Abstract
The purpose of this study was to identify risk factors for falls in postmenopausal women and provide evidence for the primary prevention of falls in postmenopausal women. The protocol for this meta-analysis is registered with PROSPERO (CRD42020170927). We searched PubMed, the Cochrane Library and EMBASE for observational studies on the risk factors for falls in postmenopausal women. Review Manager 5.3 was used to calculate the relative risk (RR) or weighted mean difference (WMD) of potential risk factors related to falls. STATA 14.0 was used for the quantitative evaluation of publication bias. Eleven studies with 42,429 patients from 7 countries were included. The main risk factors for falls in postmenopausal women were patient sociodemographic risk factors (age: WMD = 0.37, 95% CI 0.07 to 0.68; body weight: WMD = 0.88, 95% CI 0.56 to 1.12; BMI: WMD = 0.34, 95% CI 0.21 to 0.46; exercise: RR = 0.97, 95% CI 0.94 to 0.99; and FES-I: WMD = 6.60, 95% CI 0.72 to 12.47) and medical risk factors (dietary calcium intake: WMD = − 16.91, 95% CI − 25.80 to − 8.01; previous fracture history: RR = 1.21, 95% CI 1.13 to 1.29; previous falls: RR = 2.02, 95% CI 1.91 to 2.14; number of diseases, ˃ 2: RR = 1.17, 95% CI 1.11 to 1.23; and number of reported chronic health disorders: WMD = 0.30, 95% CI 0.10 to 0.49). Knowledge of the many risk factors associated with falls in postmenopausal women can aid in fall prevention. However, we cannot rule out some additional potential risk factors (age at the onset of menopause, years since last menstruation, hormone therapy and BMD) that need further clinical research.
Electronic supplementary material
The online version of this article (10.1007/s00198-020-05508-8) contains supplementary material, which is available to authorized users.
Keywords: Falls, Meta-analysis, Postmenopausal women, Risk factors
Introduction
Falls are a major public health issue, and the international guidelines for fall prevention for elderly people define a fall as a sudden, involuntary change in posture that causes one to fall to the ground or some lower level but is not caused by violence, loss of consciousness or hemiplegia [1]. As global ageing accelerates, the incidence of age-related health problems, including falls, is increasing [2]. Approximately one-third of elderly people over the age of 65 years fall one or more times each year, and the number increases with increasing age; the annual incidence of falls in elderly people over 80 years old is as high as 50% [3]. A retrospective cohort study of 2094 women from 2005 to 2008 showed that the probability of falls for women over 65 years is 31.9%, which is very harmful in terms of the disability and mortality of older women [4]. Falling can cause severe soft tissue damage and even death in postmenopausal women, making falls a serious health risk in this group [5]. The associated long-term activity limitations eventually cause elderly people to experience negative emotions such as apathy and irritability, which affect their physical and mental health. At the same time, falls and fall-related injuries can cause significant social and economic burdens [6, 7].
In women, the fear of falling (FOF) increases the likelihood of falls [8]. Because of this FOF, they reduce their activities, leading to a decline in their ability to move and in turn increasing their risk of falling [9]. Postmenopausal women undergo a special physiological phase in menopause, and menopause-related changes in metabolic rate, weight and body composition may also lead to limitations in physical function [10]. Menopause is related to some inherent risk factors for falls, such as decreased physical function, increased FOF and postural sway [11], and studies have suggested that obesity and muscle dysfunction are risk factors for falls in postmenopausal women [12]. Many published studies have suggested that independent risk factors for falls in postmenopausal women may be related to smoking, menopausal age, bone density, drug use and previous medical history [11, 13]. However, since the published literature contains mostly single-centre cohort studies, they differ in the identification of certain risk factors (such as alcoholism and hormone use) [14–16], and further research is urgently needed to identify the risk factors for falls in postmenopausal women.
Defining the risk factors for falls can provide evidence for the primary prevention of falls in postmenopausal women. The purpose of this meta-analysis is to quantitatively analyse these risk factors in postmenopausal women. Identifying high-risk factors can be used to target preventive measures to reduce the risk of falling after menopause.
Methods
This meta-analysis was performed in strict accordance with the relevant requirements of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) statement [17]. Additionally, this study was registered on the PROSPERO website https://www.crd.york.ac.uk/PROSPERO/(registration number CRD42020170927).
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) published observational studies, including case-control studies, retrospective cohort studies and prospective cohort studies; (2) study subjects who were postmenopausal women, regardless of age or nationality; (3) studies that clearly involved fall groups and non-fall groups; (4) studies that analysed data on risk factors for falls in postmenopausal women; and (5) studies that were published only in the English language.
The exclusion criteria were as follows: (1) reviews, meeting abstracts and case reports; (2) duplicate publications or studies with identical data; and (3) studies that did not have sufficient data to calculate the means and SDs or with data that were not available to the authors.
Literature retrieval strategy
The PubMed, Cochrane Library and EMBASE databases were searched, and observational studies meeting the inclusion criteria were included. To perform a qualitative analysis and reduce the possibility of missed articles, this study also searched the Cochrane Library (which mainly includes clinical trials). The retrieval time was from the establishment of each database to February 2020, see supplement 1 for the retrieval strategy for each database. We also manually searched all the references of the included studies to identify other studies that might be eligible for inclusion.
Literature screening and data extraction
Two orthopaedic surgeons retrieved the studies, and the preliminary and secondary screening of the studies was conducted in strict accordance with the preestablished inclusion and exclusion criteria. Two researchers independently extracted the data, and a third researcher performed the comparisons. In cases of errors or differences, the third researcher and corresponding author assisted in the final determination.
