Abstract
Objective
There is rising interest in digital health in preventive cardiology, particularly for blood pressure (BP) management. In a digital health study of early BP assessment following acute myocardial infarction (AMI), we sought to examine feasibility and the (1) proportion of post-AMI patients with controlled BP and hypotension, and (2) association between prior cardiovascular disease (CVD) and BP post-AMI.
Methods
In this substudy of the parent Myocardial infarction, COmbined-device, Recovery Enhancement (MiCORE) study, type 1 AMI patients were enrolled between October 2017 and April 2019. Participants self-monitored their BP through 30 days after hospital discharge using an FDA-approved wireless BP monitor connected with a smartphone application. Linear mixed-effects models assessed the association between prior CVD and BP trajectory post-discharge, adjusting for antihypertensive medications and a propensity score inclusive of CVD risk factors.
Results
Sixty-eight AMI patients (mean age 58 ± 10 years, 75% male, 68% white race, 68% history of hypertension, 24% prior CVD) provided 2638 measurements over 30 days. The percentage of BP control <130/80 mmHg was 59.6% (95% CI: 54.3–64.9%) and <140/90 mmHg was 83.7% (95% CI: 80.3–87.2%). The percentage of systolic BP <90 mmHg was 1.1% (95% CI: 0.17–2.0%) and the percentage of diastolic BP <60 mmHg was 3.9% (95% CI: 2.6–5.2%). Prior CVD was associated with 12.2 mmHg higher mean daily systolic BP during admission (95% CI: 3.5–20.9 mmHg), which persisted over follow-up. There was no association between prior CVD and diastolic BP.
Conclusion
The digital health program was feasible and ~40% of post-AMI patients who engaged in it had uncontrolled BP according to recent guideline cutpoints, while hypotension occurred rarely. The gap in BP control was especially large in patients in whom AMI represented recurrent CVD. These data suggest an opportunity for more aggressive secondary prevention early after MI as care models integrate digital health.
Keywords: Blood pressure, Myocardial infarction, Secondary prevention, Digital health, Smartphone
Highlights
-
•
Digital health reveals home blood pressure trends during early recovery after an event.
-
•
~40% of early MI patients had mean daily blood pressure exceeding the guideline goal of <130/80 mmHg.
-
•
Hypotension occurred rarely over 30 days post-MI.
-
•
The gap in BP control was especially large in patients in whom MI represented recurrent CVD.
-
•
There is opportunity for more aggressive secondary prevention early after MI as care models integrate digital health.
1. Introduction
Every year in the United States, approximately 605,000 individuals experience their first acute myocardial infarction (AMI), and about 200,000 suffer recurrent AMIs [1]. Secondary prevention strategies, which include smoking cessation, adherence to cardioprotective medications, cardiac rehabilitation, and management of weight, lipids, diabetes, and blood pressure (BP), reduce the recurrence of cardiovascular events [2]. BP monitoring is one important component of management following AMI, and self-monitored BP has potential to improve the diagnosis and management of hypertension [3]. The 2011 American Heart Association/American College of Cardiology (AHA/ACC) secondary prevention guidelines for patients with atherosclerotic cardiovascular disease (CVD) emphasized BP control to <140 mmHg systolic and <90 mmHg diastolic [2]. The 2017 AHA/ACC Clinical Practice Guidelines for hypertension lowered the BP goal to <130 mmHg systolic and <80 mmHg diastolic [4,5], The early post-discharge period is a particularly vulnerable time, in which patients transition from having inpatient vitals monitored multiple times a day with close titration of antihypertensives to outpatient care with fewer assessments of vitals and adjustment of antihypertensives. Digital health interventions (DHIs) have shown early potential to increase patient adherence to guideline-based therapies and attainment of BP targets, allowing for collection of more ambulatory data to assess whether treatment goals are met [6,7]. Given the importance of secondary prevention in reducing AMI recurrence, we hypothesized that there would be differences in early BP trajectories between participants with and without CVD prior to the index AMI. Greater atherosclerotic burden in recurrent CVD may contribute to these differences. In this feasibility study, we investigated BP patterns among AMI patients who self-monitored their BP using Corrie, a DHI that educates patients on secondary prevention and facilitates BP monitoring and medication tracking, over 30 days post-hospital discharge. In this descriptive analysis of post-AMI patients, we examined the (1) prevalence of controlled BP as defined by 2017 and 2011 AHA/ACC guidelines [2,4], (2) prevalence of systolic hypotension (<90 mmHg) or diastolic hypotension (<60 mmHg) [8,9], and (3) associations between prior CVD and BP during admission and trajectories after discharge.
2. Methods
This analysis included a subset of participants enrolled in the Myocardial infarction, Combined-device, Recovery Enhancement (MiCORE) study, which has been previously described [10], who engaged with the Corrie Health digital platform and FDA-approved iHealth Bluetooth BP monitor. In brief, MiCORE was a multicenter, prospective study with a historical comparison group, which aimed to assess Corrie’s feasibility, usability, and effectiveness in reducing 30-day readmissions after AMI (NCT03760796). Patients admitted to Johns Hopkins Hospital, Johns Hopkins Bayview Medical Center, Massachusetts General Hospital, and Reading Hospital for an ST elevation myocardial infarction (STEMI) or Type 1 non ST-elevation myocardial infarction (NSTEMI) were enrolled with the Corrie intervention during admission and were followed for 30 days post-discharge from the hospital. Inclusion criteria in addition to type 1 AMI were: (1) 18 years or older, (2) owned a smartphone, and (3) approved to participate by their inpatient care team. Exclusion criteria were: (1) non-English speaking, (2) had a visual, auditory, cognitive, or motor impairment that would interfere with smartphone usage, or (3) hemodynamically unstable. To reduce selection bias, participants who did not own an iPhone were given a loaner phone to use for the duration of the study [11,12]. Data exclusion criteria for this sub-study analysis were: (1) BP values outside of the manufacturer-specified measurement ranges of 60–260 mmHg systolic BP or 40–199 mmHg diastolic BP [13], (2) participants who were not given an iHealth BP monitor (due to participation during the pilot phase or lack of cuff availability in appropriate size) to minimize erroneous values [10], and (3) participants who only recorded BP values on a single day that would preclude assessment of trends over time. Ethical approval was obtained from the Johns Hopkins University School of Medicine Institutional Review Board and other participating sites. All participants provided informed consent.
