Abstract
Background
Traditional tumor thermal ablations, such as radiofrequency ablation (RFA) and cryoablation, can result in good local control of tumor, but traditional tumor thermal ablations are limited by poor long-term survival due to the failure of control of distal metastasis. Our previous studies developed a novel cryo-thermal therapy to treat the B16F10 melanoma mouse model. Long-term survival and T-cell-mediated durable antitumor immunity were achieved after cryo-thermal therapy, but whether tumor antigen-specific T-cells were augmented by cryo-thermal therapy was not determined.
Methods
The long-term antitumor therapeutic efficacy of cryo-thermal therapy was performed in B16F10 murine melanoma models. Splenocytes derived from mice treated with RFA or cryo-thermal therapy were coincubated with tumor antigen peptides to detect the frequency of antigen specific CD4+ and CD8+ T-cells by flow cytometry. Splenocytes were then stimulated and expanded by αCD3 or peptides and adoptive T-cell therapy experiments were performed to identify the antitumor efficacy of T-cells induced by RFA and cryo-thermal therapy. Naïve mice and tumor-bearing mice were used as control groups.
Results
Local cryo-thermal therapy generated a stronger systematic antitumor immune response than RFA and a long-lasting antitumor immunity that protected against tumor rechallenge. In vitro studies showed that the antigen-specific CD8+ T-cell response was induced by both cryo-thermal therapy and RFA, but the strong neoantigen-specific CD4+ T-cell response was only induced by cryo-thermal therapy. Cryo-thermal therapy-induced strong antitumor immune response was mainly mediated by CD4+ T-cells, particularly neoantigen-specific CD4+ T-cells.
Conclusion
Cryo-thermal therapy induced a stronger and broader antigen-specific memory T-cells. Specifically, cryo-thermal therapy, but not RFA, led to a strong neoantigen-specific CD4+ T-cell response that mediated the resistance to tumor challenge.
Keywords: immunology, oncology, tumors, adaptive immunity, CD4-positive T-lymphocytes
Background
It is increasingly acknowledged that the induction of long-lasting antitumor immunity is critical in cancer treatment.1 Modulating the immune system to enhance the antitumor response has improved cancer survival.2 Immunotherapy is a powerful, developing cancer treatment modality that can be combined with chemotherapy, radiotherapy and other traditional treatments from preclinical development to clinical application.3 However, immunotherapy is still limited by the low rate of response, unpredictable efficacy and off-target side effects.4
With the development of modern imaging, local thermal ablation is increasingly used for clinical cancer treatment. Radiofrequency ablation (RFA) and cryoablation are two main energy-based approaches. In the central zone of RFA, where the temperature can be increased by more than 60℃, the tumor cells undergo coagulative necrosis.5 6 Neutrophils, macrophages, dendritic cells (DCs), natural killer cells (NK cells), B cells and T-cells infiltrate into the transitional zone, where tumor cells are either undergoing apoptosis or recovering from reversible injury after RFA.7 Immunogenic intracellular substrates, including antigens and danger signals, are also released to activate innate immunity.8 However, coagulative necrosis induced by RFA increases the tissue impedance and therefore limits further electrical conduction through the remaining tissue.9 As a result, intracellular substrates from apoptotic tumor cells are not fully released, which may decrease the extent of the immunological response and even induce immunosuppression.10 In cryoablation, the lethal temperature of tumor cells is considered to be between −40℃ and −20℃.11 Although an antitumor immunological response is induced in cryoablation, such as production of tumor antigen-specific antibodies and T-cell and NK cell activation, the immunosuppressive effect of cryoablation limits its clinical therapeutic effect.11 12
To avoid the disadvantages of RFA and cryoablation, we combined these two therapies to develop a novel tumor cryo-thermal therapy through the alternative cooling and heating of tumor tissue in preclinical animal models.13 14 The long-term survival rate was significantly improved in 4T1 murine mammary cancer and B16F10 murine melanoma models.15–17 The acute proinflammatory cytokines and danger signals released after cryo-thermal therapy efficiently activated innate immunity.15 16 18 19 As a result, a long-lasting CD4+ T-cells-dependent antitumor immune memory response was triggered.15 17 19 However, whether tumor antigen-specific T-cells, especially neoantigen-specific T-cells, can be induced by cryo-thermal therapy has still not been investigated.
