Abstract
Introduction
Folic acid is the most important dietary determinant of homocysteine (Hcy). Hcy serves as a critical intermediate in methylation reactions. It is created from methionine and either converted back to methionine or transformed into cysteine. This process is aided through several enzymes and three vitamins, folic acid, B12, and B6. Daily supplementation with 0.5–5.0 mg of folic acid typically lowers plasma Hcy levels by approximately 25%. Hyperhomocysteinemia is a known risk factor for coronary artery disease. In this regard, elevated levels of Hcy have been found in a majority of patients with vascular disease.
Methods
A literature review of folic acid supplementation for various disease states including cardiovascular disease was conducted. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
In this review, we discuss the biochemistry of folic acid, Hcy biosynthesis, Hcy and hydrogen sulfide bioavailability, pathogenesis of hyperhomocysteinemia and its role as a risk factor for disease, and treatment studies with folic acid supplementation in disease states.
Conclusion
Folic acid supplementation should be recommended to any patient who has an elevated Hcy level, and this level should be measured and treated at an early age, since folic acid is easily obtained and may likely reduce vascular disease and other deleterious pathologic processes in high-risk populations.
Keywords: Coronary artery disease, Folic acid, Heart disease, Homocysteine, Hyperhomocysteinemia
Key Summary Points
| Hyperhomocysteinemia is a known risk factor for coronary artery disease. |
| Elevated levels of homocysteine have been found in a majority of patients with vascular disease. |
| Folic acid supplementation should be recommended to any patient who has an elevated homocysteine level. |
| Folic acid is easily obtained and may likely reduce vascular disease and other deleterious pathologic processes in high-risk populations. |
Introduction
Recently, homocysteine (Hcy), an amino acid, has garnered much attention in the medical community. The earliest known report of Hcy was in 1932 by DuVignaued and Butz, who created it through transmethylation of methionine (Met) [1, 2]. At the time, the role of this molecule was undetermined; however, later it was discovered that Hcy acted as an intermediate component in the biosynthesis of cysteine and Met [2]. It was not until the 1960s that elevation in Hcy levels was linked to various disease states, especially cardiovascular disease [2].
In the early 1990s, homocystinemia became a recognized risk factor and later an independent risk factor for atherosclerotic disease [2, 3]. Levels of Hcy > 15 micromoles/L was defined as hyperhomocysteinemia, and in one study it was reported to be found in “16 of 38 patients with cerebrovascular disease (42%), 7 of 25 with peripheral vascular disease (28%), and 18 of 60 with coronary vascular disease (30%), but in none of the 27 normal subjects” [4].
Considering that cardiovascular disease is responsible for nearly 1/3rd of all deaths worldwide, understanding the pathogenesis and treatment of homocystinemia is of clinical relevance [2].
Hcy serves as a key intermediate in methylation reactions. It is created from Met and either converted back to Met or transformed into cysteine. This process is aided through several enzymes and three vitamins, folic acid, B12, and B6. A dysfunction in these enzymes or a deficiency in these vitamins can lead to a rise in the blood levels of Hcy. Given this relationship, several studies have shown evidence that daily supplementation of folic acid lowers Hcy levels, more so than the addition of B12 and B6 [6]. In fact, it has been shown that folic acid supplementation of 0.5–5.0 mg can lower Hcy levels by 25% and, thus, may decrease the risk of cardiovascular disease [7]. It should also be stated that there are many available preparations, not only of folic acid but which additionally include B12 and B6.
Several mechanisms have been proposed for Hcy’s pathogenesis related to vascular disease. Hcy can cause endothelial injury, dysfunction of DNA, proliferation of smooth muscle cells, oxidative stress, decreased function of glutathione peroxidase, impaired nitric oxide synthase, and inflammation [8].
Given that Hcy is associated with vascular disease, there has been growing research on hydrogen sulfide (H2S). A deficiency of H2S is associated with Hcy and increased cardiovascular disease [3]. Interestingly, H2S is produced through cystathionine β-synthase (CBS) and cystathionine γ-lyase (CTH), key enzymes that metabolize Hcy to cysteine [3]. There is evidence to suggest that H2S and Hcy regulate each other, and that the ratio of H2S/Hcy is a reliable marker for cardiovascular disease risk [3]. H2S also has a protective effect against Hcy-induced vascular injury, especially with eNOS signaling and reducing oxidative stress [3].
In patients undergoing coronary artery bypass grafting (CABG), there is an increased risk of subsequent ischemic events, either through worsening of coronary artery disease or decrease in vein graft patency by atherosclerosis [9]. To reduce adverse cardiovascular events, CABG patients are given antiplatelet and lipid-lowering agents as a secondary prevention measure [9]. Furthermore, there is evidence that folic acid can increase bioavailability of tetrahydrobiopterin (BH4), which improves endothelial function; however, its effectiveness in decreasing adverse cardiovascular events in CABG has not yet been determined [10].
In this review, we discuss the biochemistry of folic acid, Hcy biosynthesis, Hcy and H2S bioavailability, pathogenesis of hyperhomocysteinemia and its role as a risk factor for disease, and treatment studies with folic acid supplementation in disease states.
Biochemistry of Folic Acid
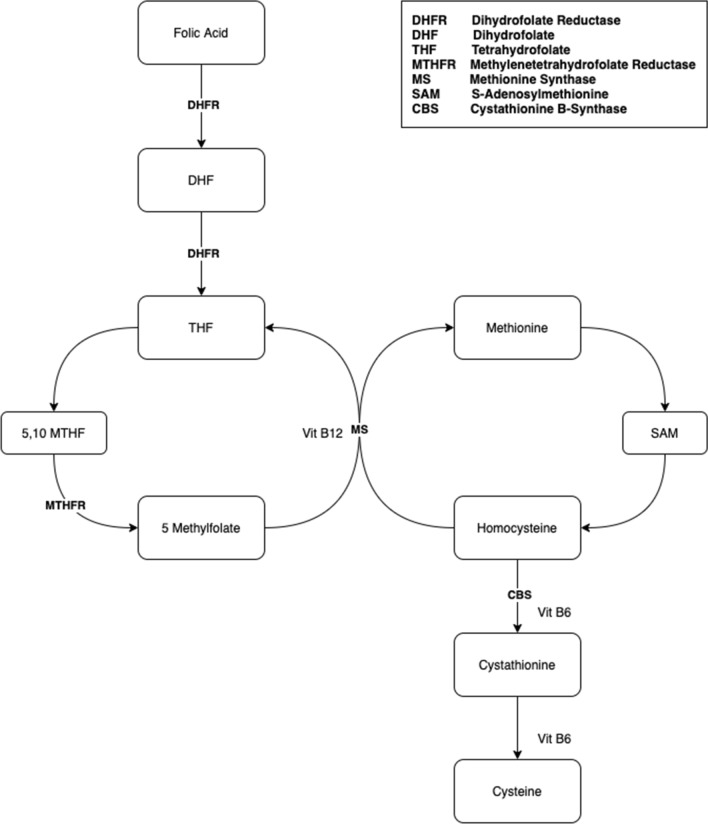
Folate is an essential nutrient from the B group of vitamins and is present naturally in foods such as fruits and green leafy vegetables, kidney, and liver [11, 12]. Tetrahydrofolate (THF), an important derivative of folate, plays an essential role in one-carbon metabolism, which involves the transfer of single-carbon units from donor molecules into biosynthetic pathways such as purine and pyrimidine biosynthesis [12, 13]. Additionally, one-carbon metabolism is important for biosynthesis of Met, interconversion of serine and glycine, and catabolism of histidine [12, 14].