The data extracted for this study included the title, first author, publication year, country, sample size, mean age, fall definition, fall ascertainment, fall reference period, identified significant risk factors, items relevant for the evaluation of the quality of the study and all possible associated risk factors.
Quality assessment of the included studies
The Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of the included observational studies. The NOS evaluation includes 4 items (4 points) for the evaluation of selection, 1 item (2 points) for the evaluation of the comparability of groups and 3 items (3 points) for the evaluation of the outcome of interest; the highest possible score is 9 points. Studies with a score greater than or equal to 6 are considered high quality, and those with a score less than 6 are considered low quality. The NOS uses a semiquantitative star system to evaluate the quality of studies. If a study meets the standard, it has received 1 star per item, with a total score of 9 points. The higher the score, the higher the quality of the study [18]. Two researchers independently completed the quality evaluations of the included studies. If there were inconsistencies, they were resolved by consultation with the corresponding author.
Statistical analysis
The relative risk (RR) was used to evaluate the effects of binary variables, and the weighted mean difference (WMD) was used to evaluate the effects of continuous variables; the 95% confidence intervals (CIs) of the RR and WMD were calculated. Review Manager 5.3.5 software (Cochrane Collaboration, Oxford, UK) was used to calculate the efficacy and safety indicators and their 95% confidence intervals. In addition, for homogeneous data sets, P > 0.1 and I2 < 50% were considered the test standards. When the above two statistical conditions were met, a fixed-effect model was used for the meta-analysis because the pooled effect sizes were relatively homogenous. If one of the above standards was not met, the homogeneity of the pooled effect size was not ideal, and the random effects model was applied. If there was a significant difference, a RR ≥ 2 was considered a high-risk factor, 1 < RR < 2 was a medium risk factor, and RR < 1 was a protective factor.
For heterogeneous risk factor indicators, the process was as follows: if the heterogeneity was large, Review Manager 5.3.5 software was used to evaluate the heterogeneity using the leave one out method. If the source of heterogeneity was identified, the analysis was performed again after the elimination of that study.
To quantitatively assess whether there were publication biases in different risk factor indicators, this study used Stata 14.0 (STATA Corporation, Lakeway, Texas, USA) software to perform Egger and Begg linear regression tests on the outcome indicators included in the combined analysis of 4 or more studies.
Results
Literature screening process and results
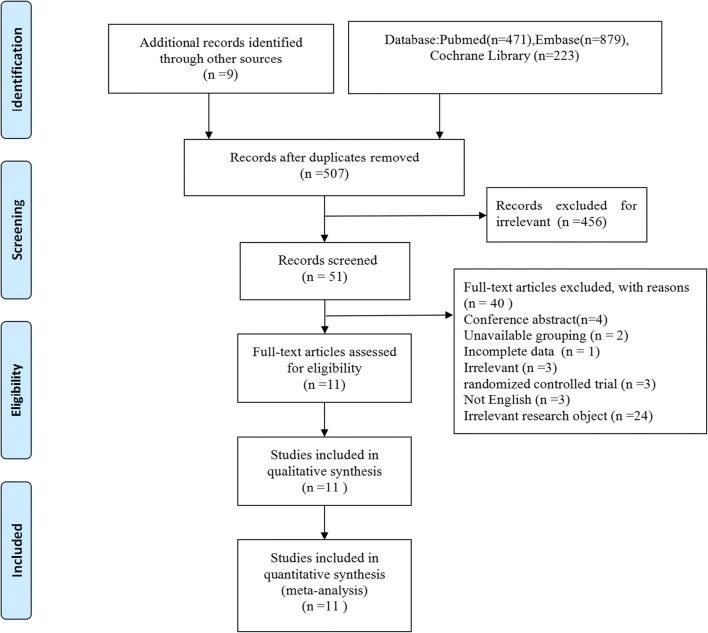
A total of 1582 relevant documents were obtained during the preliminary searches of the PubMed (n = 471), EMBASE (n = 879) and Cochrane Library (n = 223) databases and other manual searches (n = 9). After excluding duplicate studies, 507 articles were retained. After reading the titles and abstracts, excluding irrelevant studies and applying the inclusion and exclusion criteria, this meta-analysis included 11 prospective cohort studies with 42,429 patients from 7 countries. The study screening process and results are shown in Fig. 1, and basic information about the included studies is presented in Table 1.
Fig. 1.
Flow diagram of the study selection
Table 1.