Neoantigens have been proven to be high immunogenic and used as potent tumor vaccines for melanoma patients.20 21 Accumulating evidence implies that the tumor regression initiated by immunotherapy is achieved via the activation of cytotoxic T-cells targeting neoantigens.22 Some studies show that DNA mutation-derived peptides are more frequently used to induce neoantigen-specific CD4+ T-cells than CD8+ T-cells.20 23–25 Endogenous CD4+ T-cells recognizing neoantigens have been found in patients with cancer.26 27 Neoantigen-specific CD4+ T-cell responses can mediate the regression of metastatic epithelial cancer.28 CD4+ T-cell neoepitopes-based vaccination in tumor-bearing mice resulted in rejection of established B16F10 melanoma and CT26 colon tumor models.21
In this study, we examined the tumor antigen-specific T-cells induced by cryo-thermal therapy in a B16F10 murine melanoma model and investigated the role of tumor antigen-specific T-cells in cryo-thermal therapy-induced strong antitumor immunity. We found that cryo-thermal therapy induced a stronger systematic and long-term antitumor immunity compared with RFA. Both cryo-thermal therapy and RFA could induce tumor antigen-specific CD8+ T-cells; however, neoantigen-specific CD4+ T-cells were more efficiently induced after cryo-thermal therapy compared with RFA. Most importantly, we documented that neoantigen-specific CD4+ T-cells played an indispensable role in cryo-thermal therapy-induced long-term antitumor immunity. Thus, this study identified and elucidated the underlining mechanisms through which the local cryo-thermal therapy to induce neoantigen specific CD4 T-cell immune responses eradicate tumors and inhibit metastasis.
Methods
Cell culture
B16F10 mouse melanoma tumor cell line was donated by Weihai Yin at Med-X Research Institute, Shanghai Jiao Tong University. CT26 colon carcinoma cell line was donated by Yan Zhang at Med-X Research Institute, Shanghai Jiao Tong University. B16F10 cells were cultured in Dulbecco’s Modified Eagle’s Medium (GE Healthcare, Logan, Utah, USA) supplemented with 10% fetal bovine serum (FBS, Gemini Bio-Products, West Sacramento, California, USA), 100 units/mL penicillin and 100 µg/mL streptomycin at 37°C in a humidified 5% CO2 incubator. CT26 cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, California, USA) supplemented with 10% FBS (Gemini Bio-Products), 100 units/mL penicillin and 100 µg/mL streptomycin at 37°C in a humidified 5% CO2 incubator.
Animal models
The female C57BL/6 and BALB/c mice were obtained from the Shanghai Slaccas Experimental Animal (China) and used for experimental study at the age of 6–8 weeks. Mice were housed in isolated cages and a 12-hour light/dark cycle environment, feeding with sterile food and acidified water with pH value kept at 7.5–7.8. To prepare the tumor-bearing mice, approximately 5×105 cells were injected subcutaneously into the right flank of each mouse. To investigate the distal effect of the treatment, approximately 1×105 B16F10 cells were injected subcutaneously on the left flank 7 days after primary injection.
The cryo-thermal therapy and RFA procedures
The system developed in our laboratory was composed of liquid nitrogen for cooling and RF for heating. To reduce the effect of contact thermal resistance and obtain a continuous thermal delivery during the treatment, a probe was designed with a cylinder-shaped tip of 1 mm in diameter for the thermal therapy of subcutaneous tumor. Subcutaneous injection of B16F10 melanoma cells into C57BL/6 mice leads to form primary tumors in 7–9 days and spontaneous metastasis in lungs. Twelve days after tumor inoculation, when the tumor volume reached about 0.2 cm3, the mice were divided into three groups: tumor-bearing group without the treatment (control), the cryo-thermal group with freezing at the temperature of −20°C for 5 min, followed by RF heating at the temperature of 50℃ for 10 min on primary tumor and the RFA group with RF heating at the temperature of 60℃ for 15 min. The mice were anesthetized with intraperitoneal injection (i.p.) of 1.6% pentobarbital sodium (0.5 mL/100 g, Sigma-Aldrich, St. Louis, Missouri, USA). The tumor site was sanitized with 75% alcohol before the treatment. All the procedures were performed aseptically.
