Abstract
Objective
To investigate olfactory and gustatory dysfunction in patients with coronavirus disease 2019 (COVID-19) in Wuhan using a telephone interview.
Methods
This retrospective telephone survey investigated 196 consecutive patients with COVID-19 at 3 months after discharge from two hospitals in Wuhan, China. The characteristics of the patient’s disease course and time to recovery from olfactory and/or gustatory dysfunction (OD and/or GD) were collected by telephone interview. Demographic data were collected from the patient medical records.
Results
A total of 196 patients with COVID-19 completed the study. The most prevalent general symptoms were fever, cough, and fatigue. Overall, 19.9% of the patients reported OD and/or GD. In 87.2% of these cases, OD or GD appeared after the general symptoms. The time to recovery from OD and/or GD was more than 4 weeks in 51.4% of the patients. Patients with COVID-19 and OD and/or GD had significantly higher rates of cardiovascular disease than patients without OD and/or GD (p = 0.002).
Conclusions
Recovery from chemosensory dysfunction (OD and/or GD) was slow, with over half of the patients taking more than 4 weeks to recover. Cardiovascular disease might be related to the development of olfactory or taste disorders in patients with COVID-19.
Keywords: COVID-19, SARS-CoV-2, Olfactory disorders, Gustatory disorders, Recovery
Introduction
The coronavirus disease 2019 (COVID-19) pandemic is still spreading around the world at an exponential rate. At the time of writing, the virus causing this disease, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has infected millions of people worldwide and has killed more than 450 000. Early in the pandemic, Chinese clinicians reported typical symptoms of the disease as fever, fatigue, cough, and dyspnea (Guan et al., 2020). However, the spread of COVID-19 in the United States and Europe revealed some atypical symptoms of the disease, such as olfactory and gustatory dysfunction (OD and GD) (Lechien et al., 2020a, Speth et al., 2020).
Few Chinese studies have focused on OD and GD in patients with COVID19. It appears that only one study has described these two symptoms. In their study of neurological symptoms of SARS-CoV-2 infection, Mao et al. (2020) found that 5.6% of patients had hypogeusia and 5.2% had hyposmia, and these were the most common peripheral nervous system symptoms. These rates are much lower than those reported from Europe and the United States.
Two hypotheses might explain the low prevalence of OD and/or GD reported in the Chinese study. First, the number of Chinese patients with COVID-19 who exhibit olfactory or gustatory disorders is indeed lower. Second, most early research in Wuhan was based on the hospital medical records, and the patients’ sense of smell or taste might have been ignored by doctors during inquiries into their medical history or were not recorded due to the relative scarcity of medical resources during the early outbreak in Wuhan. Furthermore, all previous studies were limited to the acute phase with a short follow-up period, and this period might not reflect the regularity of recovery of olfactory and/or gustatory impairment in patients with COVID-19.
In otolaryngology, OD and GD following viral infection is not uncommon. Unfortunately, in China, there is no professional group of ENT physicians that has studied this condition. Currently, the mechanisms by which patients with COVID-19 develop OD and/or GD remain unclear. It has been hypothesized that the development of OD after SARS-CoV-2 infection may be related to direct damage to the olfactory bulb, to damage to olfactory receptor neurons in the olfactory epithelium, or both. The ensuing change in taste may depend largely on olfactory impairment (Ralli et al., 2020). Exploring the clinical features of these chemosensory disorders would help us to gain insight into the mechanisms behind them. Therefore, it was decided to collect detailed information about the OD and/or GD of patients with COVID-19 via telephone interview.
The aim of this study was to investigate the occurrence and time to recovery of OD and/or GD in patients with COVID-19 in China, at 3 months after discharge from hospital units other than intensive care units.
Methods
Study design and population
A total of 206 consecutive patients with COVID-19, discharged from two hospitals in Wuhan, China (Renmin Hospital of Wuhan University and Wuchang Mobile Cabin Hospital) between March 1 and March 16, 2020, were followed up. All adult patients hospitalized and diagnosed as having COVID-19 by reverse transcriptase PCR (RT-PCR) test for SARS-CoV-2 from nasopharyngeal swabs were identified from the computerized databases of these hospitals. The exclusion criteria were as follows: patients under 18 years of age, patients with a history of cognitive disorders, and patients with OD and/or GD known before the epidemic.
