Abstract
Background
Concentrations of endogenous sex hormones fluctuate across the menstrual cycle (MC), which could have implications for exercise performance in women. At present, data are conflicting, with no consensus on whether exercise performance is affected by MC phase.
Objective
To determine the effects of the MC on exercise performance and provide evidence-based, practical, performance recommendations to eumenorrheic women.
Methods
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Four databases were searched for published experimental studies that investigated the effects of the MC on exercise performance, which included at least one outcome measure taken in two or more defined MC phases. All data were meta-analysed using multilevel models grounded in Bayesian principles. The initial meta-analysis pooled pairwise effect sizes comparing exercise performance during the early follicular phase with all other phases (late follicular, ovulation, early luteal, mid-luteal and late luteal) amalgamated. A more comprehensive analysis was then conducted, comparing exercise performance between all phases with direct and indirect pairwise effect sizes through a network meta-analysis. Results from the network meta-analysis were summarised by calculating the Surface Under the Cumulative Ranking curve (SUCRA). Study quality was assessed using a modified Downs and Black checklist and a strategy based on the recommendations of the Grading of Recommendations Assessment Development and Evaluation (GRADE) working group.
Results
Of the 78 included studies, data from 51 studies were eligible for inclusion in the initial pairwise meta-analysis. The three-level hierarchical model indicated a trivial effect for both endurance- and strength-based outcomes, with reduced exercise performance observed in the early follicular phase of the MC, based on the median pooled effect size (ES0.5 = − 0.06 [95% credible interval (CrI): − 0.16 to 0.04]). Seventy-three studies had enough data to be included in the network meta-analysis. The largest effect was identified between the early follicular and the late follicular phases of the MC (ES0.5 = − 0.14 [95% CrI: − 0.26 to − 0.03]). The lowest SUCRA value, which represents the likelihood that exercise performance is poor, or among the poorest, relative to other MC phases, was obtained for the early follicular phase (30%), with values for all other phases ranging between 53 and 55%. The quality of evidence for this review was classified as “low” (42%).
Conclusion
The results from this systematic review and meta-analysis indicate that exercise performance might be trivially reduced during the early follicular phase of the MC, compared to all other phases. Due to the trivial effect size, the large between-study variation and the number of poor-quality studies included in this review, general guidelines on exercise performance across the MC cannot be formed; rather, it is recommended that a personalised approach should be taken based on each individual's response to exercise performance across the MC.
Electronic supplementary material
The online version of this article (10.1007/s40279-020-01319-3) contains supplementary material, which is available to authorized users.
Key Points
| In women, exercise performance might be reduced by a trivial amount during the early follicular phase of the menstrual cycle when compared with other phases. However, large between-study variance was identified, indicating that research design, participant characteristics and choice of outcome measure might influence any group-level effect. |
| Practically, the current evidence does not warrant general guidance on modulating exercise across the menstrual cycle. As such, we recommend that a personalised approach should be taken based on each individual's response to exercise performance across the menstrual cycle. |
| The quality of evidence for this review was mostly classified as “low” quality, which can be attributed to a range of methodological issues. Future studies need to improve methodological quality and limit confounders to facilitate a deeper understanding of the effects of the menstrual cycle on exercise performance. |
Background
Over the last three decades, there has been a rise in the number of women participating in exercise, from physical activity to elite sport, attributable to the increasing development of, and investment in, women’s professional sport [1–4]. Specifically, the percentage of women competing at the Olympic Games has increased from 26% in Seoul in 1988 to 45% in Rio de Janeiro in 2016 [5]. Furthermore, Tokyo 2021 is set to be the most sex-balanced Games in history, with the same number of medals available for men and women, which is projected to see women participation in the Games rise to 49% [5]. Performance-based research in women has not kept pace with the exponential rise in participation [6, 7]. Indeed, it would be naive to assume that all research in men can be directly applied to women, given the anatomical, physiological and endocrinological differences between the sexes [4, 8–10]. As such, sportswomen will benefit from sex-specific research and guidelines, which consider the effects of women’s physiology, such as the menstrual cycle (MC), on performance [8, 11].
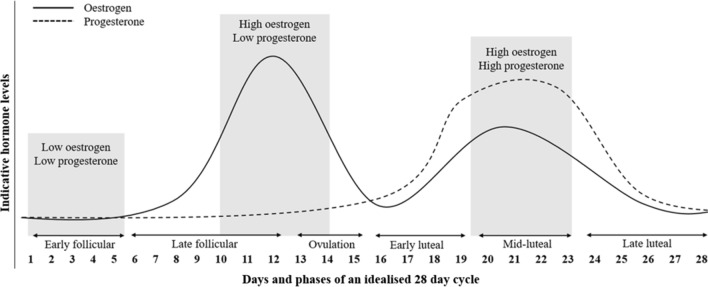
The MC is an important biological rhythm, whereby large cyclic fluctuations in endogenous sex hormones, such as oestrogen and progesterone, are observed [12–14]. The fairly predictable (and measurable) fluctuations in oestrogen and progesterone across the MC create significantly different transient hormonal profiles, which are used to differentiate between MC phases [15, 16]. As such, the MC is commonly divided into three phases, (1) the early follicular phase, characterised by low oestrogen and progesterone, (2) the ovulatory phase, characterised by high oestrogen and low progesterone, and (3) the mid-luteal phase, characterised by high oestrogen and progesterone [17]. Although the primary function of these hormones is to support reproduction, research has highlighted that the changing concentrations of oestrogen and progesterone across the MC also exert a myriad of diverse and complex effects on multiple physiological systems, including cardiovascular, respiratory, metabolic and neuromuscular parameters [12, 18, 19], which could have subsequent implications for exercise performance [15, 20–23].
There are a range of suggested mechanisms by which the cyclical fluctuations in oestrogen and progesterone across the MC might affect performance. Specifically, oestrogen is thought to have an anabolic effect on skeletal muscle [24, 25] and has been shown to play a role in substrate metabolism changes through increased muscle glycogen storage and increased fat utilisation [26]. Additionally, progesterone is thought to have anti-oestrogenic effects [21]. As such, it is plausible that changes in exercise performance might be observed due to the different hormonal profiles across the MC [15, 20–23]. To date, the effects of fluctuations in oestrogen and progesterone across the MC on exercise performance are conflicting, with studies reporting improved performance outcomes during the early follicular [27–29], ovulatory [30] and mid-luteal [31, 32] phases; whereas, others have shown no changes in exercise performance between MC phases [33–39]. Therefore, it is evident that a consensus is yet to be reached regarding the effects of the MC on exercise performance. Subsequently, no evidence-based guidelines for managing exercise performance across the MC currently exist for either exercising women, nor for practitioners working with elite sportswomen.
Given the recent increase in the number of women participating in exercise and the lack of consensus regarding the effects of the MC on exercise performance, there is a growing need to determine the effects of the fluctuations in oestrogen and progesterone across the MC on exercise performance. To our knowledge, this is the first meta-analysis to critically examine existing studies investigating changes in exercise performance across the MC, in eumenorrheic women. Additionally, this review is the first of its kind to appraise the quality of previous studies using robust assurance tools. The information provided by this meta-analysis can be used to inform practical recommendations for athletes, practitioners and researchers interested in managing exercise performance across the MC.
Methods
This review conforms to the PRISMA statement guidelines (see Electronic Supplementary Material Appendix S1) [40].
Study Inclusion and Exclusion Criteria
Consideration of Population, Intervention, Comparator, Outcomes and Study design (PICOS) was used to determine the parameters within which the review was conducted:
Population
Participants included healthy women who were (a) between the ages of 18 and 40 years; (b) eumenorrheic; (c) not taking any hormonal contraceptives or medication known to affect the hypothalamic–pituitary–ovarian (HPO) axis; (d) free from any menstrual-related dysfunctions (such as, amenorrhea) or any other conditions (e.g., pregnancy, eating disorders or disordered eating) known to affect the HPO axis; and (e) free from any injury that would affect participation. No restrictions were placed on activity level or training status.
Intervention
No specific intervention was investigated, but all participants were required to have a normal MC, defined as having a minimum of nine cycles per calendar year and a MC that ranged between 21 and 35 days in length.
Comparator
Comparisons were made between the early follicular phase (acting as a ‘control’ phase) of the MC and all other MC phases, in line with the following predetermined MC phase classification as shown in Fig. 1: early follicular (days 1–5), late follicular (days 6–12), ovulation (days 13–15), early luteal (days 16–19), mid-luteal (days 20– 23) and late luteal (days 24-28).
Fig. 1.
Schematic displaying the hormonal fluctuations across an idealised 28-day menstrual cycle, with ovulation occurring on day 14
Adapted from Pitchers and Elliott-Sale [8]
Outcomes
The primary outcome was exercise test performance. For the purposes of this review, exercise test performance was defined as total work done, time to completion, time to exhaustion, mean, peak and ratio outputs, rate of force production and decline, and indices of fatigue. Although maximum oxygen uptake (maximal [O2max] or peak [O2peak]) is not a performance test, this physiology-based outcome was included as it can be used as an indicator of performance. A full list of considered outcomes can be found in Electronic Supplementary Material Appendix S2. Performance outcome data were allocated into broad categories to allow for subgroup analysis; namely endurance (power and capacity) and strength (maximal expression of force and rate of force development). All exercise outcomes were extracted, and effect size duplication of multiple outcomes from the same test accounted for within the statistical analysis, as described below.