Tumor rechallenge analysis
Study of rechallenge with the B16F10 melanoma tumor cells was performed in survivors 45 days after treatment. Mice were intravenously infused with 1×105 B16F10 melanoma cells, and lung tumor nodules were enumerated 21 days later.
Synthetic Peptides
gp10025-33 (EGSRNQDWL) (Purity: % peak area by HPLC ≥95%, purchased from ANASPEC), TYR368–376(YMDGTMSQV) (Purity: % peak area by HPLC ≥95%, purchased from ANASPEC), TRP2180-188 (SVYDFFVWL) (Purity: % peak area by HPLC ≥95%, purchased from ANASPEC), AH16-14(SPSYVYHQF) (Purity: % peak area by HPLC ≥95%, purchased from ANASPEC), B16-M20 (FRRKAFLHWYTGEAMDEMEFTEAESNM) (Purity: % peak area by HPLC ≥99%, synthesis by ChinaPeptides), B16-M30(PSKPSFQEFVDWENVSPELNSTDQPFL) (Purity: % peak area by HPLC ≥99%, synthesis by ChinaPeptides) were used CT26-M13(AGTQCEYWASRALDSEHSIGSMIQLPQ) (Purity: % peak area by HPLC ≥90%, synthesis by ChinaPeptides), CT26-M55(EGDPCLRSSDCIDEFCCARHFWTKICK) (Purity: % peak area by HPLC ≥90%, synthesis by ChinaPeptides).21
Preparation of a single-cell suspension of spleen and flow cytometry analysis
Mice were sacrificed after the cryo-thermal therapy, and the spleens were collected (n=4 per group at each time point). A single-cell suspension of splenocytes was prepared using GentleMACS dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany), and then treated with erythrocyte-lysing reagent containing 0.15 M NH4Cl, 1.0 M KHCO3 and 0.1 mM Na2EDTA (ethylenediaminetetraacetic acid) to remove the red blood cells. The cells were dispersed using 70 µm mesh screens and used for analysis of antigen-specific T-cells and T-cell expansion.
For cell surface staining, the cells were stained with fluorescence conjugated antibodies which could bind specific surface marker at 4 °C for 30 min. For intercellular cytokine staining, cells were cultured with with Brefeldin A (BFA), surface staining, fixed, permeabilized and incubated with anti-interferon-γ (IFN-γ) monoclonal antibody (mAb). Foxp3 staining were conducted by True-Nuclea Transcription Factor Buffer Set (Biolegend). Data were acquired using BD FACS Aria II cytometer (BD Biosciences) and analyzed using FlowJo V.10 software (FlowJo LLC, Ashland, Oregon, USA). Fixation Buffer, Intracellular Staining Permeabilization Wash Buffer and BFA were purchased from Biolegend (San Diego, CA). Fluorochrome-conjugated mAb: CD3-FITC (clone 145–2 C11), CD3-PerCP/Cy5.5 (clone 145–2 C11), CD4-PE/Cy7 (clone GK1.5), CD8-APC/Cy7 (clone 53–6.7), IFN-γ-PE (clone XMG1.2), CD25-FITC (clone 3C7), CD69-Brilliant Violet 421 (clone H1.2F3), perforin-PE (clone S16009A), granzyme B-APC (clone QA16A02), PD-1-PE/Cy7 (clone RMP1-30), Foxp3-PE (clone MF-14) were all purchased from Biolegend. Zombie Violet Fixable Viability Kit was purchased from Biolegend to assess live vs dead status of cells.
