Abstract
The potential health consequences of the Trinity nuclear weapon test of 16 July 1945 at Alamogordo, New Mexico, are challenging to assess. Population data are available for mortality but not for cancer incidence for New Mexico residents for the first 25 y after the test, and the estimates of radiation dose to the nearby population are lower than the cumulative dose received from ubiquitous natural background radiation. Despite the estimates of low population exposures, it is believed by some that cancer rates in counties near the Trinity test site (located in Socorro County) are elevated compared with other locations across the state. Further, there is a concern about adverse pregnancy outcomes and genetic diseases (transgenerational or heritable effects) related to population exposure to fallout radiation. The possibility of an intergenerational effect has long been a concern of exposed populations, e.g., Japanese atomic bomb survivors, survivors of childhood and adolescent cancer, radiation workers, and environmentally exposed groups. In this paper, the likelihood of discernible transgenerational effects is discounted because (1) in all large-scale comprehensive studies of exposed populations, no heritable genetic effects have been demonstrated in children of exposed parents; (2) the distribution of estimated doses from Trinity is much lower than in other studied populations where no transgenerational effects have been observed; and (3) there is no evidence of increased cancer rates among the scientific, military, and professional participants at the Trinity test and at other nuclear weapons tests who received much higher doses than New Mexico residents living downwind of the Trinity site.
Key words: epidemiology, fallout, health effects, nuclear weapons
INTRODUCTION
On 16 July 1945, the first detonation of a nuclear device occurred at the Trinity site near Alamogordo, New Mexico (in Socorro County), on what is now part of White Sands Missile Range. Robert Oppenheimer, the director of Los Alamos National Laboratory at the time, was inspired by the poetry of John Donne to assign the code name “Trinity” to the test. The test was of an implosion-design plutonium device, informally nicknamed “The Gadget.” Among those present at the test, 396 were commissioned officers or enlisted men in the US Army; they have been studied for late effects as nuclear weapons test participants (Till et al. 2014; Boice et al. 2019a).
Population data are available for mortality for New Mexico residents (but not for cancer incidence) for the first 25 y after the test, and the estimates of radiation dose to the population (except that for the thyroid gland) are practically all <10 mGy (Simon et al. 2020), lower than the cumulative external dose received from ubiquitous natural background radiation of approximately 80 mGy over a 40 y period. Despite these estimates of low population exposures, concerns have been raised by the citizens living in the vicinity of the Trinity test that the fallout radiation has caused increased rates of cancer and transgenerational effects, i.e., genetic and adverse pregnancy outcomes (APOs) (TBDC 2017).
The possibility of intergenerational effects has long been a concern of exposed populations, e.g., Japanese atomic bomb survivors and other exposed groups. However, there is little to no convincing or consistent evidence among the offspring of environmentally exposed populations; childhood, adolescent, and young adult cancer survivors; Japanese atomic bomb survivors; or radiation-exposed workers for an excess of malformations, stillbirths, neonatal deaths, cancer, cytogenetic syndromes, single-gene disorders, or cytogenetic markers that would indicate an increase of heritable genetic mutations in the exposed parents (UNSCEAR 2001; COMARE 2004, 2016; Nakamura 2006; NA/NRC 2006; Fujiwara 2008; Winther and Olsen 2012; NCRP 2013; Brent 2015; Grant et al. 2015).
Radiation clearly induces mutations in somatic cells of rodents and humans, and transgenerational (heritable) effects are established from experimental studies conducted in the 1950s and 1960s of irradiated Drosophila and mice (UNSCEAR 2001; NA/NRC 2006; NCRP 2013). Thus, the possibility of human germ-cell mutation following radiation is recognized and considered by radiation protection committees (ICRP 2007; NCRP 2018b). However, the ability to establish an association between parental exposure and transgenerational effects in humans, if one exists, is in the future and would be related to advances in genetic technologies (NCRP 2013; Brent 2015). It is noteworthy that the “mega-mice” studies involved nearly 7 million rodents, which suggest the enormity of a comparable human investigation. Further, the lack of clear and convincing evidence for transgenerational effects in human studies conducted since the 1960s has reduced the level of concern of heritable effects (Fig. 1) (Hall 2009), and radiation protection committees have reduced the genetic component assigned to the radiation health detriment (ICRP 2007; NCRP 2018b). The experimental and human studies support the notion that if transgenerational effects occur in humans, they are too small to be detected by epidemiologic study (IOM 1995).
Fig. 1.
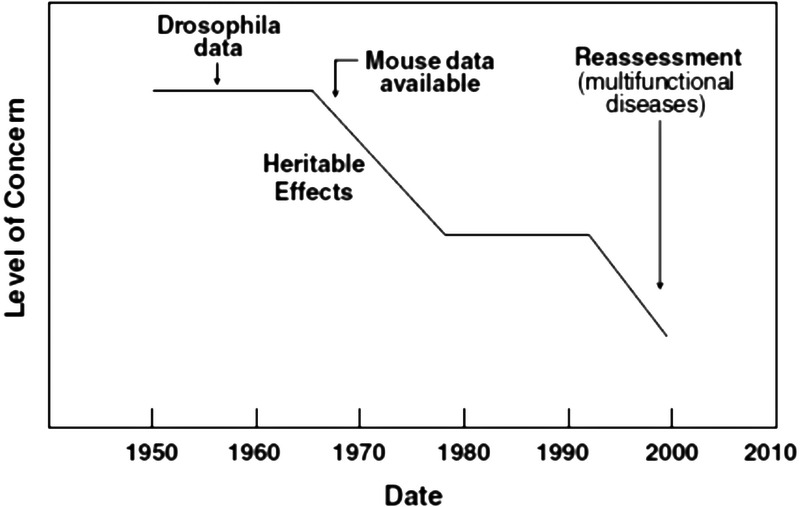
Schematic of how the level of concern about transgenerational (heritable) risks has decreased from the 1950s to the present as more information from human studies has become available (Hall et al. 2009).
METHODS
Human studies of the children of radiation-exposed parents are discussed; specifically, studies of the offspring of environmentally exposed populations; childhood, adolescent, and young adult cancer survivors; atomic bomb survivors; and radiation-exposed workers. The studies sought to identify any excess of malformations, stillbirths, neonatal deaths, cancer, cytogenetic syndromes, single-gene disorders, or cytogenetic markers that would indicate an increase of heritable genetic mutations in the exposed parents.
The section begins, however, with an overview of studies of cancer risk among environmentally exposed populations in New Mexico and among nuclear weapons test participants present at the Trinity detonation. It is generally accepted that a cancer risk in exposed populations is much more likely to be detected than a transgenerational (inheritable) risk, which has not been seen in the children of exposed parents (IOM 1995; NCRP 2013; Brent 2015; NA/NRC 2006).
Studies of cancer risk among environmentally exposed populations
Studies of environmentally exposed populations and cancer risk in New Mexico
The studies described below are of New Mexico residents who lived near radiation facilities such as Los Alamos National Laboratory or the uranium mill in Grants, New Mexico. The potential for exposure was to any atmospheric release of radioactive material during plant operation or to environmental contamination or ingestion of any waste associated with uranium milling.
Stebbings and Voelz (1981) examined both cancer mortality and incidence data from the New Mexico Tumor Registry for Los Alamos County, New Mexico, where Los Alamos National Laboratory is located. They found a suggestive excess mortality from leukemia, but there was no parallel increase in leukemia incidence. There were suggestions of excesses in neoplasms of the reticuloendothelial system in the early years and of the colon and rectum; the latter were thought to be explainable in terms of socioeconomic factors. There was no conclusive evidence of cancer risk among the residents near these radiation facilities.
