–379. doi: 10.1111/j.1478-3231.2005.01033.x. [DOI] [PubMed] [Google Scholar]
- 37.Nagai M, Furihata T, Matsumoto S, Ishii S, Motohashi S, Yoshino I, Ugajin M, Miyajima A, Matsumoto S, Chiba K. Identification of a new organic anion transporting polypeptide 1B3 mRNA isoform primarily expressed in human cancerous tissues and cells. Biochemical and biophysical research communications. 2012;418(4):818–823. doi: 10.1016/j.bbrc.2012.01.115. [DOI] [PubMed] [Google Scholar]
- 38.Kindla J, Rau TT, Jung R, Fasching PA, Strick R, Stoehr R, Hartmann A, Fromm MF, König J. Expression and localization of the uptake transporters OATP2B1, OATP3A1 and OATP5A1 in non-malignant and malignant breast tissue. Cancer biology & therapy. 2011;11(6):584–591. doi: 10.4161/cbt.11.6.14533. [DOI] [PubMed] [Google Scholar]
- 39.Bleasby K, Castle JC, Roberts CJ, Cheng C, Bailey WJ, Sina JF, Kulkarni AV, Hafey MJ, Evers R, Johnson JM, Ulrich RG, Slatter JG. Expression profiles of 50 xenobiotic transporter genes in humans and pre-clinical species: a resource for investigations into drug disposition. Xenobiotica. 2006;36(10–11):963–988. doi: 10.1080/00498250600861751. [DOI] [PubMed] [Google Scholar]
- 40.Banerjee N, Allen C, Bendayan R. Differential role of organic anion-transporting polypeptides in estrone-3-Sulphate uptake by breast epithelial cells and breast cancer cells. Journal of Pharmacology and Experimental Therapeutics. 2012;342(2):510–519. doi: 10.1124/jpet.112.192344. [DOI] [PubMed] [Google Scholar]
- 41.Maeda T, Irokawa M, Arakawa H, Kuraoka E, Nozawa T, Tateoka R, Itoh Y, Nakanishi T, Tamai I. Uptake transporter organic anion transporting polypeptide 1B3 contributes to the growth of estrogen-dependent breast cancer. The Journal of steroid biochemistry and molecular biology. 2010;122(4):180–185. doi: 10.1016/j.jsbmb.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Iusuf, D., Hendrikx, J. J., van Esch, A., van de Steeg, E., Wagenaar, E., Rosing, H., et al. (2015). Human OATP1B1, OATP1B3 and OATP1A2 can mediate the in vivo uptake and clearance of docetaxel. International journal of cancer., 136(1), 225–233. [DOI] [PubMed]
- 43.Leonhardt M, Keiser M, Oswald S, Kühn J, Jia J, Grube M, Kroemer HK, Siegmund W, Weitschies W. Hepatic uptake of the magnetic resonance imaging contrast agent Gd-EOB-DTPA: Role of human organic anion transporters. Drug Metabolism and Disposition. 2010;38(7):1024–1028. doi: 10.1124/dmd.110.032862. [DOI] [PubMed] [Google Scholar]
- 44.van de Steeg E, van Esch A, Wagenaar E, Kenworthy KE, Schinkel AH. Influence of human OATP1B1, OATP1B3, and OATP1A2 on the pharmacokinetics of methotrexate and paclitaxel in humanized transgenic mice. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(4):821–832. doi: 10.1158/1078-0432.CCR-12-2080. [DOI] [PubMed] [Google Scholar]
- 45.Pizzagalli F, Hagenbuch B, Stieger B, Klenk U, Folkers G, Meier PJ. Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter. Molecular endocrinology (Baltimore, Md) 2002;16(10):2283–2296. doi: 10.1210/me.2001-0309. [DOI] [PubMed] [Google Scholar]
- 46.Hagenbuch B. Cellular entry of thyroid hormones by organic anion transporting polypeptides. Best Practice & Research Clinical Endocrinology & Metabolism. 2007;21(2):209–221. doi: 10.1016/j.beem.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Holla VR, Backlund MG, Yang P, Newman RA, DuBois RN. Regulation of prostaglandin transporters in colorectal neoplasia. Cancer prevention research (Philadelphia, Pa) 2008;](2):93–99. doi: 10.1158/1940-6207.CAPR-07-0009. [DOI] [PubMed] [Google Scholar]