Characteristics of the included studies
| Author | Year | Country | Study design | Sample size | Age, years | Fall definition | Fall ascertainment | Fall reference period | Identified significant risk factors | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fall | Non-fall | Fall | Non-fall | ||||||||
| Chu-Hsu Lin 2016 [19] | 2016 | Taiwan, China | Co, P | 183 | 770 | 68.8 ± 8.3 | 66.4 ± 8.4 | Falling with landing on the ground or lower level | Telephone interview | Previous 24 months | Age, hypertension, diabetes |
| Wojciech Pluskiewicz 2016 [20] | 2016 | Poland | Co, P | 328 | 650 | 67.2 ± 7.7 | 65.2 ± 7.5 | “From an upright position” | Interviews | Previous 12 months | Age, rural stay, prior fracture, diabetes type 1, bronchial asthma, and depression |
| Nadia Afrin 2016 [21] | 2016 | Finland | Co, P | 3593 | 7001 | 52.2 ± 2.9 | 52.3 ± 2.9 | “From an upright position” | Self-report | Previous 12 months | Weight, BMI, surgery, current use of prescribed medications, smoking, previous fracture |
| Nadia Afrin 2018 [22] | 2018 | Finland | Co, P | 3397 | 5259 | 62.1 ± 2.9 | 62.2 ± 2.9 | “From an upright position” | Self-report | Previous 12 months | BMI, number of prescribed medications, number of chronic diseases, mobility, smoker, alcohol, number of women with musculoskeletal disorders |
| B.Drozdzowska 2013 [23] | 2013 | Poland | Co, P | 211 | 407 | 67.6 ± 7.9 | 65.6 ± 7.7 | NR | Interviews | Previous 12 months | Age, height, menopause, instrumental activity of daily living |
| Yuksel Ersoy 2009 [24] | 2009 | Turkey | Co, P | 35 | 90 | 64.5 ± 8.1 | 60.2 ± 7.5 | Unintentionally coming to rest on the ground, floor, or other lower level | Self-report | Previous 12 months | Age, Berg Balance Scale, TUG test, FES-I, fear |
| F. Hita-Contreras 2013 [25] | 2013 | Spain | Co, P | 20 | 76 | 56.6 ± 4.2 | 57.9 ± 3.9 | An unexpected event in which the participants come to rest on the ground, floor, or lower level | Interviews | Previous 12 months | FES-I, velocity, sway area |
| Kerri M. Winters-Stone 2011 [26] | 2011 | USA | Co, P | 34 | 25 | 59.2 ± 7.1 | 57.4 ± 12.4 | Unintentionally coming to rest on the ground or at some other lower level, not as a result of a major intrinsic event or overwhelming hazard | Self-report | Previous 6 months | Balance disturbances of vestibular origin, delays in detecting low contrast visual stimuli |
| Shawna Follis 2018 [27] | 2018 | USA | Co, P | 2509 | 7415 | 50–79 | 50–79 | Fall and land on the floor or ground | Self-report | Previous 12 months | Sarcopenic obesity |
| Kaisa M. Randell 2001 [28] | 2001 | Finland | Co, P | 3049 | 6743 | 57.5 ± 2.8 | 57.6 ± 2.9 | NR | Self-report | Previous 12 months | Weight, number of disorders, dairy Ca intake, physically active, smoking |
| Abdulrahim A. Rouzi 2015 [29] | 2015 | Saudi Arabia | Co, P | 91 | 543 | 61.64 ± 5.69 | 59.6 ± 5.95 | Asudden unintentional change in position causing an individual to land at a lower level on an object, on the floor, or on the ground | Interviews | Previous 12 months | Age, time since menopause, bone mineral density, handgrip strength, TUG test |
Co, cohort study; P, prospective study; NR, not report; TUG, timed up and go; BMI, body mass index; FES-I, Falls Efficacy Scale International
Quality evaluation of the included studies
The 11 observational studies included in this meta-analysis are all prospective cohort studies, with study quality evaluation scores of 6–9. According to the NOS criteria, 2 studies received 9 points, 4 studies received 8 points, 4 studies received 7 points and 1 study received 6 points. Each article included in this study had an NOS score greater than or equal to 6 points, indicating that the quality of the studies was very high (Table 2).
Table 2.
Newcastle-Ottawa Scale for risk of bias assessment of cohort studies included in the meta-analysis
| Study | Selection | Comparability | Outcome | Overall | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of exposed cohort | Selection of non-exposed | Ascertainment of exposure | Outcome not present at start | Assessment of outcome | Adequate follow-up length | Adequacy of follow-up | |||
| Chu-Hsu Lin 2016 [19] | ☆ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 |
| Wojciech Pluskiewicz 2016 [20] | ☆ | ★ | ★ | ☆ | ★★ | ★ | ★ | ★ | 7 |
| Nadia Afrin 2016 [21] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Nadia Afrin 2018 [22] | ★ | ★ | ★ | ☆ | ★ | ★ | ★ | ★ | 7 |
| B. Drozdzowska 2013 [23] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Yuksel Ersoy 2009 [24] | ☆ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 |
| F. Hita-Contreras 2013 [25] | ☆ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 |
| Kerri M. Winters-Stone 2011 [26] | ☆ | ★ | ★ | ★ | ★ | ★ | ★ | ☆ | 6 |
| Shawna Follis 2018 [27] | ☆ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 |
| Kaisa M. Randell 2001 [28] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Abdulrahim A. Rouzi 2015 [29] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
★, score of 1; ★★, score of 2; ☆, score of 0
Findings from the meta-analysis
Sociodemographic risk factors
We performed a meta-analysis of twelve sociodemographic risk factors: age, body weight, body height, body mass index (BMI), age at the onset of menopause, years since last menstruation, exercise, smoking, and alcohol use, Falls Efficacy Scale International (FES-I) scores, timed up and go test (TUG test) results and five-times sit-to-stand test (5-STS) results (Table 3). Based on the statistical results of the meta-analysis, we identified the following risk factors for falls in postmenopausal women: age (WMD 0.37, 95% CI 0.07 to 0.68), body weight (WMD 0.88, 95% CI 0.56 to 1.12), BMI (WMD 0.34, 95% CI 0.21 to 0.46), exercise (RR 0.97, 95% CI 0.94 to 0.99), smoking (RR 0.78, 95% CI 0.72 to 0.85) and FES-I scores (WMD 6.60, 95% CI 0.72 to 12.47). Among the above statistically significant risk factors, the higher the FES-I score, the more likely a fall event is; thus, this score is a high-risk factor. Interestingly, smoking is a protective factor in postmenopausal women. Older age, lack of physical exercise, weight gain and high BMI all increase the risk of falls in postmenopausal women. However, body height, age at the onset of menopause, years since last menstruation, alcohol use, TUG time and 5-STS time were not found to be direct risk factors for falls in postmenopausal women (P > 0.05).