In humans, folate is obtained from the diet and is transported across the enterocyte brush border membrane in the jejunum. After absorption, it is released into portal circulation and taken up by the liver, which is the main regulator of folate homeostasis. Within the plasma, up to 40% of folate is bound to carrier proteins such as albumin and transferrin [12]. Transport into cells is accomplished via both receptor-mediated and carrier-mediated mechanisms [14].
A polyglutamate form of folate known as THF is the central acceptor molecule in the one-carbon cycle. Folate is sequentially reduced into dihydrofolate and THF by the enzyme dihydrofolate reductase [14, 15]. Methotrexate is a folic acid antagonist used in cancer treatment, autoimmune disease, and non-surgical treatment of ectopic pregnancy. By irreversibly inhibiting dihydrofolate reductase, methotrexate decreases the formation of THF, which ultimately hinders the biosynthesis of DNA and RNA [15, 16].
Once THF is formed, a single carbon group is then transferred from serine to THF in a reaction mediated by serine hydroxymethyltransferase, forming 5,10-methylene-THF and glycine in the process. Then, 5,10-methylene-THF together with the enzyme thymidylate synthase participate in reductive methylation of deoxyuridine monophosphate, resulting in the formation of deoxythymidine monophosphate (dTMP) and dihydrofolate (DHF) [17, 18]. Consequently, dTMP may go on to participate in DNA biosynthesis, while DHF may be reduced to THF and 5,10-methylene-THF [19, 20].
Alternatively, 5,10-methylene-THF may be further reduced to 5-methyl-THF by methylene tetrahydrofolate reductase (MTHFR). In a subsequent step, methionine synthase catalyzes the formation of THF from 5-methyl-THF in a step that simultaneously recycles Hcy into Met. Met is involved in the metabolism of S-adenosyl-methionine (SAM) and Hcy, which are discussed below.
Hcy Biosynthesis
Hcy is a non-essential, sulfur-containing amino acid [21, 22]. Although it is not used directly in the synthesis of proteins, Hcy is required as an intermediate in the metabolism of the essential amino acid Met, which is derived from the diet. Hcy represents an intersection of a series of metabolic pathways: it may be resynthesized into S-adenosyl-l-homocysteine (SAH); it may undergo remethylation into methionine through the remethylation pathway; or it may be irreversibly degraded to cysteine via the transsulphuration pathway. Hcy is an important molecule in the synthesis of SAM, a universal methyl donor involved in transferring methyl groups to various acceptor molecules in biosynthesis of numerous biochemical compounds [22, 23].
As mentioned previously, Hcy is not obtained from the diet but rather is derived from Met. In a reaction involving ATP, the enzyme SAM synthetase/l-methionine adenosyltransferase activates Met and leads to the formation of SAM. As a universal donor of methyl groups, SAM is utilized in a wide variety of biosynthetic pathways, including the synthesis of DNA, RNA, amino acids, polyamines, proteins, creatine, epinephrine, carnitine, and phospholipids [21, 24]. Additionally, SAM has been shown to play an important role in epigenetic mechanisms, such as the regulation of DNA methylation, the remodeling of chromatin, editing of RNA, and post-translational modification of histone proteins [24]. Inappropriately high levels of SAM have been linked to the hypermethylation of tumor suppressor genes and, subsequently, cancer [25, 26].
The transfer of methyl groups from SAM to acceptor molecules is mediated or modulated by methyltransferase enzymes, and the result of every SAM-dependent methyltransferase reaction is the formation of SAH [21, 24]. This is true regardless of the biosynthetic pathway or specific methyltransferase enzyme involved. Several methyltransferases have been described in mammals. It is estimated, however, that approximately 85% of SAM-dependent methyltransferase reactions are mediated by phosphatidylethanolamine N-methyltransferase and guanidine-acetate N-methyltransferase in the liver, which are involved the synthesis of phosphatidylcholine and creatinine, respectively [24, 27]. The cytosolic enzyme glycine N-methyltransferase is another important example of SAM-dependent methyltransferase. Within the liver, this enzyme helps in the optimization of transmethylation reactions by regulating the ratio of SAM to SAH [24].
Following SAM-dependent transmethylation, SAH hydrolase metabolizes SAH into adenosine and Hcy. However, SAH hydrolase is a reversible enzyme; in states of elevated Hcy levels, SAH hydrolase catalyzes the reverse reaction of Hcy to SAH. High levels of SAH have an inhibitory effect of methyltransferase enzymes, which are largely regulated by the ratio of SAM to SAH [28]. Consequently, dysregulation of these biochemical pathways leading to reduced synthesis of SAM is thought to be one of the mechanisms behind the vascular and neurodegenerative effects of hyperhomocysteinemia [29].
Under normal circumstances, approximately half of Hcy undergoes remethylation into Met [24]. This is accomplished via one of two pathways: a vitamin B12- and folate-dependent pathway, or a betaine-dependent pathway. Additionally, Hcy may undergo irreversible transsulfuration to ultimately become cysteine [21, 22, 24]. The Hcy and folic acid metabolisms are illustrated in Fig. 1.
Fig. 1.