The Corrie Health digital platform integrated with the FDA-approved iHealth Bluetooth BP monitor (iHealth Lab Inc., BP3L), which allowed participants to monitor, save, and review BP recordings in the hospital and post-discharge with Corrie. A description of the Corrie smartphone application (app) and screenshots are available in the Supplemental Figure. The app provided participants the opportunity to check their blood pressure during their hospitalization and at home via self-monitoring. At the time of enrollment, patients were instructed to input their vitals regularly (preferably daily), but the frequency and timing of the self-monitored BPs were left to their own discretion. During the enrollment process, participants had their arm circumference measured and were given the iHealth BP3L monitor with the appropriately sized cuff (standard 22–36 cm, large 30–42 cm, extra-large 42–48 cm). A research team member assisted the participant with setup and device pairing and trained the participant on the proper technique for BP self-measurement, which included taking the measurement at a seated position with the arm supported and legs uncrossed. Participants were also instructed to relax for 5 min prior and avoid caffeine. Time-stamped BP measurements were exported from the backend at the completion of the MiCORE study. The first 60 participants were enrolled during the pilot phase of MiCORE (October 1, 2016–September 30, 2017) and were not provided BP monitors. The remaining 140 participants were enrolled during the prospective phase (October 1, 2017–April 12, 2019), during which BP monitors were available. The MiCORE study researchers did not inform clinical decision-making based on this data, but the patient-facing education portion of the Corrie app included instruction on the recommended BP goals. Participants could also choose to share their self-monitored BP measurements with clinicians on their own. After hospital discharge, participants had no scheduled appointments with the study team.
Clinical and demographic characteristics were obtained through chart review from the electronic medical records. History of prior CVD was defined as ≥1 of the following: AMI, coronary revascularization including percutaneous coronary intervention and coronary artery bypass graft, stroke/transient ischemic attack, or peripheral artery disease prior to index AMI.
BP measurements were analyzed by time period and by mean daily values. Time periods reflected the scheduled time slots for BP entry inherent to the Corrie interface: Morning (0800–1159), Noon/Afternoon (1200–1559), Late Afternoon/Evening (1600–1959), and Evening/Night (2000–2359). The last time period, Night/Early Morning (0000–0759) contained any measurements outside of the 4 time slots in Corrie. The mean value was taken if there were multiple measurements within each of the 5 time periods. The mean daily BP was calculated if there were multiple time periods in which participants had entered BP values.
BP control by the 2011 AHA/ACC guidelines [2] was defined as systolic BP < 140 mmHg and diastolic BP < 90 mmHg. BP control by the 2017 AHA/ACC guidelines [4] was defined as systolic BP < 130 mmHg and diastolic BP < 80 mmHg. We also assessed the proportion of hypotension, analyzing the proportion of mean systolic BP < 90 mmHg [8] and mean diastolic BP < 60 mmHg [9] separately. These proportions in the presence of binary measures repeated within a day in turn nested within persons were estimated by performing a linear regression on a constant and using the cluster-robust estimator of variance to relax the assumption of the independence of the observations. Using a double cluster identifier, we grouped the observations by participant and time of day. Additionally, we assessed whether any measurements were within hypertensive crisis range (systolic BP > 180 mmHg and/or diastolic BP > 120 mmHg) [4].
We used random-intercept and random-slope multivariable linear mixed models to estimate whether the association between history of prior CVD and mean daily BP changed across time during the 30 days post-discharge. To account for within-patient correlations of repeated BP measures, we used the random-coefficient structure, in which the covariances depend on the random effects and the correlation between intercept and slope. To test whether longitudinal within-patient changes in BP varied between patients with and without prior CVD, the model included the interaction between prior CVD and continuous study days (Day 0–30, with Day 0 representing mean BP measures during admission and Day 1 corresponding to the first day after hospital discharge). This assumed linear changes over time. The models controlled for CVD risk factors and number of antihypertensive medications prescribed at discharge (inclusive of beta blockers, calcium channel blockers, diuretics, and angiotensin converting enzyme inhibitors/angiotensin receptor blockers). In order not to overfit the statistical models, we used logistic regression to compute propensity scores for prior CVD risk. In this model, binary prior CVD status was the dependent variable, and the following cardiovascular risk factors were independent covariates: age, sex, race, hypertension, type 2 diabetes, hyperlipidemia, and ever smoker [14]. The estimated propensity score was the derived predicted value of this equation and all models were adjusted for quintiles of the propensity score. To account for the potential effect of self-monitoring on BP outcomes, we ran a sensitivity analysis that added number of BP measurements as a covariate. To investigate associations between prior CVD and change in BP measured at specific times of the day over follow-up, the model was also applied to individual time period measurements of systolic and diastolic BP. Differences with p-values <0.05 were considered statistically significant. All analyses were performed using Stata version 15.1.
