Abstract
Cell sorting is a commonly used technology to isolate highly purified cell populations for downstream applications. Because the sorted cells are destined for further analysis, i.e., gene expression assays or functional assays, ensuring that the sorting process itself has little effect on the cells is of utmost importance. Previous studies examining the effects of sorting on cellular function have primarily focused on a specific cell type or condition. One of the goals of the Flow Cytometry Research Group of the Association of Biomolecular Resource Facilities is to establish best practice guidelines for cell sorting conditions that minimize cell stress, perturbation, or injury to the sorted cell population. In this study, the effects of nozzle size, sample pressure, UV exposure, and instrument type were evaluated for their effects on gene expression and cell cycle using both established cell lines and primary cells across several flow cytometry shared facilities. Results indicate that nozzle size and pressure, as well as UV exposure and instrument type, have only minor effects on gene expression, which were diminished by subsequent culturing of the sorted cells. In this assessment, these data demonstrate that cell sorting itself, regardless of instrumentation used, has minimal effects on downstream cellular applications.
Keywords: flow cytometry, RNA microarray, immune cells
INTRODUCTION
Investigations into the effects that different purification technologies have on cell phenotype, health, stress response, and function in downstream applications have spurred a growing interest in studies that determine best practices for cell isolation. With the increased diversity of applications utilizing sorted cells such as next-generation sequencing, cell culture, RNA sequencing (RNA-seq), single cell assays, and stem cell transplantation, there is a strong interest in understanding the effects of cell sorting on a cell’s biology and the degree to which a cell’s health, function, and differentiation status may be affected by the sorting process.
Fluorescence-activated cell sorting, commonly referred to as cell sorting or, more technically, electrostatic cell sorting, is a well-accepted approach to isolate purified cell populations to study the functionality of a wide variety of cells including B and T lymphocytes, tumor cells, and primary dendritic cells.1 Several studies have observed little impact of cell sorting on cell health and function. One study indicated that gene expression profiles of isolated blood lymphocytes and monocytes were affected to a greater extent by positive and negative immunomagnetic selection than by flow cytometric cell sorting.2 In another study, Richardson et al.3 observed minimal gene expression changes in different populations of mouse mammary cell subsets when examined immediately after cell sorting. Sadreddini et al.4 demonstrated that magnetically activated cell sorting resulted in a higher transformation efficiency of memory B cells by Epstein-Barr virus when compared with traditional cell sorting. A study that examined the effect of sorting on human peripheral blood CD8+ cells observed sorting-induced activation of MAPK p38 with minimal functional changes.5 Despite the evidence presented in these studies, there is still concern that cell sorting can induce functional changes in cells that can affect the results of downstream applications.
Several studies have examined the metabolic state of sorted cells and found it to be altered. One study by Llufrio et al.6 examined the redox state and cellular metabolome of astrocytes and found that cell sorting introduced oxidative stress and subsequently altered the metabolic state of the cells. Another study by Binek et al.7 observed significant changes in the metabolome of mouse peritoneal macrophages that was attributed to the effects of cell sorting. These studies, along with anecdotal data, clearly suggest cell sorting may trigger a certain gene or pathway response worthy of further investigation in some cell types.
Because electrostatic cell sorting has become an important step prior to single cell analysis and other advanced applications, understanding the contribution of shear forces, osmotic forces, laser excitation, and electrical charges on cell health is critical. Shear force is defined as an external force that moves parallel to the plane of an object or surface. Shear force has been demonstrated to induce cell damage, and even cell death, in fluid systems including electrostatic cell sorters.8 Another parameter used to define hydrodynamic stress on cells in fluids is the energy dissipation rate (EDR).9 The EDR is defined as the rate at which energy is applied to a fluid or, in flow sorting, a cell, and takes into account shear and extensional forces. In a study by Mollet et al.9 the EDR was modeled based on various sheath pressures and 2 nozzle sizes (70 and 100 μm) in a FACSVantage (BD Biosciences, Franklin Lakes, NJ, USA) jet-in-air type of cell sorter. The researchers demonstrated that significant EDR resulted in damage to Chinese hamster ovary cells that had been cultured in serum-free medium and sorted at 17 psi with a 100 μm nozzle. Notably, the data presented in this study9 are from cells sorted into high serum collection buffer, which has been demonstrated to increase cell survival after sorting.10 Although the Mollet study addresses concerns with the jet-in-air sorters, examination of hydrodynamic forces in a cuvette system has not been reported.
Very little information exists comparing cellular function from sorted cells using different instrument types, nozzle sizes, pressure, and parameters. In addition, no single study has evaluated the effects of sorting on cell state across multiple instruments and sites. Using microarrays, RNA-seq, cell cycle analysis, and multiplexed bead-based gene expression assays, our study was designed to evaluate the transcriptional response and proliferative state among sorted cells using a range of instrument types and configurations located in multiple shared resource facilities (SRLs) across the United States.
MATERIALS AND METHODS
Study sites and instrument setup
Cell sorting for each experiment was performed at 1–7 flow cytometry SRLs located across the United States. Table 1 lists the instrument type and setup for the different cell types tested.
TABLE 1.
Instrument setup
| Sorted cell type | Instrument | Nozzle tip size, microns | Sort pressure, psi |
|---|---|---|---|
| Jurkat cells | Aria II | 70 | 70 |
| Aria II | 85 | 45 | |
| Aria II | 100 | 20 | |
| Aria II | 100 | 25 | |
| MoFlo | 50 | 60 | |
| MoFlo | 70 | 60 | |
| MoFlo Legacy | 50 | 60 | |
| MoFlo Legacy | 70 | 60 | |
| MoFlo XDP | 100 | 25 | |
| Mouse B cells | Aria II | 70 | 70 |
| Aria II | 100 | 20 | |
| Aria II | 100 | 25 | |
| Influx | 100 | 20 | |
| MoFlo Astrios | 70 | 70 | |
| MoFlo Astrios | 100 | 20 | |
| MoFlo XDP | 100 | 25 | |
| V6.5 ES cells | MoFlo Legacy | 100 | 20 |
Cell lines
The cell lines used in this study were 1) human Jurkat T lymphoblast cell line (suspension type) and 2) V6.5 mouse embryonic stem (ES) cell line, which represents an adherent cell line (Stowers Institute for Medical Research Tissue Culture Core, Kansas City, MO, USA). For experiments using Jurkat cells, each of the participating core labs cultured the cells in complete Roswell Park Memorial Institute medium (RPMI; containing 10% fetal bovine serum, 0.05 mM 2-ME, 15 mM HEPES, 1 mM sodium pyruvate, 2 mM l-glutamine, penicillin, streptomycin, and minimum essential medium (MEM) nonessential amino acids). The V6.5 ES cell line was established from cells derived from the inner cell mass of a 3.5-d-old mouse embryo from a C57BL/6 × 129/sv cross and grown using NB27+2i media [neurobasal media (21103-049; Thermo Fisher Scientific, Waltham, MA, USA), DMEM/F12 media (10565-018; Thermo Fisher Scientific), 0.5× N2 (17502-048; Thermo Fisher Scientific), 1× B27 (17504044; Thermo Fisher Scientific), 2-ME (ES-007-E; MilliporeSigma, Burlington, MA, USA), Glutamax (10378-016; Thermo Fisher Scientific), 1× non essential amino acids (07600; StemCell Technologies, Vancouver, BC, Canada), 3 μM CHIR99021 (4423; Tocris Bioscience, Bristol, United Kingdom), PD0325901 (72184; StemCell Technologies), and 0.033% bovine serum albumin (15260037; Thermo Fisher Scientific)].11, 12 Accutase (StemCell Technologies) was used as the cell release agent followed by washing with PBS without calcium.
