This study showed strong public interest for 2 hypothetical diagnostic tests for Neisseria gonorrhoeae/Chlamydia trachomatis detection and N. gonorrhoeae resistance marker identification, but highlighted some challenges relating to their adoption.
Supplemental digital content is available in the text.
Abstract
Background
To assess the potential market for 2 hypothetical diagnostic tests, one for Neisseria gonorrhoeae/Chlamydia trachomatis (NG/CT) detection and one for NG antimicrobial resistance (AMR) marker identification.
Methods
This is a qualitative interview-based study. Semistructured interviews with global- and country-level experts were performed. Interviewees were provided with simplified versions of Foundation for Innovative New Diagnostics/World Health Organization–developed target product profiles for each test. Interviewees were asked to comment on use cases, test characteristics, and factors that may influence test adoption.
Results
Twenty-one experts were interviewed, including 15 country-level experts (from South Africa, India, Zimbabwe, Ghana, China, Peru, Kenya, and Cambodia). Interviewees welcomed an NG/CT point-of-care test, with near-universal preference for a test that could detect symptomatic and asymptomatic infections. Interviewees also saw value in a test that could be used to screen high-risk populations. Factors that may drive adoption of the NG/CT test identified by interviewees included price, cost-effectiveness, evidence of public health benefit, and World Health Organization guidance. Interviewees felt that AMR test use would likely be limited to patients failing first-line treatment.
Conclusions
Although the potential target population for an NG/CT diagnostic test in low- and middle-income countries is sizeable, there are areas of uncertainty relating to the price of the test and its intended use, warranting further research to determine the most effective positioning. An NG AMR test would likely be used very selectively.
In 2016, there were an estimated 87 million new cases of gonorrhea globally.1 Gonorrhea is often asymptomatic in infected women, of whom only around 34% exhibit symptoms.2 However, infection can result in severe complications including infertility, pelvic inflammatory disease, and adverse pregnancy outcomes. Although gonorrhea is treatable with antibiotics, Neisseria gonorrhoeae (NG) has progressively developed resistance to most available antibiotics, including all those that have to date been recommended for first-line treatment, raising concern for the longevity of the remaining and future therapies.3,4 Currently, World Health Organization (WHO) guidelines recommend the use of cefixime or ceftriaxone, in combination with azithromycin, for NG infection,5 but resistance to these “last-line” antibiotics is increasing.6,7
The prevalence of gonorrhea in low- and middle-income countries (LMICs) is particularly high.1 Management of gonorrhea and other sexually transmitted infections (STIs) in LMICs is largely based on a syndromic approach,8 in which patients are identified and treated on the basis of characteristic signs and symptoms; for gonorrhea, these include urethral or vaginal discharge and lower abdominal pain. This inevitably leads to overtreatment, which is thought to be one of the drivers of antimicrobial resistance (AMR), and to missed treatment, which can facilitate further transmission and lead to severe complications in untreated patients. Syndromic management has been successful in reducing the prevalence of STIs over the years, but has now reached its limits for the aforementioned reasons.9,10 Etiological case management, in which only patients with a positive NG or Chlamydia trachomatis (CT) diagnosis receive treatment, is therefore highly desirable.11,12
Current diagnostic tests for NG include Gram staining of urethral discharge smears and bacterial culture. Smears have limited sensitivity in populations other than men with urethral discharge, whereas culture takes several days and cannot be performed at the point of care (POC).4 Nucleic acid amplification tests for NG and CT are available, but cost and operational restrictions limit these to higher levels of health care in selected countries.4 To enable effective etiological case management, a rapid and easy-to-use POC diagnostic test that can provide accurate results that immediately inform treatment is required. If sensitivity is sufficiently high, such a test could also be used to screen high-risk groups to identify asymptomatic infections, which are estimated to make up a substantial proportion of overall cases.