Table 3.
The main outcomes of meta-analysis and subgroup analysis
| Risk factors | No. of studies | RR or WMD | LL 95% CI | UL 95% CI | P value | I2 (%) | Analysis model |
|---|---|---|---|---|---|---|---|
| Sociodemographic risk factors | |||||||
| Age, years | 10 | 0.37† | 0.07 | 0.68 | 0.02 | 85 | IV, random |
| Age (eliminate heterogeneous sources) | 7 | 1.83† | 0.93 | 2.73 | < 0.0001 | 52 | IV, random |
| Body weight, kg | 6 | 0.88† | 0.56 | 1.21 | < 0.00001 | 0 | IV, fixed |
| Body height, cm | 7 | − 0.16† | − 0.40 | 0.08 | 0.20 | 56 | IV, random |
| BMI (kg/m2) | 9 | 0.34† | 0.21 | 0.46 | < 0.00001 | 0 | IV, fixed |
| Age of menopause, years | 5 | − 0.31† | − 0.91 | 0.29 | 0.31 | 64 | IV, random |
| Years since last menstruation, years | 4 | 0.01† | − 0.15 | 0.16 | 0.94 | 37 | IV, fixed |
| Exercise (yes vs. no) | 4 | 0.97* | 0.94 | 0.99 | 0.01 | 0 | M-H, fixed |
| Smoking (yes vs. no) | 3 | 0.78* | 0.72 | 0.85 | < 0.00001 | 0 | M-H, fixed |
| Alcohol use, heavy use, > 30 drinks/month (yes vs. no) | 2 | 0.98* | 0.85 | 1.13 | 0.77 | 43 | M-H, fixed |
| FES-I, point | 2 | 6.60† | 0.72 | 12.47 | 0.03 | 79 | IV, random |
| TUG test, second | 2 | 1.32† | − 1.21 | 3.86 | 0.31 | 90 | IV, random |
| 5-STS, second | 3 | 0.30† | − 0.36 | 0.96 | 0.37 | 0 | IV, fixed |
| Medical risk factors | |||||||
| Dietary calcium intake, mg/day | 3 | − 16.91† | − 25.80 | − 8.01 | 0.0002 | 7 | IV, fixed |
| Previous fracture history (yes vs. no) | 3 | 1.21* | 1.13 | 1.29 | < 0.00001 | 0 | M-H, fixed |
| Previous fallers (yes vs. no) | 3 | 2.02* | 1.91 | 2.14 | < 0.00001 | 0 | M-H, fixed |
| Hormone therapy (yes vs. no) | 4 | 1.00* | 0.98 | 1.03 | 0.80 | 0 | M-H, fixed |
| Number of diseases, ˃ 2 (yes vs. no) | 2 | 1.17* | 1.11 | 1.23 | < 0.00001 | 0 | M-H, fixed |
| Number of reported chronic health disorders | 2 | 0.30† | 0.10 | 0.49 | 0.003 | 94 | IV, random |
| BMD, g/cm3, neck femur | 2 | 0.00† | − 0.02 | 0.02 | 1.00 | 0 | IV, fixed |
Note: The forest map of all risk factors is shown in Supplementary appendix 2
TUG, timed up and go; 5-STS, five-times sit-to-stand; BMI, body mass index; FES-I, Falls Efficacy Scale International; BMD, bone mineral density; RR, relative risk; WMD, standardized mean differences; LL, lower limit; UL, upper limit; M-H, Mantel Haenszel test; IV, inverse variance
*RR
†WMD
Medical risk factors
We analysed 7 medical-related risk factors, including dietary calcium intake (mg/day), previous fracture history, previous falls, hormone therapy, number of diseases (˃ 2), number of reported chronic health disorders and bone mineral density (BMD) of the neck femur (g/cm3) (Table 3). Based on the combined RRs or WMDs, we identified the following risk factors: previous fracture history (RR 1.21, 95% CI 1.13 to 1.29), previous falls (RR 2.02, 95% CI 1.91 to 2.14), number of diseases, ˃ 2 (RR 1.17, 95% CI 1.11 to 1.23) and number of reported chronic health disorders (WMD 0.30, 95% CI 0.10 to 0.49). In addition, the meta-analysis showed that postmenopausal women who had a fall event had lower levels of calcium intake (low-calcium diet) than postmenopausal women who had not fallen (WMD − 16.91, 95% CI − 25.80 to − 8.01). Considering all the significant risk factors, postmenopausal women who have experienced previous falls have a higher risk of falling again. Postmenopausal women who have a low-calcium diet, have a history of fractures or have more chronic health disorders also have a relatively higher risk of falling. However, hormone use (RR 1.00, 95% CI 0.98 to 1.03) and femoral neck bone density (WMD 0.00, 95% CI − 0.02 to 0.02) are not risk factors for falls.
Evaluation of publication bias
To quantitatively analyse whether there was publication bias in the relevant outcome indicators of this study, we conducted Egger’s and Begg’s tests on the outcome indicators appearing in 4 or more studies. Begg’s tests suggested no significant publication bias in the following risk factors: age (P = 0.721), body weight (P = 0.707), body height (P = 0.368), BMI (P = 0.348), age at the onset of menopause (P ˃ 0.05), years since last menstruation (P ˃ 0.05), exercise (P = 0.734) and hormone therapy (P ˃ 0.05). The data analysis process and statistical results of the evaluation of publication bias are shown in Supplementary 3.