Study Design
Experimental studies were considered for analysis if they met the following inclusion criteria: (a) published, in full, in a peer-reviewed journal, (b) had the primary or secondary objective of assessing changes in exercise performance across the MC, (c) included within-group comparisons and (d) outcome measure(s) were taken in two or more defined MC phases. As such, case studies, review articles, study protocol papers and conference abstracts were excluded. Moreover, only full texts that were published in English or had an existing translation were retrieved and examined. There was no limit on date of publication.
Search Strategy for Identification of Studies
A systematic electronic literature search was conducted by KLM to identify all relevant articles using four online databases (PubMed, CENTRAL, SPORTDiscus and ProQuest). The searches were performed using medical subject headings terms, free-text and thesaurus terms, as well as, keywords from existing relevant papers [15, 20–23]. The following search terms and their combinations were used: (‘menstrual cycle’, OR ‘menstrual phase’, OR ‘follicular phase’, OR ‘luteal phase’) AND (‘strength’, OR ‘power’, OR ‘torque’, OR ‘force’, OR ‘neuromuscular’, OR ‘max* voluntary contraction’, OR ‘isometric’, OR ‘isokinetic’, OR ‘skeletal muscle’ OR ‘muscular performance’, OR ‘aerobic’, OR ‘aerobic power’, OR ‘aerobic capacity’, OR ‘endurance’, OR ‘endurance power’, OR ‘endurance capacity’, OR ‘anaerobic’, OR ‘anaerobic power’, OR ‘anaerobic capacity’, OR ‘athletic performance’, OR ‘sports performance’). An example of a full electronic search for one database (PubMed: 14/01/2019) is presented in Electronic Supplementary Material Appendix S3. Databases were searched from inception until February 2019. The reference lists of obtained relevant articles and review articles were hand-searched to identify any further studies and were added in manually. Following the same search criteria and strategy, an updated electronic and manual hand-search for relevant literature was subsequently conducted in April 2020 to identify any further articles published between February 2019 and April 2020.
Data Selection, Extraction and Study Quality Assessment
Selection of Studies
Three reviewers (KLM, KMH and KES) independently reviewed the titles, abstracts and full-text paper of the identified articles for inclusion and any duplicates were removed, using Covidence systematic review software (v1251, Veritas Health Innovation, Australia). All searches followed a two-phase screening strategy. Phase one assessed the eligibility of the title and abstract of every manuscript generated from the electronic searches and hand-searching against the predetermined inclusion and exclusion criteria. Studies that either clearly did not meet the inclusion criteria or met at least one exclusion criterion were excluded at this phase. In phase two, the full-text paper was retrieved for the articles identified in stage one and assessed against the predetermined inclusion and exclusion criteria. Any conflicts between the reviewers relating to study eligibility were resolved in consensus meetings (KLM, KMH and KES).
Data Extraction and Management
Data extraction was conducted by one reviewer (KLM), using a pre-piloted data extraction form, and independently verified by two members of the review team (KMH and KES). Any discrepancies were resolved by reviewing the original article and consensus achieved by discussion during consensus meetings (KLM, KMH and KES), or, if needed, in consultation with a fourth reviewer (ED). When data were presented in graphical and not in numerical format, DigitizeIt software (v2.3, DigitizeIt, Germany) was used to convert the relevant data. Further, where data were incomplete, authors were contacted to obtain the relevant information. Authors were given 4 weeks to respond; if the authors failed to respond after this date, the papers were excluded if no relevant data could be extracted from the published version of the paper.
Quality Assessment of Included Studies
Study quality was assessed by one reviewer (KLM) and independently verified by two members of the review team (KMH and KES), using a strategy based on the recommendations of the Grading of Recommendations Assessment Development and Evaluation (GRADE) working group [41]. This strategy considers quality of evidence for any one outcome based on five domains, namely risk of bias, indirectness, inconsistency, imprecision or evidence of publication bias. Both risk of bias and indirectness were initially conducted at the individual study level, with mode ratings used to describe whole outcomes. The initial appraisal tool used was based on the Downs and Black checklist for measuring study quality [42] and was specifically modified for use in this review (see Electronic Supplementary Material Appendix S4). The modified Downs and Black checklist comprised 15 outcomes, from five domains: (1) reporting; (2) external validity; (3) internal validity—bias; (4) internal validity—confounding; and (5) power. A maximum attainable score of 16 could be awarded, whereby study quality was categorised as follows: “high” (14–16); “moderate” (10–13); “low” (6–9); or “very low” (0–5). The results of the Downs and Black assessment were used to assign an a priori quality rating to each study. This a priori rating was then either maintained, or downgraded a level, based on the response to two questions that were considered key to the directness of these research studies: Q.1) was the MC phase confirmed using blood samples? If the authors reported using blood samples to confirm MC phase, the a priori rating was maintained and if not, the study was downgraded a level (e.g., a study that started out as “high” in quality, but did not confirm MC phase using a blood sample, drops to “moderate” in quality); and Q.2) was the MC phase confirmed using urinary ovulation detection kits? If the authors reported the use of an urinary ovulation detection kit to identify MC phase, the Q.1 rating was maintained; if not, the study was downgraded a level (as such, the maximum rating for any study that does not use serum analysis or urinary ovulation detection kits to identify and verify MC phase is “low”). The inclusion of these specific questions was based on the methodological conclusions made in previous studies [10, 17]. Consistency was ascertained using the meta-analysis results and was based on visual inspection of effect size estimates, whether or not confidence intervals overlapped, and on statistical tests for heterogeneity. Precision was judged based on the number of outcomes available (with outcomes based on < 5 data points downgraded) and on visual analysis of the width of the confidence intervals. Publication bias was assessed using Egger’s test along with visual inspection of funnel plots. Overall, this procedure allowed the final quality of evidence for each outcome to be categorised as either “high”, “moderate”, “low” or “very low” in quality. This quality appraisal was not used to exclude any study, although a sensitivity analysis was conducted using only those individual studies deemed to be of “high” or “moderate” quality, based on the risk of bias and directness assessments. Any differences between the reviewers were resolved by discussion during consensus meetings (KLM, KMH and KES), or, if needed, in consultation with a fourth reviewer (ED).
Data Synthesis
Data were extracted from studies comprising both between- and within-group designs. Pairwise effect sizes were calculated by dividing mean differences by pooled standard deviations. At the study level, variance of effect sizes was calculated according to standard distributional assumptions [43]. All meta-analyses were conducted within a Bayesian framework enabling the results to be interpreted more intuitively compared to a standard frequentist approach through use of subjective probabilities [44]. With a Bayesian framework, dichotomous interpretations of the results of a meta-analysis with regards to the presence or absence of an effect (e.g. with p values) can be avoided, and greater emphasis placed on describing the most likely values for the average effect and addressing practical questions such as, the probability the average effect is beyond a certain threshold [44]. The Bayesian framework is also particularly suited to hierarchical models and sharing information within and across studies to improve estimates [44]. In the present meta-analysis, three-level hierarchical models were conducted to account for covariance in multiple outcomes presented in the same study [45]. For the initial analysis, individual effect sizes were calculated by comparing exercise performance in the early follicular phase (acting as a ‘control’ phase) with all other phases of the MC (late follicular, ovulation, early luteal, mid-luteal and late luteal). Meta-regression was performed to assess whether the pooled effect size estimate was influenced by testing category (endurance or strength outcomes). Where no evidence of a difference was identified, the model was re-run combining both categories of outcomes to increase data to better estimate model parameters. Given the expectation of relatively small effect sizes, an a priori threshold of ± 2 was identified for outliers. Primary analyses were completed with outliers removed, but results were also presented from the full complement of studies as sensitivity analyses. A sensitivity analysis was also conducted on data obtained from studies categorised as “high” or “moderate” in quality. Assessment of publication bias was made using a multilevel extension of Egger’s test with effect sizes regressed on the inverse of standard errors [46]. Inferences from all analyses were performed on posterior samples generated by Markov Chain Monte Carlo with Bayesian 95% credible intervals (CrIs) constructed to enable probabilistic interpretations of parameter values. Interpretations were based on visual inspection of the posterior sample, the median value (ES0.5: 0.5 quantile) and 95% CrIs. Cohen's [47] standard threshold value of 0.8 was used to describe effect size as large, values between 0.5 and 0.8 as medium, values between 0.2 and 0.5 as small, and values between 0 and 0.2 as trivial.
Subsequent to this initial analysis, a network meta-analysis approach was used to compare exercise performance measured across all MC phases (early follicular, late follicular, ovulation, early luteal, mid-luteal and late luteal) with each other. Network meta-analyses are becoming increasingly common in evidence synthesis and are most commonly used to compare multiple experimental treatments where individual studies are unlikely to directly compare all relevant treatments [48]. The technique calculates pairwise effect sizes from studies comparing two treatments (direct evidence), and generates indirect evidence comparing other treatments through a common comparator [48]. The technique was adopted in the present review to supplement the initial pairwise meta-analysis and synthesise additional data comparing exercise performance using different combinations of MC phases. Study-specific treatment effects were drawn from multivariate normal distributions with up to five arms included. To test the consistency assumption of the network meta-analysis, the fit of the base-case model was compared to that of the inconsistency model. To summarise potential differences in exercise performance outcomes across all MC phases, results from the network meta-analysis were used to calculate the Surface Under the Cumulative Ranking curve (SUCRA; [49]). For each MC phase, a SUCRA value expressed as a percentage was calculated representing the likelihood that exercise performance was maximised or near maximised relative to other MC phases. More formally, the SUCRA value can be interpreted as the average proportion of phases where exercise performance is lower than the phase considered, with the mean SUCRA value equal to 50% [50]. Analyses were performed using the R packages R2WinBUGS [51] and brms [52]. Convergence of parameter estimates was checked with Gelman–Rubin R-hat values [53].