Generation of effector T-cells from spleen
Single splenocytes were resuspended at 2×106 cells per mL in RPMI 1640 medium (Hyclone, USA) supplemented with 15% FBS, 100 units/mL penicillin and 100 µg/mL streptomycin and cultured in 24 well plates. For antibody stimulation, the plates were precoated with 10 µg/mL anti-CD3 mAb (Biolegend, clone 2c11) at 4℃ overnight. For peptides stimulation, 10 µg/mL peptides were added in culture medium. After 2 days of stimulation, the T-cells were harvested and expanded in RPMI 1640 medium supplemented with 15% FBS, 100 units/mL penicillin, 100 µg/mL streptomycin, 60 IU/mL recombinant human interleukin 2 (rhIL-2) and 50 µM beta-mercaptoethanol and cultured in six well plates for three additional days. T-cells were then harvested, washed twice in PBS, counted and used for adoptive T-cell therapy and analysis of antigen-specific T-cells.
Analysis of antigen-specific T-cells
Splenocytes or expanded T-cells were cocultured with 1 µg/mL peptide, respectively, for 12 hours, and BFA was added in the last 4 hours to enhance intracellular cytokine staining signals. Intracellular analysis of IFN-γ production was measured by flow cytometry.
Depletion of CD4+ and CD8+ T-cell subsets in vivo
For T-cell-depleting experiments, the treated mice (n=4 mice per group) were depleted with anti-CD4 or anti-CD8 mAb (Sungene Biotech), respectively (mice were injected i.p. with 300 µg mAb against CD4 or CD8, on day 1, 4, 7, 10 and 13 after cryo-thermal therapy). The effect of mAb depletion was confirmed in vivo.
Adoptive T-cell therapy
Naïve mice were fused with 1×105 B16F10 cells via tail vein injection. Three days later, 4×107 expanded T-cells from donors of naïve, untreated, cryo-thermal therapy and RFA were adopted to these mice via tail vein injection. After T-cell adoption, 90 000 IU IL-2 was given to all mice three times per day for three continuous days. On day 14 after adoptive T-cells therapy, mice were sacrificed to count the number of lung tumor nodules.
Western blot analysis
Tumors were harvested at 0, 3, 6, 12 and 24 hours after treatments. Tumor tissue was wrapped with Nylon filter paper with a pore size of 15 µm and centrifuged at 2500 rpm in a 15 mL centrifuge tube for 20 min. Tumor interstitial fluid was collected. 50 µg of total proteins were separated onto a 4%–20% gradient Tris-Glycine precast gel and transferred to a PVDF membrane. Blot was probed with anti-TRP2 (Abcam). Each shown Western blot was a representative from three separate experiments.
Statistical analysis
All data are presented as mean±SD. Significance was determined using a two-sided Student’s t-test, a one-way analysis of variance (ANOVA) with Holm-Sidak correction for multiple comparisons, or a two-way ANOVA with Holm-Sidak correction for multiple comparisons. A Mann-Whitney rank sum test was used if data did not follow a normal distribution. Significant differences in survival were determined using a log-rank (Mantel-Cox) test. GraphPad Prism V.7.0 (La Jolla, California, USA) was used for all statistical analysis.