Metabolism of homocysteine and folic acid
Hcy and H2s Bioavailability
Hcy and H2S levels are associated with a wide variety of clinical physiology, such as cardiovascular function, neuromodulation, and inflammation [32]. The downstream health effects of these molecules are antagonistic to each other. Elevated Hcy levels have been implicated as a possible cause of hypertension and coronary artery disease, whereas H2S has been shown to act as a gaseous antioxidant protector against disease [33]. Because of the competing roles in disease, bioavailability of these compounds has been of immense clinical interest. Amino acid metabolism of sulfur-containing amino acids—also known as the transsulfuration pathway (TSP)—is known to be a major pathway that determines H2S and Hcy bioavailability [34]. As discussed above, three enzymes involved in the TSP are classically known to synthesize H2S and Hcy: CBS, CTH, and 3-mercaptopyruvate sulfurtransferase (3-MST). In particular, the most common cause of inherited homocystinemia is decreased CBS enzymatic activity [35]. Under normal conditions, CBS condenses Hcy and serine to cystathionine, effectively depleting amino acid levels of Hcy. CBS is a vitamin B6 (2 pyridoxal 5′-phosphate, PLP)-dependent enzyme. Therefore, either genetic loss-of-function mutations to CBS, epigenetic promotor hypermethylation, or a deficiency of vitamin B6 levels can all drive homocystinemia [36]. Vitamin B6 is a water-soluble vitamin found in many Western diet foods (i.e., fish, beans, nuts, grains, fruits, vegetables), and thus dietary causes of deficiency are rare in the United States. Various drugs (i.e., isoniazid, levodopa, penicillamine) and autoimmune disorders (rheumatoid arthritis) can alter vitamin B6 metabolism and thereby induce PLP deficiencies [37]. The next step of amino acid metabolism is carried out by CTH and dependent upon PLP cofactor to produce H2S. The equilibrium constant of CTH favors the forward reaction to generate cysteine and α-ketobutyrate from cystathionine reactant. Therefore, CTH enzymatic activity is critical in driving forward the CBS reaction, and thus Hcy removal. Alterations in CTH expression and protein activity have been linked to numerous disease states, such as retinopathy, neuropathy, and renal fibrosis [38–40]. The final TSP enzyme known to increase bioavailability of Hcy and H2S is 3-MST [41].
The transsulfuration pathway is extraordinarily plastic in response to dietary, microbiota, and other environmental cues. Dietary interventions of high fat content that precipitate obesity, diabetes, and hypertension have a direct impact on the bioavailability of Hcy and H2S. For example, a recent experiment studying mice found that renal CBS is selectively downregulated in both genetic and diet-induced obesity [42]. Additional studies have found that liver synthesis of H2S are decreased in both the liver and kidney organs of obesogenic mouse models, albeit protein levels of CBS and CTH were variable [43]. Interestingly, exercise appears to have a negative effect on high-fat diet-altered H2S production by the CBS and CTH enzymes. In one study, it was found that exercise training in high-fat diet-induced non-alcoholic fatty liver disease resulted in increased serum and liver H2S levels corresponding with increased CBS, CTH, and 3-MST mRNA [44]. Meanwhile, other micronutrient agents have been linked to decreases in H2S bioavailability, such as a high-salt diet and aspartame, commonly used as a food sweetener [45].
Of additional importance is a new frontier of H2S bioavailability research exploring the role of gut microorganisms in various diseases of the gastrointestinal tract (i.e., inflammatory bowel syndrome and inflammatory bowel disease) [46]. Bacterial residents of the gut microbiome utilize inorganic sulfate from the diet as a terminal electron acceptor to serve as a nonenzymatic source of H2S. These bacteria are collectively called sulfate-reducing bacteria and include many species of the Desulfovibrio genus (D. piger, D. desulfuricans), Desulfobacter, Desulfobulbus, Desulfotomaculu, and Fusobacterium [47]. The H2S equilibrium is precariously U-shaped, meaning either that too high or too low concentrations can trigger detrimental health effects. Sulfate-rich diets containing nutrients like sulfated glycans stimulate growth of D. piger in mouse models but only in the presence of Bacteroides thetaiotaomicron because the latter species liberates sulfate from mucopolysacharides via sulfatases [48, 49]. Additionally, the presence of D. piger is positively correlated with Actinobacterium and Collinsella aerofaciens due to the symbiotic nature of sugar fermentation and electron donation to D. piger for H2S reduction. Despite the non-enzymatic microbiome generation of H2S, a second reservoir of H2S synthesis is derived from several anaerobic bacterial strains, including Escherichia coli, Salmonella enterica, Clostridia, and Enterobacter aerogenes [50]. Gram-negative microbiota convert cysteine to H2S, pyruvate, and ammonia by cysteine desulfhydrase. Moreover, several bacteria also conduct sulfite reduction via sulfite reductase. These microbiota include Klebsiella, Bacillus, Staphylococcus, Corynebacterium and Rhodococcus [51, 52]. On this subject, antibiotic use has clear implications for H2S bioavailability in the intestine. One experiment in mouse models showed that neomycin administration (an antibiotic that does not cross the GBB) demonstrated significant reductions in intracolonic and peripheral blood concentrations of thiosulfate and sulfane sulfur, both products of H2S oxidation. Compared to intracolonic administration of Na2S (H2S donor), the changes in H2S oxidative products were opposite [51]. In summary, antibiotic-induced gut dysbiosis can directly alter the bioavailability of H2S.
Additional environmental factors have been linked to changes in CBS, CTH, and 3-MST enzymatic activity and in corollary Hcy levels. UV radiation, alcoholic hepatitis, and particular cancers (e.g., breast cancer, liver cancer) have all been linked to decreased CBS activity [45]. In contrast, cocaine has been shown in increase CBS activity. A plethora of other environmental cues have been shown to alter CTH activity, including morphine, cigarette smoke, ketogenic diet, and air pollutants, among many others [45]. The clinical significance of these factors has posed a challenge for researchers to tease apart, but correlation between Hcy and H2S bioavailability with health span and disease is undeniable.
Pathogenesis of Hyperhomocysteinemia and Its Role as A Risk Factor for Disease
Hyperhomocysteinemia is a syndrome of elevated plasma Hcy levels resulting in dysfunction of multiple organ systems, most significantly an elevated risk for arterial thrombosis, venous thromboembolism, and premature development of cardiovascular disease [53]. Elevations in plasma Hcy levels are often multifactorial, with genetic and acquired components [54]. While the correlation between hyperhomocysteinemia and vascular disease is well established [55–57], the underlying mechanisms are under investigation. Animal and cell models have highlighted the potential mechanisms for Hcy-mediated damage to the vascular architecture, but evidence of a causal relationship between hyperhomocysteinemia and vascular disease in humans remains to be shown [58].
Severe hyperhomocysteinemia and subsequent homocystinuria is rare, and most often caused by an inherited deficiency in the enzyme CBS [54]. In this variant of the disease, also known as classic homocystinuria, plasma Hcy levels in excess of 100 μmol/L are frequently seen. In addition to an increased risk of arterial and venous thromboembolic events, which is a major cause of morbidity and mortality, classic homocystinuria is also associated with ocular defects, including ectopia lentis, myopia, glaucoma, optic atrophy, and retinal detachment; a Marfanoid habitus characterized by elongated bones, genu valgum, pectus malformation, scoliosis, and osteoporosis; and neurological defects including intellectual disability, psychiatric disorders, and seizures [53, 54].
In contrast to severe hyperhomocysteinemia, mild to moderate hyperhomocysteinemia occurs more commonly, and is typically caused by a nutritional deficiency of folate, vitamin B12, or vitamin B6 in the setting of genetic polymorphisms in MTHFR. Common polymorphisms in MTHFR include C677T and A1298C, which result in decreased enzyme activity and increased thermolability [54]. Mild elevations of serum Hcy (15–30 μmol/L) or moderate elevations (30–100 μmol/L) are commonly seen. Hyperhomocysteinemia related to MTHFR polymorphism presents with predominantly neurologic symptoms, including psychomotor retardation, abnormal gait, psychiatric disturbances, and seizures. Thromboembolic events and ectopia lentis are also seen [53].