- 48.Wlcek K, Svoboda M, Riha J, Zakaria S, Olszewski U, Dvorak Z, Sellner F, Ellinger I, Jäger W, Thalhammer T. The analysis of organic anion transporting polypeptide (OATP) mRNA and protein patterns in primary and metastatic liver cancer. Cancer biology & therapy. 2011;11(9):801–811. doi: 10.4161/cbt.11.9.15176. [DOI] [PubMed] [Google Scholar]
- 49.Wlcek K, Svoboda M, Thalhammer T, Sellner F, Krupitza G, Jaeger W. Altered expression of organic anion transporter polypeptide (OATP) genes in human breast carcinoma. Cancer biology & therapy. 2008;7(9):1450–1455. doi: 10.4161/cbt.7.9.6282. [DOI] [PubMed] [Google Scholar]
- 50.Kochel TJ, Goloubeva OG, Fulton AM. Upregulation of cyclooxygenase-2/prostaglandin E2 (COX-2/PGE2) pathway member multiple drug resistance-associated protein 4 (MRP4) and downregulation of prostaglandin transporter (PGT) and 15-prostaglandin dehydrogenase (15-PGDH) in triple-negative breast cancer. Breast cancer : basic and clinical research. 2016;10:61–70. doi: 10.4137/BCBCR.S38529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. The Journal of pharmacology and experimental therapeutics. 2004;308(2):438–445. doi: 10.1124/jpet.103.060194. [DOI] [PubMed] [Google Scholar]
- 52.Al Sarakbi W, Mokbel R, Salhab M, Jiang WG, Reed MJ, Mokbel K. The role of STS and OATP-B mRNA expression in predicting the clinical outcome in human breast cancer. Anticancer Research. 2006;26(6c):4985–4990. [PubMed] [Google Scholar]
- 53.Pizzagalli F, Varga Z, Huber RD, Folkers G, Meier PJ, St-Pierre MV. Identification of steroid sulfate transport processes in the human mammary gland. The Journal of clinical endocrinology and metabolism. 2003;88(8):3902–3912. doi: 10.1210/jc.2003-030174. [DOI] [PubMed] [Google Scholar]
- 54.Stute, P., Reichenbach, A., Szuwart, T., Kiesel, L., & Gotte, M. (2012). Impact of testosterone on the expression of organic anion transporting polypeptides (OATP-1A2, OATP-2B1, OATP-3A1) in malignant and non-malignant human breast cells in vitro. Maturitas., 71(4), 376–384. [DOI] [PubMed]
- 55.Hirano M, Maeda K, Shitara Y, Sugiyama Y. Drug-drug interaction between pitavastatin and various drugs via OATP1B1. Drug metabolism and disposition: the biological fate of chemicals. 2006;34(7):1229–1236. doi: 10.1124/dmd.106.009290. [DOI] [PubMed] [Google Scholar]
- 56.Nozawa T, Suzuki M, Takahashi K, Yabuuchi H, Maeda T, Tsuji A, Tamai I. Involvement of estrone-3-sulfate transporters in proliferation of hormone-dependent breast cancer cells. The Journal of pharmacology and experimental therapeutics. 2004;311(3):1032–1037. doi: 10.1124/jpet.104.071522. [DOI] [PubMed] [Google Scholar]
- 57.Tamai I, J-i N, Uchino H, Sai Y, Oku A, Shimane M, et al. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochemical and biophysical research communications. 2000;273(1):251–260. doi: 10.1006/bbrc.2000.2922. [DOI] [PubMed] [Google Scholar]
- 58.Huber RD, Gao B, Sidler Pfandler MA, Zhang-Fu W, Leuthold S, Hagenbuch B, et al. Characterization of two splice variants of human organic anion transporting polypeptide 3A1 isolated from human brain. American journal of physiology Cell physiology. 2007;292(2):C795–C806. doi: 10.1152/ajpcell.00597.2005. [DOI] [PubMed] [Google Scholar]
- 59.Nozawa T, Suzuki M, Yabuuchi H, Irokawa M, Tsuji A, Tamai I. Suppression of cell proliferation by inhibition of estrone-3-sulfate transporter in estrogen-dependent breast cancer cells. Pharmaceutical Research. 2005;22(10):1634–1641. doi: 10.1007/s11095-005-7096-0. [DOI] [PubMed] [Google Scholar]