Results
Literature Search
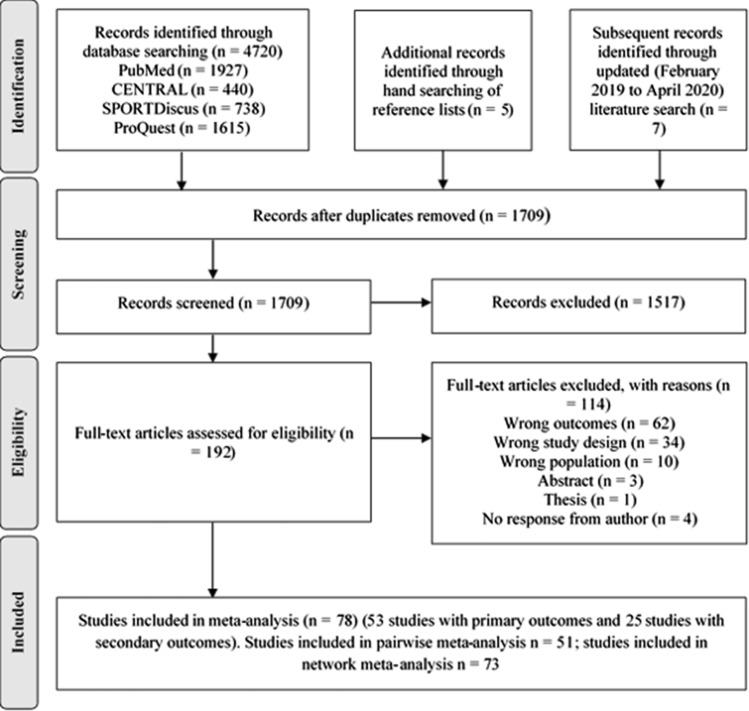
The literature search and selection of studies are presented in Fig. 2.
Fig. 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines flow chart for literature search and study selection
Study Characteristics
In total, 78 studies [19, 27–39, 54–117] with a total of 1193 participants were included in the review. Details of the included studies are shown in Electronic Supplementary Material Appendix S5.
Methodological Quality
Quality Assessment of Included Studies
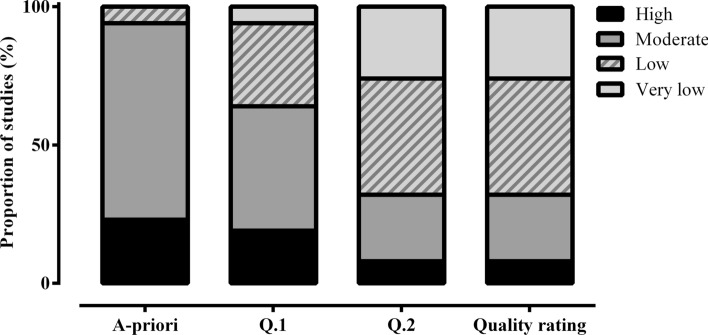
All quality classifications are presented in Fig. 3. Analysis of quality based on the entire evidence base (n = 78) was ascertained at the individual study level, and according to the Downs and Black checklist, as well as the additional questions regarding MC phase confirmation. The quality of the evidence from the 78 studies included in this review was primarily classified as “low” in quality (8% “high”; 24% “moderate”; 42% “low”; 26% “very low”; Fig. 3) such that, “our confidence in the effect estimate is limited: the true effect might be substantially different from the estimate of the effect” [118]. In particular, 71% of studies were initially allocated an a priori rating of “moderate” quality; however, following the application of questions pertaining to MC phase identification and verification, only 24% of these studies were allocated a final rating of “moderate” quality.
Fig. 3.
Quality rating of outcomes from all included studies (n = 78). Each bar represents the proportion of studies assigned a “high,” “moderate,” “low,” or “very low” quality rating. The x-axis represents the different stages of the quality appraisal process, with question one (Q. 1) and question two (Q. 2) indicating the questions asked to determine menstrual cycle phase identification and verification in each study, with the final bar representing the proportion of studies assigned to each quality rating category
Menstrual Cycle Phase Identification and Verification
In the 78 included studies, an array of methods was used to identify MC phase: (1) a combination of methods (e.g. counting of days, basal body temperature [BBT], assessment of menstrual symptoms, MC history and serial follicular scanning] without urinary ovulation detection kits (45%); (2) a combination of methods (e.g. counting of days, BBT, MC history, assessment of menstrual symptoms and urine ovulation detection kits) with urinary ovulation detection kits (31%); (3) counting of days (10%); (4) MC history (4%); (5) BBT (4%); and (vi) urinary ovulation detection kits (1%). In addition, some studies (5%) did not provide any information on how MC phases were identified. In relation to MC phase verification, out of the 78 studies included in the review, the majority of studies (59%) retrospectively verified MC phase using serum oestrogen and progesterone, a small number of studies retrospectively verified MC phase using saliva (4%) or urine (2%) oestrogen and progesterone, and the remaining studies provided no information on how they verified the identified MC phase (35%).
Outcomes
Analysis 1: Pairwise Meta-Analysis
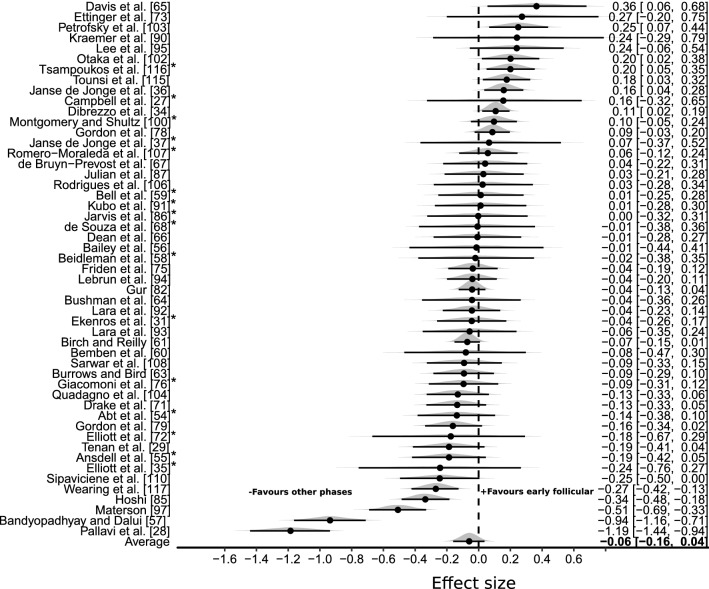
The initial meta-analysis comprised pooling of pairwise effect sizes comparing exercise performance during the early follicular phase of the MC with all other MC phases (late follicular, ovulation, early luteal, mid-luteal and late luteal). From the 78 studies that were eligible for the systematic review, 51 studies [19, 27–29, 31, 34–37, 54–60, 62–67, 70–72, 74, 75, 77, 78, 81, 84–86, 89–94, 96, 99, 101–103, 105–107, 109, 114–116] included assessment of exercise performance during the early follicular phase of the MC and included all other data required for calculations. The 51 studies (mode quality rating = “low”; 8% “high”; 24% “moderate”; 37% “low”; 31% “very low”) generated 362 pairwise effect sizes (240 strength and 122 endurance) with an average of four outcomes per study and a range from 1 to 12 outcomes. Data were obtained from 709 participants with studies comprising a mean participant size of 14 (range n = 5–100). A total of nine outliers were identified (seven studies with effect sizes less than −2 [favoring the “other MC phases”] and two studies with effect sizes greater than +2 [favoring the early follicular phase]) and subsequently removed from the analysis. The three-level hierarchical model indicated a trivial effect with reduced performance obtained in the early follicular phase of the MC, based on the median pooled effect size (ES0.5 = − 0.06 [95% CrI: − 0.16 to 0.04]; Fig. 4). Large between-study variance was identified (0.5 = 0.26 [0.18–0.38]) and interclass correlation coefficient estimates close to zero indicated little within-study correlation between outcomes. Pooling of strength and endurance outcomes was conducted as no evidence was obtained that indicated a differential effect between these performance categories (ES0.5/Endurance-Strength = − 0.01 [95% CrI: − 0.18 to 0.16]). Posterior estimates of the pooled effect size indicated close to zero probability of a small effect either in favour of the early follicular phase or all other MC phases (d ≥ 0.2; p ≤ 0.001). Egger’s regression test provided no evidence of publication bias (Egger0.5 = –0.01 [95% CrI: − 0.09 to 0.08]). Inclusion of outliers within the model had minimal influence on the average effect size (ES0.5 = − 0.08 [95% CrI: − 0.21 to 0.05]) and between-study variance ( 0.5 = 0.30 [95% CrI: 0.23–0.39]). A sensitivity analysis was completed with data obtained from studies classified as either “high” or “moderate” in quality (16 studies compromising 38 strength effect sizes and 12 endurance effect sizes from 169 participants [19, 27, 31, 35, 37, 54, 57, 58, 67, 71, 75, 85, 90, 99, 106, 115]). Compared to the primary analysis, the reduced data set resulted in a relatively symmetric credible interval around the zero value (ES0.5 = − 0.01 [95% CrI: − 0.11 to 0.08]).