These observations of cancer risks among populations living near nuclear facilities in New Mexico are based on small numbers but are consistent with the much larger study conducted by the National Cancer Institute (NCI) of cancer risk among populations living near nuclear facilities throughout the United States (Jablon et al. 1991). A special scientific advisory committee of nongovernment scientists was established by NCI to provide guidance and oversight over the study. The committee concluded “that the survey produced no evidence that an excess occurrence of cancer had resulted from living near nuclear facilities. Further, that the measurements of radioactive releases from nuclear facilities indicate that the dose from routine operations is generally much below natural background radiation, and hence are unlikely to produce observable effects on the health of surrounding populations” (Jablon et al. 1990).
Boice et al. (2010) examined both cancer incidence and mortality in populations living near uranium milling and mining operations in Grants, Cibola County, New Mexico, during 1950–2004. Lung cancer mortality and incidence were significantly increased among men but not women, and the excess was attributed to a previously reported risk of lung cancer among underground miners living in Grants and exposed to radon gas and its decay products (Boice et al. 2008). Stomach cancer mortality and incidence were both significantly increased among women but not men. These excesses seem unlikely to be related to uranium milling and mining activities since the elevated risks were greatest in the years before uranium mills and mines operated in Cibola County; furthermore, the stomach cancer rates decreased over time to normal levels.
Studies of nuclear weapons test participants at Trinity and other series, and cancer effects
To provide a different look at the possibility that adverse pregnancy outcomes or genetic disease might occur among the children of parents exposed to fallout from the Trinity detonation, the dose distributions and the mortality experience of the 396 atomic veterans present at the Trinity shot is evaluated. This study of persons who were present at the Trinity detonation in 1945 and who were followed through 2010 provides information on cancer risk at higher doses than were received by residents living near the Trinity site. Those present at the Trinity test included Robert Oppenheimer, General Leslie Groves, Hans Bethe, Enrico Fermi, Theodore Hall, Louis Hempelmann, Hymer Friedell, Richard Feynman, and Kenneth Bainbridge. The Trinity detonation was part of a larger study of 113,806 nuclear weapons test participants conducted within the Million Person Study of Low-Dose Health Effects (MPS) (Bouville et al. 2015; Boice et al. 2019a). Dose estimates for the Trinity participants and all atomic veterans were determined for all participants at one of eight test series (Till et al. 2014, 2018; Beck et al. 2017; Dauer et al. 2018; NCRP 2018a), and doses to red bone marrow are presented in Table 1. Extensive follow-up procedures located over 95% of the cohort (Mumma et al. 2018) and identified a cause of death for practically all known to have died.
Table 1.
Bone marrow dose distributions for atomic veterans at the Trinity test and for all veterans in the Eight Series Studya within the Million Person Study.b
| Bone marrow dose (2-y lag) | ||
|---|---|---|
| Dose (mGy) | Veterans at Trinity | Total number of veterans |
| <2.5 | 225 (57%) | 58,203 (51%) |
| 2.5 to ≤5 | 27 (7%) | 19,092 (17%) |
| 5 to ≤10 | 32 (8%) | 17,050 (15%) |
| 10 to ≤25 | 53 (13%) | 14,195 (12%) |
| >25 | 59 (15%) | 5,266 (5%) |
| Total | 396 | 113,806 |
a The Eight Series Study included military aboveground weapon test participants present at one of these eight test series (year): Upshot-Knothole (1953), Plumbbob (1957); Crossroads (1946); Greenhouse (1951); Castle (1954); Redwing (1956); Hardtack I (1958); Trinity (1945) (Till et al. 2014, 2018); bBeck et al. 2017.
Standardized mortality rates (SMRs) were computed for all Trinity and other test series participants (Table 1 provides a listing of the eight test series) to compare observed rates with the general population and 95% confidence intervals computed. Cox proportional hazards models were used to analyze leukemia and lung cancer dose response. Because only 3 leukemia deaths were due to leukemia other than chronic lymphocytic leukemia (CLL), a malignancy not considered to be increased following radiation exposure (UNSCEAR 2008; Leuraud et al. 2015), the internal analyses could not be conducted of Trinity participants but only of the entire cohort, which included 717 leukemia deaths other than CLL and 8,027 lung cancer deaths.
Among the 396 Trinity participants, 319 (or 81%) had died, and the all cause of death SMR was 0.71 (95% confidence interval [CI]: 0.63–0.79) (Table 2). Cancer mortality also was below expectations but not significantly so (SMR 0.95; 95% CI: 0.77–1.16). The dose distribution of Trinity participants was similar to that of all 113,806 participants (Table 1). The mean dose to red bone marrow was 9 mGy (maximum 35 mGy) and higher than the estimated red bone marrow doses received by New Mexico residents living near the Trinity site (Simon et al. 2020). No excess of leukemia, excluding CLL, or any other cancer was observed among test participants at Trinity. The internal dose-response analyses for all 113,806 test participants did not show an increase for leukemia (excess relative risk [ERR] at 95% CI for 100 mGy = −0.35 [−1.05, 0.34], n = 717) or for lung cancer (ERR at 95% CI for 100 mGy = 0.04 [−0.11, 0.19], n = 8,027) (Boice et al. 2019b).
Table 2.
Standardized mortality ratios (SMRs) and 95% confidence intervals (CIs) among 396 atomic veterans at the Trinity site followed from 1945 through 2010 with 18,884 person-y of follow-up.a
| Trinity | |||
|---|---|---|---|
| Veterans at risk | 396 | ||
| Person-y of follow-up | 18,884 | ||
| Cause of death (ICD9)a | Observed | SMR | 95% CI |
| All causes of death (001–999) | 319 | 0.71c | 0.63–0.79 |
| All malignant neoplasms (140–208) | 97 | 0.95 | 0.77–1.16 |
| Colon (153) | 9 | 0.99 | 0.45–1.88 |
| Bronchus, trachea, and lung (162) | 21 | 0.64c | 0.40–0.98 |
| Prostate (185) | 16 | 1.3 | 0.74–2.11 |
| Non-Hodgkin’s lymphoma (200, 202) | 6 | 1.59 | 0.58–3.46 |
| Leukemia and aleukemia (204–208) | 5b | 1.22 | 0.39–2.85 |
| Smoking-related cancers (140–150, 157, 161–162, 188–189) | 33 | 0.67c | 0.46–0.94 |
| Non-smoking-related cancers | 64 | 1.22 | 0.94–1.56 |
| Diabetes (250) | 8 | 0.94 | 0.40–1.85 |
| Mental and behavioral disorders (290-319) | 6 | 0.8 | 0.29–1.74 |
| Dementia and Alzheimer’s disease (290.0-290.4, 331.0) | 10 | 0.87 | 0.42–1.60 |
| Diseases of the nervous system (320–389) | 10 | 0.69 | 0.33–1.27 |
| All heart disease (390–398, 404, 410–429) | 79 | 0.47c | 0.37–0.59 |
| Ischemic heart disease (410–414) | 64 | 0.50c | 0.39–0.64 |
| Cerebrovascular disease (430–438) | 16 | 0.60c | 0.34–0.97 |
| Nonmalignant respiratory disease (460–519) | 31 | 0.69c | 0.47–0.98 |
| Bronchitis, emphysema, asthma (490–493) | 9 | 0.66 | 0.30–1.25 |
| Nephritis and nephrosis (580–589) | 6 | 1 | 0.37–2.18 |
| All external causes of death (800–999) | 10 | 0.43c | 0.21–0.79 |
| Unknown causes of death | 30 | — | — |
a SMRs shown only for causes of death with five or more cases. ICD9: International Classification of Diseases, 9th Revision.
b 2 of the 5 leukemia deaths were due to chronic lymphocytic leukemia.
c Denotes statistical significance at the p < 0.05 level, i.e., the 95% confidence limit does not contain 1.00.