- 60.Okabe M, Szakács G, Reimers MA, Suzuki T, Hall MD, Abe T, et al. Profiling SLCO and SLC22 genes in the NCI-60 cancer cell lines to identify drug uptake transporters. Molecular cancer therapeutics. 2008;7(9):3081–3091. doi: 10.1158/1535-7163.MCT-08-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuei-Ling K. Localization and functional characterization of the rat Oatp4c1 transporter in an in vitro cell system and rat tissues [doctor of philosophy (PhD)] UKnowledge: University of Kentucky; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olszewski-Hamilton U, Svoboda M, Thalhammer T, Buxhofer-Ausch V, Geissler K, Hamilton G. Organic anion transporting polypeptide 5A1 (OATP5A1) in small cell lung Cancer (SCLC) cells: Possible involvement in chemoresistance to satraplatin. Biomarkers in cancer. 2011;3:31–40. doi: 10.4137/BIC.S7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee SY, Williamson B, Caballero OL, Chen YT, Scanlan MJ, Ritter G, et al. Identification of the gonad-specific anion transporter SLCO6A1 as a cancer/testis (CT) antigen expressed in human lung cancer. Cancer immunity. 2004;4:13. [PubMed] [Google Scholar]
- 64.Oba-Shinjo SM, Caballero OL, Jungbluth AA, Rosemberg S, Old LJ, Simpson AJG, et al. Cancer-testis (CT) antigen expression in medulloblastoma. Cancer immunity. 2008;8:7. [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang L, Dresser MJ, Gray AT, Yost SC, Terashita S, Giacomini KM. Cloning and functional expression of a human liver organic cation transporter. Molecular Pharmacology. 1997;51(6):913–921. doi: 10.1124/mol.51.6.913. [DOI] [PubMed] [Google Scholar]
- 66.Nies AT, Koepsell H, Winter S, Burk O, Klein K, Kerb R, Zanger UM, Keppler D, Schwab M, Schaeffeler E. Expression of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology. 2009;50(4):1227–1240. doi: 10.1002/hep.23103. [DOI] [PubMed] [Google Scholar]
- 67.Nies, A. T., Koepsell, H., Damme, K., & Schwab, M. (2011). Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handbook of experimental pharmacology., 201, 105–167. [DOI] [PubMed]
- 68.Gupta S, Wulf G, Henjakovic M, Koepsell H, Burckhardt G, Hagos Y. Human organic cation transporter 1 is expressed in lymphoma cells and increases susceptibility to irinotecan and paclitaxel. The Journal of pharmacology and experimental therapeutics. 2012;341(1):16–23. doi: 10.1124/jpet.111.190561. [DOI] [PubMed] [Google Scholar]
- 69.Thomas J, Wang L, Clark RE, Pirmohamed M. Active transport of imatinib into and out of cells: Implications for drug resistance. Blood. 2004;104(12):3739–3745. doi: 10.1182/blood-2003-12-4276. [DOI] [PubMed] [Google Scholar]
- 70.Zhang S, Lovejoy KS, Shima JE, Lagpacan LL, Shu Y, Lapuk A, Chen Y, Komori T, Gray JW, Chen X, Lippard SJ, Giacomini KM. Organic cation transporters are determinants of oxaliplatin cytotoxicity. Cancer Research. 2006;66(17):8847–8857. doi: 10.1158/0008-5472.CAN-06-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clinical pharmacology and therapeutics. 2009;86(4):396–402. doi: 10.1038/clpt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hayer-Zillgen M, Brüss M, Bönisch H. Expression and pharmacological profile of the human organic cation transporters hOCT1, hOCT2 and hOCT3. British journal of pharmacology. 2002;136(6):829–836. doi: 10.1038/sj.bjp.0704785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burger H, Zoumaro-Djayoon A, Boersma AWM, Helleman J, Berns EMJJ, Mathijssen RHJ, Loos WJ, Wiemer EAC. Differential transport of platinum compounds by the human organic cation transporter hOCT2 (hSLC22A2) British journal of pharmacology. 2010;159(4):898–908. doi: 10.1111/j.1476-5381.2009.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu X, Huang W, Ganapathy ME, Wang H, Kekuda R, Conway SJ, Leibach FH, Ganapathy V. Structure, function, and regional distribution of the organic cation transporter OCT3 in the kidney. American journal of physiology Renal physiology. 2000;279(3):F449–F458. doi: 10.1152/ajprenal.2000.279.3.F449. [DOI] [PubMed] [Google Scholar]