Fig. 4.
Bayesian Forest Plot of multilevel meta-analysis comparing performance measured during the early follicular phase with all other menstrual cycle phases. The study-specific intervals represent individual effect size estimates and sampling error. The circle represents the pooled estimate generated with Bayesian inference along with the 95% credible interval (95% CrI). Negative values favour all other menstrual cycle phases (late follicular, ovulation, early luteal, mid-luteal and late luteal) compared to the early follicular phase. High and moderate quality studies are indicated with an asterisk (*)
Analysis 2: Network Meta-Analysis
Figure 5 shows a network diagram illustrating the pairwise effect sizes calculated across the six MC phases (early follicular, late follicular, ovulation, early luteal, mid-luteal and late luteal). Seventy-three studies (mode quality rating = “low”; 7% “high”; 26% “moderate”; 42% “low”; 25% “very low”) included enough data to be included in the network meta-analysis [19, 27–29, 31, 33–39, 54–68, 70–72, 74, 75, 77–117]. A total of 220 performance outcomes were included across 954 participants, with the number of comparisons across MC phases equal to: comparison between two phases = 87; comparison between three phases = 93; comparison between four phases = 27; comparison between five phases = 10; and comparison between six phases = 3. The most frequent comparisons made were between the early follicular and mid-luteal phase of the MC (21% of comparisons), followed by the late follicular and mid-luteal phases of the MC (18% of comparisons). Pairwise estimates including the early follicular phase as a reference are presented in Table 1. with negative median pooled effect sizes (“other MC phases”) obtained for all comparisons and the largest effect identified between the early follicular and the late follicular phase of the MC (ES0.5 = − 0.14 [95% CrI: − 0.26 to − 0.03]). The lowest SUCRA value was obtained for the early follicular phase (30%) with all other MC phase values ranging between 53 and 55%.
Fig. 5.
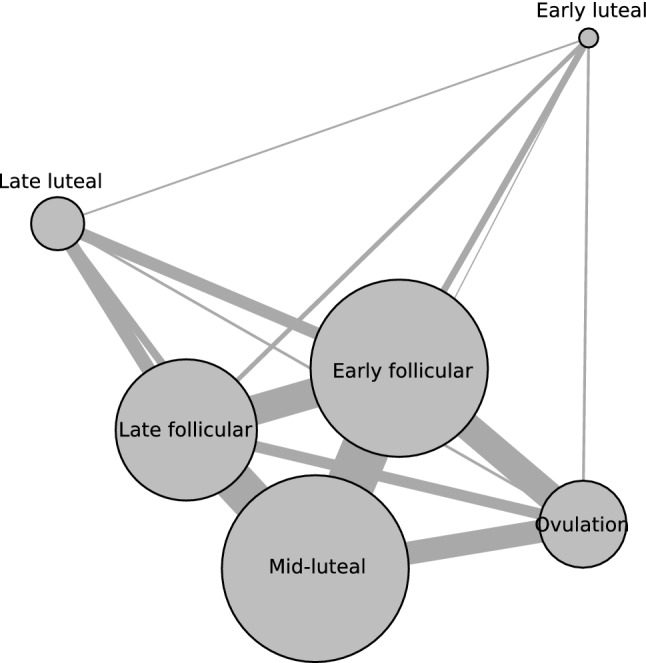
Network diagram illustrating the pairwise effect sizes calculated across the six menstrual cycle phases (early follicular, late follicular, ovulation, early luteal, mid-luteal and late luteal). The analysis included direct and indirect pairwise effect sizes from 73 studies. The relative size of nodes and relative thickness of connecting lines illustrate the frequency of outcomes measured in a given menstrual cycle phase and the number of direct comparisons between two phases, respectively
Table 1.
Summary of network meta-analysis results from 73 studies using the early follicular phase as a reference
| Comparison to early follicular phase | Effect size [95% CrI] | SUCRA (%) |
|---|---|---|
| Early follicular | − | 30 |
| Late follicular | − 0.14 [− 0.26 to − 0.03] | 54 |
| Ovulation | − 0.07 [− 0.15 to 0.07] | 55 |
| Early luteal | − 0.07 [− 0.19 to 0.16] | 54 |
| Mid-luteal | − 0.04 [− 0.11 to 0.08] | 55 |
| Late luteal | − 0.01 [− 0.18 to 0.17] | 53 |
Negative values for effect sizes favour all other menstrual cycle phases (late follicular, ovulation, early luteal, mid-luteal and late luteal) compared to the early follicular phase
SUCRA the surface under the cumulative ranking curve, CrI credible intervals
Discussion
The aim of this review was to examine if MC phase affects exercise performance in eumenorrheic women. The results indicate that on average, exercise performance might be trivially reduced during the early follicular phase of the MC when compared with all other MC phases. Performance was consistent between all other MC phases. In addition to the estimated trivial average effect, results from the meta-analysis models showed relatively large between-study variance indicating that research design, participant characteristics and type of performance measured might influence any effect. Furthermore, most studies that were included in this meta-analysis were classified as “low” in quality, and as such, the confidence in the evidence reported in this meta-analysis is also low, and should be interpreted with caution. Due to the trivial effect size, the large between-study variation and the number of poor-quality studies included in this review, general guidelines on exercise performance across the MC cannot be formed; rather, it is recommended that a personalised approach should be taken based on each individual's response to exercise performance across the MC.
There are a range of suggested mechanisms by which the lower levels of oestrogen and progesterone seen in the early follicular phase of the MC might negatively affect the exercise performance. Although a detailed mechanistic review is beyond the scope of this review, the following points can be noted. First, oestrogen is known for its anabolic effects [24, 25], as well as its role in regulating substrate metabolism through increasing glycogen uptake and sparing glycogen stores. Additionally, it has been shown to have antioxidant and membrane stabiliser properties, which might offer protection against exercise-induced muscle damage and reduce inflammatory responses [26]. Further, oestrogen is thought to have neuroexcitatory effects, whereby it reduces inhibition and increases voluntary activation [19]. Therefore, when oestrogen rises during the late follicular and ovulatory phases and remains elevated in the mid-luteal phase, it is plausible that this might affect muscular performance [24, 25] or maximal and submaximal intensity exercise performance [26]. Moreover, progesterone is thought to have anti-oestrogenic effects [21]; therefore, it could be speculated that the beneficial performance effects of oestrogen are likely to be greater in the late follicular and ovulatory phases when oestrogen is high without the interference of progesterone, compared to the mid-luteal phase when both oestrogen and progesterone are high. This speculation is supported by the finding presented here that the biggest difference in performance was between the early follicular and late follicular phases of the MC. However, the average effect calculated was trivial and there was considerable overlap between each of the pairwise comparisons with the early follicular phase. Whilst the current meta-analysis cannot identify the mechanisms responsible, it does indicate that, on average, exercise performance might be reduced by a trivial amount in the early follicular phase of the MC compared with all other phases. Interestingly, our sister meta-analysis, on the effects of oral contraceptives (OCs) on exercise performance, showed that, compared with eumenorrheic women, OC users have on average slightly inferior exercise performance [119]. Oral contraceptive use results in significantly downregulated concentrations of endogenous oestrogen and progesterone when compared with the ovulatory and mid-luteal phases of the MC [71]. Indeed, the endogenous hormonal profile of OC users is comparable to the profile seen during the early follicular phase of the MC [71]. Both meta-analyses show slightly impaired, group-level, exercise performance when both oestrogen and progesterone are at their lowest, therefore collectively suggesting that exercise performance might be mediated by the concentration of endogenous ovarian hormones in some exercising women.
Within the literature to date, the most common comparison used when investigating the effects of the MC on performance was between the early follicular and mid-luteal phase. This is not surprising, as the difference in the hormonal milieu is typically at its greatest between these phases (early follicular when both oestrogen and progesterone are low, and mid-luteal when both oestrogen and progesterone are high) [17]. As such, if performance was altered by synergistic fluctuations in oestrogen and progesterone levels, the comparison between these two phases would maximise the chance of observing an effect. This bi-phasic comparison, however, ignores the late follicular and ovulatory phases of the MC, when oestrogen is high, and progesterone is low. The network analysis indicated that the largest difference in performance might be expected between the early follicular and the late follicular phases of the MC, when both oestrogen and progesterone are low and when oestrogen rises without a concurrent increase in progesterone. Therefore, the effects of oestrogen, without the interference of progesterone, might be overlooked if the late follicular or ovulatory phases are not included within the phase comparisons. Future studies should, therefore, consider multiple phase comparisons so that the effects of different ratios of oestrogen and progesterone can be explored. It should be noted, however, that the inclusion of multiple phase comparisons will result in more variability, and as such, more participants will be needed to conclude any potential effects.