Current methods to detect AMR in patients with gonorrhea also rely on slow bacterial culture techniques that do not return results in a time frame conducive to treatment. A POC AMR test, however, could be used to inform the selection of antibiotics for treatment, allowing the most appropriate antibiotics to be prescribed for each case and potentially reducing the development of resistance. A molecular assay for the detection of NG and ciprofloxacin susceptibility has been recently approved13,14; however, utility in most African and Southeast Asian countries would be limited owing to the already high levels of resistance to this antibiotic.6 An AMR POC test that can detect resistance to ceftriaxone, cefixime, and azithromycin would be of greater value in these settings.
To drive the development of new tools for gonorrhea control and antibiotic stewardship, WHO and the Foundation for Innovative New Diagnostics have developed target product profiles (TPPs) for 2 potential diagnostic tests.15,16 The first TPP (TPP1) is for a test to detect NG (with or without the ability to detect CT infection). Two versions of the tests are being considered: a test that will improve on the performance of syndromic management by identifying NG/CT in symptomatic patients and a second, more sensitive version that could be used for screening asymptomatic populations. The second TPP (TPP2) is for a test to identify NG resistance markers (either alone or in combination with NG/CT diagnosis).
The potential market size for these tests is substantial. Using published prevalence data, it can be estimated that more than 70 million people in LMICs are symptomatic and likely to present for NG/CT testing, assuming that 40% of men and 30% of women seek care (Fig. 1).1,2,17 The total sizes of the high-risk and vulnerable populations that may be considered for asymptomatic NG/CT screening are also large, with an estimated 4 million female sex workers, 4.9 million men who have sex with men (MSM), 130,000 people enrolled in HIV preexposure prophylaxis (PrEP), 107 million pregnant women seen at antenatal care clinics, and 509 million young women/adolescents (although only a fraction of these young women would be considered high risk).17 However, despite these sizeable eligible populations, a number of factors, including affordability and operational constraints, will limit actual demand. The objective of this study was to gain qualitative insights into the potential demand for both diagnostic tests under various use scenarios. For this research, we took an exploratory approach through global- and country-level expert interviews to gain a broad range of views.
Figure 1.
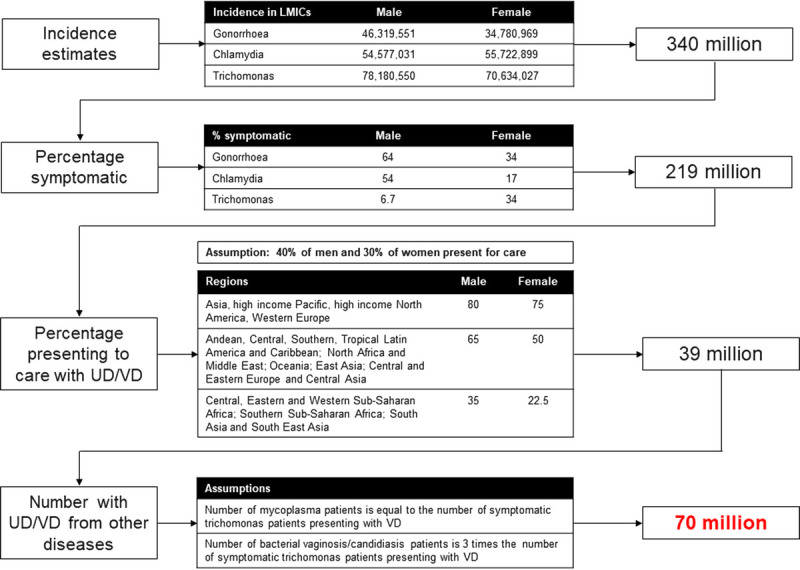
Estimating number of symptomatic patients eligible for NG/CT testing. Incidence estimates taken from Rowley et al.,1 percentage of symptomatic patients and percentage presenting to care taken from Newman et al.,2 and assumptions from Korenromp et al.17 VD, vaginal discharge; UD, urethral discharge.