- 75.Heise M, Lautem A, Knapstein J, Schattenberg JM, Hoppe-Lotichius M, Foltys D, Weiler N, Zimmermann A, Schad A, Gründemann D, Otto G, Galle PR, Schuchmann M, Zimmermann T. Downregulation of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) in human hepatocellular carcinoma and their prognostic significance. BMC Cancer. 2012;12(1):109. doi: 10.1186/1471-2407-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yokoo S, Masuda S, Yonezawa A, Terada T, Katsura T, Inui K. Significance of organic cation transporter 3 (SLC22A3) expression for the cytotoxic effect of oxaliplatin in colorectal cancer. Drug metabolism and disposition: the biological fate of chemicals. 2008;36(11):2299–2306. doi: 10.1124/dmd.108.023168. [DOI] [PubMed] [Google Scholar]
- 77.Shnitsar V, Eckardt R, Gupta S, Grottker J, Muller GA, Koepsell H, et al. Expression of human organic cation transporter 3 in kidney carcinoma cell lines increases chemosensitivity to melphalan, irinotecan, and vincristine. Cancer Research. 2009;69(4):1494–1501. doi: 10.1158/0008-5472.CAN-08-2483. [DOI] [PubMed] [Google Scholar]
- 78.Alcorn J, Lu X, Moscow JA, McNamara PJ. Transporter gene expression in lactating and nonlactating human mammary epithelial cells using real-time reverse transcription-polymerase chain reaction. Journal of Pharmacology and Experimental Therapeutics. 2002;303(2):487–496. doi: 10.1124/jpet.102.038315. [DOI] [PubMed] [Google Scholar]
- 79.Cai H, Zhang Y, Han T, Everett RS, Thakker DR. Cation-selective transporters are critical to the AMPK-mediated antiproliferative effects of metformin in human breast cancer cells. International journal of cancer. 2016;138(9):2281–2292. doi: 10.1002/ijc.29965. [DOI] [PubMed] [Google Scholar]
- 80.Tamai I, Yabuuchi H, Nezu J, Sai Y, Oku A, Shimane M, Tsuji A. Cloning and characterization of a novel human pH-dependent organic cation transporter, OCTN1. FEBS letters. 1997;419(1):107–111. doi: 10.1016/s0014-5793(97)01441-5. [DOI] [PubMed] [Google Scholar]
- 81.Jong NN, McKeage MJ. Emerging roles of metal solute carriers in cancer mechanisms and treatment. Biopharmaceutics & Drug Disposition. 2014;35(8):450–462. doi: 10.1002/bdd.1903. [DOI] [PubMed] [Google Scholar]
- 82.Tamai I, Ohashi R, Nezu J, Yabuuchi H, Oku A, Shimane M, Sai Y, Tsuji A. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. The Journal of biological chemistry. 1998;273(32):20378–20382. doi: 10.1074/jbc.273.32.20378. [DOI] [PubMed] [Google Scholar]
- 83.Scalise M, Galluccio M, Accardi R, Cornet I, Tommasino M, Indiveri C. Human OCTN2 (SLC22A5) is down-regulated in virus- and nonvirus-mediated cancer. Cell biochemistry and function. 2012;30(5):419–425. doi: 10.1002/cbf.2816. [DOI] [PubMed] [Google Scholar]
- 84.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. The Journal of general physiology. 1927;8(6):519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu S, Franke RM, Filipski KK, Hu C, Orwick SJ, de Bruijn EA, Burger H, Baker SD, Sparreboom A. Interaction of imatinib with human organic ion carriers. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(10):3141–3148. doi: 10.1158/1078-0432.CCR-07-4913. [DOI] [PubMed] [Google Scholar]
- 86.Jong NN, Nakanishi T, Liu JJ, Tamai I, McKeage MJ. Oxaliplatin transport mediated by organic cation/carnitine transporters OCTN1 and OCTN2 in overexpressing human embryonic kidney 293 cells and rat dorsal root ganglion neurons. The Journal of pharmacology and experimental therapeutics. 2011;338(2):537–547. doi: 10.1124/jpet.111.181297. [DOI] [PubMed] [Google Scholar]