Although this systematic review included 78 studies and 1193 women (range n = 5–100), there were very few studies classified as “moderate” or “high” in quality, which implies that the confidence in the evidence used in this meta-analysis should be low. Specifically, only 24% of studies were allocated a quality rating of “moderate”, and only 8% of studies were allocated a quality rating of “high”. Our quality assessment approach included consideration of the methods used to identify and verify the MC phase in the included studies, which is considered to be key to the trustworthiness of the results obtained (i.e. Q1. was the MC phase confirmed using blood samples; Q2. was the MC phase confirmed using urinary ovulation detection kits?). Across the included studies there was large variability in the methods used to identify and then verify MC phase, namely calendar-based counting, BBT, MC history questionnaires, urinary ovulation detection kits, and salivary, urinary and serum measurement of both oestrogen and progesterone. Calendar-based counting is an indirect method to identify MC phase, whereby the self-reported onset of menses is set as day one, and the phases are then established by counting days from this point [17]. This method, however, assumes that all participants with regular menstruation experience ovulatory cycles with a mid-cycle peak in oestrogen, which is not always the case [120, 121]. As such, the use of calendar-based counting methods in isolation is not recommended when accurate identification of MC phase is required [122]. Similarly, BBT is a widely used method for identifying ovulation, and the length of the follicular and luteal phases [17], but this method does not provide information regarding actual hormone concentrations [123], and temperature readings might also be influenced by a range of factors such as illness, stress, sleep patterns and medication [124]; hence BBT in isolation is not considered a reliable method for MC phase verification [17]. Studies using these aforementioned methods were downgraded on this basis. Indeed, very few studies used a combination of the recommended methods by Cable and Elliott [10] and Janse de Jonge et al. [17], which include the use of the calendar-based counting method in conjunction with urinary ovulation detection kits to assist in setting the timing of testing throughout the MC and to confirm the presence of an ovulatory cycle, followed by serum measurement of both oestrogen and progesterone levels to subsequently verify the phases of the MC. Given that the rationale for exploring the effect of the MC on performance is underpinned by changes in oestrogen and progesterone, it is essential that studies should accurately verify the acute changes in endogenous hormones during each phase of the MC to ensure that the intended phase is being examined. Overall, without blood analysis, it is unclear which hormone milieu is being investigated, thus making it difficult to draw accurate conclusions regarding changes in performance across the MC and to make direct comparisons between studies. These recommendations echo recent publications in the area of women’s physiology [10, 17], demonstrating an increasing awareness for the nuances of this type of research, and collectively provide researchers with ample tools to make methodological decisions for future investigations. To limit the influence of low quality papers on the analyses, a sensitivity analysis was conducted with data obtained from studies that were classified as either “moderate” or “high” in quality [19, 27, 31, 35, 37, 54, 57, 58, 67, 71, 75, 85, 90, 99, 106, 115]. Due to the limited amount of data available, only the pairwise meta-analysis comparing exercise performance during the early follicular phase of the MC with all other MC phases was conducted. The sensitivity analysis provided no evidence of any effect, with a relatively symmetric credible interval centred close to zero. Whilst studies that were allocated a higher quality rating were better able to identify and verify the MC phase, there was no association between study quality and average sample size. Given the reduced amount of data included within the sensitivity analysis and the low sample sizes, the result is consistent with the primary analyses and conclusion that if an average effect exists, it is likely to be trivial in magnitude.
The results from the meta-analysis models consistently showed large between-study variance, which might be attributable to several factors: (a) inconsistent research design, as shown by the network analysis that highlights the discrepancy in the number of phase comparisons made between studies; (b) poor methodological practices, as emphasised by the quality assessment, whereby the majority of studies included in the meta-analysis were classified as “low” (42%) in quality primarily due to inadequate MC phase identification and verification in many studies; (c) non-homogenous participant groups, as shown in Electronic Supplementary Material Appendix S5 participants in this meta-analysis ranged from sedentary, to healthy, to physically active to elite athletes; and (d) large variation in the type of performance outcome measured, as detailed in Electronic Supplementary Material Appendix S2. As such, the breadth of this research area, without the corresponding depth, makes it difficult to apply a meaningful, yet generalisable, interpretation of the current data.
Conclusion
This is the first systematic review with meta-analysis to examine the effect of MC phase on exercise performance in eumenorrheic women. These data provide new information that exercise performance might on average be reduced by a trivial amount during the early follicular phase of the MC, compared with all other MC phases. The current meta-analysis also identified large between-study variance in the effect of the MC on exercise performance. This might have been influenced by a range of methodological factors and small participant numbers (average n = 14) as well as associated high sampling variance. Participant characteristics, such as training history, might also have contributed to the large between-study variance observed. From a practical perspective, as the effects tended to be trivial and variable between studies, the implications of these findings are likely to be so small as to be meaningless for most of the population. These trivial effects might, however, be of greater relevance to elite athletes, where the difference between winning and losing is marginal. Specifically, we recommend that practitioners working with elite sportswomen need to consider the MC and be aware of the potential times across the cycle whereby exercise performance might be reduced (early follicular phase) or enhanced (all other MC phases), but this approach should be tailored to, and informed by, the individual athlete. In the future, it would be interesting to identify which factors might cause some women to experience reduced performance during the early follicular phase of the MC when compared with all other MC phases, and identify strategies to monitor these effects. Therefore, future studies need to improve methodological quality (e.g., appropriate biochemical outcomes to confirm MC phase) and limit confounders to facilitate a deeper understanding of the effects of the MC on exercise performance in individuals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author Contributions
KLM, KES, KMH, PA, SG and KT designed the research. KLM conducted the searches and screening and KLM, KES and KMH completed the three-phase screening process. KLM extracted the data, which were verified by KES and KMH. PAS performed all the statistical analysis. PAS, KLM, KMH, KES and ED interpreted the data analysis. KLM and KES wrote the manuscript with critical input from KMH, ED, PAS, PA, SG and KT. All authors read and approved the final manuscript.
Availability of Data and Material
Please contact the corresponding author for data requests.
Compliance with Ethical Standards
Funding
No sources of funding were used to assist in the preparation of this article. Eimear Dolan is supported by a research grant (2019/05616-6) from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Conflicts of interest
Kelly Lee McNulty, Kirsty Jayne Elliott-Sale, Eimear Dolan, Paul Alan Swinton, Paul Ansdell, Stuart Goodall, Kevin Thomas and Kirsty Marie Hicks declare that they have no potential conflicts of interest with the content of this article.
Footnotes
Kelly Lee McNulty and Kirsty Jayne Elliott-Sale: Joint first authors.
References
- 1.European Commission. Gender equality in sport: proposal for strategic actions 2014–2020. https://ec.europa.eu/sport/news/2014/gender_equality_sporten. Accessed 3 Dec 2019.
- 2.International Working Group on Women and Sport, Women Sport International. Women, gender equality and sport. New York (NY), 2007. http://www.un.org/womenwatch/daw/public/Women%20and%20Sport.pdf. Accessed 3 Dec 2019.
- 3.Fink JS. Female athletes, women’s sport, and the sport media commercial complex: have we really “come a long way, baby”? Sport Manage. Rev. 2015;18(3):331–342. doi: 10.1016/j.smr.2014.05.001. [DOI] [Google Scholar]
- 4.Forsyth J, Roberts CM. Introduction to the exercising female: science and its application. In: Forsyth J, Roberts CM, editors. the exercising female. London: Routledge; 2018. pp. 1–6. [Google Scholar]
- 5.The International Olympic Committee. Promotion of women in sport through time. https://www.olympic.org/women-in-sport/background. Accessed 3 Dec 2019.