Jurkat cell sorting and cell cycle analysis
Jurkat cells were split 3 d prior to sorting. On the day prior to the sort, cultures with a viability >80% were centrifuged at 500 g for 5 min at 4°C and resuspended at 1 × 107 cells/ml in complete RPMI. Cells were separated into 6 aliquots, 3 for each of 2 groups (experimental and control), of 2 × 106 cells each to act as “Sorted-Experimental” or “Control (not sorted)” populations for analysis. The control samples were not exposed to the flow cytometer; however, they experienced all the same treatments as the sorted samples, including tube aliquoting, pipetting, incubating on ice, and centrifugation. The sorted experimental cell populations were exposed to the pressure and nozzle size indicated in Table 1. Cells were sorted into a 12 × 75 tube containing PBS at 4°C. Sorted and control (cells not sorted) Jurkat samples were centrifuged, and the cell concentration and viability were determined using a hemocytometer and trypan blue exclusion. Cells were fixed by the dropwise addition of cold 80% ethanol, incubated on ice for 30 min, and stored at 4°C in ethanol until shipped to University of Vermont Cancer Center Flow Cytometry Lab (Burlington, VT, USA) for staining and cell cycle analysis. Cell cycle analysis was performed using propidium iodide (PI) at a final concentration of 50 μg/ml in PBS containing 0.1% Triton X-100, 0.1 mM EDTA, and 50 μg/ml RNase (50 U/mg). Cells were analyzed immediately after staining, and 1 × 105 cells were analyzed using a Beckman Coulter Epics XL (Beckman Coulter, Brea, CA, USA). The percent of cells in each stage of the cell cycle was determined using the ModFitLT v.3.0 software (Verity Software House, Topsham, ME, USA).
Jurkat cell sorting for microarray analysis
On the day of sorting, Jurkat cells were harvested from tissue culture flasks, counted, and centrifuged at 200 g for 8 min. Cells were resuspended at a concentration of 1.5 × 107 in 3 ml of complete RPMI in 3 tubes for 3 conditions—sorted, control no pressure, and control pressure-exposed. For the “Control no Pressure” sample, 2 × 106 cells were transferred to a tube containing 1 ml of a 1:1 solution of RPMI:Dulbecco’s PBS to simulate dilution of media by sheath fluid in sorted samples. The remaining cells were sorted as 2 × 106 cell aliquots into tubes containing 1 ml complete RPMI. These samples were labeled as “Sorted with Pressure.” For the “No Sort with Pressure” samples, the tube that had been placed on the sorter (exposed to pressure in the sample port) was removed, and 2 × 106 cells were transferred to a new tube with 300 μl Dulbecco’s PBS. All samples were centrifuged, resuspended in fresh complete RPMI, split into 3 aliquots, and incubated at 37°C, 5% CO2 for 0, 4, or 8 h. At each time point post-sort, 1 aliquot of each sample was extracted with Trizol LS (Thermo Fisher Scientific) and stored at −80°C until the samples were shipped to the Center for Functional Genomics at State University of New York Albany for RNA isolation and analysis.
Jurkat exposure to UV laser excitation
Jurkat cells were analyzed by flow cytometry and interrogated by a standard 365 nm UV laser at 200 mW power with a spot size approximately of 20 × 10 μM ellipsoid beam profile. Because laser power was not adjustable, instrument pressure change was used as a surrogate for adjusting dosage of UV because sorting at lower pressures results in longer exposure times because of the lower velocity of fluid flow; the cell spends longer time in the laser beam. In this experiment, the difference of exposure time was ~2-fold based on analysis of pulse widths using an instrument equipped with an oscilloscope. The same sample of Jurkat cells was sorted using high and low pressure (70 and 20 psi, respectively) and collected with the UV laser shutter either open or closed (4 conditions total). After sorting, cells were cultured in complete RPMI with 10% serum at 37°C with 5% C02 for 3 h before RNA extraction and microarray analysis.
Microarray analysis of sorted Jurkat cells
RNA was isolated from the Jurkat cell samples at the Center for Functional Genomics at State University of New York Albany using Qiagen RNeasy Micro Kit (74004) with DNase treatment (Qiagen, Germantown, MD, USA) per manufacturer’s instructions. Microarray target synthesis was performed using NuGen Ovation Pico whole transcriptome analysis reagents following manufacturer’s instructions (NuGen Technologies, Redwood City, CA, USA) and hybridized to PrimeView GeneChip microarrays (Affymetrix, Thermo Fisher Scientific) following the manufacturer’s protocol. Gene expression data were analyzed using Partek Genomic Suite (Partek, St. Louis, MO, USA), Transcriptome Analysis Console (TAC; Thermo Fisher Scientific), and Genespring GX v.12.6.1 (Santa Clara, CA, USA).
Mice
For the B-cell sorting experiments, 2–6-mo-old male C57Bl/6 mice were selected. Mice were housed following the procedures and protocols of each participating core facility’s institutional animal facility. Mice were euthanized with CO2 in accordance with the facility’s protocol. Each core facility used a single C57Bl/6 mouse spleen as a source of B cells.