MATERIALS AND METHODS
Both global- and country-level experts were interviewed. Country-level experts were persons involved in national STI programs or surveillance, local academics, or persons involved in nongovernmental implementation program. Global experts were from WHO or other health care organizations with interest in diagnostics and/or STIs. The interviews were performed by a single interviewer by telephone, according to a semistructured interview guide. The interviewer was an experienced global health markets expert. Separate guides were developed for global-level experts (Supplementary Figure 1A, http://links.lww.com/OLQ/A517) and country-level experts (Supplementary Figure 1B, http://links.lww.com/OLQ/A518). Interviews were carried out between February and March 2019.
The interviews centered around simplified versions of the draft TPPs that were available at the time of the study (Table 1) and shown to the interviewees. Four potential primary use scenarios were described: (a) the NG/CT diagnostic test in symptomatic patients only, (b) the NG/CT diagnostic test for testing symptomatic patients and screening asymptomatic patients, (c) an AMR test for use in confirmed NG cases, and (d) a comprehensive NG/CT diagnosis and AMR test.
TABLE 1.
Simplified TPPs Used During Expert Interviews
| TPP1: Diagnosis of NG and CT in Symptomatic Patients | |
|---|---|
| Purpose | Diagnosis of gonorrhea and chlamydia |
| Use scenario | Primarily for use in symptomatic patients at primary health care level |
| Sample type | Vaginal swab (female) and urethral swab (male; possibly urine, rectal, pharyngeal) |
| Time to result | 10–30 min |
| Sensitivity | Better than syndromic management; not high enough for asymptomatic cases |
| Cost | ~US$1 |
| TPP1: Diagnosis of NG and CT in Symptomatic and Asymptomatic Patients | |
|---|---|
| Purpose | Diagnosis of gonorrhea and chlamydia |
| Use scenario | In symptomatic patients in primary health care and for screening of asymptomatic patients |
| Sample type | Vaginal swab (female) and urethral swab (male; possibly urine, rectal, pharyngeal) |
| Time to result | 10–30 min |
| Sensitivity | Sufficiently high to detect asymptomatic cases of gonorrhea |
| Cost | ~US$5 |
| TPP2 (Resistance Only): Individualized Treatment for Confirmed NG Cases (Already Diagnosed) | |
|---|---|
| Purpose | Identify resistance to 2 common antibiotics in confirmed gonorrhea cases |
| Use scenario | In patients testing positive for gonorrhea, most likely at facilities with a small laboratory area |
| Sample type | Vaginal swab (female) and urethral swab (male; possibly urine, rectal, pharyngeal) |
| Time to result | 20–60 min |
| Format | Either an easy to use, sample-in answer-out, POC instrument with disposable cartridges or a disposable cartridge ± a reader |
| Cost | ~$US5 to $US10 per test cartridge; ± an instrument costing US$5000–US$7000 |
| TPP2 (Comprehensive): Diagnosis of NG and CT (High Sensitivity) and Individualized Treatment | |
|---|---|
| Purpose | Diagnosis of NG and CT (symptomatic and asymptomatic) and identification of resistance to 3 commonly used treatments (genetic markers) |
| Use scenario | Most likely use at facilities with a small laboratory area (possibility of use at primary health care level if disposable cartridge format) |
| Sample type | Vaginal swab (female) and urethral swab (male; possibly urine, rectal, pharyngeal) |
| Time to result | 20–60 min |
| Format | Either an easy to use, sample-in answer-out, POC instrument with disposable cartridges or a disposable cartridge ± a reader |
| Cost | ~$US5 to $US10 per test cartridge; ± an instrument costing US$5000–$US7000 |
CT indicates Chlamydia trachomatis; NG, Neisseria gonorrhoeae; POC, point of care; TPP, target product profile.
The interviews focused on understanding the local context in terms of gonorrhea and AMR control (for country-level experts), prioritization of test options and potential use cases, key test characteristics, and identification of factors that could influence test adoption. Interviewees were asked which of the tests were most important from a public health perspective and why, and to comment on which use cases would be most likely to be adopted. Country-level experts were also asked about current guidelines, practice, and structure of care in their countries.