To place these analyses in perspective with regard to transgenerational effects, the Institute of Medicine (IOM 1995) evaluated the likelihood that an epidemiologic study could detect an increase in heritable genetic effects among the children of atomic veterans (had there been an increase) and concluded that it was not possible. In the absence of any radiation effects, 15,000 newborn children with major birth defects would be expected to be diagnosed at birth among the estimated 500,000 offspring of 210,000 atomic veterans. An additional 3–5% of these children would be expected to be diagnosed with a major congenital anomaly in the first 10 y of life. Thus, the study size would have to be enormous, and controlling for confounding influences would be nearly impossible. Further, the gonadal doses to produce a possible increase in transgenerational effects also would have to be very high. “Relatively high doses of radiation (greater than 2,000 mSv [200 rem]) would add only a small number of additional cases of genetic disorders to the large number that are expected to occur as a result of spontaneous mutations, most of which have existed in the population for many generations” (IOM 1995). Such high doses are not observed among atomic veterans (if they had occurred, deterministic effects would have been evident) nor among the residents near the Trinity site exposed to fallout radiation.
In summary, nuclear weapons test participants received the highest radiation doses of any population from nuclear test detonations in the United States. These doses are still relatively low but higher than those estimated for the populations living near or downwind of the Trinity site (Simon et al. 2020). No dose-related increases in cancer were observed among the Trinity participants or among the large series of 113,806 atomic veterans. The low doses estimated for the smaller number of New Mexico residents near or downwind of the Trinity detonation site point to the implausibility that cancer or transgenerational (heritable) effects occurred in excess or could be observed in any epidemiologic investigation.
Studies of environmentally exposed populations and transgenerational effects
Transgenerational studies have been conducted in areas of high natural background radiation in India (Jaikrishan et al. 1999) and China (Wei et al. 1990) and in areas in Ireland exposed to airborne releases from a nuclear fuel reprocessing plant (Dean et al. 2000). The radiation sources in the environment include thorium-containing monazite sands and effluents from nuclear facilities (NCRP 2013). No transgenerational effects have been demonstrated among people exposed to fallout from the Chernobyl reactor accident (WHO 2006).
Studies of Down syndrome and other genetic anomalies in populations living in high background radiation areas are mostly ecological and are limited because individual doses and potential confounding influences are unknown. An increased rate of Down syndrome among residents in areas of high background radiation in China was later attributed to increased maternal age at birth and to better case ascertainment in the high background radiation areas compared with the control areas (UNSCEAR 1993; Wei et al. 1990). Airborne radiation released from the Sellafield nuclear fuel reprocessing plant in England was claimed to have caused a cluster of Down syndrome on the coast of Ireland but was later discounted (Dean et al. 2000). Studies of 140,000 inhabitants residing in Kerala, India, in areas of high natural background radiation (15 to 25 mGy annual whole-body dose) reported increased rates of Down syndrome (Kochupillai et al. 1976), which were not confirmed in subsequent studies that used more reliable sources of information (Kesavan 1997). No correlation between increased levels of natural background radiation and malformation, stillbirth, or twinning was found in a comprehensive study of over 40,000 newborn children and stillbirths in Kerala (Jaikrishan et al. 1999). High natural background radiation levels in Kerala also were not correlated with increases in mental retardation, cleft lip, or cleft palate (Koya et al. 2012). Clusters of Down syndrome in Germany were reported just after the Chernobyl accident, but low-dose radiation was not considered a contributing cause (Little 1993; Burkart et al. 1997). Offspring of residents in Kerala were reported to have certain inherited genomic changes to mitochondrial DNA (Forster et al. 2002) and to the Y chromosome (Premi et al. 2009), but results have not been replicated, and there is uncertainty as to the gonadal dose received by parents and the adequacy of the control groups.
Reproductive and hereditary effects have not been demonstrated among people exposed to fallout from the Chernobyl accident nor are any expected (Little 1993; WHO 2006). No effects on fertility, numbers of stillbirths, or adverse pregnancy outcomes have been attributed to radiation, in large part because of the low radiation doses received. A modest but steady increase in reported congenital malformations in both contaminated and uncontaminated areas of Belarus appeared related to improved reporting and not to radiation exposure (WHO 2006). “An increased frequency of trisomy 21 in Berlin in January 1987, and increases in the frequency of neural tube defects in several small hospital-based series in Turkey, were not confirmed in larger and more representative series in Europe. No clear changes in the prevalence at birth of anomalies which might be associated with the accident are apparent in Byelorussia or the Ukraine, the republics with the highest exposure to fallout” (Little 1993).
These studies of environmentally exposed populations provide little to no evidence for transgenerational effects.
Studies of the offspring of childhood, adolescent, and young adult cancer survivors treated with radiation and transgenerational effects
In 2006, the Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation (Biological Effects of Ionizing Radiation [BEIR] VII committee) concluded that studies on the genetic effects of radiotherapy on childhood cancer should be encouraged (NA/NRC 2006). Subsequently, heritable disease among the children of cancer survivors treated with radiation in four countries was extensively evaluated in a large-scale international collaboration, the genetic consequences of cancer therapy study (Boice et al. 2003; NCRP 2013). Over 35,800 children of 21,205 cancer survivors were conceived after therapy had ended. The parents in Denmark and Finland were cancer survivors diagnosed under 35 y of age; the parents of US and Canadian cancer survivors were under 20 y of age at cancer diagnosis. Estimates of gonadal doses of radiation were based on original radiation-therapy records and phantom reconstructions (Stovall et al. 2004). No associations between birth defects and gonadal doses were found (Mulvihill et al. 2009; Green et al. 2009; Signorello et al. 2010; Winther et al. 2012). The mean testicular dose for men was 500 mGy, and the mean ovarian dose for women was 1,200 mGy. High therapeutic doses to the uterus of female cancer survivors was found to increase the rates of spontaneous abortions (miscarriages), preterm births, and stillbirths; these adverse pregnancy outcomes were attributed to a somatic (deterministic) effect from a damaged uterus and not to a genetic or heritable effect of the radiation exposure (Signorello et al. 2006; Winther et al. 2008). A small difference was reported for cytogenetic abnormalities (e.g., Down syndrome [relative risk, RR = 1.1] and Turner syndrome [RR = 1.3]) among the children of Danish cancer survivors compared with the children of their siblings but was not statistically meaningful (Winther et al. 2004). An altered sex ratio among the live-born children of cancer survivors treated with radiation therapy was not observed and provided no support for a possible transgenerational or germline effect (Winther et al. 2003).