Hyperhomocysteinemia can also be acquired as a side effect of medications, lifestyle, or chronic disease (Table 1). Hyperhomocysteinemia may result from vitamin B6 deficiency following isoniazid therapy. Folate and B12 deficiencies are seen in alcoholics and pregnant women [53]. Drugs such as methotrexate, theophylline, phenytoin, and cyclosporine are also associated with acquired hyperhomocysteinemia. Finally, hyperhomocysteinemia is associated with chronic diseases such as end-stage renal disease, severe hepatic dysfunction, diabetes mellitus, and hypothyroidism [53, 54].
Table 1.
Etiologies of hyperhomocysteinemia
| Mild to moderate hyperhomocsyteinemia | Severe hyperhomocsyteinemia |
|---|---|
| Genetic polymorphism of MTHFR (commonly C677T and A1298C) | Classic homocystinuria (CBS deficiency) |
| Medication side effects (methotrexate, theophylline, phenytoin, and cyclosporine) | |
| Lifestyle (alcoholism, pregnancy) | |
| Chronic diseases (end-stage renal disease, severe hepatic dysfunction, diabetes mellitus, and hypothyroidism) |
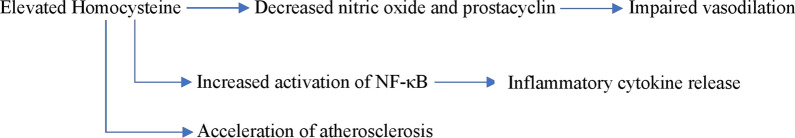
The vascular endothelium plays an important role in maintaining vascular homeostasis by regulating vascular tone, inflammation, and cell growth [58]. Endothelial dysfunction caused by hyperhomocysteinemia can trigger inflammation, apoptosis, and the subsequent formation of atherosclerotic lesions [59]. Studies involving cell cultures and animal models have shown that Hcy impairs the ability of endothelial cells to produce nitric oxide and prostacyclin, both potent endogenous vasodilators [60–62]. There is also evidence that Hcy induces inflammation in endothelial cells, resulting in increased activation of NF-κB and subsequent release of inflammatory cytokines, including interleukin-6, interleukin-8, and tumor necrosis factor-α [63–66]. Additionally, Hcy has been shown to induce apoptosis in endothelial cells [58, 61]. A proposed mechanism for Hcy-mediated damage to endothelium and other vascular cells is via irreversible protein homocysteinylation. In the setting of elevated Hcy, Hcy is incorporated into proteins, affecting their biological activity [58, 67].
The medial layer of arteries comprises smooth muscle cells interspersed with elastin filaments, collagen, and proteoglycans. This layer is responsible for the elastic properties of the artery. Healthy vascular smooth muscle cells are contractile and non-proliferative, allowing for the elasticity and structural integrity of the vascular media [58]. In cell and animal studies, aortic smooth muscle cells exposed to Hcy underwent proliferation and exhibited the formation of fatty streaks [68–70], changes also associated with atherosclerosis [58].
Finally, Hcy has been shown to induce atherosclerotic changes in the outermost layer of blood vessels, the adventitia [58]. In animal studies, elevated Hcy levels caused inflammation in the adventitia [71] and increased deposition of adventitial collagen [72]. The pathophysiologic changes resulting from hyperhomocysteinemia are illustrated in Fig. 2.
Fig. 2.
Pathophysiologic changes related to hyperhomocysteinemia
Treatment Studies with Folic Acid Supplementation in Disease States
The effect of folate supplementation on lowering serum Hcy levels in other disease states is well established. For example, a 2019 study of major depressive disorder found that l-methylfolate improved symptoms in treatment-resistant major depressive disorder, in particular patients with SSRI-resistant depression and subgroups of patients with biomarkers of inflammation, metabolic disorders, or folate metabolism-related genetic polymorphisms [73]. There is also evidence for its effectiveness in treating rheumatoid arthritis [74]. In 1998, the Homocysteine Lowering Trialists’ Collaboration conducted a meta-analysis to assess the effect of folate supplementation on serum Hcy levels, both with and without the addition of vitamins B6 and B12. All published and unpublished randomized trials were considered, and, after applying exclusion criteria, the analysis identified 12 suitable trials including 1114 subjects in total. Subject demographics were notable for a mean age of 52 years, with a range of trial means from 23 to 75 years, and a mean treatment duration of 6 weeks, with a range from 3 to 12 weeks. Folate supplementation resulted in a decrease in serum Hcy levels, although this reduction was dependent on pretreatment serum levels of Hcy and folate. The highest proportional and absolute reductions in serum Hcy occurred at higher pretreatment serum Hcy levels (P < 0.001) and at lower pretreatment serum folate concentrations (P < 0.001). A proportional reduction of serum Hcy of 16% was seen in subjects in the bottom fifth of pretreatment serum Hcy levels, while a reduction of 39% was seen in subjects in the top fifth. Overall, folate supplementation resulted in a 25% decrease in serum Hcy levels (95% confidence interval 23–28%; P < 0.001). Combined vitamin B12 supplementation produced a modest additional 7% reduction in serum Hcy levels (95% confidence interval 3–10%), while combined vitamin B6 supplementation did not have a significant additional effect [75]. This effect of folate supplementation on serum Hcy levels has been replicated in subsequent studies [76]. Furthermore, a 2019 systematic review and dose–response meta-analysis of prospective cohort studies investigating the intake of vitamin B6, folate, and vitamin B12 and the risk of coronary heart disease found that higher intake of folate and vitamin B6 is associated with a lower risk of coronary heart disease in the general population [77]. Another 2019 prospective cohort study investigating dietary vitamin B6 intake found that a higher intake level of vitamin B6 was associated with a reduced cardiovascular disease risk in Korean men [78]. These data are supported in several other studies [79, 80].
Of note, a trial conducted in 2006 among Indian soldiers working in a high-altitude environment showed that daily supplementation with folate, vitamin B6, and vitamin B12 reduced thrombotic events over a period of 2 years. Rapid ascent to high altitude is a risk factor for thrombotic events, including cerebral thrombosis. This study specifically looked at the impact on homocysteine and other thrombotic factors and vasodilators. The study participants consisted of 12,000 soldiers entering a high-altitude region in northern India at an altitude of 3500 m above mean sea level for a period of 2 years. Participants were randomly assigned to receive a supplement of 5 mg folic acid, 3 mg vitamin B6, and 1 mg vitamin B12 daily, or a placebo. All cases of thrombotic events were reported throughout the duration of the study. At the conclusion of the study, 5 thrombotic events occurred in the intervention group and 17 occurred in the control group (relative risk 0.29; 95% CI 0.11–0.80). The study concluded that supplementation with folate, vitamin B6, and vitamin B12 had a protective effect on thrombotic events at high altitude [81].