Mouse splenic B-cell sorting
Single cell suspensions were generated from the mouse spleens by grinding spleens through 70-μm filter mesh baskets using the frosted end of glass slides dipped in 70% ethanol and flamed. Red blood cells were removed using Histopaque specific gravity 1.083 (MilliporeSigma, Burlington, MA, USA) following manufacturer’s instructions. A total of 2 × 107 splenocytes were stained with anti-CD19 conjugated to eFluor660 according to manufacturer instructions (clone: eBio 1D3; eBioscience, Thermo Fisher Scientific) to identify and sort B cells. Also, 2 μg/ml PI was added to the sample to identify and exclude the dead cells. Stained cells were washed, resuspended in sorting buffer (PBS without Ca2+ and Mg2+, 1 mM EDTA, 25 mM HEPES pH 7.0, and 1% heat-inactivated fetal bovine serum), and filtered through a 70-μm filter prior to sorting. Details about the instrument setup and sorting conditions are presented in Table 1. Both the unsorted samples and the sorted cells were maintained at 4°C. Sorted cells were collected in 12 × 75 polypropylene tubes containing 1 ml fetal bovine serum. For 0-, 4-, and 8-h post-sort time points, each sorted sample was split into 3 tubes of 2.5 × 105 cells and tested for purity by reanalyzing a small aliquot (20 μl) from each sorted tube on the sorter instrument.
Sorted cells were centrifuged at 500 g for 5 min at 4°C and resuspended in complete RPMI prior to being placed at 37°C, 5% CO2 for either 0, 4, or 8 h. At the appropriate time point post-sort, the samples were removed from the incubator and centrifuged, and the cell pellet was resuspended in 200 μl PBS. To each sample, 5 μl of a RNase inhibitor (RiboLock, E0381; Thermo Fisher Scientific) was added followed by centrifugation to prevent RNA degradation. Supernatant was removed, and sample pellets were stored as frozen pellets at −80°C until shipping. Samples were shipped on dry ice to the Center for Functional Genomics at State University of New York Albany for RNA isolation and analysis.
Microarray analysis of B cells
RNA was isolated and analyzed from all sorted B-cell samples as previously stated above with the exception that Mouse Gene ST 2.0 microarrays (Affymetrix Thermo Fisher) were used. Data analysis criteria were as follows: 1) probe signal at the bottom 20th percentile across all samples were filtered; 2) 2-way ANOVA was run to select entities that showed differential expression either between 4 and 8 h when compared with the 0-h time point within each instrument and both pressures or between the different pressures at 0-h time points within each instrument; 3) 2-fold cutoff was applied to each comparison; and 4) differentially expressed gene (DEG) lists were generated for the following comparisons: (a) 0-h low pressure vs. 0-h high pressure (within each instrument), (b) 4 vs. 0 h (within each instrument at a given pressure, and (c) 8 vs. 0 h (within each instrument at a given pressure). T-stochastic neighbor embedding (tSNE) plots were generated by normalizing probe set intensity values from all samples and analyzed in R using Rtsne package and plotted using ggplot2.
Mouse V6.5 ES cell sorting
V6.5 ES cells were harvested, centrifuged at 350 g for 5 min, manually counted, and sorted on a Beckman Coulter MoFlo Legacy flow cytometer at a rate of 2300 events per second (~25 μl/min) using a 100-μm nozzle at 20 psi. Cells (2.0 × 105) were sorted into 3 wells of a 6-well plate containing ES cell media using a large forward scatter/side scatter gate. For unsorted controls, the same number of cells were pipetted into the other 3 wells of the 6-well plate, and 600 μl Dulbecco’s PBS was added to account for the dilution of the media by sheath fluid in the sorted samples. All samples were incubated at 37°C with 5% CO2 overnight and examined at 18 h to confirm cellular growth and viability for each well. Growth media were replaced, and cells were incubated for 4 additional hours and washed with PBS (without Ca2+, Mg2+, or fetal bovine serum). One set of replicates were used for RNA-seq. RNA was extracted at the Stowers Institute for Medical Research using Sigma GenElute total RNA extraction kit (St. Louis, MO, USA); libraries were constructed and sequenced using the TruSeq RNA library reagents and sequenced with paired end 2 × 50 bp sequencing-by-synthesis v.4 reagents with an Illumina HiSeq2500 (Illumina, San Diego, CA, USA). The remaining replicate cell pellets were shipped on dry ice to the Cincinnati Children’s Hospital Research Flow Cytometry Core (Cincinnati, OH, USA) for QuantiGene Plex (QGP) analysis.
QuantiGene assay of microarray candidate genes
Sorted mouse B cells and V6.5 ES cells were evaluated for gene expression changes using Affymetrix microarrays as stated above. A custom QuantiGene (Thermo Fisher Scientific) 60-plex assay kit was designed to include genes previously found to be differentially expressed in B cells after sorting at different pressures (microarray candidate genes), from microarray data of the same material, in addition to genes involved in cell stress pathways (Table 2). Total RNA was extracted using Qiagen RNeasy and ribo-depleted using RiboZero (Illumina) and converted to RNA-seq libraries using the QGP (Thermo Fisher Scientific) and analyzed using a BioPlex200 (Bio-Rad, Hercules, CA, USA) at Cincinnati Children’s Hospital Research Flow Cytometry Core. Data were analyzed using Excel and R software with edgeR, ggplot2, and limma packages.
TABLE 2.