Molecular analyses of cancer family blood samples (blood taken from the irradiated cancer survivor, the spouse or partner, and at least one child) have been used to study a number of mechanistic processes possibly related to transgenerational effects and cancer susceptibility. Analyses of unstable chromosome aberrations, however, provided no evidence of radiotherapy-related induction of persistent genomic instability (Tawn et al. 2005). G2 chromosomal radiosensitivity evaluations were inconclusive but confirmed that the radiosensitivity phenotype is heritable (Curwen et al. 2010). Polymorphic variation in DNA repair genes showed statistically significant genotype differences between survivors and their partners for the APEX Asp148Glu site, but this initial observation was not confirmed in subsequent studies (Curwen et al. 2011; Wilding et al. 2007). No transgenerational effects of maternal exposure to cancer treatment were seen in an evaluation of mutations in mitochondrial DNA, but the size of the population was small (Guo et al. 2012).
Family blood studies also were used to examine possible radiation-induced germline minisatellite mutations. Minisatellite mutations at hypervariable loci are tandemly repeated regions of DNA which occur at a high frequency throughout the genome. Some repeat DNA sequences exhibit high frequencies of spontaneous germline mutations to new allele lengths (up to 1,000 times more frequent than mutations in genes that code for proteins), and screening for length changes may indicate radiation-induced germline mutations using relatively small population samples. No convincing or consistent evidence has been found, however, that radiation causes germline mutations based on changes in minisatellite lengths among cancer survivor families (mean parental gonadal dose ~500 mGy) or other exposed populations (Tawn et al. 2011, 2015; Little et al. 2013; NCRP 2013, 2015).
These and other studies (Byrne et al. 1998; Green et al. 2009) of the children of childhood, adolescent, and young adult cancer survivors treated with radiation provide little to no evidence for transgenerational effects.
Studies of the offspring of Japanese atomic bomb survivors and transgenerational effects
The Japanese atomic bomb survivor study was initially focused on evaluating and quantifying the risk of genetic disease associated with parental exposures received during the 1945 bombings of Hiroshima and Nagasaki (Neel and Schull 1991). Nearly 80,000 children born to parents exposed to the atomic bombs were evaluated. The measures of possible transgenerational effects included malformations, chromosomal abnormalities, stillbirths, neonatal deaths, cancer, chromosomal translocations, mutations in minisatellites, and multifactorial disease (Schull 2003; Fujiwara et al. 2008). Because experimental studies of Drosophila and rodents had established that radiation can cause heritable effects, it was surprising that there was no evidence for any significantly increased risk for any measure of genetic disease. Although there were no statistically significant findings, most of the measures of transgenerational effects were in the direction of a positive effect. The mean conjoined gonadal dose was of the order of 360 mGy, and it was estimated that the doubling dose (DD, the dose to a population that would produce the same amount of genetic damage as occurs spontaneously each generation) is of the order of 2 Gy for acute exposures and of the order of 4 Gy for chronic exposures, i.e., quite high doses (Neel 1998, 1999a; Schull 2003). Over the years, there has been a shift from concern over genetic effects (to future generations) to concern about the individual and the subsequent development of cancer, a somatic effect (Fig. 1).
The Japanese atomic bomb survivor study of heritable effects is the most comprehensive of all human studies engaged in examining the consequences of preconception irradiation (Schull et al. 1981; Neel and Schull 1991; Satoh et al. 1996; Neel 1998; Izumi et al. 2003; Schull 2003; NA/NRC 2006; Nakamura 2006; Fujiwara et al. 2008; Grant et al. 2015). A broad range of gonadal doses were examined with respect to many indicators of genetic damage (NCRP 2013): (1) untoward pregnancy outcomes (i.e., stillborn, neonatal death, major congenital malformation); (2) cancer in the offspring; (3) early death among offspring (Grant et al. 2015); (4) chromosomal aberrations; (5) frequency of sex-chromosome aneuploids, i.e., having one or more chromosomes above or below the normal number; (6) frequency of mutation-altering protein change or function (electrophoretic mutations); (7) growth and development of the F1 offspring population; (8) inherited mutations in minisatellite DNA (Kodeira et al. 2004); and (9) multifactorial disease (Fujiwara et al. 2008).
These comprehensive studies of the children of Japanese atomic bomb survivors provide little to no evidence for transgenerational effects. The absence of detectable increases in any measure of transgenerational effect is notable in light of similar findings in large-scale studies of the children of cancer survivors and to a lesser effect, in the studies of environmentally exposed and radiation-exposed workers.
Studies of the offspring of radiation-exposed workers and transgenerational effects
Studies of the children of nuclear radiation workers and x-ray technologists are described but are limited by small sample sizes, low gonadal doses, minimal dosimetric information, or inadequate comparison groups (NCRP 2013).
A cluster of leukemia and non-Hodgkin’s lymphoma in young people living in the village of Seascale, Cumbria, UK, was reported in 1983 by a team of investigative television reporters (Black 1984). A subsequent case-control study by Gardner et al. (1990) reported an association between preconception irradiation and leukemia and non-Hodgkin’s lymphoma in children of male workers at the Sellafield nuclear fuel reprocessing plant adjacent to Seascale. Further studies failed to confirm that low doses to the testes received before conception is a cause of cancer (Doll et al. 1994; Kinlen 1993; Kinlen et al. 1993; Little et al. 1996; Neel 1999b; Tawn 1995; UNSCEAR 1994; Wakeford 2000; COMARE 2004, 2016). Then a cohort study confirmed the statistical association between preconception radiation of Sellafield workers and leukemia and lymphoma (Dickinson and Parker 2002), but it was not an independent test of the hypothesis since it included the same cases previously studied by Gardner et al. (1990). Dickinson et al. (2003) had raised a number of important concerns about the original case-control study by Gardner et al. (1990). An infectious agent associated with a high level of population mixing was raised as a possible explanation (Kinlen 1995, 2015; Sorahan et al. 2003).
The possibility that an increase in minisatellite germline mutations following parental exposure could be related to leukemia was discounted when no increase in inherited germline minisatellite mutations were found in children with leukemia (Davies et al. 2007) nor among workers at the Sellafied nuclear fuel reprocessing plant (Tawn et al. 2015). There was no convincing evidence that parental occupational exposure was related to increases in childhood cancer in the children of US radiologic technologists (Johnson et al. 2008).
Studies of workers at the Sellafield nuclear fuel reprocessing plant have reported a statistical association between paternal preconception exposure and stillbirth (Parker et al. 1999), which was questioned by Abrahamson and Tawn (2001) at the time but also was not consistent with a larger study of workers in the UK nuclear industry (Doyle et al. 2000) or with the atomic bomb survivors study (Little 1999; Otake et al. 1990) or with studies of the children of cancer survivors (Mulvihill et al. 2009; Signorello et al. 2010; Winther et al. 2012). Maternal factors were not considered (Boice et al. 2000). Further, an increase in minisatellite germline mutations following worker exposures was not found in their children (Tawn et al. 2015). No association was found between preconception dose and congenital malformations among the children of workers in the Canadian nuclear power industry (Green et al. 1997). A study of preconception radiation among Hanford workers evaluated 12 major congenital anomalies, including Down syndrome (Sever et al. 1988a). There was no evidence for a radiation association overall, except for neural tube defects, which was based on only three cases and which was not confirmed by Doyle and colleagues in the United Kingdom (2000). To further test the Gardner hypothesis, Sever et al. (1997) evaluated childhood cancers around three US Department of Energy nuclear facilities: Hanford Site; Idaho National Engineering Laboratory; and the K-25, Y-12, and X-10 plants at Oak Ridge National Laboratory. No statistically meaningful associations were found between paternal exposure and childhood leukemia, leukemia plus non-Hodgkin’s lymphoma, central nervous system, or all childhood cancers. Studies of medical radiographers are in large part negative with respect to adverse inherited outcomes but are hampered by inadequate dosimetry (Boice et al. 1992; Roman et al. 1996). The low gonadal doses in most occupational studies preclude statistically powerful evaluations.