3. Results
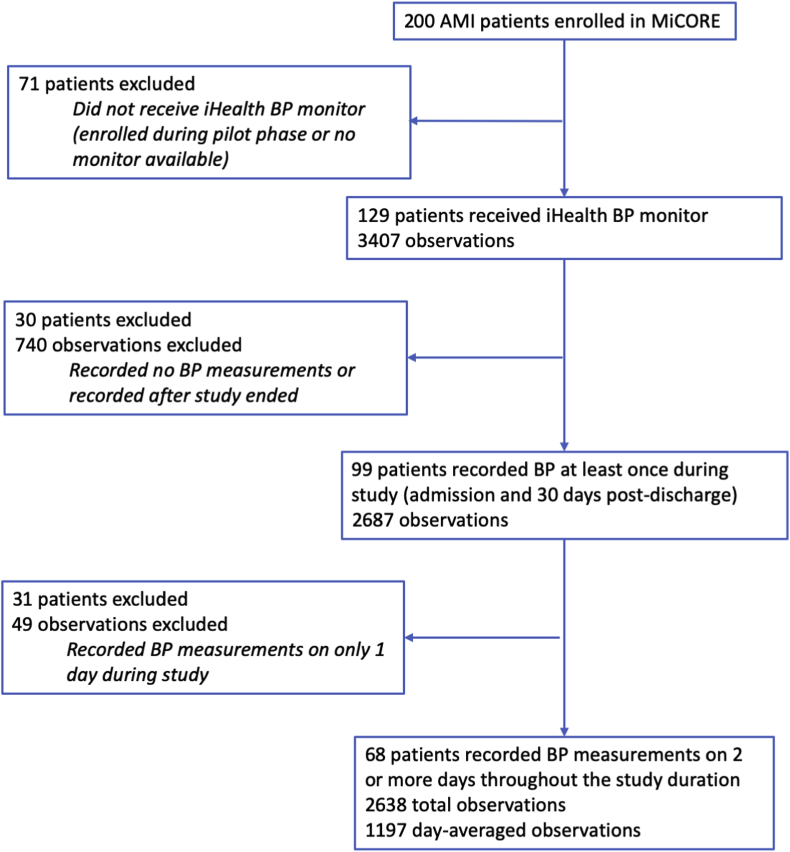
Of the 200 MiCORE participants, 129 received an iHealth BP monitor. For the BP data, we first excluded 12 observations from 8 individuals which were outside of the manufacturer-specified measurement ranges [13] or for which systolic BP was less than diastolic BP. After applying further data exclusion criteria, there were 68 participants with BP values measured on at least 2 days in the 30-day period after discharge who were included in the current analysis (Fig. 1). These 68 participants were enrolled between November 17, 2017 to April 7, 2019, and baseline demographic and clinical characteristics are summarized in Table 1. They were mean age 58 ± 10 years, 25% women, 68% white race, 44% with bachelor’s degree or higher education level, and 40% had Medicare or Medicaid insurance. Patients admitted for STEMI comprised 49% of participants, while the remainder were admitted for Type 1 NSTEMI, with 24% having a prior history of CVD and 68% having a history of hypertension. The frequency of antihypertensive medications prescribed at the time of discharge were: 92.7% beta blockers, 54.4% angiotensin converting enzyme inhibitors/angiotensin receptor blockers, 33.8% diuretics, and 10.3% calcium channel blockers. Compared with participants who received an iHealth BP monitor but did not provide sufficient data (n = 61), the participants included in this analysis (n = 68) were less likely to have prior CVD or MI, and more likely to have complete revascularization during admission. Although not statistically significant, participants included in the analysis were less often on Medicare or Medicaid insurance (Table 1).
Fig. 1.
Data flow. MiCORE denotes Myocardial infarction, COmbined-device, Recovery Enhancement (the parent study for this analysis); BP blood pressure; AMI acute myocardial infarction.
Table 1.
Participant characteristics. Participants who contributed sufficient data to be included in analysis are compared with those who did not. Values are presented as n (%) except where otherwise indicated. P-values were obtained using the Chi [2] test.
| Characteristic | Participants included in analysis (n = 68) n (%) | Participants excluded due to not contributing sufficient data (n = 61) n (%) | P-value | |
| Age | mean ± SD | 58 ± 10 | 59 ± 13 | – |
| ≥65 years | 19 (27.9) | 24 (39.3) | 0.17 | |
| Sex | Female | 17 (25.0) | 18 (29.5) | 0.57 |
| Race | White | 46 (67.7) | 40 (65.6) | 0.78 |
| Black or African American | 14 (20.6) | 13 (21.3) | ||
| Hispanic/Latino | 1 (1.5) | 3 (4.9) | ||
| Asian | 4 (5.9) | 2 (3.3) | ||
| Other/Unknown | 3 (4.4) | 3 (4.9) | ||
| Education | Bachelor’s degree or above | 30 (44.1) | 21 (34.4) | 0.26 |
| Insurance | Private | 39 (57.4) | 21 (34.4) | 0.06 |
| Medicare | 21 (30.9) | 26 (42.6) | ||
| Medicaid | 6 (8.8) | 12 (19.7) | ||
| Self-pay | 2 (2.9) | 2 (3.3) | ||
| Type of MI | STEMI | 33 (48.5) | 23 (37.7) | 0.22 |
| NSTEMI type 1 | 35 (51.5) | 38 (62.3) | ||
| Conditions prior to admission | Cerebrovascular disease or stroke | 6 (8.8) | 7 (11.5) | 0.62 |
| Peripheral arterial disease | 2 (2.9) | 2 (3.3) | 0.91 | |
| Diabetes mellitus | 26 (38.2) | 30 (49.2) | 0.21 | |
| Hyperlipidemia | 35 (51.5) | 38 (62.3) | 0.22 | |
| Hypertension | 46 (67.7) | 45 (73.8) | 0.45 | |
| CABG | 4 (5.9) | 7 (11.5) | 0.26 | |
| PCI | 8 (11.8) | 15 (24.6) | 0.06 | |
| MI | 5 (7.4) | 19 (31.2) | <0.01 | |
| Ever smoker | 38 (55.9) | 34 (55.7) | 0.99 | |
| Cardiovascular disease (prior stroke/TIA, PAD, CABG, PCI, MI) | 16 (23.5) | 29 (47.5) | <0.01 | |
| Admission Characteristics | CABG | 13 (19.1) | 18 (29.5) | 0.17 |
| PCI | 53 (77.9) | 42 (68.9) | 0.24 | |
| Complete revascularization | 62 (91.2) | 43 (70.5) | <0.01 | |
| Heart failure | 8 (11.8) | 9 (14.8) | 0.62 | |
| Length of stay median (IQR) | 4.5 (3.0–10.0) | 5.0 (3.0–10.5) | – | |
| Antihypertensive Medications prescribed at discharge | ACEi/ARB | 37 (54.4) | 38 (62.3) | 0.37 |
| Beta blocker | 63 (92.7) | 57 (93.4) | 0.86 | |
| Diuretic | 23 (33.8) | 22 (36.1) | 0.79 | |
| Calcium-channel blocker | 7 (10.3) | 11 (18.0) | 0.21 | |
| Number of antihypertensive medications at discharge | 0–1 | 15 (22.1) | 9 (14.8) | 0.36 |
| 2 | 42 (61.8) | 37 (60.7) | ||
| 3–4 | 11 (16.2) | 15 (24.6) | ||
ACEi/ARB angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker; CABG coronary artery bypass graft; MI myocardial infarction; NSTEMI non-ST-elevation myocardial infarction; PAD peripheral arterial disease; PCI percutaneous coronary intervention; STEMI ST-elevation myocardial infarction; TIA transient ischemic attack.