Demographic data including sex, age, and patient comorbidities and general symptoms were collected from the electronic medical records systems of these hospitals. Information about OD and/or GD of each patient, including the duration of the symptoms and the time to recovery were obtained by telephone interview. Three trained otolaryngologists conducted the telephone interviews with all participants using a standard questionnaire. All patients were then contacted by telephone up to three times to complete the study. The study follow-up was stopped on June 20, 2020. This study was approved by the Ethics Committee of Renmin Hospital of Wuhan University (No. WDRY2020-K148).
Statistical analysis
IBM SPSS Statistics software version 24.0 (IBM Corp, Armonk, NY, USA) was used to perform all statistical analyses. Data are presented as arithmetical mean values with the standard deviation (SD). The statistical significance of differences between data was evaluated using an independent samples t-test. The Chi-square test was used to evaluate the constituent ratios in the ‘COVID-19 with OD and/or GD’ group and the ‘COVID-19 without OD and/or GD’ group. A level of significance of p < 0.05 was used.
Results
Characteristics of the study participants
Two hundred and six patients were interviewed by telephone. Seven patients were excluded from the study because they could not be reached by phone three times. A further three patients were unable to complete the questionnaire. Hence, a total of 196 patients (95.1%) completed the survey. The mean age of the patients was 50.6 ± 13.8 years; 108 were female and 88 were male. The most common comorbidities in these patients were hypertension, diabetes, and cardiovascular disease. Table 1 shows the clinical and demographic characteristics of the patients. Patients with COVID-19 and OD and/or GD had significantly higher rates of cardiovascular disease than those without OD and/or GD (p = 0.002). The two groups did not differ significantly in their general symptoms or other comorbidities (p > 0.05).
Table 1.
Epidemiological characteristics of patients with COVID-19.
| Total | With OD or GD | Without OD or GD | p-Value | |
|---|---|---|---|---|
| n = 196 | n = 39 | n = 157 | ||
| Age (years) | 50.6 ± 13.8 | 50.3 ± 13.2 | 50.7 ± 14.0 | 0.874 |
| 20–40 | 46 (23.5) | 8 (20.5) | 38 (24.2) | 0.626 |
| 40–60 | 86 (43.9) | 19 (48.7) | 67 (42.7) | 0.496 |
| >60 | 64 (32.7) | 12 (30.8) | 52 (33.1) | 0.779 |
| Sex | ||||
| Female | 108 (55.1) | 25 (64.1) | 83 (52.9) | 0.207 |
| Male | 88 (44.9) | 14 (35.9) | 74 (47.1) | |
| Symptoms | ||||
| Fever | 167 (85.2) | 35 (89.7) | 132 (84.1) | 0.372 |
| Cough | 106 (54.1) | 20 (51.3) | 86 (54.8) | 0.695 |
| Fatigue | 50 (25.5) | 9 (23.1) | 41 (26.1) | 0.697 |
| Chills | 14 (7.1) | 2 (5.1) | 12 (7.6) | 0.585 |
| Headache | 11 (5.6) | 1 (2.6) | 10 (6.4) | 0.355 |
| Myalgia | 26 (13.3) | 2 (5.1) | 24 (15.3) | 0.094 |
| Diarrhea | 24 (12.2) | 3 (7.7) | 21 (13.4) | 0.333 |
| Nausea, vomiting | 8 (4.1) | 2 (5.1) | 6 (3.8) | 0.712 |
| Dyspnea | 11 (5.6) | 4 (10.3) | 7 (4.5) | 0.159 |
| Chest pain | 1 (0.5) | 0 (0) | 1 (0.6) | 0.617 |
| Comorbidities | ||||
| Hypertension | 28 (14.3) | 3 (7.7) | 25 (15.9) | 0.189 |
| Diabetes | 14 (7.1) | 3 (7.7) | 11 (7.0) | 0.882 |
| Cardiovascular disease | 8 (4.1) | 5 (12.8) | 3 (1.9) | 0.002 |
| Chronic lung disease | 8 (4.1) | 0 (0) | 8 (5.1) | 0.15 |
| Neurologic disease | 2 (1.0) | 0 (0) | 2 (1.3) | 0.479 |
| Digestive system diseases | 9 (4.6) | 2 (5.1) | 7 (4.5) | 0.858 |
| Urinary system diseases | 3 (1.5) | 0 (0) | 3 (1.9) | 0.384 |
COVID-19, coronavirus disease 2019; OD, olfactory dysfunction; GD, gustatory dysfunction. Results are reported as the mean ± standard deviation, or as the number (percentage) of patients.