Even though supplementation with dietary folate decreases serum Hcy levels, no corresponding mortality benefit has been shown. Beginning in 2000, the Heart Outcomes Prevention Evaluation 2 trial was conducted to assess the effect of long-term supplementation with folate, vitamin B6, and vitamin B12 on the risk of major cardiovascular events in patients with vascular disease. The study included 5522 men and women age 55 or older with a history of vascular disease, including coronary artery disease, cerebrovascular disease, or peripheral vascular disease, or a history of diabetes mellitus and additional risk factors for atherosclerosis. Patients were randomly assigned to receive a supplement of 2.5 mg folic acid, 50 mg vitamin B6, and 1 mg vitamin B12 daily, or a placebo. All study participants and investigators were blinded. Serum levels of folate, vitamin B6, vitamin B12, and Hcy were measured at intervals throughout the study, which had an average length of 5 years. At the conclusion of the study, 519 patients (18.8%) of the active treatment group had experienced a major cardiovascular event, defined as myocardial infarction, stroke, or death related to cardiovascular causes, compared with 547 patients (19.8%) in the placebo group (relative risk 0.95; 95% CI 0.84–1.07; P = 0.41). The risk of death related to any cause was similar in the active treatment group and the placebo group (relative risk 0.99; 95% CI 0.88–1.13; P = 0.94). The study concluded that supplementation with folate, vitamin B6, and vitamin B12 had no beneficial effects on major vascular events in high-risk patients with a history of vascular disease [82].
These conclusions have been supported by subsequent investigations. The Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine trial, a double-blind randomized controlled trial of 12,064 patients with a history of myocardial infarction conducted between 1998 and 2008, showed no benefit of folate and vitamin B12 supplementation on major cardiovascular events [83]. A meta-analysis published in 2011 investigating 16 trials and 44,841 patients came to similar conclusions [84].
Several explanations have been proposed for the lack of morbidity and mortality benefit shown in most studies of folate supplementation. Despite promising mechanisms of Hcy-mediated vascular damage [58], elevated Hcy may simply be a marker of vascular disease, and not a cause [82]. Alternatively, the potential benefit of exogenous folate may be offset by a direct toxic effect in the body, causing increased inflammation and proliferation of existing atherosclerotic lesions [58, 85]. Finally, vascular damage may be mediated solely by intracellular or tissue-bound Hcy, irrespective of changes in serum Hcy levels [58]. It should also be considered that many patients with cardiovascular disease have comorbidities (diabetes mellitus, hypertension, sedentary lifestyle, etc.) which may complicate the perceived effectiveness of folate as a treatment for high levels of Hcy. Furthermore, the progression of cardiovascular disease and comorbidities in these patients should also be considered, as folic acid alone, or combined with vitamins B6 and B12, may not be enough to essentially reverse long-standing, established health issues in these patients. Overall, high levels of Hcy in cardiovascular disease patients is a simple blood test that does have established, statistically significant benefits; therefore, folic acid supplementation should be considered in these patients, as it has been shown to reduce Hcy levels and could help improve their symptoms and potentially may have significant long-term benefits. Table 2 summarizes clinical trials investigating the associations between hyperhomocysteinemia and cardiovascular health.
Table 2.
Folate supplementation trials in hyperhomocysteinemia and cardiovascular health
| Source | Study type | No. of pts | Mean age (years) | Population | Mean homocysteine (µmol/L) | Benefit |
|---|---|---|---|---|---|---|
| Clarke et al. (1998) [6] | Meta-analysis | 1114 | 52 | Varied | 12 | Yes |
| Kotwal et al. (2015) [81] | Randomized controlled trial | 12,000 | Not reported | Indian Soldiers at high altitudes | 8.2 | Yes |
| Lonn et al. (2006) [82] | Randomized controlled trial | 5,522 | 68.85 | Vascular disease (coronary, cerebrovascular, peripheral vascular) | 12.2 | No |
| Armitage et al. (2010) [83] | Randomized controlled trial | 12,064 | 64 | Previous MI | 13 | No |
| Zhou et al. (2011) [84] | Meta-analysis | 44,841 | Varied by trial. Means ranged from 48.5–65.8 | Cardiovascular events | Did not study | No |
Conclusions
Hcy has widely been accepted as an independent risk for cardiovascular disease. This has been demonstrated in multiple studies. Given that hyperhomocysteinemia was reported to be found in greater than 60% of patients with vascular disease, there needs to be further research on treatment and management of homocystinemia [86]. One such option is folic acid supplementation. Having shown promising results with reductions of blood levels of Hcy, the results were greatest among patients with high blood Hcy levels or lower blood folate levels prior to treatment [6]. Although low-dose folic acid has been shown to improve vascular function by increasing bioavailability of BH4 and enhancing eNOS coupling, its effect in reducing adverse cardiovascular events in patients undergoing CABG has not yet yielded promising results [9, 10]. Further studies must be conducted in this regard to better ascertain best practice strategies in different pathological states.
Understanding the biosynthesis of Hcy and folic acid is tantamount towards treating hyperhomocysteinemia. Although it has been identified that deficiencies in folic acid, B12, and B6 cause elevations in Hcy, folic acid treatment has shown to significantly reduce Hcy compared to its B vitamin counterparts [8, 67]. Furthermore, H2S, a byproduct of Hcy metabolism, has been shown to antagonize Hcy’s effects and is vascular protective [8]. Knowing this, the study of H2S is medically relevant and should be further investigated.
Folic acid supplementation of 0.5–5.0 mg can lower Hcy levels by 25% and therefore may decrease the risk of cardiovascular disease [7]. Prior meta-analysis of observational studies have suggested that lowering Hcy to 3–4 μmol/l corresponds to about 30–40% less vascular disease [6]. Given that folic acid is cheap and effective, this should be a viable option for patients with high risk for cardiovascular adverse events. Although there is still ongoing research on folic acid (and other B vitamins) and homocystinemia, the present review provides a strong basic science rationale to advocate for the identification/screening of all patients with elevated levels of Hcy and the therapeutic delivery of folic acid supplementation as a strategy, in part, to reduce vascular pathogenesis and other deleterious sequalae from numerous disease states.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Acknowledgements
Funding
This work was supported by an Institutional Development Award from the National Institutes of General Medical Sciences of the National Institutes of Health under grant number P20GM121307 to C.G. Kevil. No Rapid Service Fee was received by the journal for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Alan Kaye, George Jeha, Alex Pham, Mitchell Fuller, Zachary I. Lerner, Elyse M. Cornett, Ivan Urits, Omar Viswanath, Christopher G. Kevil, and Gerald Sibley have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Footnotes
Digital Features
This article is published with digital features to facilitate understanding of the article. You can access the digital features on the article’s associated Figshare page. To view digital features for this article go to 10.6084/m9.figshare.12788843.