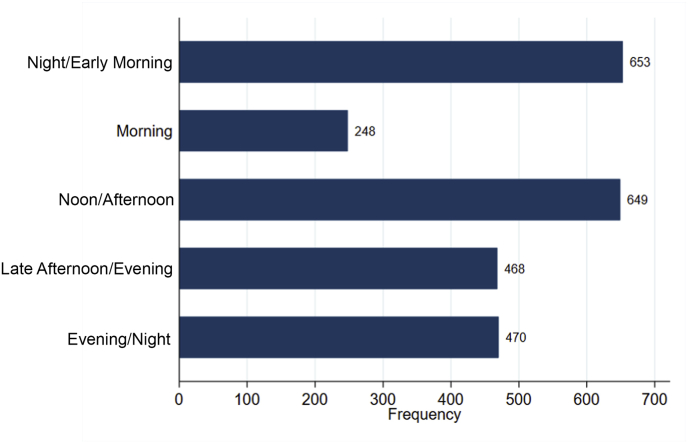
Overall, there were a total of 150 time period-averaged BP measurements (mean 2.2 per individual) during admission and 2488 time period-averaged BP measurements (mean 37 per individual) during the 30 days after discharge. The most common self-selected time periods in which patients measured their BP were at night/early morning (26.3%, 653/2488 observations) and noon/afternoon (26.1%, 649/2488 observations). The least common time period of self-selected BP measurement was morning (10%, 248/2488 observations, Fig. 2). Participants contributed 1197 days of BP measurements, for an average of 0.56 measurements per person per day. Participants who had prior CVD had a median of 20 time-period measurements per participant over the study duration (IQR 6–41), which was slightly lower than those without prior CVD (median 33 measurements, IQR 11–68), though this was not significant by the Mann-Whitney test (p = 0.16).
Fig. 2.
Distribution of time of day of self-selected home blood pressure recording. Total number of recordings = 2488. Time periods are defined as follows: Night/Early Morning (0000–0759), Morning (0800–1159), Noon/Afternoon (1200–1559), Late Afternoon/Evening (1600–1959), and Evening/Night (2000–2359).
When looking at mean BP over the study duration per patient, the percentage of BP control to <130/80 mmHg was 59.6% (95% CI: 54.3–64.9%), and the percentage of BP control to <140/90 mmHg was 83.7% (95% CI: 80.3–87.2%, Table 2). Among the participants with a history of hypertension, the percentage of BP control to <130/80 mmHg was 53.4% (95% CI: 47.3–59.5%), and the percentage of BP control to <140/90 mmHg was 79.9% (95% CI: 75.3–84.5%). There were no time period-averaged systolic BP values above 180 mmHg and no diastolic BP values above 120 mmHg. With regards to post-discharge hypotension, the percentage of systolic BP < 90 mmHg was 1.1% (95% CI: 0.17–2.0%), or 27/2488 measurements contributed by 5 participants. The percentage of diastolic BP < 60 mmHg was 3.9% (95% CI: 2.6–5.2%), or 98/2488 measurements, contributed by 24 participants.
Table 2.
Estimated percentage of mean BP control among patients with and without history of hypertension.
| BP Goal | All participants n/N Percentage (95% CI) | History of hypertension n/N Percentage (95% CI) | No history of hypertension n/N Percentage (95% CI) |
|---|---|---|---|
| <130/80 mmHg | 1483/2488 59.6% (54.3–64.9%) |
850/1592 53.4% (47.3–59.5%) |
633/896 70.6% (61.1–80.2%) |
| <140/90 mmHg | 2083/2488 83.7% (80.3–87.2%) |
1272/1592 79.9% (75.3–84.5%) |
811/896 90.5% (86.1–94.9%) |
BP blood pressure.