Discussion
Falling often leads to restricted activities, social isolation, increased disability and death. Therefore, it is important to identify the risk factors for falls and implement preventive measures early. For the first time, this meta-analysis systematically and quantitatively analysed the correlations between falls and some potential risk factors in postmenopausal women, which may be helpful for informing strategies for the prevention of falls in this group in the future. We analysed 19 sociodemographic risk factors and medical risk factors in postmenopausal women. The meta-analysis results showed that older age, higher body weight, higher BMI, lack of physical exercise, higher FES-I scores, previous fracture history, previous falls and more coexisting diseases are risk factors for falls in postmenopausal women.
Sociodemographic risk factors
We found that older age is a major risk factor for falling in postmenopausal women. When we combined the results from 10 studies, the level of heterogeneity was 85%, and the difference was statistically significant (P = 0.02). When we removed the source of heterogeneity through a sensitivity analysis and performed the statistical analysis again, the level of heterogeneity was 52%, and the difference was statistically significant (P < 0.0001). The meta-analysis showed that older age is a risk factor for falls, and the statistical results are stable and reliable. During menopause, women’s hormone levels decrease each year, and their physical function declines more rapidly than that of men [30, 31]. Therefore, as menopausal women grow older, their physical functions, muscle functions and stability may weaken, thus increasing the risk of falling. Being overweight and having a higher BMI also increase the risk of falls in postmenopausal women. In an 8-year cohort study, Christine L et al. [32] found that weight gain was positively correlated with the risk of falling. Studies have shown that a higher BMI in older women increases their risk of falling; weight gain is closely related to hormonal changes in postmenopausal women, an increase in the incidence of flatfoot and a decline in lower-limb muscle quality [33, 34]. Obese elderly women have high lean leg masses but lack muscle strength, so they have poor balance and stability and are prone to falls [35]. Additionally, this meta-analysis shows that body height is not a risk factor for falls in postmenopausal women (WMD − 0.16, 95% CI − 0.40 to 0.08). It can be concluded that obesity and weight gain are associated with a higher risk of falling. We found that a lack of physical exercise also leads to an increased risk of falls. Research has shown that strengthening physical exercises constitutes an important means of enhancing balance, improving physical fitness and reducing falls in elderly people [36]. Strengthening physical exercises can help increase muscle strength and enhance the stability and balance of the body, thus reducing the risk of falls [37]. The higher the FES-I score is, the greater the risk of falling and the FOF. A large-scale clinical study [8] showed that the FOF is common in elderly women, with falls mainly being caused by obstacles to balance and movement. Because of the FOF, older women tend to restrict their activities, which leads to a decline in their mobility and loss of physical independence, in turn increasing their actual risk of falling [9]. Therefore, psychological interventions for postmenopausal women to help them overcome the FOF are very helpful to prevent falling. Interestingly, smoking is a protective factor rather than a risk factor for falling in postmenopausal women. Smoking is extremely harmful to the human cardiac and respiratory systems, and the deterioration of cardiopulmonary function theoretically affects people’s daily lives, including reducing activity [38, 39], which may in turn reduce the risk of falling. In addition, smoking itself is a behavioural intermediary, and this behaviour does not directly reduce the risk of falling. However, the decline in physical function caused by smoking, which reduces body activity, may reduce the risk of falling. Furthermore, we found no evidence to suggest that the risk of falls in postmenopausal women is related to body height, age at the onset of menopause, years since last menstruation, alcohol use, or TUG and 5-STS test results.
Medical risk factors
The results of this meta-analysis showed that postmenopausal women on a low-calcium diet were more likely to fall than postmenopausal women who regularly consumed calcium (WMD − 16.91, 95% CI − 25.80 to − 8.01). Therefore, this study shows that calcium-containing diets may be helpful for preventing falls in postmenopausal women. However, whether supplemental calcium is necessary and the means of quantifying the intake of calcium need further research. Postmenopausal women’s oestrogen levels decrease, their bone mass decreases significantly, and osteoporosis is likely to occur, which in turn affects bone resistance to fractures [40, 41]. Therefore, proper postmenopausal calcium supplementation (a calcium-containing diet) is helpful for preventing falls. Postmenopausal women with a history of fractures and falls are more likely to fall again. In fact, the fracture history of elderly individuals, especially elderly women, is mostly a result of falling, making it a causal factor [42, 43]. People who have suffered fractures tend to limit their daily activities and simultaneously experience negative emotions such as depression, anxiety and decreased self-confidence. The FOF reduces flexibility, independence and balance of the body, thereby increasing the risk of falls [44, 45]. We also found that the more chronic health disorders postmenopausal women have, the more likely they are to fall. Indeed, studies have shown that chronic health disorders have important psychological and physical effects on older people [46]. Discomfort caused by chronic health disorders, such as chronic obstructive pulmonary disease and knee osteoarthritis, can reduce a patient’s mobility and ability to cope with potential fall hazards in the environment, which increase the risk of falls in elderly patients [47, 48]. This meta-analysis found that the risk of falls in postmenopausal women was not associated with hormone use or femoral neck bone density. Despite these findings, we cannot exclude these two variables as potential risk factors, as studies have suggested that hormone use and bone mineral density are closely related to the fall risk in postmenopausal women [29, 49, 50]. Therefore, we recommend further clinical studies of these variables.
Limitations
Several study limitations were unavoidable. First, the sample size of 11 studies included in this analysis means that the differences among the studies were relatively large, potentially leading to heterogeneity and affecting the results. Second, some of the risk factors that we expected to have a positive association, such as bone density and menopausal age, had a negative association in our findings, which may be related to the small number of studies we included. Third, due to limitations of the original data, we still lack research on potential risk factors such as drug use, family-related factors and serum levels of 25-hydroxyvitamin D, which are factors that need attention in future research. Fourth, since the risk of falling is a universal problem, the restriction of the included studies to those published in English papers is a major limitation.