References
- 1.Warren CJ. What is Homocysteine? Am J Nurs. 2020;99(10):39–41. https://www.jstor.org/stable/3521915. Accessed 1 Mar 2020. [PubMed]
- 2.Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14(1):1–10. doi: 10.1186/1475-2891-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Q, He GW. Imbalance of homocysteine and H2S: significance, mechanisms, and therapeutic promise in vascular injury. Oxid Med Cell Longev. 2019 doi: 10.1155/2019/7629673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke R, Daly L, Robinson K, et al. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324(17):1149–1155. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- 5.Krause’s Food and the Nutrition Care Process-E-Book—L. Kathleen Mahan, Janice L Raymond—Google Books. https://books.google.com/books?id=DXIwDAAAQBAJ&pg=PA639&lpg=PA639&dq=A+dysfunction+in+these+enzymes+or+deficiency+in+these+vitamins+can+lead+to+a+rise+in+blood+levels+of+homocysteine5&source=bl&ots=W6GZTC02E1&sig=ACfU3U1uLKtFvIb_IEig-nGZLdWAaYt2tw&hl=en&sa=X&ved=2ahUKEwiPxpeKgbnqAhUFSq0KHaE9AjAQ6AEwAHoECAkQAQ#v=onepage&q=5&f=falseAdysfunctionintheseenzymes ordeficiencyinthesevitaminscanleadtoariseinbloodlevelsofhomocysteine. Accessed 1 Mar 2020.
- 6.Clarke R, Brattström L, Landgren F, et al. Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. BMJ. 1998;316(7135):894–898. doi: 10.1136/bmj.316.7135.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liakishev AA. Homocysteine lowering with folic acid and B vitamins in vascular disease. Kardiologiia. 2006;46(5):70. doi: 10.1016/s0749-4041(08)70686-9. [DOI] [PubMed] [Google Scholar]
- 8.Pushpakumar S, Kundu S, Sen U. Endothelial dysfunction: the link between homocysteine and hydrogen sulfide. Ingenta Connect. 2014;21(32):3662–3672. doi: 10.2174/0929867321666140706142335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulik A, Ruel M, Jneid H, et al. Secondary prevention after coronary artery bypass graft surgery. Circulation. 2015;131(10):927–964. doi: 10.1161/CIR.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 10.Shirodaria C, Antoniades C, Lee J, et al. Global improvement of vascular function and redox state with low-dose folic acid. Circulation. 2007;115:2262–2270. doi: 10.1161/CIRCULATIONAHA.106.679084. [DOI] [PubMed] [Google Scholar]
- 11.Attia AAA, Amer MAEM, Hassan M, El DSFG. Low serum folic acid can be a potential independent risk factor for erectile dysfunction: a prospective case–control study. Int Urol Nephrol. 2019;51(2):223–229. doi: 10.1007/s11255-018-2055-y. [DOI] [PubMed] [Google Scholar]
- 12.Lucock M. Folic acid: Nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab. 2000;71(1–2):121–138. doi: 10.1006/mgme.2000.3027. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly JG. Folic acid. Crit Rev Clin Lab Sci. 2001;38(3):183–223. doi: 10.1080/20014091084209. [DOI] [PubMed] [Google Scholar]
- 14.Fowler B. The folate cycle and disease in humans. Kidney Int Suppl. 2001 doi: 10.1046/j.1523-1755.2001.59780221.x. [DOI] [PubMed] [Google Scholar]
- 15.Milman N. Intestinal absorption of folic acid—new physiologic and molecular aspects. Indian J Med Res. 2012;136(5):725–728. [PMC free article] [PubMed] [Google Scholar]
- 16.Funk RS, Van Haandel L, Becker ML, Steven LJ. Low-dose methotrexate results in the selective accumulation of aminoimidazole carboxamide ribotide in an erythroblastoid cell line. J Pharmacol Exp Ther. 2013;347(1):154–163. doi: 10.1124/jpet.113.206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stover PJ. One-carbon metabolism-genome interactions in folate-associated pathologies. J Nutr. 2009;139(12):2402–2405. doi: 10.3945/jn.109.113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer. 2013;13(8):572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frewin R. Biochemical aspects of anaemia. In: Clinical biochemistry: metabolic and clinical aspects. 3. Amsterdam: Elsevier; 2014. pp. 515–532. [Google Scholar]
- 20.Avendaño C, Menéndez JC, Avendaño C, Menéndez JC (2008) Chapter 2—Antimetabolites. Med Chem Anticancer Drugs.10.1016/B978-0-444-52824-7.00002-0
- 21.Kumar A, Palfrey HA, Pathak R, Kadowitz PJ, Gettys TW, Murthy SN. The metabolism and significance of homocysteine in nutrition and health. Nutr Metab. 2017 doi: 10.1186/s12986-017-0233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blom HJ, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34(1):75–81. doi: 10.1007/s10545-010-9177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19(1):217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 24.Škovierová H, Vidomanová E, Mahmood S, et al. The molecular and cellular effect of homocysteine metabolism imbalance on human health. Int J Mol Sci. 2016 doi: 10.3390/ijms17101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stirzaker C, Song JZ, Ng W, et al. Methyl-CpG-binding protein MBD2 plays a key role in maintenance and spread of DNA methylation at CpG islands and shores in cancer. Oncogene. 2017;36(10):1328–1338. doi: 10.1038/onc.2016.297. [DOI] [PubMed] [Google Scholar]
- 26.Warnecke PM, Bestor TH. Cytosine methylation and human cancer. Curr Opin Oncol. 2000;12(1):68–73. doi: 10.1097/00001622-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Mudd SH, Ebert MH, Scriver CR. Labile methyl group balances in the human: the role of sarcosine. Metabolism. 1980;29(8):707–720. doi: 10.1016/0026-0495(80)90192-4. [DOI] [PubMed] [Google Scholar]
- 28.Kerr S. Completing methyltransferase systems. J Biol Chem. 1972;247:4248–4252. [PubMed] [Google Scholar]
- 29.Jung M, Pfeifer GP. Aging and DNA methylation. BMC Biol. 2015 doi: 10.1186/s12915-015-0118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McRae MP. Betaine supplementation decreases plasma homocysteine in healthy adult participants: a meta-analysis. J Chiropr Med. 2013;12(1):20–25. doi: 10.1016/j.jcm.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng Q, Kalari K, Fridley BL, et al. Betaine-homocysteine methyltransferase: human liver genotype-phenotype correlation. Mol Genet Metab. 2011;102(2):126–133. doi: 10.1016/j.ymgme.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber GJ, Pushpakumar S, Tyagi SC, Sen U. Homocysteine and hydrogen sulfide in epigenetic, metabolic and microbiota related renovascular hypertension. Pharmacol Res. 2016;113:300–312. doi: 10.1016/j.phrs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kar S, Shahshahan HR, Kambis TN, et al. Hydrogen sulfide ameliorates homocysteine-induced cardiac remodeling and dysfunction. Front Physiol. 2019 doi: 10.3389/fphys.2019.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J, Minkler P, Grove D, et al. Non-enzymatic hydrogen sulfide production from cysteine in blood is catalyzed by iron and vitamin B6. Commun Biol. 2019 doi: 10.1038/s42003-019-0431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sacharow SJ, Picker JD, Levy HL. Homocystinuria caused by cystathionine beta-synthase deficiency. Seattle: University of Washington; 1993. [PubMed] [Google Scholar]
- 36.Yudkoff M. Transsulfuration Pathway—an overview|ScienceDirect Topics. Basic Neurochemistry. 2012. https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/transsulfuration-pathway. Accessed 1 Mar 2020.