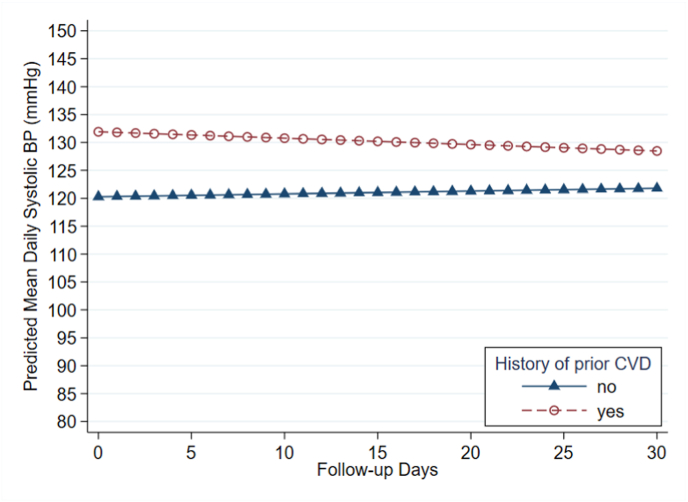
When controlling for CVD risk factors and number of antihypertensive medications prescribed at discharge, a history of prior CVD was associated with 12.2 mmHg higher systolic BP during admission (95% CI: 3.5, 20.9 mmHg, p = 0.006). This admission period encompassed a median of length of stay of 4.5 days (IQR 3–10 days) (Table 1). The interaction between history of CVD and study day was not significant (Table 3). Thus, we did not detect a difference in change in mean daily systolic BP over 30 days between those who had prior CVD and those who did not. Fig. 3 shows the predicted trajectories of mean daily systolic BP values during admission and over 30 days of follow-up, based on the mixed effects model. There was no association between prior CVD and diastolic BP during admission or with regards to change over 30 days of follow-up (Table 3). In the sensitivity analysis, adding the total number of BP measurements during the study as a covariate in the above model revealed similar results: a history of prior CVD was associated with 11.8 mmHg higher systolic BP during admission (95% CI 3.1–20.4), and the interaction term between history of CVD and study day remained non-significant.
Table 3.
Prior cardiovascular disease (CVD) and subsequent day-averaged blood pressure levels during 30-day post-discharge. Results were determined using linear mixed-effects modelling, adjusting for a propensity score inclusive of CVD risk factors, number of antihypertensive medications prescribed at discharge, and the interaction between prior CVD and time. Number of participants = 68; number of observations = 1197.
| Systolic BP Beta coefficient (95% CI) | Diastolic BP Beta coefficient (95% CI) | |
|---|---|---|
| Prior CVD | 12.2 (3.5, 20.9) | 2.9 (−2.8, 8.5) |
| Study days | 0.0 (−0.2, 0.2) | 0.0 (−0.1, 0.1) |
| Prior CVD x Study days | −0.2 (−0.6, 0.2) | 0.0 (−0.2, 0.3) |
BP: blood pressure; CVD: cardiovascular disease.
Fig. 3.
Predicted trajectories of average daily systolic blood pressure (BP) values over 30-day post discharge, by prior cardiovascular disease (CVD) status. Study day 0 represents mean systolic BP during the admission period. Trajectories of mean BP values on each follow-up day were predicted using a linear mixed-effects model, which was adjusted for CVD propensity score, number of antihypertensive medications, and the interaction term between prior CVD and study day. Number of participants = 68; number of observations = 1197.
When looking at the trends in systolic BP by time period, the same pattern of higher systolic BP during admission in patients with prior CVD than those without prior CVD held for the BP measurements taken at night and noon, but not for the morning, afternoon, or evening measurements. There was no evidence of interaction between history of CVD and study day on the outcome of systolic BP measurements over any of the time periods. Table 4 summarizes the results of the models and interactions for each time period. There were no significant associations between prior CVD and diastolic BP within the same time periods during admission or over follow-up.
Table 4.
Association between history of cardiovascular disease (CVD) and systolic blood pressure (BP) during admission and over 30 days post-discharge after acute myocardial infarction (AMI), by time period of BP measurement. Results were determined using linear mixed-effects modelling, adjusting for a propensity score inclusive of CVD risk factors, number of antihypertensive medications prescribed at discharge, and the interaction between prior CVD and time.
| Time Period | n | Beta coefficient for prior CVD, mmHg (95% CI) | P-value for prior CVD | Beta coefficient for prior CVD-study day interaction, mmHg (95% CI) | P-value for interaction |
|---|---|---|---|---|---|
| Night/Early Morning (0000–0759) | 57 | 16.20 (6.41, 25.99) | <0.01 | −0.39 (−0.87, 0.08.) | 0.10 |
| Morning (0800–1159) | 40 | 7.12 (−3.78, 18.02) | 0.20 | −0.13 (−0.65, 0.39) | 0.62 |
| Noon/Afternoon (1200–1559) | 60 | 11.32 (2.63, 20.01) | 0.01 | −0.17 (−0.63, 0.30) | 0.48 |
| Late Afternoon/Evening (1600–1959) | 64 | 7.92 (−2.32, 18.15) | 0.13 | −0.40 (-0.86, 0.07) | 0.10 |
| Evening/Night (2000–2359) | 60 | 8.68 (−1.73, 19.09) | 0.10 | −0.37 (−0.79, 0.05) | 0.09 |
CVD cardiovascular disease.
4. Discussion
This study demonstrates that, in an older and higher-risk sample, self-monitored BP using a DHI was feasible, reflects clinical characteristics, and can provide insight into quality of care. In the early post-AMI recovery period, we found suboptimal rates of BP control (59.6%) by the 2017 AHA/ACC hypertension guidelines and higher rates of control (83.7%) by the 2011 AHA/ACC secondary prevention guidelines. The proportion of systolic or diastolic BP in the hypotensive range was relatively low, and no participants recorded time period averaged values greater than the hypertensive crisis threshold of 180/120 mmHg. A history of prior CVD, which suggests inadequate secondary prevention in the past and greater vasculopathy, was associated with higher mean systolic BP during admission but not with change rate over 30 days post-discharge.
The suboptimal rates of BP control in the early post-AMI recovery period represents an addressable target for CVD risk modification. Specifically, as patients transition from frequent vitals monitoring in the hospital to outpatient care, there is a need to provide early identification of patients with uncontrolled BP who may need nonadherence counselling, lifestyle modification, or intensification of antihypertensive drug therapy. Furthermore, because these data are from 2017 to 2019, the gap between 59.6% of participants meeting the 2017 guidelines versus 83.7% of participants meeting the 2011 guidelines may represent a delay in adoption, confusion around the guidelines, or simply that the newer target is harder to attain. Additionally, the suboptimal rates of BP control may represent clinical caution to avoid overtreatment [[15], [16], [17], [18]]. Nevertheless, the ability of DHIs to provide home BP monitoring can help catch episodes of hypotension and support individualized therapeutic goals [15].