Prevalence and timing of olfactory and gustatory dysfunction in patients with COVID-19
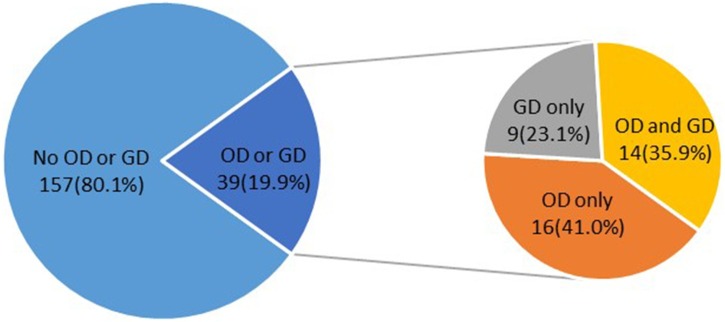
Of the 196 patients, 39 (9.7%) reported smell and/or taste disorders. Among them, 16 (41.0%) reported only smell disorders and nine (23.1%) reported only taste disorders, while 14 (35.9%) reported both smell and taste disorders (Figure 1 ).
Figure 1.
Proportion of olfactory dysfunction (OD) and/or gustatory dysfunction (GD) in patients with COVID-19.
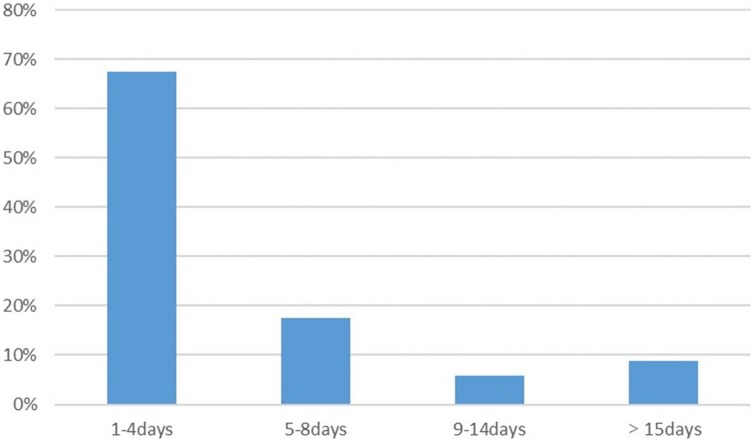
Five of the 39 patients (12.8%) reported that OD or GD was their first symptom of COVID-19. The five patients stated that they had exhibited typical symptoms, such as fever and cough, about 1 week after the onset of OD or GD. Thirty-four patients (87.2%) began to experience olfactory or gustatory disturbances following the appearance of general symptoms of COVID-19 (Figure 2 ). Among them, 85.3% (29/34) reported chemosensory disorders (OD and/or GD) within 8 days of the onset of general symptoms (Figure 2). The median time to develop OD and/or GD after the onset of the typical symptoms was 3 days.
Figure 2.
Time from first onset of typical symptoms to onset of olfactory dysfunction (OD) and/or gustatory dysfunction (GD).