- 87.Ling B, Alcorn J. Acute administration of cefepime lowers L-carnitine concentrations in early lactation stage rat milk. The Journal of nutrition. 2008;138(7):1317–1322. doi: 10.1093/jn/138.7.1317. [DOI] [PubMed] [Google Scholar]
- 88.Wang C, Uray IP, Mazumdar A, Mayer JA, Brown PH. SLC22A5/OCTN2 expression in breast cancer is induced by estrogen via a novel intronic estrogen-response element (ERE) Breast Cancer Research and Treatment. 2012;134(1):101–115. doi: 10.1007/s10549-011-1925-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koepsell H, Endou H. The SLC22 drug transporter family. Pflügers Archiv. 2004;447(5):666–676. doi: 10.1007/s00424-003-1089-9. [DOI] [PubMed] [Google Scholar]
- 90.Gong S, Lu X, Xu Y, Swiderski CF, Jordan CT, Moscow JA. Identification of OCT6 as a novel organic cation transporter preferentially expressed in hematopoietic cells and leukemias. Experimental Hematology. 2002;30(10):1162–1169. doi: 10.1016/s0301-472x(02)00901-3. [DOI] [PubMed] [Google Scholar]
- 91.Okabe M, Unno M, Harigae H, Kaku M, Okitsu Y, Sasaki T, Mizoi T, Shiiba K, Takanaga H, Terasaki T, Matsuno S, Sasaki I, Ito S, Abe T Characterization of the organic cation transporter SLC22A16: a doxorubicin importer. Biochemical and biophysical research communications. 2005;333(3):754–762. [DOI] [PubMed]
- 92.Aouida M, Poulin R, Ramotar D. The human carnitine transporter SLC22A16 mediates high affinity uptake of the anticancer polyamine analogue bleomycin-A5. The Journal of biological chemistry. 2010;285(9):6275–6284. doi: 10.1074/jbc.M109.046151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nishino S, Itoh A, Matsuoka H, Maeda K, Kamoshida S. Immunohistochemical analysis of organic anion transporter 2 and reduced folate carrier 1 in colorectal cancer: Significance as a predictor of response to oral uracil/ftorafur plus leucovorin chemotherapy. Molecular and clinical oncology. 2013;1(4):661–667. doi: 10.3892/mco.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sweet DH. Organic anion transporter (Slc22a) family members as mediators of toxicity. Toxicology and Applied Pharmacology. 2005;204(3):198–215. doi: 10.1016/j.taap.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 95.Enomoto A, Endou H. Roles of organic anion transporters (OATs) and a urate transporter (URAT1) in the pathophysiology of human disease. Clinical and Experimental Nephrology. 2005;9(3):195–205. doi: 10.1007/s10157-005-0368-5. [DOI] [PubMed] [Google Scholar]
- 96.Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Ho Cha S, Hosoyamada M, Takeda M, Sekine T, Igarashi T, Matsuo H, Kikuchi Y, Oda T, Ichida K, Hosoya T, Shimokata K, Niwa T, Kanai Y, Endou H. Molecular identification of a renal urate–anion exchanger that regulates blood urate levels. Nature. 2002;417(6887):447–452. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 97.Monte JC, Nagle MA, Eraly SA, Nigam SK. Identification of a novel murine organic anion transporter family member, OAT6, expressed in olfactory mucosa. Biochemical and biophysical research communications. 2004;323(2):429–436. doi: 10.1016/j.bbrc.2004.08.112. [DOI] [PubMed] [Google Scholar]