DISCUSSION
Transgenerational studies in humans
The possibility of transgenerational effects following radiation exposure has been a concern for over 70 y and has been studied in atomic bomb survivors, survivors of childhood and adolescent cancer, radiation workers, and environmentally exposed groups. No radiation-related transgenerational effects, hereditary diseases, adverse pregnancy outcomes, germline minisatellite mutations, stillbirths, neonatal deaths, cancer, early death, chromosome aberrations, mitochondrial DNA changes, cytogenetic abnormalities, single-gene disorders, or any measure of transgenerational effect has been convincingly or consistently demonstrated in any human population exposed to ionizing radiation before conception (UNSCEAR 2001; NA/NRC 2006; NCRP 2013; Brent 2015).
The average gonadal doses have been large, of the order of 300 mGy among atomic bomb survivors, 500 mGy among male cancer survivors, and 1,200 mGy among female cancer survivors. These doses are much higher than those estimated for participants at the Trinity test site or for the New Mexico residents exposed to fallout from the Trinity detonation.
The experimental and human studies support the notion that if radiation causes transgenerational effects in human populations, they are too small to detect by epidemiologic study (IOM 1995; NA/NRC 2006). Many birth anomalies occur (about 3%) from all causes, so that even high gonadal doses of the order of 2,000 mGy would be expected to add only a small number of additional cases of genetic disorders to the large number that are expected to occur as a result of spontaneous mutations or other factors. Recognizing that it would be close to impossible to control for the known, much less the unknown, confounding influences for birth anomalies, the Institute of Medicine (1995) concluded that a valid epidemiologic study of atomic veterans on transgenerational effects could not be conducted.
One of several possible explanations for the absence of radiation-related genetic effects in humans is a biological filtering process; i.e., the germ cells that produce sperm or ova are so damaged by radiation that the body’s natural processes filter out any defective embryo, leading to only a low chance of children being born with birth defects (Brent 1994; NCRP 2013).
CONCLUSION
The Trinity nuclear test of 16 July 1945 resulted in a much lower dose from radioactive fallout to the surrounding population than was experienced by Japanese survivors of the atomic bombs, cancer survivors treated with radiotherapy who were able to have children, and the nuclear weapons test participants present at the Trinity and other tests. Given the absence of evidence for transgenerational effects among the ~80,000 children of Japanese atomic bomb survivors and among the ~36,000 children of cancer survivors in the United States, Canada, Denmark, and Finland, and the enormity of the dose needed to detect an effect had there been one (noting that the birth prevalence of major congenital malformation is ~3%), it is not scientifically or biologically plausible that the low doses experienced from the Trinity fallout could result in transgenerational effects in the children of exposed residents near the Trinity site.
Acknowledgments
The research related to nuclear weapons test participants was supported in part by contracts and grants from NCI (grant no. U01 CA137026) and the US Department of Energy (grant no. DE-SC0008944 awarded to the National Council on Radiation Protection and Measurements), and a discovery grant from the Vanderbilt-Ingram Cancer Center (grant no. 404-3 57-9682). Publication costs for this paper were supported by Intramural Research Program of the NCI, National Institutes of Health, and the Intra-Agency Agreement between the National Institute of Allergy and Infectious Diseases (NIAID) and NCI (NIAID agreement #Y2-Al-5077 and NCI agreement #Y3-CO-5117).
Footnotes
The author declares no conflicts of interest.
REFERENCES
- Abrahamson S, Tawn EJ. Risk of stillbirth in offspring of men exposed to ionising radiation. J Radiol Protect 21:133–144; 2001. [DOI] [PubMed] [Google Scholar]
- Beck HL, Till JE, Grogan HA, Aanenson JW, Mohler HJ, Mohler SS, Voillequé PG. Red bone marrow and male breast doses for a cohort of atomic veterans. Radiat Res 187:221–228; 2017. [DOI] [PubMed] [Google Scholar]
- Black D. Investigation of the possible increased incidence of cancer in West Cumbria. Report of the Independent Advisory Group. London: HMSO; 1984. Available at https://archive.org/details/op1278204-1001. Accessed 27 July 2019. [Google Scholar]
- Boice JD, Jr, Mandel JS, Doody MM, Yoder RC, McGowan R. A health survey of radiologic technologists. Cancer 69:586–598; 1992. [DOI] [PubMed] [Google Scholar]
- Boice JD, Jr, Robison LL, Mertens A, Stovall M, Green DM, Mulvihill JJ. Stillbirths and male irradiation. J Radiol Protect 20:321–322; 2000. [DOI] [PubMed] [Google Scholar]
- Boice JD, Jr, Tawn EJ, Winther JF, Donaldson SS, Green DM, Mertens AC, Mulvihill JJ, Olsen JH, Robison LL, Stovall M. Genetic effects of radiotherapy for childhood cancer. Health Phys 85:65–80; 2003. [DOI] [PubMed] [Google Scholar]
- Boice JD, Jr, Cohen SS, Mumma MT, Chadda B, Blot WJ. A cohort study of uranium millers and miners of Grants, New Mexico, 1979–2005. J Radiol Protect 28:303–325; 2008. [DOI] [PubMed] [Google Scholar]
- Boice JD, Jr, Mumma MT, Blot WJ. Cancer incidence and mortality in populations living near uranium milling and mining operations in Grants, New Mexico, 1950–2004. Radiat Res 174:624–636; 2010. [DOI] [PubMed] [Google Scholar]
- Boice JD, Jr, Cohen SS, Mumma MT, Ellis ED. The Million Person Study, whence it came and why. Int J Radiat Biol [online]. 2019a. Available at https://www.tandfonline.com/doi/abs/10.1080/09553002.2019.1589015?journalCode=irab20. Accessed 28 September 2019. [DOI] [PubMed]
- Boice JD, Jr, Ellis ED, Golden AP, Zablotska LB, Mumma MT, Cohen SS. Sex-specific lung cancer risk among radiation workers in the Million Person Study and among TB-fluoroscopy patients. Int J Radiat Biol [online]. 2019b. Available at https://www.tandfonline.com/doi/abs/10.1080/09553002.2018.1547441?af=R&journalCode=irab20. Accessed 28 September 2019. [DOI] [PubMed]
- Bouville A, Toohey RE, Boice JD, Jr, Beck HL, Dauer LT, Eckerman KF, Hagemeyer D, Leggett RW, Mumma M, Napier B, Pryor KH, Rosenstein M, Schauer DA, Sherbini S, Stram DO, Thompson JL, Till JE, Yoder C, Zeitlin C. Dose reconstruction for the million worker study: status and guidelines. Health Phys 108:206–220; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent RL. Biological factors related to male mediated reproductive and developmental toxicity. In: Olshan AF, Mattison DR, eds. Male-mediated developmental toxicity. New York: Plenum Press; 1994: 209–242. [Google Scholar]
- Brent RL. Protection of the gametes embryo/fetus from prenatal radiation exposure. Health Phys 108:242–274; 2015. [DOI] [PubMed] [Google Scholar]
- Burkart W, Grosche B, Schoetzau A. Down syndrome clusters in Germany after the Chernobyl accident. Radiat Res 147:321–328; 1997. [PubMed] [Google Scholar]
- Byrne J, Rasmussen SA, Steinhorn SC, Connelly RR, Myers MH, Lynch CF, Flannery J, Austin DF, Holmes FF, Holmes GE, Strong LC, Mulvihill JJ. Genetic disease in offspring of long-term survivors of childhood and adolescent cancer. Am J Hum Genet 62:45–52; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Medical Aspects of Radiation in the Environment (COMARE). Review of pregnancy outcomes following preconceptional exposure to radiation, 8th report. Chilton, Didcot, Oxon, UK: Health Protection Agency; 2004. Available at https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/304586/COMARE8thReport.pdf. Accessed 27 July 2019.