- 37.Brown MJ, Beier K. Vitamin B6 Deficiency (Pyridoxine). Treasure Island (FL): StatPearls; 2018. https://www.ncbi.nlm.nih.gov/books/NBK470579/. Accessed 1 Mar 2020.
- 38.Gersztenkorn D. The traditionally protective cystathionine gamma-lyase/hydrogen sulfide pathway contributes to pathological retinal neovascularization in oxygen-induced retinopathy. Abstract. UTMB Health. https://utmb-ir.tdl.org/handle/2152.3/11202.
- 39.Jung KJ, Jang HS, Kim JI, Han SJ, Park JW, Park KM. Involvement of hydrogen sulfide and homocysteine transsulfuration pathway in the progression of kidney fibrosis after ureteral obstruction. Biochim Biophys Acta Mol Basis Dis. 2013;1832(12):1989–1997. doi: 10.1016/j.bbadis.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 40.Paul BD, Sbodio JI, Snyder SH. Cysteine metabolism in neuronal redox homeostasis. Trends Pharmacol Sci. 2018;39(5):513–524. doi: 10.1016/j.tips.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karmin O, Siow YL. Metabolic imbalance of homocysteine and hydrogen sulfide in kidney disease. Curr Med Chem. 2017 doi: 10.2174/0929867324666170509145240. [DOI] [PubMed] [Google Scholar]
- 42.Liu M, Deng M, Su J, et al. Specific downregulation of cystathionine β -synthase expression in the kidney during obesity. Physiol Rep. 2018;6(13):e13630. doi: 10.14814/phy2.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peh MT, Anwar AB, Ng DSW, Shirhan BMAM, Kumar SD, Moore PK. Effect of feeding a high fat diet on hydrogen sulfide (H2S) metabolism in the mouse. Nitric Oxide Biol Chem. 2014;41:138–145. doi: 10.1016/j.niox.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Wang B, Zeng J, Gu Q. Exercise restores bioavailability of hydrogen sulfide and promotes autophagy influx in livers of mice fed with high-fat diet. Can J Physiol Pharmacol. 2017;95(6):667–674. doi: 10.1139/cjpp-2016-0611. [DOI] [PubMed] [Google Scholar]
- 45.Hine C, Zhu Y, Hollenberg AN, Mitchell JR. Dietary and endocrine regulation of endogenous hydrogen sulfide production: implications for longevity. Antioxid Redox Signal. 2018;28(16):1483–1502. doi: 10.1089/ars.2017.7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace JL, Motta J-P, Buret AG. Hydrogen sulfide: an agent of stability at the microbiome-mucosa interface. Am J Physiol Liver Physiol. 2018;314(2):G143–G149. doi: 10.1152/ajpgi.00249.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blachier F, Beaumont M, Kim E. Cysteine-derived hydrogen sulfide and gut health. Curr Opin Clin Nutr Metab Care. 2018 doi: 10.1097/MCO.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 48.Croix JA, Carbonero F, Nava GM, Russell M, Greenberg E, Gaskins HR. On the relationship between sialomucin and sulfomucin expression and hydrogenotrophic microbes in the human colonic mucosa. PLoS ONE. 2011 doi: 10.1371/journal.pone.0024447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rey FE, Gonzalez MD, Cheng J, Wu M, Ahern PP, Gordon JI. Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc Natl Acad Sci USA. 2013;110(33):13582–13587. doi: 10.1073/pnas.1312524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barton LL, Ritz NL, Fauque GD, Lin HC. Sulfur cycling and the intestinal microbiome. Dig Dis Sci. 2017;62(9):2241–2257. doi: 10.1007/s10620-017-4689-5. [DOI] [PubMed] [Google Scholar]
- 51.Tomasova L, Konopelski P, Ufnal M. Gut bacteria and hydrogen sulfide: the new old players in circulatory system homeostasis. Molecules. 2016;21(11):1558. doi: 10.3390/molecules21111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng Y, Stams AJM, de Vos WM, Sánchez-Andrea I. Enrichment of sulfidogenic bacteria from the human intestinal tract. FEMS Microbiol Lett. 2017 doi: 10.1093/femsle/fnx028. [DOI] [PubMed] [Google Scholar]
- 53.Johnson A, Glass M. Hyper-homocysteinemia. Clinical Advisor website. https://www.clinicaladvisor.com/home/decision-support-in-medicine/hospital-medicine/hyper-homocysteinemia/. Accessed 28 Feb 2020.