Because this was an interview study only, no institutional review board or ethics committee approval was required.
RESULTS
Expert Demographics
Twenty-one experts were interviewed, including 15 country-level experts (5 from South Africa, 4 from India, and 1 each from Zimbabwe, Ghana, China, Peru, Kenya, and Cambodia; Table 2). Six interviewees either worked directly for a national STI/HIV program or advised the programs at the national or state level, 2 worked for the national disease surveillance institute, 4 were local academics, and 3 were from local nongovernmental organizations or implementation programs that deliver services and conduct research. Country-level experts' feedback related to knowledge of their local setting, including operational and financial challenges, whereas global experts' opinions were primarily related to national implementation of global policies and guidelines.
TABLE 2.
Affiliations and Countries of Expert Interviewees
| No. | Organization | Country |
|---|---|---|
| 1 | World Health Organization | Global |
| 2 | Global Antibiotic Research and Development Partnership | Global |
| 3 | Foundation for Innovative New Diagnostics (FIND) | Global |
| 4 | Sweden National Reference Laboratory for STIs | Global |
| 5 | Western Sydney Sexual Health Centre/University of Sydney | Global |
| 6 | University College London, Centre for Gender and Global Health | Global |
| 7 | Wits Reproductive Health and HIV Institute, University of the Witwatersrand | South Africa |
| 8 | National Health Laboratory System | South Africa |
| 9 | Anova Health Institute | South Africa |
| 10 | Foundation for Professional Development | South Africa |
| 11 | National Institute for Communicable Diseases | South Africa |
| 12 | National AIDS Control Organization | India |
| 13 | STI advisor, formally National AIDS Control Organization | India |
| 14 | Private practice, former national STI advisor | India |
| 15 | Centers for Disease Control and Prevention/Christian Medical Association of India | India/Sri Lanka |
| 16 | US Centers for Disease Control and Prevention | Cambodia |
| 17 | National Center for AIDS/STD Control and Prevention | China |
| 18 | Kwame Nkrumah University of Science and Technology | Ghana |
| 19 | Kenya Medical Research Institute | Kenya |
| 20 | School of Public Health | Peru |
| 21 | Harare Skin & Genito-Urinary Medicine Clinic | Zimbabwe |
AIDS indicates acquired immunodeficiency virus; STD, sexually transmitted disease; STI, sexually transmitted infection.
NG/CT Test: Use Scenarios
In general, interviewees stated that they would welcome an NG/CT POC test. There was near-universal preference for a test that could detect symptomatic and asymptomatic infections.
There were differing views between and within countries regarding the primary use of the NG/CT POC test. Some interviewees prioritized testing of symptomatic patients to reduce antibiotic misuse, given the current evidence of increased overtreatment rates with syndromic management approaches. Indian experts in particular believed that local prevalence has reduced, thus syndromic management was severely overtreating and should be replaced with a test-and-treat strategy. However, in settings with a higher burden of STIs and HIV such as Kenya and Southern Africa, there was a greater focus on screening of asymptomatic patients. Some experts felt that HIV cannot be tackled without attention to other STIs and were concerned about the large asymptomatic population, especially with regard to infections in young women and adolescents, and about prevalence and potential resistance in MSM and female sex worker populations. South African interviewees generally placed less emphasis on testing of symptomatic people, considering testing instead of syndromic management as a “nice to have,” because there was already a well-validated syndromic system. The evidence base for a public health benefit of introducing diagnostics into syndromic management was described as “thin,” although a recent review has suggested otherwise.9
There were differences with respect to prioritization of testing among symptomatic men versus women. Several interviewees implied that testing was needed for women because they are significantly overtreated, because of providers often neglecting to inquire about risk factors or perform speculum examinations. That said, a couple of interviewees worried about the sustainability of testing all symptomatic women and suggested that filters would still be needed to target testing. Asymptomatic infections in women were also a major concern in some settings.