- Committee on Medical Aspects of Radiation in the Environment (COMARE). Further consideration of the incidence of cancers around the nuclear installations at Sellafield and Dounreay, 17th report. Chilton, Didcot, Oxon, UK: Health Protection Agency; 2016. Available at https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/554981/COMARE_17th_Report.pdf. Accessed 27 July 2019. [Google Scholar]
- Curwen GB, Cadwell KK, Winther JF, Tawn JE, Rees GS, Olsen JH, Rechnitzer C, Schroeder H, Guldberg P, Cordell HJ, Boice JD., Jr The heritability of G(2) chromosomal radiosensitivity and its association with cancer in Danish cancer survivors and their offspring. Int J Radiat Biol 86:986–995; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curwen GB, Murphy S, Tawn EJ, Winther JF, Boice JD., Jr A study of DNA damage recognition and repair gene polymorphisms in relation to cancer predisposition and G2 chromosomal radiosensitivity. Environ Mol Mutagen 52:72–76; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer LT, Bouville A, Toohey RE, Boice JD, Jr, Beck HL, Eckerman KF, Hagemeyer D, Leggett RW, Mumma MT, Napier B, Pryor KH, Rosenstein M, Schauer DA, Sherbini S, Stram DO, Thompson JL, Till JE, Yoder RC, Zeitlin C. Dosimetry and uncertainty approaches for the million-worker study of radiation workers and veterans: overview of the recommendations in NCRP Report No. 178. Review. Int J Rad Biol [online]. 2018. Available at https://www.tandfonline.com/doi/abs/10.1080/09553002.2018.1536299?journalCode=irab20. Accessed 28 September 2019. [DOI] [PubMed]
- Davies BG, Hussain A, Ring SM, Birch JM, Eden TO, Reeves M, Dubrova YE, Taylor GM. New germline mutations in the hypervariable minisatellite CEB1 in the parents of children with leukaemia. Br J Cancer 96:1265–1271; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean G, Nevin NC, Mikkelsen M, Karadima G, Petersen MB, O’Sullivan J. Investigation of a cluster of children with Down’s syndrome born to mothers who had attended a school in Dundalk, Ireland. Occup Environ Med 57:793–804; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson HO, Parker L. Leukaemia and non-Hodgkin’s lymphoma in children of male Sellafield radiation workers. Int J Cancer 99:436–443; 2002. [DOI] [PubMed] [Google Scholar]
- Dickinson HO, Hodgson JT, Parker L. Comparison of Health and Safety Executive and Cumbrian birth cohort studies of risk of leukaemia/non-Hodgkin’s lymphoma in relation to paternal preconceptional irradiation. J Radiol Protect 23:385–403; 2003. [DOI] [PubMed] [Google Scholar]
- Doll R, Evans HJ, Darby SC. Paternal exposure not to blame. Nature 367:678–680; 1994. [DOI] [PubMed] [Google Scholar]
- Doyle P, Maconochie N, Roman E, Davies G, Smith PG, Beral V. Fetal death and congenital malformation in babies born to nuclear industry employees: report from the nuclear industry family study. Lancet 356:1293–1299; 2000. [DOI] [PubMed] [Google Scholar]
- Forster L, Forster P, Lutz-Bonengel S, Willkomm H, Brinkmann B. Natural radioactivity and human mitochondrial DNA mutations. Proc Natl Acad Sci USA 99:13950–13954; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S, Suyama A, Cologne JB, Akahoshi M, Yamada M, Suzuki G, Koyama K, Takahashi N, Kasagi E, Grant EJ, Lagarde E, Hsu WL, Furukawa K, Ohishi W, Tatsukawa Y, Neriishi K, Takahashi I, Ashizawa K, Hida A, Imaizumi M, Nagano J, Cullings HM, Katayama H, Ross NP, Kodama K, Shore RE. Prevalence of adult-onset multifactorial disease among offspring of atomic bomb survivors. Radiat Res 170:451–457; 2008. [DOI] [PubMed] [Google Scholar]
- Gardner MJ, Snee MP, Hall AJ, Powell CA, Downes S, Terrell JD. Results of case-control study of leukaemia and lymphoma among young people near Sellafield nuclear plant in West Cumbria. BMJ 300:423–434; 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant EJ, Furukawa K, Sakata R, Sugiyama H, Sadakane A, Takahashi I, Utada M, Shimizu Y, Ozasa K. Risk of death among children of atomic bomb survivors after 62 years of follow-up: a cohort study. Lancet Oncol 16:1316–1323; 2015. [DOI] [PubMed] [Google Scholar]
- Green L, Dodds L, Miller A, Tomkins D, Jiehui L, Escobar M. Risk of congenital anomalies in children of parents occupationally exposed to low level ionising radiation. Occup Environ Med 54:629–635; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Sklar CA, Boice JD, Jr, Mulvihill JJ, Whitton JA, Stovall M, Yasui Y. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the childhood cancer survivor study. J Clin Oncol 27:2374–2381; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Cai Q, Samuels DC, Ye F, Long J, Li CI, Winther JF, Tawn EJ, Stovall M, Lahteenmaki P, Malia N, Levy S, Shaffer C, Shyr Y, Shu XO, Boice JD., Jr The use of next generation sequencing technology to study the effect of radiation therapy on mitochondrial DNA mutation. Mutat Res 744:154–160; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall EJ. Radiation biology for pediatric radiologists. Pediatr Radiol 39(Suppl 1):S57–S64; 2009. [DOI] [PubMed] [Google Scholar]
- International Commission on Radiological Protection. The 2007 recommendations of the International Commission on Radiological Protection. Oxford: Pergamon Press; ICRP Publication 103; 2007. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Committee to study the feasibility of, and need for, epidemiologic studies of adverse reproductive outcomes in the families of atomic veterans. Adverse reproductive outcomes in families of atomic veterans: the feasibility of epidemiologic studies. Washington, DC: National Academy Press; 1995. Available at http://www.nap.edu/catalog/4992.html. Accessed 13 January 2019. [PubMed] [Google Scholar]
- Izumi S, Syuama A, Koyama K. Radiation-related mortality among offspring of atomic bomb survivors: a half-century of follow-up. Int J Cancer 107:292–297; 2003. [DOI] [PubMed] [Google Scholar]
- Jablon S, Hrubec Z, Boice JD, Jr, Stone BJ. Cancer in populations living near nuclear facilities. Washington, DC: US Government Printing Office; NIH Publ 90-874; 1990. [Google Scholar]
- Jablon S, Hrubec Z, Boice JD., Jr Cancer in populations living near nuclear facilities. A survey of mortality nationwide and incidence in two states. JAMA 265:1403–1408; 1991. [PubMed] [Google Scholar]
- Jaikrishan G, Andrews VJ, Thampi MV, Koya PK, Rajan VK, Chauhan PS. Genetic monitoring of the human population from high-level natural radiation areas of Kerala on the southwest coast of India. I. Prevalence of congenital malformations in newborns. Radiat Res 152(6 Suppl):S149–S153; 1999. [PubMed] [Google Scholar]
- Johnson KJ, Alexander BH, Doody MM, Sigurdson AJ, Linet MS, Spector LG, Hoffbeck RW, Simon SL, Weinstock RM, Ross JA. Childhood cancer in the offspring born in 1921–1984 to US radiologic technologists. Br J Cancer 99:545–550; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavan PC. Indian research on high levels of natural radiation: pertinent observations for further studies. In: Wei L, Sugahara T, Tao Z, eds. High levels of natural radiation, 1996: radiation dose and health effects. Amsterdam: Elsevier; 1997: 112–117. [Google Scholar]