- 54.Anderson JA, Hogg KE, Weitz JI. Hypercoagulable states. Hematology: basic principles and practice. Amsterdam: Elsevier; 2018. pp. 2076–2087. [Google Scholar]
- 55.Selhub J, Jacques PF, Bostom AG, et al. Association between plasma homocysteine concentrations and extracranial carotid-artery stenosis. N Engl J Med. 1995;332(5):286–291. doi: 10.1056/NEJM199502023320502. [DOI] [PubMed] [Google Scholar]
- 56.Majors A, Allen Ehrhart L, Pezacka EH. Homocysteine as a risk factor for vascular disease: enhanced collagen production and accumulation by smooth muscle cells. Arterioscler Thromb Vasc Biol. 1997;17(10):2074–2081. doi: 10.1161/01.ATV.17.10.2074. [DOI] [PubMed] [Google Scholar]
- 57.Liu J, Quan J, Li Y, Wu Y, Yang L. Blood homocysteine levels could predict major adverse cardiac events in patients with acute coronary syndrome: a STROBE-compliant observational study. Med (USA) 2018;97(40):e12626. doi: 10.1097/MD.0000000000012626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balint B, Jepchumba VK, Guéant J-L, Guéant-Rodriguez R-M. Mechanisms of homocysteine-induced damage to the endothelial, medial and adventitial layers of the arterial wall. Biochimie. 2020 doi: 10.1016/j.biochi.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 59.Esse R, Barroso M, De Almeida IT, Castro R. The contribution of homocysteine metabolism disruption to endothelial dysfunction: state-of-the-art. Int J Mol Sci. 2019 doi: 10.3390/ijms20040867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu X, Zhang L, Miao Y, et al. Homocysteine causes vascular endothelial dysfunction by disrupting endoplasmic reticulum redox homeostasis. Redox Biol. 2019;20:46–59. doi: 10.1016/j.redox.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Z, Wei C, Zhou Y, et al. Homocysteine induces apoptosis of human umbilical vein endothelial cells via mitochondrial dysfunction and endoplasmic reticulum stress. Oxid Med Cell Longev. 2017;2017:5736506. doi: 10.1155/2017/5736506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitchell JA, Ali F, Bailey L, Moreno L, Harrington LS. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp Physiol. 2008;93(1):141–147. doi: 10.1113/expphysiol.2007.038588. [DOI] [PubMed] [Google Scholar]
- 63.Kamat PK, Kalani A, Givvimani S, Sathnur PB, Tyagi SC, Tyagi N. Hydrogen sulfide attenuates neurodegeneration and neurovascular dysfunction induced by intracerebral-administered homocysteine in mice. Neuroscience. 2013;252:302–319. doi: 10.1016/j.neuroscience.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han S, Wu H, Li W, Gao P. Protective effects of genistein in homocysteine-induced endothelial cell inflammatory injury. Mol Cell Biochem. 2015;403(1–2):43–49. doi: 10.1007/s11010-015-2335-0. [DOI] [PubMed] [Google Scholar]
- 65.Li J, Luo M, Xie N, Wang J, Chen L. Curcumin protects endothelial cells against homocysteine induced injury through inhibiting inflammation. Am J Transl Res. 2016;8(11):4598–4604. [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao J, Chen H, Liu N, et al. Role of hyperhomocysteinemia and hyperuricemia in pathogenesis of atherosclerosis. J Stroke Cerebrovasc Dis. 2017;26(12):2695–2699. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 67.Jakubowski H. Protein homocysteinylation: possible mechanism underlying pathological consequences of elevated homocysteine levels. FASEB J. 1999;13(15):2277–2283. doi: 10.1096/fasebj.13.15.2277. [DOI] [PubMed] [Google Scholar]
- 68.Tsai JC, Perrella MA, Yoshizumi M, et al. Promotion of vascular smooth muscle cell growth by homocysteine: a link to atherosclerosis. Proc Natl Acad Sci U S A. 1994;91(14):6369–6373. doi: 10.1073/pnas.91.14.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buemi M, Marino D, Di Pasquale G, et al. Effects of homocysteine on proliferation, necrosis, and apoptosis of vascular smooth muscle cells in culture and influence of folic acid. Thromb Res. 2001;104(3):207–213. doi: 10.1016/S0049-3848(01)00363-2. [DOI] [PubMed] [Google Scholar]
- 70.Küskü-Kiraz Z, Genc S, Bekpınar S, et al. Effects of betaine supplementation on nitric oxide metabolism, atherosclerotic parameters, and fatty liver in guinea pigs fed a high cholesterol plus methionine diet. Nutrition. 2018;45:41–48. doi: 10.1016/j.nut.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 71.Liu Z, Luo H, Zhang L, et al. Hyperhomocysteinemia exaggerates adventitial inflammation and angiotensin II-induced abdominal aortic aneurysm in mice. Circ Res. 2012;111(10):1261–1273. doi: 10.1161/CIRCRESAHA.112.270520. [DOI] [PubMed] [Google Scholar]
- 72.Yao D, Sun N-L. Hyperhomocysteinemia accelerates collagen accumulation in the adventitia of balloon-injured rat carotid arteries via angiotensin II type 1 receptor. Int J Mol Sci. 2014;15(11):19487–19498. doi: 10.3390/ijms151119487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jain R, Manning S, Cutler AJ. Good, better, best: clinical scenarios for the use of L-methylfolate in patients with MDD. CNS Spectr. 2019 doi: 10.1017/S1092852919001469. [DOI] [PubMed] [Google Scholar]
- 74.Essoumac M, Noubiap JJN. Therapeutic potential of folic acid supplementation for cardiovascular disease prevention through homocysteine lowering and blockade in rheumatoid arthritis patients. Biomark Res. 2015;3(24). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4559887/. Accessed March 10, 2020. [DOI] [PMC free article] [PubMed]
- 75.Collaboration HLT. Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. Homocysteine Lowering Trialists’ Collaboration. BMJ. 1998;316(7135):894–898. doi: 10.1136/bmj.316.7135.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wald DS, Bishop L, Wald NJ, et al. Randomized trial of folic acid supplementation and serum homocysteine levels. Arch Intern Med. 2001;161(5):695–700. doi: 10.1001/archinte.161.5.695. [DOI] [PubMed] [Google Scholar]
- 77.Jayedi A, Zargar MS. Intake of vitamin B6, folate, and vitamin B12 and risk of coronary heart disease: a systematic review and dose-response meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr. 2019;59(16):2697–2707. doi: 10.1080/10408398.2018.1511967. [DOI] [PubMed] [Google Scholar]
- 78.Jeon J, Park K. Dietary vitamin B6 intake associated with a decreased risk of cardiovascular disease: a prospective cohort study. Nutrients. 2019 doi: 10.3390/nu11071484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cui R, Iso H, Date C, Kikuchi S, Tamakoshi A. Dietary folate and vitamin B6 and B12 intake in relation to mortality from cardiovascular diseases: Japan collaborative cohort study. Stroke. 2010;41(6):1285–1289. doi: 10.1161/STROKEAHA.110.578906. [DOI] [PubMed] [Google Scholar]
- 80.Li Y, Huang T, Zheng Y, Muka T, Troup J, Hu FB. Folic Acid Supplementation and the Risk of Cardiovascular Diseases: A Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2016 doi: 10.1161/JAHA.116.003768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kotwal J, Kotwal A, Bhalla S, Singh PK, Nair V. Effectiveness of homocysteine lowering vitamins in prevention of thrombotic tendency at high altitude area: a randomized field trial. Thromb Res. 2015;136(4):758–762. doi: 10.1016/j.thromres.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and b vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 83.Armitage JM, Bowman L, Clarke RJ, et al. Effects of homocysteine-lowering with folic acid plus vitamin B 12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. J Am Med Assoc. 2010;303(24):2486–2494. doi: 10.1001/jama.2010.840. [DOI] [PubMed] [Google Scholar]
- 84.Zhou YH, Tang JY, Wu MJ, et al. Effect of folic acid supplementation on cardiovascular outcomes: a systematic review and meta-analysis. PLoS ONE. 2011;6(9):e25142. doi: 10.1371/journal.pone.0025142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smulders YM, Blom HJ. The homocysteine controversy. J Inherit Metab Dis. 2011;34(1):93–99. doi: 10.1007/s10545-010-9151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shahbazian N, Jafari RM, Haghnia S. The evaluation of serum homocysteine, folic acid, and vitamin B12 in patients complicated with preeclampsia. Electron Physician. 2016;8(10):3057–3061. doi: 10.19082/3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.