By suggesting a relationship between prior atherosclerotic disease and higher systolic BP during admission after AMI, without difference in rate of change over 30 days post-discharge, this study underscores the importance of secondary prevention. Although the predicted values of mean daily systolic BP in the CVD and non-CVD group appear to converge over time (Fig. 3), this relationship was non-significant, which may be due to variability, small sample size, or an attenuating effect of the DHI. Given that atherosclerosis and age are associated with increased pulse pressure [19] with lower diastolic BP and higher systolic BP, it is unsurprising that we did not find any association between diastolic BP and prior CVD. This also aligns with the relatively higher percentage of diastolic hypotension (3.9%) compared with systolic hypotension (1.1%).
Our finding that the association between prior CVD and higher systolic BP was most pronounced in measurements between the hours of 0000–0759 fits with known patterns in the circadian rhythm of BP [20]. Specifically, the difference in systolic BP between participants with and without prior CVD was of a greater magnitude in the night/early morning hours (0000–0759), coinciding with awakening from overnight sleep. This enhanced difference could also reflect the increased risk of cardiovascular events seen in patients who lack nocturnal BP dipping [20]. It is important to note that these findings are exploratory, and the lack of significance in morning, afternoon, and evening measurements may represent insufficient power and/or less variation in BP compared to nighttime/early morning measurements. These time period patterns underscore the importance of examining BP longitudinally and at different times of day, which is a unique strength of DHIs. Future studies using digital health data should assess both population-based and individual-based longitudinal trends, as well as account for within-day variation.
The Corrie Health Digital Platform is undergoing clinical validation [10], and the iHealth Bluetooth BP monitor has been validated for measurement accuracy [13]. This is an important strength in the nascent digital health field, as many commercially available DHIs for BP have not been clinically studied and some home BP measurement devices may be of unclear accuracy [21,22]. This study is in line with the overall progression towards using digital health to collect more frequent and real-time data to inform clinical decisions. For an individual patient, their home BP trend is likely more valuable for medication titration than single in-clinic values, which have been used in DHIs for hypertension [7,23].
In the post-AMI population, a randomized clinical trial from the Netherlands [24] found no difference in proportion of BP control by the 140/90 goal among patients using DHI-enabled BP self-monitoring at 1 year after AMI compared with usual care. However, this study only incorporated 4 follow-up visits over 1 year, while more frequent medication titration based on home BP trends may be necessary to fully utilize the benefits of BP self-monitoring. This trial found 76% control by the 140/90 mmHg goal, as measured in clinic 1 year after AMI among patients in their DHI arm. While this value is lower than our finding of 83.7% control by the 140/90 mmHg goal in the first 30 days post-AMI, this difference may be attributed to factors such as time after AMI, clinical and demographic differences, and type of BP measurement (in-clinic in the trial by Treskes and colleagues [24] versus at-home in our study).
The BP measurements in this study are limited by possible inaccuracies from patient self-monitoring. We were unable to enforce proper measurement techniques, but we ameliorated this by restricting the analysis to only iHealth users, so the measurements would flow directly from the monitoring device. Research staff also instructed participants on the proper methods of BP measurement. It is also possible that the iHealth BP3L monitors overestimated BP, as a comparison study in 43 post-AMI patients found a +5.0 mmHg mean difference in systolic BP in the iHealth BP5 compared to a manual sphygmomanometer [25].
Additionally, this study was limited by a small sample size and selection bias, as participants self-selected whether to monitor their BP and were not required to use the BP monitor daily. This is reflected in the relatively high percentage of missing data and in the differences between participants included in this analysis and participants who did not contribute sufficient data. The association between prior CVD and systolic BP after AMI should be considered in the context of the sample population having lower proportion of prior CVD and higher proportion of first-time AMI and complete revascularization (perhaps indicating lower burden of disease) than those who did not provide sufficient BP measures. First-time AMI patients may have needed more resources during recovery and thus self-selected to use the Corrie DHI more frequently. Notably, Hispanic/Latino patients were underrepresented in our sample, in part due to the Corrie app being only available in English at the time of this study. However, since the start of this study, Corrie has been translated into Spanish with plans for future versions of Corrie to include other languages.
Nevertheless, given the increasing prevalence of mobile device ownership, DHIs have the potential to improve access to healthcare among underserved patients, some of whom rely on mobile devices to obtain access to the internet [26]. For some patients with limited transportation, insurance coverage, or time, a mobile app may especially increase access to care over in-person sessions [12]. In future practice, the integration of digital heath into BP evaluation requires systems that would allow clinicians to prescribe DHIs and for it to be reimbursed by payers. This is especially pertinent in the COVID-19 pandemic, as many health systems have increasingly adopted telehealth models [27]. By providing devices and support to patients of all demographics, we can leverage the benefits of DHIs for health equity.
5. Conclusions
Only 59.6% of early AMI patients had mean daily BP measurements meeting the guideline-based goal of 130/80 mmHg, while hypotension occurred more rarely. This may represent (1) a gap in care surrounding BP monitoring and titration of antihypertensives after AMI, (2) delays in implementation of new guidelines, or (3) clinical caution to avoid hypotension. Early implementation of preventive management sets the stage for long-term prevention, and DHIs can both help reduce clinical inertia and catch hypotension episodes to increase the safety of antihypertensive medication up-titration. A history of CVD prior to index AMI admission, suggesting greater vasculopathy and inadequate secondary prevention in the past, was associated with higher mean systolic BP during admission, which persisted over 30 days post-discharge. The exploratory findings of this study may guide future work in interpreting longitudinal data from DHIs and best practices for tailoring therapy based on this data.