- Kinlen LJ. Can paternal preconceptional radiation account for the increase of leukaemia and non-Hodgkin’s lymphoma in Seascale? BMJ 306:1718–1721; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlen LJ. Epidemiological evidence for an infective basis in childhood leukaemia. Br J Cancer 71:1–5; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlen L. Population mixing and childhood leukaemia. Eur J Epidemiol 2015 30:1331; 2015. [DOI] [PubMed] [Google Scholar]
- Kinlen LJ, Clarke K, Balkwill A. Paternal preconceptional radiation exposure in the nuclear industry and leukaemia and non-Hodgkin’s lymphoma in young people in Scotland. BMJ 306:1153–1158; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochupillai N, Verma IC, Grewal MS, Ramalingaswami V. Down’s syndrome and related abnormalities in an area of high background radiation in coastal Kerala. Nature 262:60–61; 1976. [DOI] [PubMed] [Google Scholar]
- Kodeira M, Izumi S, Takahashi N, Nakamura N. No evidence of radiation effect on mutation rates at hypervariable minisatellite loci in the germ cells of atomic bomb survivors. Radiat Res 162:350–356; 2004. [DOI] [PubMed] [Google Scholar]
- Koya PK, Chougaonkar MP, Predeep P, Jojo PJ, Cheriyan VD, Mayya YS, Seshadri M. Effect of low and chronic radiation exposure: a case-control study of mental retardation and cleft lip/palate in the monazite-bearing coastal areas of southern Kerala. Radiat Res 177:109–116; 2012. [DOI] [PubMed] [Google Scholar]
- Leuraud K, Richardson DB, Cardis E, Daniels RD, Gillies M, O’Hagan JA, Hamra GB, Haylock R, Laurier D, Moissonnier M, Schubauer-Berigan MK, Thierry-Chef I, Kesminiene A. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol 2:e276–281; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. The Chernobyl accident, congenital anomalies and other reproductive outcomes. Paediatr Perinat Epidemiol 7:121–151;1993. [DOI] [PubMed] [Google Scholar]
- Little MP. A comparison of the risk of stillbirth associated with paternal pre-conception irradiation in the Sellafield workforce with that of stillbirth and untoward pregnancy outcome among Japanese atomic bomb survivors. J Radiol Protect 19:361–373; 1999. [DOI] [PubMed] [Google Scholar]
- Little MP, Wakeford R, Charles MW, Andersson M. A comparison of the risks of leukaemia and non-Hodgkin’s lymphoma in the first generation offspring of the Danish Thorotrast patients with those observed in other studies of parental pre-conception irradiation. J Radiol Protect 16:25–36; 1996. [Google Scholar]
- Little MP, Goodhead DT, Bridges BA, Bouffler SD. Evidence relevant to untargeted and transgenerational effects in the offspring of irradiated parents. Mutat Res 753:50–67; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumma MT, Cohen SS, Sirko JL, Ellis ED, Boice JD., Jr Obtaining vital status and cause of death on a million persons. Int J Radiat Biol [online]. 2018. Available at https://www.ncbi.nlm.nih.gov/pubmed/30412007. Accessed 9 November 2018. [DOI] [PubMed]
- Mulvihill JJ Boice JD Winther JF Malila N Olsen JH Lähteenmäki PM Madanat-Harjuoja LM Sankila R Stovall M Tawn EJ, the GCCT Study Group. Germ cell mutations: genetic disease in offspring of cancer survivors.. American Society of Human Genetics Meeting, Honolulu, HI, 20–24 October 2009, Platform Abstract 111 [online]. 2009. Available at http://www.ashg.org/2009meeting/abstracts/fulltext/f21783.htm. Accessed 28 September 2019.
- Nakamura N. Genetic effects of radiation in atomic-bomb survivors and their children: past, present and future. J Radiat Res 47(Suppl. B):B67–B73; 2006. [DOI] [PubMed] [Google Scholar]
- National Academies/National Research Council. Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- National Council on Radiation Protection and Measurements. Preconception and prenatal radiation exposure: health effects and protective guidance. Bethesda, MD: NCRP; Report 174; 2013. [Google Scholar]
- National Council on Radiation Protection and Measurements. Health effects of low doses of radiation: perspectives on integrating radiation biology and epidemiology. Bethesda, MD: NCRP; Commentary 24; 2015. [Google Scholar]
- National Council on Radiation Protection and Measurements. Deriving organ doses and their uncertainty for epidemiologic studies (with a focus on the one million US workers and veterans study of low-dose radiation health effects). Bethesda, MD: NCRP; Report 178; 2018a. [Google Scholar]
- National Council on Radiation Protection and Measurements. Management of exposure to ionizing radiation: radiation protection guidance for the United States (2018). Bethesda, MD: NCRP; Report 180; 2018b. [Google Scholar]
- Neel JV. Genetic studies at the Atomic Bomb Casualty Commission-Radiation Effects Research Foundation: 1946–1997. Proc Natl Acad Sci USA 95:5432–5436; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel JV. Changing perspectives on the genetic doubling dose of ionizing radiation for humans, mice, and Drosophila. Teratol 59:216–221; 1999a. [DOI] [PubMed] [Google Scholar]
- Neel JV. Two recent radiation-related genetic false alarms: leukemia in West Cumbria, England, and minisatellite mutations in Belarus. Teratol 59:302–306; 1999b. [DOI] [PubMed] [Google Scholar]
- Neel JV, Schull WJ. The children of atomic bomb survivors. Washington, DC: National Academy Press; 1991. [PubMed] [Google Scholar]
- Otake M, Schull WJ, Neel JV: Congenital malformations, stillbirths, and early mortality among the children of atomic bomb survivors: a reanalysis. Radiat Res 122:1–11; 1990. [PubMed] [Google Scholar]
- Parker L, Pearce MS, Dickinson HO, Aitkin M, Craft AW. Stillbirths among offspring of male radiation workers at Sellafield nuclear reprocessing plant. Lancet 354(9188):1407–1414; 1999. [DOI] [PubMed] [Google Scholar]
- Premi S, Srivastava J, Chandy SP, Ali S. Unique signatures of natural background radiation on human Y chromosomes from Kerala, India. PLOS ONE 4:e4541; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman E, Doyle P, Ansell P, Bull D, Beral V. Health of children born to medical radiographers. Occup Environ Med 53:73–77; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh C, Takahashi N, Asakawa J, Kodaira M, Kuick R, Hanash SM, Neel JV. Genetic analysis of children of atomic bomb survivors. Environ Health Perspect 104(Suppl 3):511–519; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schull WJ. The children of atomic bomb survivors: a synopsis. J Radiol Protect 23:369–384; 2003. [DOI] [PubMed] [Google Scholar]
- Schull WJ, Otake M, Neel JV. Genetic effects of the atomic bombs: a reappraisal. Science 213:1220–1227; 1981. [DOI] [PubMed] [Google Scholar]
- Sever LE, Gilbert ES, Tucker K, Greaves JA, Greaves C, Buchanan J. Epidemiologic evaluation of childhood leukemia and paternal exposure to ionizing radiation. Springfield, VA: National Technical Information Service; U50/CCU012545-01; 1997. Available at https://www.cdc.gov/niosh/oerp/pdfs/2001-133g7.pdf. Accessed 27 July 2019. [Google Scholar]