Conclusion
Our findings provide some guidance for postmenopausal women and healthcare workers seeking to prevent falls. This meta-analysis found that sociodemographic risk factors (age, body weight, BMI, lack of exercise, FES-I scores) and medical risk factors (dietary calcium intake, previous fracture history, previous falls, number of diseases (˃ 2), number of reported chronic health disorders) are risk factors for falls in postmenopausal women.
Despite these findings, we still cannot rule out the possibility that some other variables are also risk factors (age at the onset of menopause, years since last menstruation, hormone therapy and bone mineral density). We recommend further clinical studies of these variables to obtain more persuasive evidence.
Electronic supplementary material
(DOCX 11 kb)
(DOCX 211 kb)
(DOCX 14 kb)
(PDF 76 kb)
Acknowledgements
We thank American Journal Experts for linguistic assistance during the preparation of this manuscript.
Availability of data and material
All data and materials are contained within the manuscript.
Funding information
This work was funded by the Project of Guangdong Provincial Department of Finance (No. [2014]157, No. [2018]8), the Key scientific research platforms and research projects of universities in Guangdong Province(No.2018KQNCX041), the China Postdoctoral Science Foundation (No. 2018M633036), the Medical Science Research Foundation of Guangdong Province (No. B2019091), the Project of Administration of Traditional Chinese Medicine of Guangdong Province (No.20201129), and the Science and Technology Research Project of Guangdong Provincial Hospital of Chinese Medicine (No. YN2019ML08, No. YK2013B2N19 and No. YN2015MS15).
Compliance with ethical standards
Conflicts of interest
None.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
J. Zhao, G. Liang and H. Huang contributed equally to this work.
Contributor Information
J. Zhao, Email: zhaojinlong41@163.com
G. Liang, Email: liangguihonghe@163.com
H. Huang, Email: ynhtsmile@126.com
L. Zeng, Email: lingfengzeng0202@163.com
W. Yang, Email: czyangwy@163.com
J. Pan, Email: panjianke0324@126.com
J. Liu, Email: liujun.gdtcm@hotmail.com
References
- 1.Feder G, Cryer C, Donovan S, Carter Y. Guidelines for the prevention of falls in people over 65. The Guidelines’ Development Group. BMJ. 2000;321:1007–1011. doi: 10.1136/bmj.321.7267.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartholt KA, van Beeck EF, Polinder S, van der Velde N, van Lieshout EM, Panneman MJ, van der Cammen TJ, Patka P. Societal consequences of falls in the older population: injuries, healthcare costs, and long-term reduced quality of life. J Trauma. 2011;71:748–753. doi: 10.1097/TA.0b013e3181f6f5e5. [DOI] [PubMed] [Google Scholar]
- 3.Stalenhoef PA, Diederiks JP, Knottnerus JA, de Witte LP, Crebolder HF. The construction of a patient record-based risk model for recurrent falls among elderly people living in the community. Fam Pract. 2000;17:490–496. doi: 10.1093/fampra/17.6.490. [DOI] [PubMed] [Google Scholar]
- 4.Sayyah M, Khosravi G, Bigdeli M. Frequency of fall-related injuries of female patients referred to the trauma center in the city of Kashan from years 2005 to 2008. Chin J Traumatol. 2013;16:46–50. doi: 10.3760/cma.j.issn.1008-1275.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Mackintosh SF, Hill K, Dodd KJ, Goldie P, Culham E. Falls and injury prevention should be part of every stroke rehabilitation plan. Clin Rehabil. 2005;19:441–451. doi: 10.1191/0269215505cr796oa. [DOI] [PubMed] [Google Scholar]
- 6.Xu T, Clemson L, O'Loughlin K, Lannin NA, Dean C, Koh G. Risk factors for falls in community stroke survivors: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2018;99:563–573. doi: 10.1016/j.apmr.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 7.Thaler HW, Oudshoorn C, Hartholt KA, van der Cammen TJM. Parameters of bone health and fracture risk in older female fall victims: what do they tell us? Z Gerontol Geriatr. 2015;48:539–542. doi: 10.1007/s00391-014-0843-2. [DOI] [PubMed] [Google Scholar]
- 8.Austin N, Devine A, Dick I, Prince R, Bruce D. Fear of falling in older women: a longitudinal study of incidence, persistence, and predictors. J Am Geriatr Soc. 2007;55:1598–1603. doi: 10.1111/j.1532-5415.2007.01317.x. [DOI] [PubMed] [Google Scholar]
- 9.Scheffer AC, Schuurmans MJ, van Dijk N, van der Hooft T, de Rooij SE. Fear of falling: measurement strategy, prevalence, risk factors and consequences among older persons. Age Ageing. 2008;37:19–24. doi: 10.1093/ageing/afm169. [DOI] [PubMed] [Google Scholar]
- 10.Gregorio L, Brindisi J, Kleppinger A, Sullivan R, Mangano KM, Bihuniak JD, Kenny AM, Kerstetter JE, Insogna KL. Adequate dietary protein is associated with better physical performance among post-menopausal women 60-90 years. J Nutr Health Aging. 2014;18:155–160. doi: 10.1007/s12603-013-0391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hita-Contreras F, Martínez-Amat A, Cruz-Díaz D, Pérez-López FR. Fall prevention in postmenopausal women: the role of Pilates exercise training. Climacteric. 2016;19:229–233. doi: 10.3109/13697137.2016.1139564. [DOI] [PubMed] [Google Scholar]