Results
Cryo-thermal therapy-induced strong and long-lasting immune-mediated rejection of contralateral tumor growth and lung metastasis
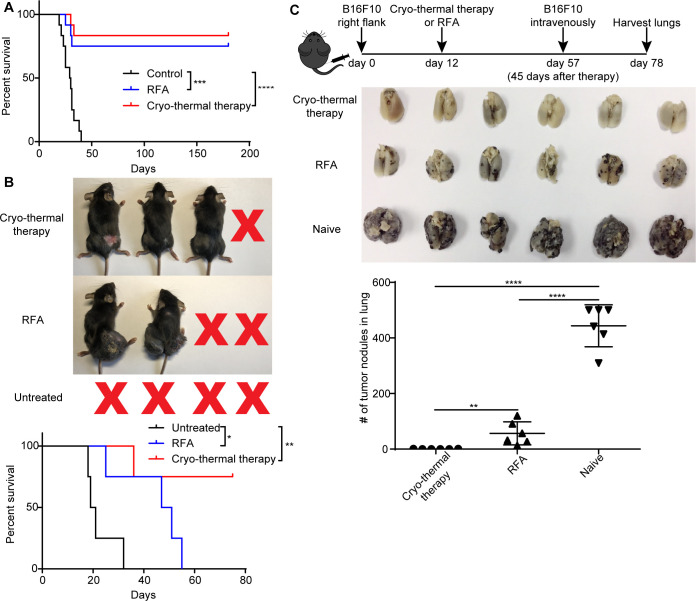
The antitumor therapeutic efficacy of cryo-thermal therapy was verified in several independent experiments using both 4T1 murine mammary cancer and B16F10 murine melanoma models.15–17 Here, we performed new experiments to demonstrate the long-term survival benefit of cryo-thermal therapy in the B16F10 murine melanoma model. RFA (60 °C for 15 min) and cryo-thermal therapy (prefreezing at −20°C for 5 mi, followed by RF heating at 50 °C for 10 min) were performed on primary tumors 12 days after tumor inoculation. As shown in figure 1A, during the observation period of over 180 days, all 12 mice in the tumor-bearing control group died, but 10 of 12 (~83.3%) mice in the cryo-thermal therapy group and 9 of 12 (75%) mice in the RFA group survived. To determine whether cryo-thermal therapy or RFA-induced strong systematic or long-term antitumor immunity, contralateral tumors were used to estimate the strength of the immune response after treatments. One week after local tumor inoculation with 5×105 B16F10 cells, 1×105 B16F10 cells were inoculated into the contralateral flank. All mice receiving RFA therapy could not suppress the contralateral tumor growth and died in 60 days, while three of four (75%) mice treated with cryo-thermal therapy rejected the contralateral tumor growth (figure 1B). This result suggested that a stronger systematic antitumor immune response was induced by cryo-thermal therapy to reject contralateral tumor growth. To investigate whether long-term antitumor immunity was induced after treatments, 45 days later, both naïve and survived mice were intravenously infused with 1×105 B16F10 melanoma cells and lung metastatic nodules were enumerated on day 78 after tumor inoculation. All control mice developed tumor nodules in the lungs. Substantial number of lung tumor nodules were observed in the RFA group. In contrast, no lung metastasis was found in long-term survivors treated with cryo-thermal therapy (figure 1C). The results indicated that local cryo-thermal therapy generated a strong systematic antitumor immune response and long-term antitumor immunity that protected against tumor rechallenge and inhibited the lung metastases.
Figure 1.
Cryo-thermal therapy-induced systematic and long-term antitumor immunity. (A) Kaplan-Meier survival curve. Cryo-thermal therapy and RFA improved long-term survival in comparison with untreated group. Approximately 5×105 B16F10 cells were injected subcutaneously into the right flank of each mouse. Twelve days later, mice were treated with RFA or cryo-thermal therapy, and untreated mice were as control. Kaplan-Meier survival curve was compared using log-rank tests. ***P<0.001, ****p<0.0001. n=12 for each group. (B) Distant tumor growth of mice treated with cryo-thermal therapy or RFA. Seven days after 5×105 B16F10 cells implanted subcutaneously into the right flank, 1×105 B16F10 cells were injected subcutaneously into the left flank. Another 5 days later, mice were treated with RFA or cryo-thermal therapy, and untreated mice were set as control. Kaplan-Meier survival curve was compared using log-rank tests. *P<0.05, **p<0.01. n=4 for each group. (C) B16F10 rechallenge on mice treated with cryo-thermal therapy or RFA. Upper: schematic of experimental design. Mid: photographic images of lungs from cryo-thermal or RFA-treated and untreated mice, respectively. Lower: quantitative statistics of tumor nodules in lung. All data were shown as mean±SD. n=6 per group. **P< 0.01, ****p<0.0001. Data for graphs were calculated using one-way ANOVA. ANOVA, analysis of variance; RFA, radiofrequency ablation.