Overall, interviewees saw great value in screening high-risk populations. For example, in South Africa, the following use scenarios were prioritized for a screening test: (1) screening of pregnant women, (2) incorporation of screening into PrEP programs, (3) screening of sex workers and MSM populations, and (4) screening of anyone testing HIV positive. Screening of adolescent and young women was also considered to be important in Southern Africa, but given the large size of this population and the difficulties in accessing them, some doubted whether an effort like this would be feasible or worthwhile. Indian interviewees also highlighted a clear role for screening of high-risk groups, as sex workers who visit clinics are often presumptively treated for NG/CT; testing would therefore reduce overtreatment and save on treatment costs.
NG/CT Test: Key Test Characteristics
Key test characteristics mentioned by interviewees included turnaround time, sample collection methods, and the ability to test for multiple STIs. Turnaround time was considered critical, as it is relevant both in reducing overtreatment and in screening asymptomatic individuals who may not be inclined to wait for a result. Interviewees suggested that turnaround times in the 10- to 30-minute range would be ideal. Minimally invasive sampling methods, including urine or self-swabs, were preferred owing to the challenges related to performing speculum examinations in crowded, underequipped clinics. Interviewees suggested that health worker acceptance of tests would be higher if additional STIs were included in the test.
NG/CT Test: Factors Driving Adoption
The test price was the most frequently mentioned factor driving adoption of any new test. One interviewee explained that because gonorrhea is not a fatal disease, other conditions are often prioritized above it. Some interviewees referenced first-line treatment prices, that is, around US$1 to US$2, as the upper limits of an acceptable price for a diagnostic test. One country-level expert considered the proposed test prices (US$1–$US5) as a big deterrent to test adoption, suggesting that “the diagnostic cost cannot be higher than the treatment cost.” However, other experts were open to higher prices, suggesting that US$3 was acceptable (US$5 was acceptable only to the Chinese expert, likely because the first-line treatment cost is relatively high in China).
Other factors mentioned as important for adoption of testing included evidence of cost effectiveness, evidence of public health benefit, and WHO guidance (Fig. 2). A few interviewees also suggested that the cost of gonorrhea treatments would be critical to prioritization of syndromic management versus a test-and-treat approach, as when treatments are inexpensive, syndromic management with etiological validation is considered acceptable, but as treatment costs increase, there is more scope for diagnostic testing.
Figure 2.
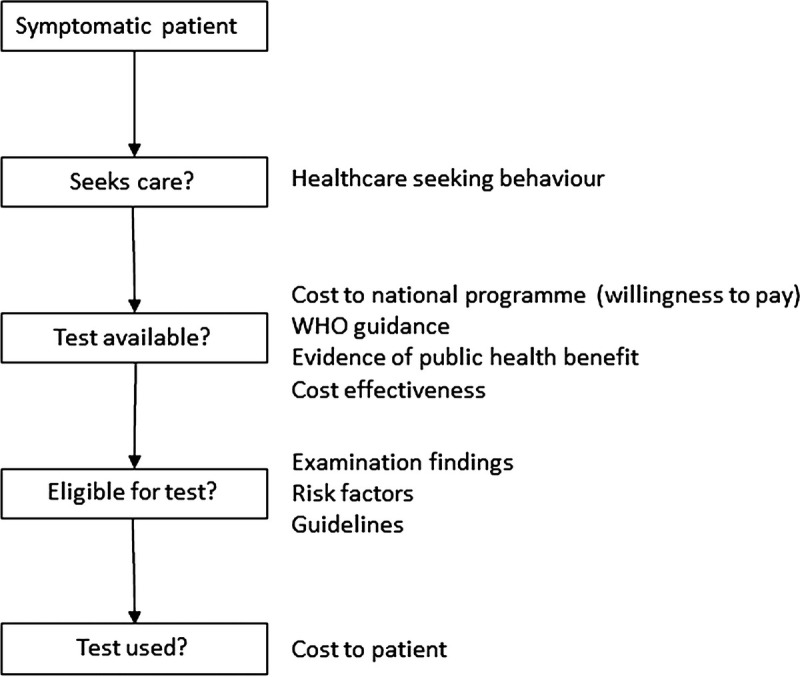
Drivers of NG/CT test adoption in symptomatic patients identified from expert interviews.