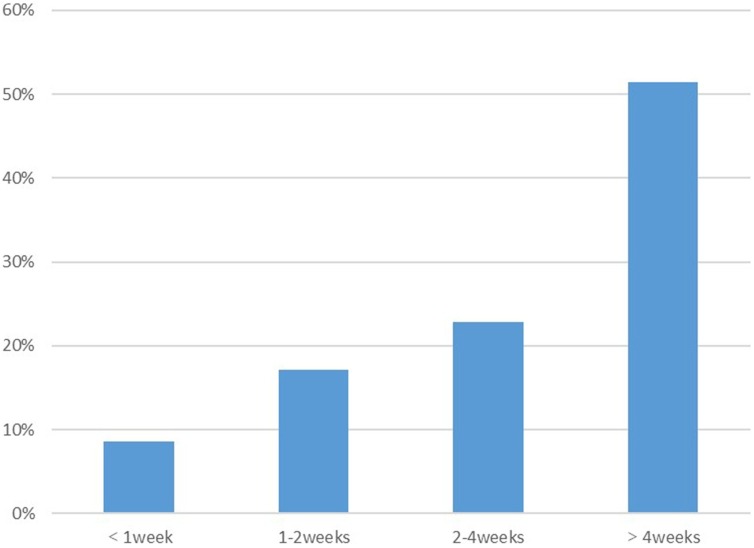
By the end of follow-up, 10.3% of patients (4/39) indicated that their olfactory and/or gustatory function had not returned to normal, while 89.7% of patients (35/39) reported that their sense of smell and/or taste function was restored. Figure 3 shows the pattern of the time to recovery for the remaining 35 patients. OD and/or GD recovered within 1 week of onset in only 8.6% of patients (3/35). It took more than 4 weeks for OD and/or GD to return to normal in 51.4% of patients (18/35). Two of the patients reported a recovery time of 2 months, which was the longest recovery time during the study follow-up.
Figure 3.
Pattern of the recovery time of olfactory dysfunction (OD) and/or gustatory dysfunction (GD) in patients with COVID-19.
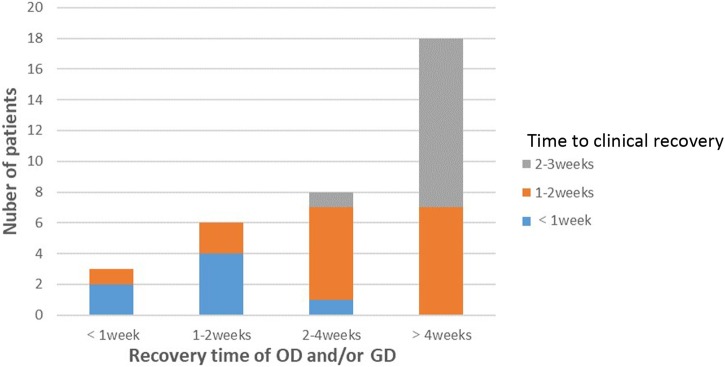
Figure 4 demonstrates the relationship between the time to recovery from OD and/or GD in patients with COVID-19 and overall patient-reported clinical improvement. The recovery of the sense of smell and taste in most patients with COVID19 was correlated temporally with overall clinical improvement of the disease. Finally, the clinical characteristics of patients with a recovered sense of smell and/or taste were compared to those of patients who had not recovered their sense of smell and/or taste. There was no statistically significant difference in age, sex composition, or length of hospital stay between the two groups of patients (Table 2 ).
Figure 4.
Relationship between recovery time of olfactory dysfunction (OD) and/or gustatory dysfunction (GD) and clinical improvement following COVID-19 onset.
Table 2.
Characteristics of patients with partial or complete recovery.
| Complete recovery (n = 35) | Partial recovery (n = 4) | p-Value | |
|---|---|---|---|
| Age (years) | 50.3 ± 12.4 | 43.3 ± 14.2 | 0.298 |
| Sex | |||
| Female | 21 (60.0) | 4 (100.0) | 0.114 |
| Male | 14 (40.0) | 0 (0) | |
| Hospitalization (days) | 12.6 ± 4.6 | 9.3 ± 4.6 | 0.184 |
Results are reported as the mean ± standard deviation, or as the number (percentage) of patientsa.