- 12.Tinetti ME, Richman D, Powell L. Falls efficacy as a measure of fear of falling. J Gerontol. 1990;45:239–243. doi: 10.1093/geronj/45.6.p239. [DOI] [PubMed] [Google Scholar]
- 13.Ma C, Liu A, Sun M, Zhu H, Wu H. Effect of whole-body vibration on reduction of bone loss and fall prevention in postmenopausal women: a meta-analysis and systematic review. J Orthop Surg Res. 2016;11:24. doi: 10.1186/s13018-016-0357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maas AH, van der Schouw YT, Grobbee DE, van der Graaf Y. Rise and fall of hormone therapy in postmenopausal women with cardiovascular disease. Menopause. 2004;11:228–235. doi: 10.1097/01.gme.0000087980.28957.86. [DOI] [PubMed] [Google Scholar]
- 15.Downey PA, Perry SB, Anderson JM. Screening postmenopausal women for fall and fracture prevention. J Geriatr Phys Ther. 2013;36:138–145. doi: 10.1519/JPT.0b013e31827bc497. [DOI] [PubMed] [Google Scholar]
- 16.Jang HJ, Ahn S. A predictive model of fall prevention behaviors in postmenopausal women. J Korean Acad Nurs. 2014;44:525–533. doi: 10.4040/jkan.2014.44.5.525. [DOI] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 18.Lo CK, Mertz D, Loeb M. Newcastle-Ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin C, Chen K, Chen C, Chang C, Huang T, Hsu H. Low vegetable intake increases the risk of fall-related fragility fracture in postmenopausal Taiwanese women, a prospective pilot study in the community. Biomed J. 2016;39:214–222. doi: 10.1016/j.bj.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wojciech P, Piotr A, Aleksandra C, Władysław G, Bogna D. Falls in RAC-OST-POL study: epidemiological study in postmenopausal women aged over 55 years. Endokrynol Pol. 2016;67:185–189. doi: 10.5603/EP.a2016.0015. [DOI] [PubMed] [Google Scholar]
- 21.Afrin N, Honkanen R, Koivumaa-Honkanen H, Lukkala P, Rikkonen T, Sirola J. Multimorbidity predicts falls differentially according to the type of fall in postmenopausal women. Maturitas. 2016;91:19–24. doi: 10.1016/j.maturitas.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Afrin N, Honkanen R, Koivumaa-Honkanen H, Sund R, Rikkonen T, Williams L. Role of musculoskeletal disorders in falls of postmenopausal women. Osteoporos Int. 2018;29:2419–2426. doi: 10.1007/s00198-018-4631-5. [DOI] [PubMed] [Google Scholar]
- 23.Drozdzowska B, Wiktor K, Pluskiewicz W. Functional status and prevalence of falls and fractures in population-based sample of postmenopausal women from the RAC-OST-POL study. Int J Clin Pract. 2013;67:673–681. doi: 10.1111/ijcp.12118. [DOI] [PubMed] [Google Scholar]
- 24.Ersoy Y, MacWalter RS, Durmus B, Altay ZE, Baysal O. Predictive effects of different clinical balance measures and the fear of falling on falls in postmenopausal women aged 50 years and over. Gerontology. 2009;55:660–665. doi: 10.1159/000235652. [DOI] [PubMed] [Google Scholar]
- 25.Hita-Contreras F, Martínez-Amat A, Lomas-Vega R, Álvarez P, Aránega A, Martínez-López E. Predictive value of stabilometry and fear of falling on falls in postmenopausal women. Climacteric. 2013;16:584–589. doi: 10.3109/13697137.2012.733464. [DOI] [PubMed] [Google Scholar]
- 26.Winters-Stone KM, Torgrimson B, Horak F, Eisner A, Nail L, Leo MC. Identifying factors associated with falls in postmenopausal breast cancer survivors: a multi-disciplinary approach. Arch Phys Med Rehab. 2011;92:646–652. doi: 10.1016/j.apmr.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Follis S, Cook A, Bea JW, Going SB, Laddu D, Cauley JA. Association between sarcopenic obesity and falls in a multiethnic cohort of postmenopausal women. J Am Geriatr Soc. 2018;66:2314–2320. doi: 10.1111/jgs.15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Randell KM, Honkanen RJ, Komulainen MH, Tuppurainen MT, Kroger H, Saarikoski S. Hormone replacement therapy and risk of falling in early postmenopausal women a population-based study. Clin Endocrinol. 2001;54:769–774. doi: 10.1046/j.1365-2265.2001.01300.x. [DOI] [PubMed] [Google Scholar]
- 29.Rouzi AA, Ardawi MM, Qari MH, Bahksh TM, Raddadi RM, Ali AY. Risk factors for falls in a longitudinal cohort study of Saudi postmenopausal women: the Center of Excellence for Osteoporosis Research Study. Menopause. 2015;22:1012–1020. doi: 10.1097/GME.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 30.Abbs E, Brown R, Guzman D, Kaplan L, Kushel M. Risk factors for falls in older adults experiencing homelessness: results from the HOPE HOME cohort study. J Gen Intern Med. 2020;35:1813–1820. doi: 10.1007/s11606-020-05637-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neri Silvia GR, Oliveira JS, Dario Amabile B, Lima Ricardo M, Anne T. Does obesity increase the risk and severity of falls in people aged 60 years and older? A systematic review and meta-analysis of observational studies. J Gerontol A Biol Sci Med Sci. 2019;75:952–960. doi: 10.1093/gerona/glz272. [DOI] [PubMed] [Google Scholar]