List of the QGP assay genes and their functional category
| Gene identifier | Functional category |
|---|---|
| Aen..42. | Apoptosis |
| Apaf1..26. | Apoptosis |
| Bax..54. | Apoptosis |
| Bbc3..12. | Apoptosis |
| Cflar..25. | Apoptosis |
| Cyfip2..68. | Apoptosis |
| Fas..20. | Apoptosis |
| Phlda3..18. | Apoptosis |
| Tnfrsf10b..13. | Apoptosis |
| Traf4..72. | Apoptosis |
| Unc5b..65. | Apoptosis |
| Xiap..94. | Apoptosis |
| Dram1..51. | Autophagy |
| Prkab1..67. | Autophagy |
| Prdm1..52. | cAMP and MAPK pathway |
| Btg2..34. | Cell cycle arrest |
| Cdkn1a..28. | Cell cycle arrest |
| Fbxw7..73. | Cell cycle arrest |
| Actb..48. | Control |
| Gapdh..70. | Control |
| Hprt..46. | Control |
| Tbp..22. | Control |
| Ubc..29. | Control |
| Ywhaz..63. | Control |
| Ddb2..81. | DNA repair |
| Polh..64. | DNA repair |
| Rrm2b..57. | DNA repair |
| Xpc..80. | DNA repair |
| Atf4..38. | Heat shock and UPR |
| Atf6..82. | Heat shock and UPR |
| Atf6b..55. | Heat shock and UPR |
| Bid..45. | Heat shock and UPR |
| Calr..76. | Heat shock and UPR |
| Ddit3..88. | Heat shock and UPR |
| Dnajc3..44. | Heat shock and UPR |
| Hsp90aa1..78. | Heat shock and UPR |
| Hsp90b1..35. | Heat shock and UPR |
| Hspa4..79. | Heat shock and UPR |
| Hspa5..15. | Heat shock and UPR |
| X.Xbp1..47. | Heat shock and UPR |
| Pank1..37. | Metabolism |
| Abcg1..14. | Microarray candidate |
| Fos..33. | Microarray candidate |
| Gm129..43. | Microarray candidate |
| Klf4..74. | Microarray candidate |
| Plk2..58. | Microarray candidate |
| Rgs1..53. | Microarray candidate |
| S1pr3..56. | Microarray candidate |
| Il6..36. | NF-κB pathway |
| IL.1A..7. | NF-κB pathway |
| Tnf..27. | NF-κB pathway |
| Alox5..62. | ROS control |
| Fdxr..61. | ROS control |
| Ppib..19. | ROS control |
| Sesn1..66. | ROS control |
| Sesn2..77. | ROS control |
| Egr1..21. | Shear stress responsive |
| Gpr87..75. | Survival |
| Tnfsf13b..30. | Survival |
| Triap1..39. | Survival |
ROS, reactive oxygen species; UPR, unfolded protein response.
RESULTS
Sorting does not select for cells cycle stage or decrease viability of Jurkat cells
For the initial study, Jurkat cells were chosen as a prototypical immune cell. Because this was a multi-site study, all participants used the same source of cells and similar passage numbers to rule out cell source variability. The Jurkat cells were cultured, expanded, and frozen at one of the participating SRLs. Frozen aliquots were sent to all participating SRLs where they were thawed, cultured, and prepared for sorting.
We hypothesized that cells at certain stages of the cell cycle may be more sensitive to the effects of sorting, possibly as a function of reorganization of the cytoskeleton during mitosis or changes in cell signaling, which may increase the likelihood of cell death or damage. To test this hypothesis, Jurkat cells were sorted and immediately fixed and stained for cell cycle analysis with aliquots set aside for viability analysis. All sorts were performed using either a jet-in-air (MoFlo, Beckman Coulter, Indianapolis, IN, USA) or cuvette flow cell (Aria, BD Biosciences) type sorter. The sorts were performed using a 50-, 70-, or 100-μm nozzle with pressures ranging from 20 to 70 psi (Table 1). All sorts were performed in SRLs across the United States with sample preparation and sorting performed by SRL staff. As an unsorted negative control, an aliquot of cells at each site was placed in the sample chamber and pressurized but not sorted. This unsorted control served to normalize the results at each site and to control for slight individual differences in the initial cell cycle status from site to site.
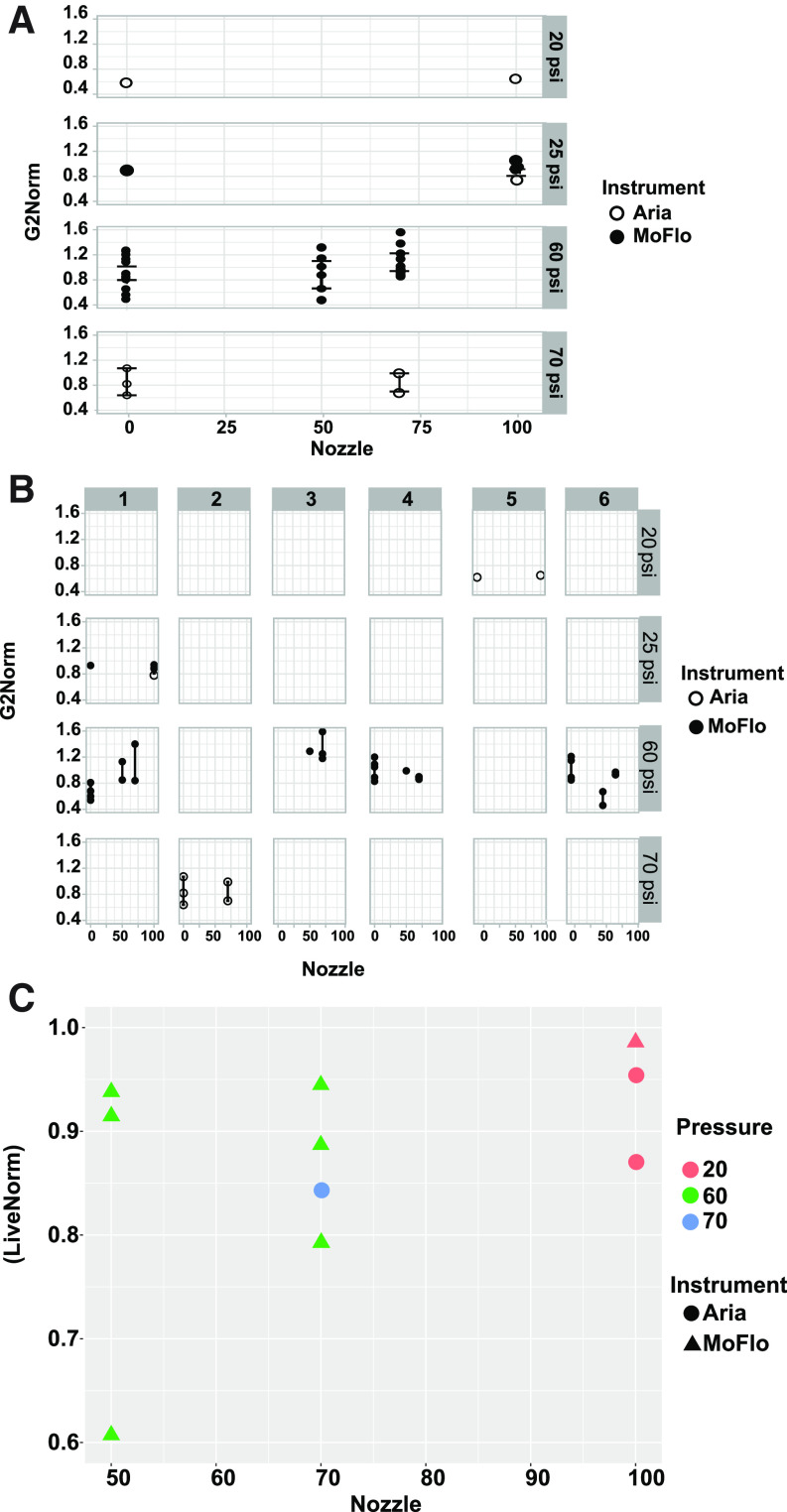
Figure 1A compares the ratio of the frequency of cells in G2/M for each sort condition normalized with that of the site-specific unsorted no pressure control. Values lower than 1.0 indicate a loss of cells that are actively cycling and could signal damage caused by cell sorting. Segregating the results by site (Fig. 1B, columns 1–6 corresponding to different sites) revealed that changes in the normalized frequency of G2/M cells relative to controls did not illustrate any common trends among sorting conditions and cell cycle status postsorting. Instead, the data suggest experimental noise, from sources such as cell health or handling, may have a larger influence than sorting or pressure. Post-sorting analysis indicated no statistically significant difference or trend in viability of the cells regardless of nozzle size or pressure used during the sort (Fig. 1C). However, a nonsignificant trend toward slightly higher viability with lower pressure (larger nozzle) may indicate a low magnitude effect that is overwhelmed by other experimental noise in the data. Taken together these data indicate that sorting does not result in cell cycle stage-specific effects or viability loss in Jurkat cells.