- Sever LE, Hessol NA, Gilbert ES, McIntyre JM. The prevalence at birth of congenital malformations in communities near the Hanford site. Am J Epidemiol 127:243–254; 1988a. [DOI] [PubMed] [Google Scholar]
- Sever LE, Gilbert ES, Hessol NA, McIntyre JM. A case-control study of congenital malformations and occupational exposure to low-level ionizing radiation. Am J Epidemiol 127:226–242;1988b. [DOI] [PubMed] [Google Scholar]
- Signorello LB, Cohen SS, Bossetti C, Stovall M, Kasper CE, Weathers RE, Whitton JA, Green DM, Donaldson SS, Mertens AC, Robison LL, Boice JD., Jr Female survivors of childhood cancer: preterm birth and low birth weight among their children. J Natl Cancer Inst 98:1453–1461; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorello LB, Mulvihill JJ, Green DM, Munro HM, Stovall M, Weathers RE, Mertens AC, Whitton JA, Robison LL, Boice JD., Jr Stillbirth and neonatal death in relation to radiation exposure before conception: a retrospective cohort study. Lancet 376:624–630; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorello LB, Mulvihill JJ, Green DM, Munro HM, Stovall M, Tawn EJ, Weathers RE, Mertens AC, Whitton JA, Robison LL, Boice JD., Jr Congenital anomalies in the children of cancer survivors: a report from the childhood cancer survivor study. J Clin Oncol 30:239–245; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Bouville A, Beck H, Melo D. Estimated radiation doses received from the 1945 Trinity nuclear test. Health Phys 119:428–477; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorahan T, Haylock RG, Muirhead CR, Bunch KJ, Kinlen LJ, Little MP, Draper GJ, Kendall GM, Lancashire RJ, English MA. Cancer in the offspring of radiation workers: an investigation of employment timing and a reanalysis using updated dose information. Br J Cancer 89:1215–1220; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbings JH, Jr, Voelz GL. Morbidity and mortality in Los Alamos County, New Mexico. I. Methodological issues and preliminary results. Environ Res 25:86–105; 1981. [DOI] [PubMed] [Google Scholar]
- Stovall M, Donaldson SS, Weathers RE, Robison LL, Mertens AC, Winther JF, Olsen JH, Boice JD., Jr Genetic effects of radiotherapy for childhood cancer: gonadal dose reconstruction. Int J Radiat Oncol Biol Phys 60:542–552; 2004. [DOI] [PubMed] [Google Scholar]
- Tawn EJ. Leukaemia and Sellafield: is there a heritable link? J Med Genet 32:251–256; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawn EJ, Curwen GB, Rees GS, Jonas P. Germline minisatellite mutations in workers occupationally exposed to radiation at the Sellafield nuclear facility. J Radiat Protect 35:21–36; 2015. [DOI] [PubMed] [Google Scholar]
- Tawn EJ, Rees GS, Leith C, Winther JF, Curwen GB, Stovall M, Olsen JH, Rechnitzer C, Schroeder H, Guldberg P, Boice JD., Jr Germline minisatellite mutations in survivors of childhood and young adult cancer treated with radiation. Int J Radiat Biol 87:330–340; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawn EJ, Whitehouse CA, Winther JF, Curwen GB, Stovall M, Olsen JH, Guldberg P, Rechnitzer C, Schrøder H, Boice JD., Jr Chromosome analysis in childhood cancer survivors and their offspring—no evidence for radiotherapy-induced persistent genomic instability. Mutat Res 583:198–206; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till JE, Beck HL, Aanenson JW, Grogan HA, Mohler HJ, Mohler SS, Voillequé PG. Military participants at U.S. atmospheric nuclear weapons testing—methodology for estimating dose and uncertainty. Radiat Res 181:471–484; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till JE, Beck HL, Aanenson JW, Grogan HA, Mohler HJ, Mohler SS, Voillequé PG. Dosimetry associated with veterans who participated in nuclear weapons testing. Int J Radiat Biol [online]. 2018. Available at https://www.tandfonline.com/doi/abs/10.1080/09553002.2018.1551639?journalCode=irab20. Accessed 28 September 2019. [DOI] [PubMed]
- Tularosa Basin Downwinders Consortium. Unknowing, unwilling, and uncompensated: the effects of the Trinity test on New Mexicans and the potential benefits of Radiation Exposure Compensation Act (RECA) amendments [online]. 2017. Available at https://www.kob.com/kobtvimages/repository/cs/files/TBDC%20HIA%20final%20report%202-8-2017.pdf. Accessed 15 March 2019.
- United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and effects of ionizing radiation. New York: United Nations; 1993. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and effects of ionizing radiation. New York: United Nations; 1994. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation. Report to the General Assembly, with scientific annexes. Hereditary effects of radiation, UNSCEAR 2001. New York: United Nations; 2001. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation. Effects of ionizing radiation, UNSCEAR 2006. Scientific annex A: epidemiological studies of radiation and cancer; Vol I. New York: United Nations; 2008. [Google Scholar]
- Wakeford R. Study of childhood cancer and paternal preconceptional irradiation at USA nuclear facilities. J Radiol Protect 20:331–332; 2000. [DOI] [PubMed] [Google Scholar]
- Wei L, Zha Y, Tao Z, He W, Chen D, Yuan Y. Epidemiological investigation of radiological effects in high background radiation areas of Yangjiang, China. J Radiat Res 31:119–136; 1990. [PubMed] [Google Scholar]
- Wilding CS, Curwen GB, Tawn EJ, Sheng X, Winther JF, Chakraborty R, Boice JD., Jr Influence of polymorphisms at loci encoding DNA repair proteins on G2 chromosomal radiosensitivity. Environ Mol Mutagen 48:48–57; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winther JF, Olsen JH. Does cancer treatment in childhood induce transgenerational genetic damage? J Clin Oncol 30:225–226; 2012. [DOI] [PubMed] [Google Scholar]
- Winther JF, Boice JD, Jr, Thomsen BL, Schull WJ, Stovall M, Olsen JH. Sex ratio among offspring of childhood cancer survivors treated with radiotherapy. Br J Cancer 88:382–387; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winther JF, Boice JD, Jr, Mulvihill JJ, Stovall M, Frederiksen K, Tawn EJ, Olsen JH. Chromosomal abnormalities among offspring of childhood cancer survivors in Denmark: population-based study. Am J Hum Genet 74:1282–1285; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winther JF, Boice JD, Jr, Svendsen AL, Frederiksen K, Stovall M, Olsen JH. Spontaneous abortion after radiotherapy for childhood cancer: a population-based cohort study. J Clin Oncol 26:4340–4346; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winther JF, Olsen JH, Wu H, Shyr Y, Mulvihill JJ, Stovall M, Nielsen A, Schmiegelow M, Boice JD., Jr Genetic disease in the children of Danish survivors of childhood and adolescent cancer. J Clin Oncol 30:27–33; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Health effects of the Chernobyl accident: n overview [online]. 2006. Available at https://www.who.int/ionizing_radiation/chernobyl/backgrounder/en/. Accessed 1 August 2019.