Discussion
The spread of COVID-19 is now accelerating worldwide, putting enormous pressure on every country’s epidemic prevention efforts. Studies have suggested OD and/or GD as a screening criterion to identify patients with mild symptoms. However, the epidemiological characteristics and pathogenesis of these chemosensory disorders remain unclear. Especially in China, there are few relevant studies. In this study, subjective olfactory and gustatory evaluations were performed in patients with COVID-19 via telephone interviews. In addition, the relationship between other symptoms of COVID and chemosensory dysfunction were analyzed using the patient electronic medical records and the telephone interviews.
In this study, 8.2% (16/196) of patients reported olfactory disorders without taste disorders and 4.6% (9/196) reported taste disorders without olfactory disorders. The low rates of OD and/or GD in the study population are clearly contrary to those reported in European and American studies (Lechien et al., 2020b, Speth et al., 2020, Chary et al., 2020). In addition, a study from Korea showed that 15% of patients with COVID-19 had anosmia or ageusia, which is similar to the present study results (Lee et al., 2020). However, a recent study showed that 38% of patients with COVID-19 who self-reported as having an olfactory disorder showed normal results in an objective olfactory test (Lechien et al., 2020a). Therefore, the prevalence of COVID-19-related olfactory disorders might have been overestimated in studies based on subjective reports.
In this study, most of the patients had OD and/or GD following general symptoms such as fever, cough, and fatigue. The median time to onset of OD and/or GD after general symptoms was 3 days. This meant that chemosensory disorders usually appeared early in the course of COVID-19. Notably, in five of the 39 patients with chemosensory disorders, OD and GD appeared before the other symptoms. In the context of the current pandemic, it is important for physicians to pay attention to patients who develop sudden OD and GD, which are important for the early detection and isolation of patients with COVID-19.
Similar to some previous studies (Lechien et al., 2020b, Chary et al., 2020), women appeared to be more susceptible to OD or GD. In the present study cohort 64.1% of patients with chemosensory disorders were female. The gender difference between the 2 groups was not statistically significant. However, Meini et al. (2020) showed that women were less likely to develop chemosensory disorders than men. Clearly, sex differences in patients with COVID-19 with OD or GD still require further study.
One of the biggest concerns for all ENT physicians and patients is the recovery time for OD and/or GD. In the present study, recovery from the chemosensory disorders took longer than 4 weeks in over half of the patients. However, this result contradicts those of previous studies. Klopfenstein et al. (2020) reported a mean duration of anosmia of 9 days, with a complete recovery occurring in almost all patients within 4 weeks. Lechien et al. (2020a) also reported that 72.6% of patients recovered their olfactory and gustatory functions completely within the first 8 days following resolution of the disease. In the present study, all patients had complete recovery of overall disease symptoms within 3 weeks post-diagnosis. In contrast, more than half of the patients did not recover their olfactory or gustatory function during that time frame. Although the study results are preliminary, it is concluded that the time to recovery of chemosensory disorders in patients with COVID-19 in the Wuhan area is slow.
It was also sought to explore the differences in clinical characteristics between patients with a fully recovered sense of smell and/or taste and those with a partially recovered sense of smell and/or taste. No statistically significant difference in age, sex composition, or length of hospital stay was found between the two groups of patients. Notably, all four patients who had not regained their sense of smell and/or taste by the end of follow-up were female.
The pathophysiological mechanisms by which SARS-CoV-2 infection causes OD or GD are unclear. A number of recent studies have explored the mechanisms that may lead to OD or GD. Zou et al. (2020) found that SARS-CoV-2 replicated particularly well in the nose, where a high viral load was detected shortly after the onset of symptoms. In addition, in the upper respiratory tract, nasal mucosal epithelial cells exhibited the highest expression of the SARS-CoV-2 receptor angiotensin I converting enzyme 2 (ACE2), which increased the chance of viruses invading these cells and causing OD (Sungnak et al., 2020). Likewise, high expression of ACE2 has been found in the tongue and oral mucosa, which might contribute to GD (Xu et al., 2020).