FIGURE 1.
Cell cycle and viability across sites and instruments. A) The normalized frequency of G2/M phase cells analyzed by staining with PI is plotted against nozzle size used for cell sorting. Nozzle size 0 indicates samples were exposed to the indicated pressure but not sorted. For each sample set, frequency of G2/M was normalized to an untreated cell sample such that 1.0 indicates no change from the untreated control. For 70- and 100-μm nozzles, cells were sorted at 2 different system pressures. Error bars represent 95% confidence intervals. B) The normalized frequency of G/2 M phase cells is depicted by site (panels 1–6 correspond to different sites). Error bars represent 95% confidence intervals. C) Frequency of live cells is plotted against the nozzle size used for cell sorting. For each sample set, the frequency of live cells was normalized to an untreated cell sample such that 1.0 indicates no change from the control.
Cell sorting has minimal effects on Jurkat cell transcriptome
To further examine the effects of sorting on the biology of Jurkat cells, transcriptome analysis of sorted and unsorted Jurkat cells was performed using microarray. Three experimental groups were used for this study: 1) control, 2) pressure, and 3) sorted. The control group consisted of cells that were not sorted or exposed to any pressure; however, they were subjected to the same treatments as the sorted cells, including buffers, centrifugation, and time on ice. The pressure group contained cells that were not sorted but were exposed to 70 psi of pressure and the same conditions as the control group outlined above. The sorted group used a 70-μm nozzle and 70 psi of pressure to sort cells into RPMI media with 10% serum. The purpose of the pressure group was to ascertain whether exposure of cells to pressure has a significant impact on the transcriptional profile separate from the effects of sorting itself. After collection, cells from each treatment group were cultured at 37°C, 5% CO2 for 4 h or 8 h, after which the RNA was extracted and subjected to microarray analysis with Affymetrix HuGene2.0 GeneChips. For each time point, the microarray data were compared as follows: Pressure Group vs. Control Group, Sorted Group vs. Control Group, and Sorted Group vs. Pressure Group to delineate the effects of pressure, sorting and pressure combined, and sorting, respectively, when compared with the unsorted control.
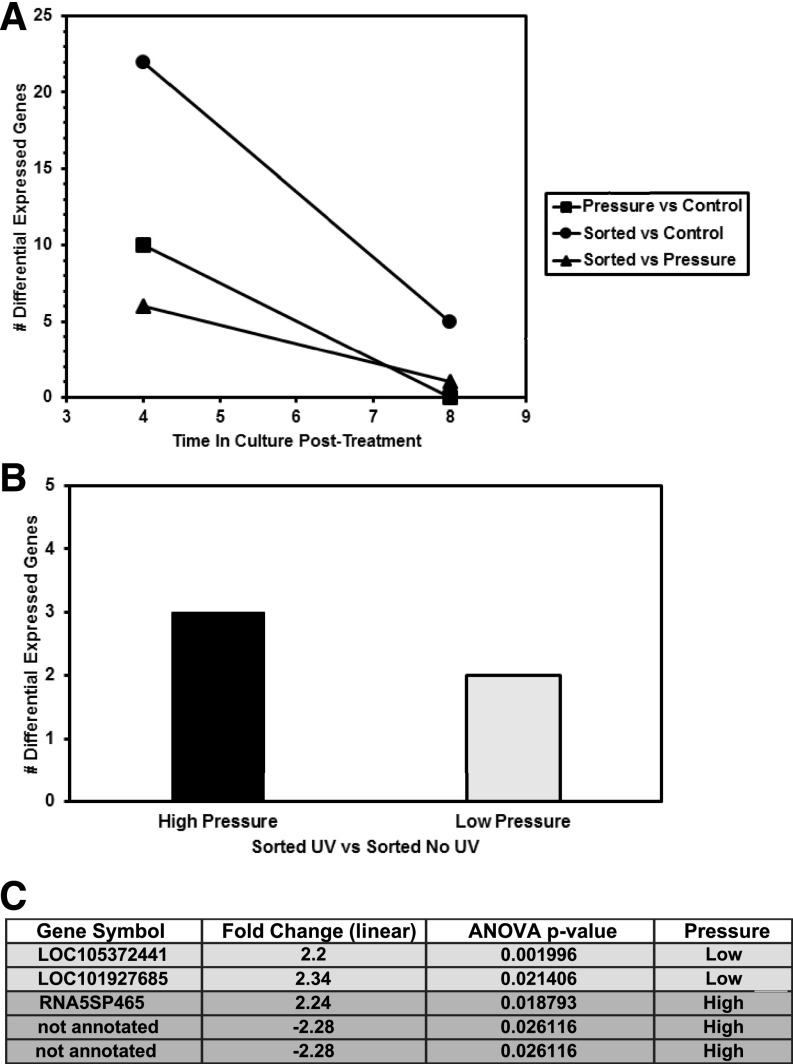
Data analysis was performed using 3 microarray-specific software packages. Results are presented as DEGs. Figure 2A shows the number of transcript changes (#DEGs) that were observed at the 4- and 8-h post-sort time points for each comparison. These results demonstrate only minimal transcriptome changes in cells as a function of pressure, sorting, or both. For a complete table of DEG identities, see Supplemental Fig. S1. The number of DEGs found for each comparison is low, and pathway analysis (TAC software) does not indicate significant involvement of any pathways annotated within the WikiPathways database (https://www.wikipathways.org/index.php/WikiPathways), including those involved in T-cell activation, cell stress response, or apoptotic pathways. Also, it was observed that continued incubation in culture post-sorting resolves most gene expression changes detected at the 4-h time point, indicating that any perturbations to the transcriptome are transient. However, it is interesting that in the sorted vs. control comparison, the highest down-regulated genes are from the heat shock protein A family.