- 98.Wu W, Bush KT, Liu HC, Zhu C, Abagyan R, Nigam SK. Shared ligands between organic anion transporters (OAT1 and OAT6) and odorant receptors. Drug metabolism and disposition: the biological fate of chemicals. 2015;43(12):1855–1863. doi: 10.1124/dmd.115.065250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schnabolk GW, Youngblood GL, Sweet DH. Transport of estrone sulfate by the novel organic anion transporter Oat6 (Slc22a20) American journal of physiology Renal physiology. 2006;291(2):F314–FF21. doi: 10.1152/ajprenal.00497.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zimmerman EI, Gibson AA, Hu S, Vasilyeva A, Orwick SJ, Du G, et al. Multikinase inhibitors induce cutaneous toxicity through OAT6-mediated uptake and MAP3K7-driven cell death. Cancer Research. 2016;76(1):117–126. doi: 10.1158/0008-5472.CAN-15-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sauter ER. Reliable biomarkers to identify new and recurrent cancer. European journal of breast health. 2017;13(4):162–167. doi: 10.5152/ejbh.2017.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karakus E, Zahner D, Grosser G, Leidolf R, Gundogdu C, Sánchez-Guijo A, et al. Estrone-3-sulfate stimulates the proliferation of T47D breast cancer cells stably transfected with the sodium-dependent organic anion transporter SOAT (SLC10A6). Frontiers in pharmacology. 2018;9:941-. [DOI] [PMC free article] [PubMed]
- 103.Suzuki T, Miki Y, Nakamura Y, Moriya T, Ito K, Ohuchi N, Sasano H. Sex steroid-producing enzymes in human breast cancer. Endocrine-related cancer. 2005;12(4):701–720. doi: 10.1677/erc.1.00834. [DOI] [PubMed] [Google Scholar]
- 104.Zhang H, Rakha EA, Ball GR, Spiteri I, Aleskandarany M, Paish EC, Powe DG, Macmillan RD, Caldas C, Ellis IO, Green AR. The proteins FABP7 and OATP2 are associated with the basal phenotype and patient outcome in human breast cancer. Breast Cancer Research and Treatment. 2010;121(1):41–51. doi: 10.1007/s10549-009-0450-x. [DOI] [PubMed] [Google Scholar]
- 105.Muto M, Onogawa T, Suzuki T, Ishida T, Rikiyama T, Katayose Y, Ohuchi N, Sasano H, Abe T, Unno M. Human liver-specific organic anion transporter-2 is a potent prognostic factor for human breast carcinoma. Cancer science. 2007;98(10):1570–1576. doi: 10.1111/j.1349-7006.2007.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Justenhoven C, Schaeffeler E, Winter S, Baisch C, Hamann U, Harth V, Rabstein S, Spickenheuer A, Pesch B, Brüning T, Ko YD, Schwab M, Brauch H. Polymorphisms of the nuclear receptor pregnane X receptor and organic anion transporter polypeptides 1A2, 1B1, 1B3, and 2B1 are not associated with breast cancer risk. Breast Cancer Research and Treatment. 2011;125(2):563–569. doi: 10.1007/s10549-010-1046-1. [DOI] [PubMed] [Google Scholar]
- 107.Zhang X, Pu Z, Ge J, Shen J, Yuan X, Xie H. Association of CYP2D6*10, OATP1B1 A388G, and OATP1B1 T521C polymorphisms and overall survival of breast cancer patients after tamoxifen therapy. Medical science monitor : international medical journal of experimental and clinical research. 2015;21:563–569. doi: 10.12659/MSM.893473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hashimoto Y, Tatsumi S, Takeda R, Naka A, Ogane N, Kameda Y, Kawachi K, Shimizu S, Sakai M, Kamoshida S. Expression of organic anion-transporting polypeptide 1A2 and organic cation transporter 6 as a predictor of pathologic response to neoadjuvant chemotherapy in triple negative breast cancer. Breast Cancer Research and Treatment. 2014;145(1):101–111. doi: 10.1007/s10549-014-2913-y. [DOI] [PubMed] [Google Scholar]
- 109.Burckhardt G. Drug transport by organic anion transporters (OATs) Pharmacology & Therapeutics. 2012;136(1):106–130. doi: 10.1016/j.pharmthera.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 110.Brenner S, Klameth L, Riha J, Schölm M, Hamilton G, Bajna E, et al. (2015) Specific expression of OATPs in primary small cell lung cancer (SCLC) cells as novel biomarkers for diagnosis and therapy. Cancer Letters. 356(2, Part B):517–24. [DOI] [PubMed]