Disclosures
Corrie Health, as described in this work, was developed by F.A.M., M.A.L., and S.S.M. They are also founders of and hold equity in Corrie Health, which intends to further develop the digital platform. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. S.S.M. has served as a consultant to Akcea, Amgen, Astra Zeneca, Esperion, Kaneka, Novo Nordisk, Quest Diagnostics, Sanofi, Regeneron, and REGENXBIO.
Sources of funding
This study has received material support from Apple and iHealth and funding from the Maryland Innovation Initiative, Wallace H. Coulter Translational Research Partnership, Louis B. Thalheimer Fund, and the Johns Hopkins Individualized Health Initiative. These funding sources were not involved in the study design; collection, analysis, and interpretation of data; in writing the report; nor in the decision to submit the article for publication. R.S. received support from the Aetna Foundation. E.M.S. has received the following financial support for the research, authorship, and publication of this article: NIH/NINR F31 NR017328, Ruth L. Kirschstein National Research Service Award and NIH/NINR T32 NR012704, Pre-Doctoral Fellowship in Interdisciplinary Cardiovascular Health Research. S.W. is supported by the Johns Hopkins School of Medicine Medical Scientist Training Program (National Institutes of Health: Institutional Predoctoral Training Grant - T32) and the National Institutes of Health: Ruth L. Kirschstein Individual Predoctoral NRSA for MD/PhD: F30 Training Grant. S.S.M has current research support from the American Heart Association Strategically Focused Research Network for Health Technology and Innovation (20SFRN35380046), Aetna Foundation, National Institutes of Health (P01 HL108800), the David and June Trone Family Foundation, and CASCADE FH.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Corrie Health, as described in this work, was developed by FAM, MAL, and SSM. They are also founders of and hold equity in Corrie Health, which intends to further develop the digital platform. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. SSM has served as a consultant to Akcea, Amgen, Astra Zeneca, Esperion, Kaneka, Novo Nordisk, Quest Diagnostics, Sanofi, Regeneron, and REGENXBIO.
Acknowledgements
We thank the participants of the MiCORE study. Additionally, we thank Nucleus Medical Media for providing educational medical animations and video content for use in the Corrie application, the Apple Health and Apple CareKit team for donation of Apple Watches and guidance with Corrie design, and iHealth for donation of Bluetooth blood pressure monitors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajpc.2020.100089.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P. On behalf of the AHAC on E and PS, Association. C and SSS. Heart disease and stroke statistics- 2020 update: a report from the American heart association. Circulation. 2020;141:e1–e45. doi: 10.1161/CIR.0000000000000757. Available at: doi: [DOI] [PubMed] [Google Scholar]
- 2.Smith S.C., Benjamin E.J., Bonow R.O., Braun L.T., Creager M.A., Franklin B.A. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update. Circulation. 2011;124:2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. https://www.ahajournals.org/doi/10.1161/CIR.0b013e318235eb4d Available at: [DOI] [PubMed] [Google Scholar]
- 3.Shimbo D., Artinian N.T., Basile J.N., Krakoff L.R., Margolis K.L., Rakotz M.K. Self-measured blood pressure monitoring at home: a joint policy statement from the American heart association and American medical association. Circulation. 2020 doi: 10.1161/CIR.0000000000000803. https://www.ahajournals.org/doi/10.1161/CIR.0000000000000803 Available at: [DOI] [PubMed] [Google Scholar]
- 4.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Collins K.J., Dennison Himmelfarb C. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018:71. doi: 10.1161/HYP.0000000000000065. https://www.ahajournals.org/doi/10.1161/HYP.0000000000000065 Available at: [DOI] [PubMed] [Google Scholar]
- 5.Casey D.E., Thomas R.J., Bhalla V., Commodore-Mensah Y., Heidenreich P.A., Kolte D. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure: a report of the American College of cardiology/American heart association task force on performance measures. Circ Cardiovasc Qual Outcomes. 2019;12 doi: 10.1161/HCQ.0000000000000057. http://www.ncbi.nlm.nih.gov/pubmed/31714813 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandhi S., Chen S., Hong L., Sun K., Gong E., Li C. Effect of mobile health interventions on the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Can J Cardiol. 2017;33:219–231. doi: 10.1016/j.cjca.2016.08.017. https://www.sciencedirect.com/science/article/pii/S0828282X1630931X?via%3Dihub Available at: [DOI] [PubMed] [Google Scholar]
- 7.Uhlig K., Patel K., Ip S., Kitsios G.D., Balk E.M. Self-measured blood pressure monitoring in the management of hypertension A systematic review and meta-analysis. Ann Intern Med. 2013;159:185–194. doi: 10.7326/0003-4819-159-3-201308060-00008. https://www.acpjournals.org/doi/10.7326/0003-4819-159-3-201308060-00008 Available at: [DOI] [PubMed] [Google Scholar]
- 8.Franklin G.A., Boaz P.W., Spain D.A., Lukan J.K., Carrillo E.H., Richardson J.D. Vol. 48. Lippincott Williams and Wilkins; 2000. Prehospital hypotension as a valid indicator of trauma team activation; pp. 1034–1039. (Journal of trauma - injury, infection and critical care). [DOI] [PubMed] [Google Scholar]
- 9.Rahman F., McEvoy J.W. The J-shaped curve for blood pressure and cardiovascular disease risk: historical context and recent updates. Curr Atherosclerosis Rep. 2017;19 doi: 10.1007/s11883-017-0670-1. [DOI] [PubMed] [Google Scholar]