FIGURE 2.
Effects of cell sorting on Jurkat transcriptional profile. A) Transcriptome changes (#DEGs) reported for 3 sets of comparisons each at 4- or 8-h time points after sorting at high pressure (70 psi). Pressure effects (squares) were determined by comparing cells exposed to sorter pressure but not sorted with untreated controls. Effects from sorting alone (triangles) compared with pressure-only treated samples. Effects from sorting and pressure combined (circles) were determined by comparing expression profiles of sorted samples with unsorted and unpressured treated controls. B) Number of DEGs are minimal when comparing cells sorted with and without UV laser exposure using high- and low-pressure conditions. C) Genes and fold changes are listed and grouped by corresponding sort pressure.
Exposure to UV laser light during sorting has no effect on Jurkat cell transcriptomes
UV light has been demonstrated to alter a cell’s proliferative capabilities by inducing DNA damage and prolonged UV exposure can result in cell death.11,12 UV laser wavelengths are increasingly used in flow cytometry and cell sorting to excite not only DNA dyes such as Hoechst and DAPI but also a broad range of fluorescently tagged antibodies and other reagents. For this reason, we tested the hypothesis that exposure to a UV laser while sorting induces changes to gene expression profiles of cells sorted under high or low pressure. In this experiment, Jurkat cells were used as the test cell type.
Contrasting microarray data from UV-on to UV-off for each pressure condition resulted in a (short) list of DEGs (Fig. 2B). When comparing the effect of UV-on (200 mW) with UV-off, virtually no DEGs between the 2 conditions at either pressure were noted. The few DEGs detected have negligible significance and were not clearly associated with the DNA damage response pathway (Fig. 2C); however, 1 gene, LOC101927685 (HSFX4) is predicted to be a member of the heat shock transcription factor family. It is likely that the dose of UV is far below the threshold required to induce cell damage, because even at low pressure (slower fluid velocity) cells are only exposed to the laser beam <5 μs.
Cell sorting has minor effects on mouse primary B-cell transcriptome
Although Jurkat cells reveal little to no response to sorting, cancer cell lines tend to be relatively hardy when compared with primary cells because they often have lost important growth or damage checkpoints after immortalization and years of propagation. To determine if primary cells are more sensitive to the effects of sorting than Jurkat cells, murine C57Bl/6 splenic CD19+ B cells were sorted using 2 different instrument conditions (high pressure and small nozzle vs. low pressure and large nozzle) on 3 different models of sorters at 2 SRL sites as outlined in Fig. 3A, B. Results from 2 participating sites are summarized in Fig. 3C–E. Material from a third site was later utilized for gene expression validation using an alternative technology (results described in Fig. 4).
FIGURE 3.

Effects of cell sorting on primary mouse splenic B cells. A) Samples were obtained by sorting cells at multiple sites, with 2 nozzle sizes and collecting cells at 3 time points postsort. B) Illustration of experimental design. Spleens from C57Bl/6 mice were dissociated and stained, and B cells were sorted as described. Cells were placed in culture for the time indicated. Cells were then washed; cell pellets were frozen and shipped for evaluation of gene expression. C) tSNE plot of normalized gene expression data for all samples evaluated using microarray. Site, time in culture, and pressure conditions are indicated as shown in the legend. D) Gene expression changes from 4 pairwise contrasts for sorted primary mouse B cells are plotted as number of DEGs detected. Each data point shows the number of DEGs (filtered on ≥2 fold change and P ≤ 0.05) resulting from ANOVA analysis for the 2 conditions. For example, the points at time 0 compare expression levels of high pressure–sorted with low pressure–sorted cells. E) Table of DEGs and pathways detected by ANOVA in TAC software as summarized in (D).
FIGURE 4.

QGP validation of RNA microarray data. A) tSNE plot of sorted B-cell samples using normalized QGP assay data. Site indicated by colored outlines around groups of points. Site #1 red, site #2 blue, site #3 green (not included in microarray data). B) Table of “hits” from QGP assay showing fold change in high pressure (HP) relative to low pressure (LP) condition, after first normalizing to matching 0-h controls. C, D) Ratio of gene expression between cells sorting using a 70-μm nozzle at 60 psi and or a 100-μm nozzle at 20 or 25 psi for 4- or 8-h postsorting time points. Values for each gene are derived by taking the ratio of expression at 4 and 0 h for each sample and nozzle to obtain a fold change expression at 4 vs. 0 h, then dividing the resulting values for the 70-μm nozzle by those for the 100-μm nozzle. Genes that are greater than ±1 sd from the mean are annotated.
Gene expression data were normalized and plotted using tSNE to visualize similarity by clustering of samples from the different conditions tested (Fig. 3C). The most significant differences were observed between all-time 0-h samples and all 4- or 8-h time point samples. Pathways down-regulated in the 4- and 8-h samples (vs. 0-h controls) include proliferation genes (Fig. 3E) that could indicate cells are entering a senesce phase because of sorting and/or being taken out of their native environment and placed in culture without growth factors necessary for B-cell homeostasis. Interestingly, when evaluating nozzle size and pressure effects, these data did not reveal any clear evidence attributable to any specific condition used for sorting (Fig. 3C). However, several genes were altered between instruments or pressures. Further analysis using ANOVA allowed the determination of differential gene response as well as subsequent pathway involvement. This revealed an increase in the number and type of DEGs involved with apoptosis but with low significance scores for pathway involvement (Fig. 3D, E).