Of note, none of the interviewees mentioned alternative diagnostics such as Gram staining as potential competition for an NG/CT POC test. Rather they felt that any competition would arise from continued syndromic management. For example, the institutionalized nature of syndromic management in India (e.g., the drug prepackaging system, dispensing by nonmedical staff) might be a barrier, whereas in South Africa, presumptively treating all members of high-risk groups is under consideration.
AMR Test: Use Scenarios
China was the only country that believed in a scenario in which all patients testing positive for NG would be tested with the AMR test. In the other countries, experts felt that AMR test use would be very limited and only used on patients suspected of having failed first-line treatment. The scenario described by several interviewees was that of a previously treated patient returning to clinic uncured, following which an AMR test would be performed at a referral facility (STI clinic, district or higher-level facility, depending on the country). Interviewees did not envision extensive use of the resistance test or deployment at primary level (unless the test was in a simple, disposable format and at a reduced price).
Other use scenarios suggested included using the test in a way that combines patient management and surveillance, for example, routine use of an AMR POCT at sentinel sites to monitor resistance levels or routine use of the test in selected key populations with particularly high gonorrhea prevalence. Some experts could envision a situation whereby if a new, significantly more expensive gonorrhea treatment became available (i.e., >$10/treatment), it would be reserved for documented resistant infections, and hence, diagnostics would be needed both to confirm the infection and resistance. One interviewee suggested that syndromic management remain and that any patient not responding to treatment should be tested using the comprehensive resistance test (i.e., NG/CT diagnosis and resistance testing combined).
Experts were uncertain about the idea of an AMR POC test replacing culture for patient management, even though the yields, quality, and turnaround time of culture are not optimal for patient management.
DISCUSSION
The populations eligible for NG/CT testing are sizeable, and experience with the syndromic approach that has been used to date has highlighted a need for a change of paradigm in the management of STIs, particularly with the growing number of NG strains with resistance to current antibiotics. There is a growing evidence around the benefit of antibiotic stewardship to avert AMR, which will require a move toward etiological case management. However, the experts interviewed in this study highlighted a number of challenges and uncertainties relating to diagnostic test adoption, similar to those described during the introduction of other POC tests.18–20 The experts suggested that actual demand will be limited by affordability, guidelines, evidence, and willingness to pay. There was enthusiasm among the interviewees for a test that can be used for screening of high-risk groups, although opinions between and within countries differed. If forced to choose, most experts stated that they would prioritize screening high-risk/vulnerable populations over testing and treating symptomatic populations, whereas a few recommended the opposite. This divergence seems to stem from different perspectives and competing priorities: although some experts were focused on reducing overtreatment and AMR, others felt that the burden of STIs is dominated by asymptomatic cases, and therefore, a greater impact could be generated by screening these populations. This lack of consensus warrants additional exploration, as it will affect the positioning of any new diagnostic test, and has implications for the evidence base and arguments that would be needed to support test adoption. Further data from other countries would help to better understand preferences regarding screening versus test and treat.
As expected, price was noted as the strongest driver of adoption. Notably, while interviewees stressed the importance of affordability, many also preferred a test that can detect asymptomatic infections, which is likely to have higher costs than a simpler, less sensitive test for diagnosis in symptomatic patients. For such a test to be adopted, economies may need to be used, although this will only be achievable if the market is sufficiently sizeable. Interviewees noted turnaround time as a key test characteristic, and this characteristic may provide scope for using versions of these tests in high-income countries, as the time to return results will be faster than existing nucleic acid amplification test–based systems. Should the tests be viable in high-income settings, a strategy of income-based differential pricing might be possible. Further data on willingness to pay and assessment of high-income country markets are required.
An AMR test was not an immediate priority for most country-level experts. An AMR test would be likely be used very selectively, for example, for testing of treatment failures. However, the number of patients eligible for testing under this scenario would be low, as only a small fraction of confirmed NG cases will fail treatment, return to clinic, and complete the follow-up.