- 32.Himes CL, Reynolds SL. Effect of obesity on falls, injury, and disability. J Am Geriatr Soc. 2012;60:124–129. doi: 10.1111/j.1532-5415.2011.03767.x. [DOI] [PubMed] [Google Scholar]
- 33.Neri Silvia GR, Harvey LA, Anne T, Gadelha AB, Lima Ricardo M. Obesity and falls in older women: mediating effects of muscle quality, foot loads and postural control. Gait Posture. 2020;77:138–143. doi: 10.1016/j.gaitpost.2020.01.025. [DOI] [PubMed] [Google Scholar]
- 34.de Cesar NC, Godoy-Santos AL, Saito GH, Lintz F, Siegler S, O'Malley MJ, Deland JT, Ellis SJ. Subluxation of the middle facet of the subtalar joint as a marker of peritalar subluxation in adult acquired flatfoot deformity: a case-control study. J Bone Joint Surg Am. 2019;101:1838–1844. doi: 10.2106/JBJS.19.00073. [DOI] [PubMed] [Google Scholar]
- 35.Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2:819–829. doi: 10.1016/S2213-8587(14)70034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altaf S, Aftab A, Malik AN. Role of exercise in reducing risk of fall in geriatric population. J Pak Med Assoc. 2019;69:1576. doi: 10.5455/JPMA.16020. [DOI] [PubMed] [Google Scholar]
- 37.Rodrigues IB, Ponzano M, Giangregorio LM. Practical tips for prescribing exercise for fall prevention. Osteoporos Int. 2019;30:1953–1960. doi: 10.1007/s00198-019-05141-0. [DOI] [PubMed] [Google Scholar]
- 38.Archie SR, Cucullo L. Harmful effects of smoking Cannabis: a cerebrovascular and neurological perspective. Front Pharmacol. 2019;10:1481. doi: 10.3389/fphar.2019.01481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parasuraman S, Zaman AG, Egred M, Bagnall A, Broadhurst PA, Ahmed J, Edwards R, Das R, Garg D, Purcell I, Noman A (2020) Smoking status and mortality outcomes following percutaneous coronary intervention. Eur J Prev Cardiol:204748732090232. 10.1177/2047487320902325 [DOI] [PubMed]
- 40.Mariana C, Ramona OR, Felicia C, Dorina S. Correlations between the quality of life domains and clinical variables in sarcopenic osteoporotic postmenopausal women. J Clin Med. 2020;9:441. doi: 10.3390/jcm9020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu HW, Yi YY, Zhang SB, Hu T, Wang SJ, Zhao WD, Wu DS. Does vitamin D status influence lumbar disc degeneration and low back pain in postmenopausal women? A retrospective single-center study. Menopause. 2020;27:586–592. doi: 10.1097/GME.0000000000001499. [DOI] [PubMed] [Google Scholar]
- 42.Sherrington C, Fairhall N, Kirkham C, Clemson L, Tiedemann A, Vogler C, Close JCT, O'Rourke S, Moseley AM, Cameron ID, Mak JCS, Lord SR (2020) Exercise to reduce mobility disability and prevent falls after fall-related leg or pelvic fracture: RESTORE randomized controlled trial. J Gen Intern Med. 10.1007/s11606-020-05666-9 [DOI] [PMC free article] [PubMed]
- 43.Afrin N, Sund R, Honkanen R, Koivumaa-Honkanen H, Rikkonen T, Williams L, Kröger H. A fall in the previous 12 months predicts fracture in the subsequent 5 years in postmenopausal women. Osteoporos Int. 2019;31:839–847. doi: 10.1007/s00198-019-05255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crotty M, Killington M, Liu E, Cameron ID, Kurrle S, Kaambwa B, Davies O, Miller M, Chehade M, Ratcliffe J. Should we provide outreach rehabilitation to very old people living in nursing care facilities after a hip fracture? A randomised controlled trial. Age Ageing. 2019;48:373–380. doi: 10.1093/ageing/afz005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lam MT, Sing CW, Li GHY, Kung AWC, Tan KCB, Cheung CL. Development and validation of a risk score to predict the first hip fracture in the oldest old: a retrospective cohort study. J Gerontol A Biol Sci Med Sci. 2019;75:980–986. doi: 10.1093/gerona/glz178. [DOI] [PubMed] [Google Scholar]
- 46.Vadivelu R, Mathew EJ, Hadid N. Hidden diseases detected after a fall. J Postgrad Med. 2002;48:328–329. [PubMed] [Google Scholar]
- 47.Li W, Cheng R. Research on the influence factors of the fall efficiency of the hospitalized geriatric patients with cerebrovascular diseases. Pak J Pharm Sci. 2016;29:2343–2348. [PubMed] [Google Scholar]
- 48.Srulijes K, Klenk J, Schwenk M, Schatton C, Schwickert L, Teubner-Liepert K, Meyer M, Srijana KC, Maetzler W, Becker C, Synofzik M. Fall risk in relation to individual physical activity exposure in patients with different neurodegenerative diseases: a pilot study. Cerebellum. 2019;18:340–348. doi: 10.1007/s12311-018-1002-x. [DOI] [PubMed] [Google Scholar]
- 49.Barrett-Connor E, Grady D, Stefanick ML. The rise and fall of menopausal hormone therapy. Annu Rev Public Health. 2005;26:115–140. doi: 10.1146/annurev.publhealth.26.021304.144637. [DOI] [PubMed] [Google Scholar]
- 50.Hita-Contreras F, Martínez-Amat A, Cruz-Díaz D, Pérez-López FR. Osteosarcopenic obesity and fall prevention strategies. Maturitas. 2015;80:126–132. doi: 10.1016/j.maturitas.2014.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 11 kb)
(DOCX 211 kb)
(DOCX 14 kb)
(PDF 76 kb)
Data Availability Statement
All data and materials are contained within the manuscript.