Interestingly, the tSNE plots showed a clustering of the 0-h time points regardless of instrument or nozzle size/pressure. When a comparison of the 0-h high with the 0-h low pressure samples by instrument or by pressure and nozzle was done, it showed 40 (Aria), 48 (MoFlo), and 87 (Influx, BD Biosciences) DEGs (Fig. 3D, E). However, pathway analysis revealed that no specific pathways were involved, and the fold change of DEGs were slightly above a 2-fold cutoff (Supplemental Fig. S3). These results are consistent with the expectation that little variation is expected to exist among 0-h samples and provided a good control for the other conditions tested. In addition, comparisons made between sorting conditions in which results exhibit similar levels of transcriptome variance and no clear pathway involvement are likely to represent background noise.
To further investigate the gene expression changes associated with each of the sorting conditions, we examined DEGs between 4 and 8 h compared with all 0-h time points from both sites and all conditions. This analysis yielded 355 and 357 differentially expressed transcripts for the 4- or 8-h time points, respectively (Fig. 3D, E). Perhaps not surprisingly, pathway analysis of DEGs from 4- to 0-h comparisons indicated a down-regulation of MAPK pathway components (see Supplemental Fig. S2) since the cells were not expected to proliferate in the absence of native growth factors.
When comparing the 4- and 8-h time points from 2 of the sites, 236 DEGs were observed with evidence of pathway activation for TLR, IL-1, peroxisome proliferator-activated receptors, and inflammation signaling present at higher levels in site #2 samples (Fig. 3E). These results suggest that the mouse used by site #2 for the B-cell sorts may have been exposed to environmental factors such as a pathogens or endotoxin, which are known activators of B cells.13
Validation of microarray results by QGP assay
To validate the microarray results and investigate targeted pathways that could be perturbed in sorted primary murine B cells, results were collected using a customized QGP 60-Plex that included targeted genes involved in the cell stress and apoptosis pathways (Fig. 4B). Additional samples from a third site that were not previously analyzed by microarray were included to increase sample size. Figure 4A shows tSNE analysis of QGP gene expression results. As with microarray data, time 0 samples cluster together, indicating similar transcriptional starting points for the naive cells. These results demonstrate that samples at time 0 h (no culture period post-sorting, cells lysed immediately) represent the initial state of the B cells prior to sorting and culture. However, samples that were placed in culture at either 4 or 8 h cluster by site, indicating a more dramatic environmental effect than was seen by microarray. In addition, it is not apparent that pressure or type of sorter has a strong influence on gene expression. To quantify this, 4- and 8-h sample QGP data were normalized to each matching time set followed by plotting the ratio of high pressure to low pressure gene expression (Fig. 4C, D). Genes with expression >1 sd from the mean are labeled and shown in tabular form in Fig. 4B. In some cases, the same genes were differentially expressed in more than 1 condition as a function of pressure. These genes include Fas, Fos, Klf4, Pank1, Prdm1, and Triap1. However, the differential expression of those genes was not always in the same direction (up vs. down), and most were not detected by microarray. This inconsistency between the results might be attributed to low signal-to-noise of the assay platform or the lack of a strong signal from the data themselves in terms of sorting-related effects on gene expression.
Cell sorting has minimal effects on mouse ES cells
Different cell types may be more prone to cell sorter–induced damage, particularly under certain conditions. Although Jurkat and primary B cells demonstrated little to no significant response to sorting, the study was expanded to include ES cells because they are known to be sensitive to culture conditions and easily perturbed, especially with respect to their maintenance of a pluripotent state.14 To determine if sorting has an effect on the transcriptome of ES cells, the V6.5 ES cell line was sorted and compared with unsorted cells using RNA-seq and QGP assays. Figure 5A summarizes RNA-seq data with DEGs between sorted vs. unsorted samples shown in red. Six DEGs were detected as well as 1 unannotated predicted gene (Fig. 5B). Data collected from the QGP assay indicated a slight up-regulation of Fas, IL-1A, and TNF in sorted vs. unsorted samples, whereas Rgs1 was down-regulated (Fig. 5C). Although QGP data for the ES cells had good intensity indicating sufficient amounts of input RNA, the results did not agree with the RNA-seq data. Because the differences in gene expression by both assays was insignificant, results were not validated.
FIGURE 5.

QGP analysis of sorted mouse ES cells. A) MA plot (logCountsPerMillion-bp vs. log2FoldChange) from RNA-seq analysis of sorted vs. unsorted mouse ES cells. Seven transcripts are detected as differentially expressed (fold change ≥2, P ≤ 0.5, logCountsPerMillion >5). B) Table of DEGs from RNA-seq analysis of sorted vs. unsorted ES cells. C) DEGs from QGP assay. Plotted as ratio of mean expression between sorted and unsorted.
DISCUSSION
The scope of downstream applications for sorted cells has expanded in recent years to include highly sensitive techniques such as quantitative RT-PCR, single cell analysis, imaging, Western blotting, microarray, and bulk and single cell DNA and RNA-seq. With this desire to use sorted cell populations in these highly sensitive assays, a better understanding of the effects of cell sorting on the health and gene expression status of cells is needed. Few rigorous attempts to characterize and measure the effects of cell sorting on a range of sample types using modern technologies have been undertaken.2–7 However, an understanding of the effects of cell sorting on samples of various kinds can guide researchers to predict whether sorting could influence a critical result and inform how to plan for addition controls or validation. In this study, cell cycle analysis and gene expression results from 3 sorting experiments using different cell types that encompassed different sites, instrument models, nozzle diameters, pressures, and exposure to UV laser illumination were performed. In experiments using Jurkat cells, a human T-cell lymphoma cell line, no statistically significant effects were observed on cell cycle. Overall, the results clustered by site, indicating that the experimental environment had a greater effect than sorting condition. Because site-to-site variation was observed in the gene expression data, future studies should consider other environmental factors such as the cleanliness of laboratory instrumentation (pipets, tubes, PBS, etc.) and presence of contaminants in the sorter fluidics that have devastating effects on downstream molecular assays (endotoxins or RNases).13
The results of this study highlight the importance of understanding the effects of cell purification (fluorescence-activated cell sorting, magnetically activated cell sorting, etc.) in context with the specific cellular material used and applications being performed post-sorting. Although this study found that the overall sorter-induced cell response appears to be minor, every cell and tissue type is different and may have a different response to sorting stress. Thus, it is important to control and evaluate the effects of sorting on any cell population in terms of the cellular behavior when determining if cell sorting is an appropriate for cellular isolation.