- 6.Costello JT, Bieuzen F, Bleakley CM. Where are all the female participants in Sports and Exercise Medicine research? Euro J Sport Sci. 2014;14(8):847–851. doi: 10.1080/17461391.2014.911354. [DOI] [PubMed] [Google Scholar]
- 7.Emmonds S, Heyward O, Jones B. The challenge of applying and undertaking research in female sport. Sports Med-Open. 2019;5(1):51. doi: 10.1186/s40798-019-0224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitchers G, Elliot-Sale K. Considerations for coaches training female athletes. Prof Strength Cond. 2019;55:19–30. [Google Scholar]
- 9.Sheel AW. Sex differences in the physiology of exercise: an integrative perspective. Exp Physiol. 2016;101(2):211–212. doi: 10.1113/EP085371. [DOI] [PubMed] [Google Scholar]
- 10.Cable NT, Elliott KJ. The influence of reproductive hormones on muscle strength. Biol Rhythm Res. 2004;35(3):235–244. doi: 10.1080/09291010412331335788. [DOI] [Google Scholar]
- 11.Knowles OE, Aisbett B, Main LC, Drinkwater EJ, Orellana L, Lamon S. Resistance training and skeletal muscle protein metabolism in eumenorrheic females: implications for researchers and practitioners. Sports Med. 2019;12:1–4. doi: 10.1007/s40279-019-01132-7. [DOI] [PubMed] [Google Scholar]
- 12.Davis HC, Hackney AC. The hypothalamic–pituitary–ovarian axis and oral contraceptives: regulation and function. In: Hackney AC, editor. Sex hormones, exercise and women: scientific and clinical aspects. London: Springer; 2017. pp. 1–17. [Google Scholar]
- 13.Landgren BM, Unden AL, Diczfalusy E. Hormonal profile of the cycle in 68 normally menstruating women. Acta Endocrinol. 1980;94(1):89–98. doi: 10.1530/acta.0.0940089. [DOI] [PubMed] [Google Scholar]
- 14.Owen JA. Physiology of the menstrual cycle. Am J Clin Nutr. 1975;28(4):333–338. doi: 10.1093/ajcn/28.4.333. [DOI] [PubMed] [Google Scholar]
- 15.Janse de Jonge XA. Effects of the menstrual cycle on exercise performance. Sports Med. 2003;33(11):833–851. doi: 10.2165/00007256-200333110-00004. [DOI] [PubMed] [Google Scholar]
- 16.Mihm M, Gangooly S, Muttukrishna S. The normal menstrual cycle in women. Anim Reprod Sci. 2011;124(3–4):229–236. doi: 10.1016/j.anireprosci.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 17.de Jonge XAJ, Thompson B, Han A. Methodological recommendations for menstrual cycle research in sports and exercise. Med Sci Sports Exerc. 2019;51(12):2610–2617. doi: 10.1249/MSS.0000000000002073. [DOI] [PubMed] [Google Scholar]
- 18.Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med. 1998;129(3):229–240. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- 19.Ansdell P, Brownstein CG, Škarabot J, Hicks KM, Simoes DC, Thomas K, et al. Menstrual cycle-associated modulations in neuromuscular function and fatigability of the knee extensors in eumenorrheic women. J Appl Physiol. 2019;126(6):1701–1712. doi: 10.1152/japplphysiol.01041.2018. [DOI] [PubMed] [Google Scholar]
- 20.Constantini NW, Dubnov G, Lebrun CM. The menstrual cycle and sport performance. Clin Sports Med. 2005;24(2):51–82. doi: 10.1016/j.csm.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Frankovich RJ, Lebrun CM. Menstrual cycle, contraception, and performance. Clin Sports Med. 2000;19(2):251–271. doi: 10.1016/s0278-5919(05)70202-7. [DOI] [PubMed] [Google Scholar]
- 22.Lebrun CM. The effect of the phase of the menstrual cycle and the birth control pill on athletic performance. Clin Sports Med. 1994;13(2):419–441. [PubMed] [Google Scholar]
- 23.Lebrun CM, Joyce SM, Constantini NW. Endocrinology of physical activity and sport. Totowa: Humana Press; 2013. Effects of female reproductive hormones on sports performance; pp. 281–322. [Google Scholar]
- 24.Baltgalvis KA, Greising SM, Warren GL, Lowe DA. Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle. PLoS One. 2010;5(4):101–164. doi: 10.1371/journal.pone.0010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowe DA, Baltgalvis KA, Greising SM. Mechanisms behind estrogens’ beneficial effect on muscle strength in females. Exerc Sport Sci Rev. 2010;38(2):61–67. doi: 10.1097/JES.0b013e3181d496bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isacco L, Boisseau N. Sex hormones and substrate metabolism during endurance exercise. In: Hackney AC, editor. Sex hormones, exercise and women: scientific and clinical aspects. London: Springer; 2017. pp. 35–58. [Google Scholar]
- 27.Campbell SE, Angus DJ, Febbraio MA. Glucose kinetics and exercise performance during phases of the menstrual cycle: effect of glucose ingestion. Am J Physiol Endocrinol Metab. 2001;281(4):817–825. doi: 10.1152/ajpendo.2001.281.4.E817. [DOI] [PubMed] [Google Scholar]
- 28.Pallavi LC, SoUza UJ, Shivaprakash G. Assessment of musculoskeletal strength and levels of fatigue during different phases of menstrual cycle in young adults. J Clin Diagn Res. 2017;11(2):11–13. doi: 10.7860/JCDR/2017/24316.9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tenan MS, Hackney AC, Griffin L. Maximal force and tremor changes across the menstrual cycle. Eur J Appl Physiol. 2016;116(1):153–160. doi: 10.1007/s00421-015-3258-x. [DOI] [PubMed] [Google Scholar]
- 30.Bambaeichi E, Reilly T, Cable NT, Giacomoni M. The isolated and combined effects of menstrual cycle phase and time-of-day on muscle strength of eumenorrheic females. Chronobiol Int. 2004;21(4–5):645–660. doi: 10.1081/CBI-120039206. [DOI] [PubMed] [Google Scholar]
- 31.Ekenros L, Hirschberg AL, Heijne A, Fridén C. Oral contraceptives do not affect muscle strength and hop performance in active women. Clin J Sport Med. 2013;23(3):202–207. doi: 10.1097/JSM.0b013e3182625a51. [DOI] [PubMed] [Google Scholar]
- 32.Oosthuyse T, Bosch AN, Jackson S. Cycling time trial performance during different phases of the menstrual cycle. Euro J Appl Physiol. 2005;94(3):268–276. doi: 10.1007/s00421-005-1324-5. [DOI] [PubMed] [Google Scholar]
- 33.Casazza GA, Suh SH, Miller BF, Navazio FM, Brooks GA. Effects of oral contraceptives on peak exercise capacity. J Appl Physiol. 2002;93(5):1698–1702. doi: 10.1152/japplphysiol.00622.2002. [DOI] [PubMed] [Google Scholar]
- 34.Dibrezzo RO, Fort IL, Brown B. Dynamic strength and work variations during three stages of the menstrual cycle. J Orthop Sports Phys Ther. 1988;10(4):113–116. doi: 10.2519/jospt.1988.10.4.113. [DOI] [PubMed] [Google Scholar]
- 35.Elliott KJ, Cable NT, Reilly T, Diver MJ. Effect of menstrual cycle phase on the concentration of bioavailable 17-β oestradiol and testosterone and muscle strength. Clin Sci. 2003;105(6):663–669. doi: 10.1042/CS20020360. [DOI] [PubMed] [Google Scholar]
- 36.de Jonge XJ, Boot CR, Thom JM, Ruell PA, Thompson MW. The influence of menstrual cycle phase on skeletal muscle contractile characteristics in humans. J Physiol. 2001;530(1):161–166. doi: 10.1111/j.1469-7793.2001.0161m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Jonge XJ, Thompson MW, Chuter VH, Silk LN, Thom JM. Exercise performance over the menstrual cycle in temperate and hot, humid conditions. Med Sci Sports Exerc. 2012;44(11):2190–2198. doi: 10.1249/mss.0b013e3182656f13. [DOI] [PubMed] [Google Scholar]
- 38.McLay RT, Thomson CD, Williams SM, Rehrer NJ. Carbohydrate loading and female endurance athletes: effect of menstrual-cycle phase. Int J Sport Nutr Exerc Metab. 2007;17(2):189–205. doi: 10.1123/ijsnem.17.2.189. [DOI] [PubMed] [Google Scholar]
- 39.Vaiksaar S, Jürimäe J, Mäestu J, Purge P, Kalytka S, Shakhlina L, et al. No effect of menstrual cycle phase and oral contraceptive use on endurance performance in rowers. J Strength Cond Res. 2011;25(6):1571–1578. doi: 10.1519/JSC.0b013e3181df7fd2. [DOI] [PubMed] [Google Scholar]
- 40.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 41.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 42.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris S. Estimating effect sizes from pretest-posttest-control group designs. Organ Res Methods. 2008;11(2):364–386. doi: 10.1177/1094428106291059. [DOI] [Google Scholar]
- 44.Kruschke JK, Liddell TM. The Bayesian New Statistics: hypothesis testing, estimation, meta-analysis, and power analysis from a Bayesian perspective. Psychon Bull Rev. 2018;25(1):178–206. doi: 10.3758/s13423-016-1221-4. [DOI] [PubMed] [Google Scholar]
- 45.Saunders B, Elliott-Sale K, Artioli GG, Swinton PA, Dolan E, Roschel H, et al. β-alanine supplementation to improve exercise capacity and performance: a systematic review and meta-analysis. Br J Sports Med. 2017;51(8):658–669. doi: 10.1136/bjsports-2016-096396. [DOI] [PubMed] [Google Scholar]
- 46.Fernández-Castilla B, Declercq L, Jamshidi L, Beretvas SN, Onghena P, Van den Noortgate W. Detecting selection bias in meta-analyses with multiple outcomes: a simulation study. J Exp Educ. 2019;29:1–20. doi: 10.1080/00220973.2019.1582470. [DOI] [Google Scholar]