Nasal inflammation and obstruction might cause OD and/or GD. However, Mercante et al. (2020) found that most patients with a reduced sense of smell or taste did not report nasal congestion. During the telephone follow-up, the patients in the present study were also asked about their nasal symptoms, including nasal obstruction, rhinorrhea (anterior and posterior), and sneezing. Similarly, few patients indicated that they had any of these symptoms.
There are other possible causes of OD and/or GD following SARS-CoV-2 infection. The invasion of SARS-CoV2 through the peripheral olfactory neurons, resulting in damage to the central nervous system, has been considered as a possible mechanism for the development of OD (Conde Cardona et al., 2020). Brain magnetic resonance imaging of patients with COVID-19 with anosmia noted abnormalities of the olfactory bulb and olfactory nerve (Li et al., 2020; Aragão et al., 2020). Previous research on severe acute respiratory syndrome coronavirus (SARS-CoV) also supports this hypothesis. Netland et al. (2008) found that the virus can enter other areas of the brain through the olfactory bulb, creating rapid transmission across neurons. In fact, many of the clinical symptoms of COVID-19 are suspected to be related to its nerve invasiveness. For example, a previous study showed that SARS-CoV-2 might cause respiratory failure in patients with COVID-19 by attacking the respiratory center in the medulla oblongata (Li et al., 2020). The slow relief of OD and GD might be related to damage to the olfactory central nervous system.
Although the prevalence and prognosis of olfactory or gustatory disturbances in patients with COVID-19 vary worldwide, the mechanisms underlying them remain unclear. Some studies have shed some light on the mechanisms that underlie these differences. Forster et al. (2020) identified three major variants, named types A, B, and C, in a phylogenetic network analysis of 160 SARS-CoV-2 genomes. They found that in Europe and the United States, type A was predominant, followed by type C, while in East Asia, type B was most common. Phenotypic characteristics might differ between these variants, including those related to the prevalence of OD and GD. In addition, the affinity of the virus for certain tissues and individuals might partially explain the clinical differences between patients in different parts of the world. The expression level of ACE2, the receptor for SARS-CoV-2, in different tissues might be critical for the susceptibility, symptoms, and outcomes of this viral infection. A previous study on SARS-CoV showed that certain human ACE2 variants displayed reduced binding to the SARS-CoV S protein (Li et al., 2005). Similar to SARS-CoV, the spike protein (S protein) of SARSCoV-2 is responsible for entry into the host cell (Wan et al., 2020). By comparing 15 expression quantitative trait locus (eQTLs) variants of the ACE2 gene, the researchers found a large number of ACE2 polymorphisms and differences in expression levels between the European and Asian populations (Cao et al., 2020).
This study also compared the clinical characteristics of patients with COVID-19 with a chemosensory disorder and those without a chemosensory disorder. The results indicated a significantly higher incidence of cardiovascular disease in patients with COVID-19 with a chemosensory disorder than in patients with COVID-19 without a chemosensory disorder. Some studies have suggested that a decreased sense of smell is a predictor of cardiovascular disease development (Schubert et al., 2015, Siegel et al., 2019). Despite the small number of cases in the present study, it is speculated that cardiovascular disease might be related to the development of olfactory or taste disorders in patients with COVID-19.
There are some limitations to this study. The main limitation is its retrospective nature, which might have led to recall bias. However, in patients with COVID-19, olfactory or gustatory disturbances are relatively specific to other symptoms (e.g. fever, cough). In the telephone survey, the vast majority of patients were able to recall the onset and duration of the OD and/or GD. The lack of full objective methods to assess olfaction may be considered as another weakness. Considering the risk of cross-infection when performing objective tests, it was decided to use a telephone interview in this study to learn about the patients’ olfactory and gustatory functions. These shortcomings should be addressed in future research.