Over the course of this study, one of the interesting observations was that larger amounts of starting RNA than anticipated were required for a successful QGP run when a large number of targets were assayed (60 genes). Although the overall QGP data were noisy, this was not unexpected because the amount of RNA input was on the low side of the recommended input. Nevertheless, Fos and Pank1 were detected twice as up-regulated in high pressure vs. low pressure in the assay, indicating their expression may change as a function of sorting at high pressure. The QGP data support the microarray results that gene expression changes associated with a specific instrument or pressure and nozzle configuration are minimal and not sufficient to trigger clearly detectable signaling pathway activation or apoptosis.
Another important factor for assessment of cell sorting effects was defining a control for unsorted cells. For the primary cell experiments in which CD19+ cells were sorted from a mouse spleen, it was difficult to obtain an unsorted control comprising highly purified primary CD19+ cells. Therefore, a chilled 0-h control, rather than an unsorted control, was deemed most appropriate. If one assumes gene expression cannot change significantly on the time scale of quickly sorting and lysing cells, then DEGs detected among time zero samples are expected to represent technical noise and signal from sources other than cell sorting such as site-to-site variation or animal-to-animal variation (each site used an animal from its own vivarium). To explain further, the timing and control of this experiment was conducted to minimize any gene expression changes caused by handling including a short duration for sorting and lysing (<15 min) as well as all preparations strictly performed on ice. These control data were then used as a baseline for calculating DEG of the 4- and 8-h experiments to show limited noise. The validity of this approach to baselining was subsequently confirmed using orthogonal data collected with the QuantiGene system, which showed clustering based on site, not sorter type or sorting conditions. However, it should be noted that site-specific issues such as poor handling or system contamination could cause the observed result. This highlights the need for flow cytometry facilities to maintain stringent cleanliness standards and handling protocols.
Overall, the results from this study are encouraging because detrimental effects caused by cell sorting are highly undesirable. In fact, these data show that little to no significant changes are observed with sorting or UV laser exposure on 2 cell types as a function of pressure, nozzle, or sorter model. Yet, the full complement of cell and tissue types can never be tested in a single study, and our data do not rule out the possibility that other cell types may be highly sensitive to conditions resulting from flow sorting. Here we illustrate how one can begin to assess such effects; however, the community must consider the further development of methodology to evaluate the effects of cell manipulations on samples.
When performing a multisite study, additional factors must also be considered and are especially important to control, including 1) time of sample handling and procession, 2) time samples are incubated on ice, 3) cell line metabolic state, and 4) general factors including media type, centrifugal speed, and time. However, in the cases of individual flow cytometry facilities, these controls are much easier to maintain.
The importance of these studies cannot be understated because flow cytometric cell sorting is now, more than ever, a key process to many studies involving elaborate downstream molecular assays such as next-generation sequencing, single cell analysis, proteomics, and other system biology techniques. Further flow sorting studies of this type are highly encouraged to continue characterizing the effect on additional cell types.
ACKNOWLEDGMENTS
We sincerely appreciate the support of ThermoFisher/Affymetrix/eBioscience (Waltham, MA, USA) for the CD19 antibody, Mouse Gene ST 2.0, and the Prime View GeneChip microarrays, and Qiagen (Germantown, MD, USA) for the RNeasy Micro Columns and NuGen Technologies (Redwood City, CA, USA) for the Ovation Pico whole transcriptome analysis reagents. We thank Marcy Kuentzel for performing the microarrays analysis at the Center for Functional Genomics at State University of New York Albany and Stefan Jellbauer and Brian McLucas at ThermoFisher/Affymetrix/eBioscience for providing the QuantiGene Plex Assay and technical support. We also thank Alyssa Sproles at Cincinnati Children’s Hospital Research Flow Cytometry Core (Cincinnati, OH, USA) for running the QuantiGene Assay. This project was approved by the animal care committee at each institution where a mouse was used. The authors declare no conflicts of interest.
REFERENCES
- 1.Conway DE, Williams MR, Eskin SG, McIntire LV. Endothelial cell responses to atheroprone flow are driven by two separate flow components: low time-average shear stress and fluid flow reversal. Am J Physiol Heart Circ Physiol. 2010;298:H367–H374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beliakova-Bethell N, Massanella M, White C, et al. The effect of cell subset isolation method on gene expression in leukocytes. Cytometry A. 2014;85:94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson GM, Lannigan J, Macara IG. Does FACS perturb gene expression? Cytometry A. 2015;87:166–175. [DOI] [PubMed] [Google Scholar]
- 4.Sadreddini S, Jadidi-Niaragh F, Younesi V, et al. Evaluation of EBV transformation of human memory B-cells isolated by FACS and MACS techniques. J Immunotoxicol. 2016;13:490–497. [DOI] [PubMed] [Google Scholar]
- 5.Andrä I, Ulrich H, Dürr S, et al. An evaluation of T-cell functionality after flow cytometry sorting revealed p38 MAPK activation. Cytometry A. 2020;97:171–183. [DOI] [PubMed] [Google Scholar]
- 6.Llufrio EM, Wang L, Naser FJ, Patti GJ. Sorting cells alters their redox state and cellular metabolome. Redox Biol. 2018;16:381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binek A, Rojo D, Godzien J, et al. Flow cytometry has a significant impact on the cellular metabolome. J Proteome Res. 2019;18:169–181. [DOI] [PubMed] [Google Scholar]
- 8.Hua J, Erickson LE, Yiin TY, Glasgow LA. A review of the effects of shear and interfacial phenomena on cell viability. Crit Rev Biotechnol. 1993;13:305–328. [DOI] [PubMed] [Google Scholar]
- 9.Mollet M, Godoy-Silva R, Berdugo C, Chalmers JJ. Computer simulations of the energy dissipation rate in a fluorescence-activated cell sorter: implications to cells. Biotechnol Bioeng. 2008;100:260–272. [DOI] [PubMed] [Google Scholar]
- 10.Kunas KT, Papoutsakis ET. The protective effect of serum against hydrodynamic damage of hybridoma cells in agitated and surface-aerated bioreactors. J Biotechnol. 1990;15:57–69. [DOI] [PubMed] [Google Scholar]
- 11.Cadet J, Sage E, Douki T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat Res. 2005;571:3–17. [DOI] [PubMed] [Google Scholar]
- 12.Rastogi RP, Richa, Kumar A, Tyagi MB, Sinha RP. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J Nucleic Acids. 2010;2010:592980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz H, Schmittner M, Duschl A, Horejs-Hoeck J. Residual endotoxin contaminations in recombinant proteins are sufficient to activate human CD1c+ dendritic cells. PLoS One. 2014;9:e113840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. [DOI] [PubMed] [Google Scholar]