Some interviewees proposed alternative use scenarios for the AMR test, such as routine use at sentinel sites to monitor resistance levels when there is no access to microbiology laboratories and no surveillance system is in place, or in selected key populations (e.g., populations with particularly high gonorrhea or urethral discharge risk, such as MSMs, commercial sex workers, or people enrolled in PrEP). These suggested use cases seem to stem from a sense that current surveillance and resistance monitoring in high-risk populations is inadequate; many interviewees focused on the need for strengthening local surveillance systems rather than on new AMR diagnostics. Given the relative size of key populations or number of samples tested at a sentinel site, overall volumes of tests required for activities such as these would be small.
Generally, the lack of a secure market for new diagnostic tests in low-resource settings, apart from those with large programmatic donors (i.e., HIV, tuberculosis, or malaria), is a disincentive for private investors. In the era of AMR and increasing prevalence of NG worldwide, the AMR test could play a strong role in stewardship of current and new antibiotics; however, the low priority of STIs in many settings and the fact that AMR is not included in many donor portfolios represent a barrier. Although there have been some investments in research and development related to AMR, these have mostly focused on the development of new antibiotics, and few advances have been made to tackle AMR in LMICs, in particular at the primary health care level. Market interventions, including new funding and procurement models designed to support AMR efforts in low-resource settings, are urgently required.21
A limitation of this study was the small number of interviewees and number of countries covered by the interviews, although given the current dearth of data on access to gonorrhea testing, the findings represent valuable new information. Additional input from a broader set of countries and experts would help to validate these findings. Furthermore, the semistructured nature of the interviews could have introduced bias.
As with all interview-based studies, our conclusions are based on expert opinion rather than quantitative evidence. In general, the lack of epidemiological and effectiveness evidence upon which to make decisions was highlighted as a significant gap, especially at the local level, and more research will be required to justify the adoption of new tests and diagnostic strategies, especially with limited budgets and competing priorities. Other research gaps include analysis of how syndromic management might overdiagnose NG, as well as the composition of high-risk groups and the time to develop resistance with current treatments, to help inform diagnostic placement decisions. Analogous markets such as HIV and syphilis screening programs might also provide useful insights and potential funding from existing donors in such populations.
In conclusion, the potential target population for an NG/CT diagnostic test in LMICs is sizeable, but there are many areas of uncertainty, notably relating to the price of the test and its intended use. This lack of consensus warrants further research and evidence generation to determine the most effective positioning of new NG/CT diagnostic tests and to support test adoption. A more in-depth assessment of selected countries is currently underway to better understand the challenges identified in this study.
Footnotes
Acknowledgments: The authors are grateful to the experts who participated in this study. Medical writing and editorial assistance were provided by Rachel Wright, PhD, funded by FIND, according to Good Publication Practice guidelines. Publication of this article was funded by FIND.
Conflict of Interest and Sources of Funding: C.F., M.R.-J., and C.K.-C. are paid employees of FIND. J.D. is a consultant employed by FIND to perform this analysis. T.W. has no conflict of interest to report. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Our work on STIs and specifically on POC for NG and CT was enabled by the Global AMR Innovation Fund (GAMRIF).
Author contributions: C.F. conceptualized and managed the execution of the study. J.D. developed the methodology and conducted the expert interviews. M.R.-J., T.W., and C.K.-C. validated the study design. All authors critically reviewed the manuscript and approved the final version for submission.