- 47.Cohen J. Statistical power analysis for the social sciences. 2. New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 48.Greco T, Biondi-Zoccai G, Saleh O, Pasin L, Cabrini L, Zangrillo A, et al. The attractiveness of network meta-analysis: a comprehensive systematic and narrative review. Heart Lung Vessel. 2015;7(2):133. [PMC free article] [PubMed] [Google Scholar]
- 49.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 50.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15(1):58. doi: 10.1186/s12874-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sturtz S, Ligges U, Gelman AE. R2WinBUGS: a package for running WinBUGS from R. J Stat Softw. 2005 doi: 10.7916/D80C55HH. [DOI] [Google Scholar]
- 52.Bürkner PC. brms: an R package for Bayesian multilevel models using Stan. J Stat Softw. 2017;80(1):1–28. [Google Scholar]
- 53.Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian data analysis. New York: Chapman and Hall/CRC; 2013. [Google Scholar]
- 54.Abt JP, Sell TC, Laudner KG, McCrory JL, Loucks TL, Berga SL, et al. Neuromuscular and biomechanical characteristics do not vary across the menstrual cycle. Knee Surg Sports Traumatolo Arthrosc. 2007;15(7):901–907. doi: 10.1007/s00167-007-0302-3. [DOI] [PubMed] [Google Scholar]
- 55.Bailey SP, Zacher CM, Mittleman KD. Effect of menstrual cycle phase on carbohydrate supplementation during prolonged exercise to fatigue. J Appl Physiol. 2000;88(2):690–697. doi: 10.1152/jappl.2000.88.2.690. [DOI] [PubMed] [Google Scholar]
- 56.Bandyopadhyay A, Dalui R. Endurance capacity and cardiorespiratory responses in sedentary females during different phases of menstrual cycle. Kathmandu Univ Med J. 2012;10(4):25–29. doi: 10.3126/kumj.v10i4.10990. [DOI] [PubMed] [Google Scholar]
- 57.Beidleman BA, Rock PB, Muza SR, Fulco CS, Forte VA, Jr, Cymerman A. Exercise V̇e and physical performance at altitude are not affected by menstrual cycle phase. J Appl Physiol. 1999;86(5):1519–1526. doi: 10.1152/jappl.1999.86.5.1519. [DOI] [PubMed] [Google Scholar]
- 58.Bell DR, Blackburn JT, Ondrak KS, Hackney AC, Hudson JD, Norcross MF, et al. The effects of oral contraceptive use on muscle stiffness across the menstrual cycle. Clin J Sport Med. 2011;21(6):467–473. doi: 10.1097/JSM.0b013e318230f50a. [DOI] [PubMed] [Google Scholar]
- 59.Bemben DA, Salm PC, Salm AJ. Ventilatory and blood lactate responses to maximal treadmill exercise during the menstrual cycle. J Sports Med Phys Fitness. 1995;35(4):257–262. [PubMed] [Google Scholar]
- 60.Birch KM, Reilly T. Manual handling performance: the effects of menstrual cycle phase. Ergonomics. 1999;42(10):1317–1332. doi: 10.1080/001401399184974. [DOI] [PubMed] [Google Scholar]
- 61.Birch K, Reilly T. The diurnal rhythm in isometric muscular performance differs with eumenorrheic menstrual cycle phase. Chronobiol Int. 2002;19(4):731–742. doi: 10.1081/CBI-120006083. [DOI] [PubMed] [Google Scholar]
- 62.Burrows M, Bird SR. Velocity at O2max and peak treadmill velocity are not influenced within or across the phases of the menstrual cycle. Euro J Appl Physiol. 2005;93(5–6):575–580. doi: 10.1007/s00421-004-1272-5. [DOI] [PubMed] [Google Scholar]
- 63.Bushman B, Masterson G, Nelsen J. Anaerobic power performance and the menstrual cycle: eumenorrheic and oral contraceptive users. J Sports Med Phys Fitness. 2006;46(1):132. [PubMed] [Google Scholar]
- 64.Davies BN, Elford JC, Jamieson KF. Variations in performance in simple muscle tests at different phases of the menstrual cycle. J Sports Med Phys Fitness. 1991;31(4):532–537. [PubMed] [Google Scholar]
- 65.Dean TM, Perreault L, Mazzeo RS, Horton TJ. No effect of menstrual cycle phase on lactate threshold. J Appl Physiol. 2003;95(6):2537–2543. doi: 10.1152/japplphysiol.00672.2003. [DOI] [PubMed] [Google Scholar]
- 66.de Bruyn-Prevost P, Masset C, Sturbois X. Physiological response from 18-25 years women to aerobic and anaerobic physical fitness tests at different periods during the menstrual cycle. J Sports Med. 1984;24(2):144–148. [PubMed] [Google Scholar]
- 67.de Souza MS, Maguire MS, Rubin KR, Maresh CM. Effects of menstrual phase and amenorrhea on exercise performance in runners. Med Sci Sports Exerc. 1990;22(5):575–580. doi: 10.1249/00005768-199010000-00006. [DOI] [PubMed] [Google Scholar]
- 68.Dombovy ML, Bonekat HW, Williams TJ, Staats BA. Exercise performance and ventilatory response in the menstrual cycle. Med Sci Sports Exerc. 1987;19(2):111–117. [PubMed] [Google Scholar]
- 69.Doolittle TL, Engebretsen J. Performance variations during the menstrual cycle. J Sports Med Phys Fitness. 1972;12(1):54. [PubMed] [Google Scholar]
- 70.Drake SM, Evetovich T, Eschbach C, Webster M. A pilot study on the effect of oral contraceptives on electromyography and mechanomyography during isometric muscle actions. J Electromyogr Kinesiol. 2003;13(3):297–301. doi: 10.1016/S1050-6411(03)00024-5. [DOI] [PubMed] [Google Scholar]
- 71.Elliott KJ, Cable NT, Reilly T. Does oral contraceptive use affect maximum force production in women? Br J Sports Med. 2005;39(1):15–19. doi: 10.1136/bjsm.2003.009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ettinger SM, Silber DH, Gray KS, Smith MB, Yang QX, Kunselman AR, et al. Effects of the ovarian cycle on sympathetic neural outflow during static exercise. J Appl Physiol. 1998;85(6):2075–2081. doi: 10.1152/jappl.1998.85.6.2075. [DOI] [PubMed] [Google Scholar]
- 73.Frandsen J, Pistoljevic N, Quesada JP, Amaro-Gahete FJ, Ritz C, Larsen S, et al. Menstrual cycle phase does not affect whole body peak fat oxidation rate during a graded exercise test. J Appl Physiol. 2020;128(3):681–687. doi: 10.1152/japplphysiol.00774.2019. [DOI] [PubMed] [Google Scholar]
- 74.Fridén C, Hirschberg AL, Saartok T. Muscle strength and endurance do not significantly vary across 3 phases of the menstrual cycle in moderately active premenopausal women. Clin J Sport Med. 2003;13(4):238–241. doi: 10.1097/00042752-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 75.Giacomoni M, Bernard T, Gavarry O, Altare S, Falgairette G. Influence of the menstrual cycle phase and menstrual symptoms on maximal anaerobic performance. Med Sci Sports Exerc. 2000;32(2):486. doi: 10.1097/00005768-200002000-00034. [DOI] [PubMed] [Google Scholar]
- 76.Girija B, Veeraiah SH. Effect of different phases of menstrual cycle on physical working capacity in Indian population. Indian J Physiol Pharmacol. 2011;55(2):165–169. [PubMed] [Google Scholar]
- 77.Gordon D, Hughes F, Young K, Scruton A, Keiller D, Caddy O, et al. The effects of menstrual cycle phase on the development of peak torque under isokinetic conditions. Isokinet Exerc Sci. 2013;21(4):285–291. [Google Scholar]
- 78.Gordon D, Scruton A, Barnes R, Baker J, Prado L, Merzbach V. The effects of menstrual cycle phase on the incidence of plateau at and associated cardiorespiratory dynamics. Clin Physiol Funct Imaging. 2018;38(4):689–698. doi: 10.1111/cpf.12469. [DOI] [PubMed] [Google Scholar]
- 79.Grucza R, Pekkarinen H, Titov EK, Kononoff A, Hänninen O. Influence of the menstrual cycle and oral contraceptives on thermoregulatory responses to exercise in young women. Eur J Appl Physiol Occup Physiol. 1993;67(3):279–285. doi: 10.1007/BF00864229. [DOI] [PubMed] [Google Scholar]
- 80.Grucza R, Pekkarinen H, Hanninen O. Cardiorespiratory responses to bicycle incremental exercise in women taking oral contraceptives. Biol Sport. 2002;19(3):267–279. [Google Scholar]
- 81.Gür H. Concentric and eccentric isokinetic measurements in knee muscles during the menstrual cycle: a special reference to reciprocal moment ratios. Arch Phys Med Rehabil. 1997;78(5):501–505. doi: 10.1016/s0003-9993(97)90164-7. [DOI] [PubMed] [Google Scholar]
- 82.Hertel J, Williams NI, Olmsted-Kramer LC, Leidy HJ, Putukian M. Neuromuscular performance and knee laxity do not change across the menstrual cycle in female athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):817–822. doi: 10.1007/s00167-006-0047-4. [DOI] [PubMed] [Google Scholar]
- 83.Hoeger Bement MK, Rasiarmos RL, DiCapo JM, Lewis A, Keller ML, Harkins AL, et al. The role of the menstrual cycle phase in pain perception before and after an isometric fatiguing contraction. Eur J Appl Physiol. 2009;106(1):105–112. doi: 10.1007/s00421-009-0995-8. [DOI] [PubMed] [Google Scholar]
- 84.Hoshi A. Changes in muscular strength of women in different phases of the menstrual cycle. Bull Nippon Dent Univ. 1997;26:219–224. [Google Scholar]
- 85.Jarvis SS, VanGundy TB, Galbreath MM, Shibata S, Okazaki K, Reelick MF, et al. Sex differences in the modulation of vasomotor sympathetic outflow during static handgrip exercise in healthy young humans. Am J Physiol Regul Integr Comp Physiol. 2011;301(1):193–200. doi: 10.1152/ajpregu.00562.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Julian R, Hecksteden A, Fullagar HH, Meyer T. The effects of menstrual cycle phase on physical performance in female soccer players. PLoS One. 2017;12(3):e0173951. doi: 10.1371/journal.pone.0173951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jurkowski JE, Jones NL, Toews CJ, Sutton JR. Effects of menstrual cycle on blood lactate, O2 delivery, and performance during exercise. J Appl Physiol. 1981;51(6):1493–1499. doi: 10.1152/jappl.1981.51.6.1493. [DOI] [PubMed] [Google Scholar]