- 10.Spaulding E.M., Marvel F.A., Lee M.A., Yang W.E., Demo R., Wang J. Corrie health digital platform for self-management in secondary prevention after acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2019;12 doi: 10.1161/CIRCOUTCOMES.119.005509. https://www.ahajournals.org/doi/10.1161/CIRCOUTCOMES.119.005509 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang W.E., Spaulding E.M., Lumelsky D., Hung G., Huynh P.P., Knowles K. Strategies for the successful implementation of a novel iPhone Loaner System (iShare) in mHealth interventions: prospective study. JMIR mHealth uHealth. 2019;7 doi: 10.2196/16391. https://mhealth.jmir.org/2019/12/e16391/ Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung G., Yang W.E., Marvel F.A., Martin S.S. Mobile health application platform a € Corrie’ personalises and empowers the heart attack recovery patient experience in the hospital and at home for an underserved heart attack survivor. BMJ Case Rep. 2020:13. doi: 10.1136/bcr-2019-231801. https://pubmed.ncbi.nlm.nih.gov/32071124/ Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shang F., Zhu Y., Zhu Z., Liu L., Wan Y. Vol. 18. Blood Press Monit; 2013. pp. 278–281.http://www.ncbi.nlm.nih.gov/pubmed/23797053 (Validation of the iHealth BP5 wireless upper arm blood pressure monitor for self-measurement according to the European Society of Hypertension International Protocol revision 2010). Available at: [DOI] [PubMed] [Google Scholar]
- 14.Goff D.C., Lloyd-Jones D.M., Bennett G., Coady S., D’Agostino R.B., Gibbons R. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. http://circ.ahajournals.org/lookup/doi/10.1161/01.cir.0000437741.48606.98 Available at: [DOI] [PubMed] [Google Scholar]
- 15.Lee T.C., Cavalcanti R.B., McDonald E.G., Pilote L., Brophy J.M. Diastolic hypotension may attenuate benefits from intensive systolic targets: secondary analysis of a randomized controlled trial. Am J Med. 2018;131:1228–1233.e1. doi: 10.1016/j.amjmed.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Park H., Hong Y.J., Cho J.Y., Sim D.S., Yoon H.J., Kim K.H. Korean acute myocardial infarction registry investigators KAMIR. Blood pressure targets and clinical outcomes in patients with acute myocardial infarction. Korean Circ J. 2017;47:446–454. doi: 10.4070/kcj.2017.0008. http://www.ncbi.nlm.nih.gov/pubmed/28765735 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thune J.J., Signorovitch J., Kober L., Velazquez E.J., McMurray J.J.V., Califf R.M. Effect of antecedent hypertension and follow-up blood pressure on outcomes after high-risk myocardial infarction. Hypertension. 2008;51:48–54. doi: 10.1161/HYPERTENSIONAHA.107.093682. [DOI] [PubMed] [Google Scholar]
- 18.Roth D., Tulder R Van, Heidinger B., Herkner H., Schreiber W., Havel C. Admission blood pressure and 1-year mortality in acute myocardial infarction. Int J Clin Pract. 2015;69:812–819. doi: 10.1111/ijcp.12588. http://www.ncbi.nlm.nih.gov/pubmed/25657060 Available at: [DOI] [PubMed] [Google Scholar]
- 19.Denardo S.J., Gong Y., Nichols W.W., Messerli F.H., Bavry A.A., Cooper-DeHoff R.M. Blood pressure and outcomes in very old hypertensive coronary artery disease patients: an INVEST substudy. Am J Med. 2010;123:719–726. doi: 10.1016/j.amjmed.2010.02.014. http://www.ncbi.nlm.nih.gov/pubmed/20670726 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giles T.D. Circadian rhythm of blood pressure and the relation to cardiovascular events. J Hypertens. 2006;24:S11–S16. doi: 10.1097/01.hjh.0000220098.12154.88. https://insights.ovid.com/crossref?an=00004872-200604002-00003 Available at: [DOI] [PubMed] [Google Scholar]
- 21.Postel-Vinay N., Bobrie G., Savard S., Persu A., Amar L., Azizi M. Home blood pressure measurement and digital health: communication technologies create a new context. J Hypertens. 2018:1. doi: 10.1097/HJH.0000000000001860. http://insights.ovid.com/crossref?an=00004872-900000000-97371 Available at: [DOI] [PubMed] [Google Scholar]
- 22.Plante T.B., Urrea B., MacFarlane Z.T., Blumenthal R.S., Miller E.R., Appel L.J. Validation of the instant blood pressure smartphone app. JAMA Intern Med. 2016;176 doi: 10.1001/jamainternmed.2016.0157. http://www.ncbi.nlm.nih.gov/pubmed/26938174 700–2. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milani R.V., Lavie C.J., Bober R.M., Milani A.R., Ventura H.O. Improving hypertension control and patient engagement using digital tools. Am J Med. 2017;130:14–20. doi: 10.1016/j.amjmed.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 24.Treskes R.W., van Winden L.A.M., van Keulen N., van der Velde E.T., Beeres S.L.M.A., Atsma D.E. Effect of smartphone-enabled health monitoring devices vs regular follow-up on blood pressure control among patients after myocardial infarction: a randomized clinical trial. JAMA Netw Open. 2020;3:e202165. doi: 10.1001/jamanetworkopen.2020.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Treskes R.W., Wolterbeek R., van der Velde E.T., Eindhoven D.C., Schalij M.J. Comparison of the diagnostic accuracy of four smartphone-compatible blood pressure monitors in post-myocardial infarction patients. J Telemed Telecare. 2018;24:404–409. doi: 10.1177/1357633X17704092. http://www.ncbi.nlm.nih.gov/pubmed/28457182 Available at: [DOI] [PubMed] [Google Scholar]
- 26.Pew Research Center . 2019. Demographics of mobile device ownership and adoption in the United States.https://www.pewinternet.org/fact-sheet/mobile/ Available at: [Google Scholar]
- 27.Wosik J., Fudim M., Cameron B., Gellad Z.F., Cho A., Phinney D. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inf Assoc. 2020;27:957–962. doi: 10.1093/jamia/ocaa067. https://academic.oup.com/jamia/article/27/6/957/5822868 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.