Data sharing statement: All relevant data are contained within the article and supplementary information.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
REFERENCES
- 1.Rowley J Vander Hoorn S Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull World Health Organ 2019; 97:548–62P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman L Rowley J Vander Hoorn S, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10:e0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wi T Lahra MM Ndowa F, et al. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLoS Med 2017; 14:e1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: Past, evolution, and future. Clin Microbiol Rev 2014; 27:587–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) WHO guidelines for the treatment of Neisseria gonorrhoeae 2016. Available at: https://www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. Accessed October 11, 2019. [PubMed]
- 6.Unemo M Lahra MM Cole M, et al. World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): Review of new data and evidence to inform international collaborative actions and research efforts. Sex Health 2019; 16:412–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eyre DW Sanderson ND Lord E, et al. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill 2018; 23:1800323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO) Progress report of the implementation of the global strategy for prevention and control of sexually transmitted infections: 2006–2015. Available at: https://www.who.int/reproductivehealth/publications/rtis/progress-report-stis-strategy/en/. Accessed October 11, 2019.
- 9.Wi TE Ndowa FJ Ferreyra C, et al. Diagnosing sexually transmitted infections in resource-constrained settings: challenges and ways forward. J Int AIDS Soc 2019; 22(Suppl 6):e25343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Gemert C Hellard M Bradshaw CS, et al. Syndromic management of sexually transmissible infections in resource-poor settings: A systematic review with meta-analysis of the abnormal vaginal discharge flowchart for Neisseria gonorrhoeae and Chlamydia trachomatis. Sex Health 2018; 15:1–12. [DOI] [PubMed] [Google Scholar]
- 11.Turner KM Christensen H Adams EJ, et al. Analysis of the potential for point-of-care test to enable individualised treatment of infections caused by antimicrobial-resistant and susceptible strains of Neisseria gonorrhoeae: A modelling study. BMJ Open 2017; 7:e015447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivard KR Dumkow LE Draper HM, et al. Impact of rapid diagnostic testing for chlamydia and gonorrhea on appropriate antimicrobial utilization in the emergency department. Diagn Microbiol Infect Dis 2017; 87:175–179. [DOI] [PubMed] [Google Scholar]
- 13.Perera SR Khan NH Martin I, et al. Multiplex real-time PCR assay for simultaneous identification of Neisseria gonorrhoeae and its ciprofloxacin susceptibility status. J Clin Microbiol 2017; 55:3201–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allan-Blitz LT Humphries RM Hemarajata P, et al. Implementation of a rapid genotypic assay to promote targeted ciprofloxacin therapy of Neisseria gonorrhoeae in a large health system. Clin Infect Dis 2017; 64:1268–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foundation for Innovative New Diagnostics (FIND) Target product profile for a rapid, low-cost diagnostic to distinguish gonorrhoea from Chlamydia infection at primary care. 2019. Available at: https://www.finddx.org/wp-content/uploads/2019/09/NG_CT-Test-TPP_20190731_clean-who.pdf. Accessed November 5, 2019.
- 16.Foundation for Innovative New Diagnostics (FIND) Target product profile for a test to identify susceptibility/resistance of gonorrhoea to antibiotics to facilitate antibiotic stewardship. 2019. Available at: https://www.finddx.org/wp-content/uploads/2019/09/Comprehensive-NG-test-TPP_20190731_clean-who.pdf. Accessed November 5, 2019.
- 17.Korenromp EL Wi T Resch S, et al. Costing of national STI program implementation for the global STI control strategy for the health sector, 2016–2021. PLoS One 2017; 12:e0170773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boadu NY Amuasi J Ansong D, et al. Challenges with implementing malaria rapid diagnostic tests at primary care facilities in a Ghanaian district: A qualitative study. Malar J 2016; 15:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin PJ Cangelosi MJ Lee DW, et al. Willingness to pay for diagnostic technologies: A review of the contingent valuation literature. Value Health 2013; 16:797–805. [DOI] [PubMed] [Google Scholar]
- 20.Pai NP Vadnais C Denkinger C, et al. Point-of-care testing for infectious diseases: Diversity, complexity, and barriers in low- and middle-income countries. PLoS Med 2012; 9:e1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clift C. Review of progress on antimicrobial resistance: Background and analysis. 2019. Available at: https://www.chathamhouse.org/sites/default/files/publications/research/2019-10-11-AMR-Full-Paper.pdf. Accessed November 27, 2019.