- 88.Kaygisiz Z, Erkasap N, Soydan M. Cardiorespiratory responses to submaximal incremental exercise are not affected by one night’s sleep deprivation during the follicular and luteal phases of the menstrual cycle. Indian J Pharmacol. 2003;47(3):279–287. [PubMed] [Google Scholar]
- 89.Kraemer WJ, Kim SK, Bush JA, Nindl BC, Volek JS, Spiering BA, et al. Influence of the menstrual cycle on proenkephalin peptide F responses to maximal cycle exercise. Euro J Appl Physiol. 2006;96(5):581–586. doi: 10.1007/s00421-005-0114-4. [DOI] [PubMed] [Google Scholar]
- 90.Kubo K, Miyamoto M, Tanaka S, Maki A, Tsunoda N, Kanehisa H. Muscle and tendon properties during menstrual cycle. Int J Sports Med. 2009;30(2):139–143. doi: 10.1055/s-0028-1104573. [DOI] [PubMed] [Google Scholar]
- 91.Lara B, Gutiérrez Hellín J, Ruíz-Moreno C, Romero-Moraleda B, Del Coso J. Acute caffeine intake increases performance in the 15-s Wingate test during the menstrual cycle. Br J Clin Pharmacol. 2019;86:745–752. doi: 10.1111/bcp.14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lara B, Gutiérrez-Hellín J, García-Bataller A, Rodríguez-Fernández P, Romero-Moraleda B, Del Coso J. Ergogenic effects of caffeine on peak aerobic cycling power during the menstrual cycle. Euro J Nutr. 2019 doi: 10.1007/s00394-019-02100-7. [DOI] [PubMed] [Google Scholar]
- 93.Lebrun CM, McKenzie DC, Prior JC, Taunton JE. Effects of menstrual cycle phase on athletic performance. Med Sci Sports Exerc. 1995;27(3):437–444. [PubMed] [Google Scholar]
- 94.Lee H, Petrofsky JS, Daher N, Berk L, Laymon M. Differences in anterior cruciate ligament elasticity and force for knee flexion in women: oral contraceptive users versus non-oral contraceptive users. Euro J Appl Physiol. 2014;114(2):285–294. doi: 10.1007/s00421-013-2771-z. [DOI] [PubMed] [Google Scholar]
- 95.Lynch NJ, Nimmo MA. Effects of menstrual cycle phase and oral contraceptive use on intermittent exercise. Eur J Appl Physiol Occup Physiol. 1998;78(6):565–572. doi: 10.1007/s004210050462. [DOI] [PubMed] [Google Scholar]
- 96.Masterson G. The impact of menstrual phases on anaerobic power performance in collegiate women. J Strength Cond Res. 1999;13(4):325–329. [Google Scholar]
- 97.Mattu AT, Iannetta D, MacInnis MJ, Doyle-Baker PK, Murias JM. Menstrual and oral contraceptive cycle phases do not affect submaximal and maximal exercise responses. Scand J Med Sci Sports. 2019 doi: 10.1111/sms.13590. [DOI] [PubMed] [Google Scholar]
- 98.McCracken M, Ainsworth B, Hackney AC. Effects of the menstrual cycle phase on the blood lactate responses to exercise. Eur J Appl Physiol Occup Physiol. 1994;69(2):174–175. doi: 10.1007/BF00609412. [DOI] [PubMed] [Google Scholar]
- 99.Montgomery MM, Shultz SJ. Isometric knee-extension and knee-flexion torque production during early follicular and postovulatory phases in recreationally active women. J Athl Train. 2010;45(6):586–593. doi: 10.4085/1062-6050-45.6.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Okudan N, Gokbel H, Ucok K, Baltaci A. Serum leptin concentration and anaerobic performance do not change during the menstrual cycle of young females. Neuroendocrinol Lett. 2005;26(4):297–300. [PubMed] [Google Scholar]
- 101.Otaka M, Chen SM, Zhu Y, Tsai YS, Tseng CY, Fogt DL, et al. Does ovulation affect performance in tennis players? Br J Sports Med. 2018;4(1):e000305. doi: 10.1136/bmjsem-2017-000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Petrofsky J, Al Malty A, Suh HJ. Isometric endurance, body and skin temperature and limb and skin blood flow during the menstrual cycle. Med Sci Moni. 2007;13(3):111–117. [PubMed] [Google Scholar]
- 103.Quadagno D, Faquin L, Lim GN, Kuminka W, Moffatt R. The menstrual cycle: does it affect athletic performance? Physician Sports Med. 1991;19(3):121–124. [Google Scholar]
- 104.Redman LM, Scroop GC, Norman RJ. Impact of menstrual cycle phase on the exercise status of young, sedentary women. Euro J Appl Physiol. 2003;90(5–6):505–513. doi: 10.1007/s00421-003-0889-0. [DOI] [PubMed] [Google Scholar]
- 105.Rodrigues P, de Azevedo Correia M, Wharton L. Effect of menstrual cycle on muscle strength. J Exerc Physiol Online. 2019;22(5):89–96. [Google Scholar]
- 106.Romero-Moraleda B, Del Coso J, Gutiérrez-Hellín J, Ruiz-Moreno C, Grgic J, Lara B. The influence of the menstrual cycle on muscle strength and power performance. J Hum Kinet. 2019;68:123–133. doi: 10.2478/hukin-2019-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sarwar R, Niclos BB, Rutherford OM. Changes in muscle strength, relaxation rate and fatiguability during the human menstrual cycle. J Physiol. 1996;493(1):267–272. doi: 10.1113/jphysiol.1996.sp021381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shaharudin S, Ghosh AK, Ismail AA. Anaerobic capacity of physically active eumenorrheic females at mid-luteal and mid-follicular phases of ovarian cycle. J Sports Med Phys Fitness. 2011;51(4):576. [PubMed] [Google Scholar]
- 109.Sipavičienė S, Daniusevičiutė L, Klizienė I, Kamandulis S, Skurvydas A. Effects of estrogen fluctuation during the menstrual cycle on the response to stretch-shortening exercise in females. Biomed Res Int. 2013;2013:1–6. doi: 10.1155/2013/243572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smekal G, Von Duvillard SP, Frigo P, Tegelhofer T, Pokan R, Hofmann P, et al. Menstrual cycle: no effect on exercise cardiorespiratory variables or blood lactate concentration. Med Sci Sports Exerc. 2007;39(7):1098–1106. doi: 10.1249/mss.0b013e31805371e7. [DOI] [PubMed] [Google Scholar]
- 111.Sunderland C, Nevill M. Effect of the menstrual cycle on performance of intermittent, high-intensity shuttle running in a hot environment. Euro J Appl Physiol. 2003;88(4–5):345–352. doi: 10.1007/s00421-002-0722-1. [DOI] [PubMed] [Google Scholar]
- 112.Sunderland C, Tunaley V, Horner F, Harmer D, Stokes KA. Menstrual cycle and oral contraceptives’ effects on growth hormone response to sprinting. Appl Physiol Nutr Metab. 2011;36(4):495–502. doi: 10.1139/h11-039. [DOI] [PubMed] [Google Scholar]
- 113.Takase K, Nishiyasu T, Asano K. Modulating effects of the menstrual cycle on cardiorespiratory responses to exercise under acute hypobaric hypoxia. Jpn J Physiol. 2002;52(6):553–560. doi: 10.2170/jjphysiol.52.553. [DOI] [PubMed] [Google Scholar]
- 114.Tounsi M, Jaafar H, Aloui A, Souissi N. Soccer-related performance in eumenorrheic Tunisian high-level soccer players: effects of menstrual cycle phase and moment of day. J Sports Med Phys Fitness. 2018;58(4):497–502. doi: 10.23736/s0022-4707.17.06958-4. [DOI] [PubMed] [Google Scholar]
- 115.Tsampoukos A, Peckham EA, James R, Nevill ME. Effect of menstrual cycle phase on sprinting performance. Eur J Appl Physiol. 2010;109(4):659–667. doi: 10.1007/s00421-010-1384-z. [DOI] [PubMed] [Google Scholar]
- 116.Wearing MP, Yuhosz MD, Campbell R, Love EJ. The effect of the menstrual cycle on tests of physical fitness. J Sports Med Phys Fitness. 1972;12(1):38–41. [PubMed] [Google Scholar]
- 117.Wiecek M, Szymura J, Maciejczyk M, Cempla J, Szygula Z. Effect of sex and menstrual cycle in women on starting speed, anaerobic endurance and muscle power. Acta Physiol Hung. 2016;103(1):127–132. doi: 10.1556/036.103.2016.1.13. [DOI] [PubMed] [Google Scholar]
- 118.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 119.Elliot-Sale KJ, McNulty KL, Ansdell P, Goodall S, Hicks KM, Thomas K, et al. The effects of oral contraceptives on exercise performance in females: a systematic review and meta-analysis. Submitted to Sports Med 2020. [DOI] [PMC free article] [PubMed]
- 120.Schaumberg MA, Jenkins DG, de Jonge XA, Emmerton LM, Skinner TL. Three-step method for menstrual and oral contraceptive cycle verification. J Sci Med Sport. 2017;20(11):965–969. doi: 10.1016/j.jsams.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 121.Sherman BM, Korenman SG. Hormonal characteristics of the human menstrual cycle throughout reproductive life. J Clin Invest. 1975;55(4):699–706. doi: 10.1172/JCI107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wideman L, Montgomery MM, Levine BJ, Beynnon BD, Shultz SJ. Accuracy of calendar-based methods for assigning menstrual cycle phase in women. Sports Health. 2013;5(2):143–149. doi: 10.1177/1941738112469930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bauman JE. Basal body temperature: unreliable method of ovulation detection. Fertil Steril. 1981;36(6):729–733. doi: 10.1016/s0015-0282(16)45916-9. [DOI] [PubMed] [Google Scholar]
- 124.Barron ML, Fehring RJ. Basal body temperature assessment: is it useful to couples seeking pregnancy? MCN Am J Matern Child Nurs. 2005;30(5):290–296. doi: 10.1097/00005721-200509000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Please contact the corresponding author for data requests.