In conclusion, the prevalence of olfactory and gustatory disorders associated with SARS-CoV-2 infection in China was found to be much lower than that in the United States and Europe. However, it is undeniable that OD and/or GD is an early and even the first symptom of COVID-19. As such, these symptoms may help in the screening and identification of patients with atypical symptoms. Another characteristic of patients with COVID-19 in China is the long recovery time from OD and/or GD. Cardiovascular disease might be related to the development of olfactory or taste disorders in patients with COVID-19. However, because of the limited sample size, further research is needed to validate these results.
Funding source
None.
Ethical approval
This study was approved by the Ethics Committee of Renmin Hospital of Wuhan University (No. WDRY2020-K148).
Conflict of interest
None.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.09.039.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Aragão M., Leal M.C., Cartaxo Filho O.Q. Anosmia in COVID-19 Associated with Injury to the Olfactory Bulbs Evident on MRI. AJNR Am J Neuroradiol. 2020 doi: 10.3174/ajnr.A6675. Published online June 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chary E., Carsuzaa F., Trijolet J.P. Prevalence and Recovery From Olfactory and Gustatory Dysfunctions in Covid-19 Infection: A Prospective Multicenter Study. Am J Rhinol Allergy. 2020 doi: 10.1177/1945892420930954. Published online June 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde Cardona G., Quintana Pájaro L.D., Quintero Marzola I.D. Neurotropism of SARS-CoV 2: Mechanisms and manifestations. J Neurol Sci. 2020;412 doi: 10.1016/j.jns.2020.116824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Li L., Feng Z. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster P., Forster L., Renfrew C. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci U S A. 2020;117(17):9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein T., Kadiane-Oussou N.J., Toko L. Features of anosmia in COVID19. Med Mal Infect. 2020 doi: 10.1016/j.medmal.2020.04.006. Published online April 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R., Chiesa-Estomba C.M., De Siati D.R. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-05965-1. Published online April 08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Min P., Lee S. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J Korean Med Sci. 2020;35(18):e174. doi: 10.3346/jkms.2020.35.e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R., Cabaraux P., Chiesa-Estomba C.M. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck. 2020 doi: 10.1002/hed.26279. Published online May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.W., Syue L.S., Tsai Y.S. Anosmia and olfactory tract neuropathy in a case of COVID-19. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.05.017. Published online June 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Zhang C., Sui J. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24(8):1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meini S., Suardi L.R., Busoni M. Olfactory and gustatory dysfunctions in 100 patients hospitalized for COVID-19: sex differences and recovery time in real-life. Eur Arch Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-06102-8. Published online June 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercante G., Ferreli F., De Virgilio A. Prevalence of Taste and Smell Dysfunction in Coronavirus Disease 2019. JAMA Otolaryngol Head Neck Surg. 2020 doi: 10.1001/jamaoto.2020.1155. Published online June 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland J., Meyerholz D.K., Moore S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralli M., Di Stadio A., Greco A. Defining the burden of olfactory dysfunction in COVID-19 patients. Eur Rev Med Pharmacol Sci. 2020;24(7):3440–3441. doi: 10.26355/eurrev_202004_20797. [DOI] [PubMed] [Google Scholar]
- Speth M.M., Singer-Cornelius T., Obere M. Olfactory Dysfunction and Sinonasal Symptomatology in COVID-19: Prevalence, Severity, Timing, and Associated Characteristics. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820929185. Published online May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungnak W., Huang N., Bécavin C. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert C.R., Cruickshanks K.J., Fischer M.E. Inflammatory and vascular markers and olfactory impairment in older adults. Age Ageing. 2015;44(5):878–882. doi: 10.1093/ageing/afv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J.K., Wroblewski K.E., McClintock M.K. Olfactory dysfunction persists after smoking cessation and signals increased cardiovascular risk. Int Forum Allergy Rhinol. 2019;9(9):977–985. doi: 10.1002/alr.22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94(7):e00127–20. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Zhong L., Deng J. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M. SARS-CoV-2 Viral Load in Upper RespiratorySpecimens of Infected Patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.