Tumor antigen-specific CD8+ and CD4+ T-cells were generated after cryo-thermal therapy
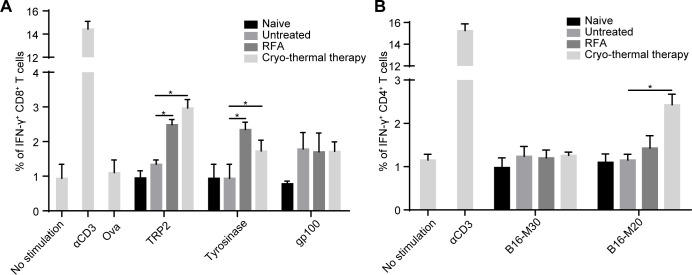
Long-term antitumor responses against tumor-associated antigens are thought to be mainly mediated by T-cells.29 The recognition of tumor cells by T-cells is dependent on the interaction of TCRs and MHC-peptide complexes.29 Our previous studies demonstrated that the percentage of CD4+ and CD8+ T-cells was significantly increased after cryo-thermal therapy.17–19 However, whether tumor antigen-specific CD4+ and CD8+ T-cells were induced by cryo-thermal therapy had not been addressed. MHC-I-restricted peptides of TRP2, tyrosinase and gp100, three known antigens of B16F10 melanoma21 were used to estimate the frequency of antigen-specific CD8+ T-cells, and MHC-II-restricted peptides of B16-M30 and B16-M20, two neoantigens of B16F10 melanoma21 were used to estimate the frequency of antigen-specific CD4+ T-cells. On day 14 after treatment, splenocytes derived from mice treated with RFA or cryo-thermal therapy were coincubated with these peptides for 24 hours. The frequency of IFN-γ-producing CD4+ and CD8+ T-cells was detected by flow cytometry (online supplemental figure S1). Naïve mice and tumor-bearing mice were used as control groups. Mice treated with RFA or cryo-thermal therapy had strong TRP2-specific and tyrosinase peptide-specific CD8+ T-cell responses compared with naïve and untreated mice, while no significant difference was found among the four groups when splenocytes were coincubated with gp100 peptides (figure 2A, online supplemental figure S2A). These data showed that both cryo-thermal therapy and RFA induced tumor antigen-specific CD8+ T-cell responses. For MHC-II-restricted neoantigen peptides, there was no evident B16-M30 specific CD4+ T-cell response induced in all groups, while the percentage of B16-M20-specific CD4+ T-cells was significantly increased after cryo-thermal therapy compared with that in the other three groups (figure 2B, online supplemental figure S2B). Meanwhile, we also analyzed antigen specific T-cell in CT26 model as shown in online supplemental figure S3, and we found that the level of specific CD8+ T-cells and CD4+ T-cells induced by cryo-thermal therapy were higher than RFA, which was very similar in B16F10 model. These data indicated that the antigen-specific CD8+ T-cell response was induced after cryo-thermal therapy and RFA, but a stronger antigen-specific CD4+ T-cell response was a distinct characteristic after cryo-thermal therapy.
Figure 2.
Frequency of IFN-γ+ cells in CD8+ and CD4+T-cells. On day 14 after RFA or cryo-thermal therapy, splenocytes were obtained from naïve mice, untreated mice and treated mice. (A) Each 1×105 splenocytes were coincubated with 10 µg/mL TRP2, tyrosinase, gp100, ova (negative control), 100 ng/mL αCD3 (positive control) and medium (blank control), respectively, to measure IFN-γ+ CD8+ T-cells. (B) Each 1×105 splenocytes were coincubated with 10 µg/mL B16-M20, B16-M30, 100 ng/mL αCD3 (positive control) and medium (blank control), respectively, to measure IFN-γ+ CD4+ T-cells. Mean percentages of IFN-γ+ CD8+ T-cells (A) and IFN-γ+ CD4+ T-cells (B) in each group were shown (data for bar graphs were calculated using two-way ANOVA, *p<0.05), n=4 for each group. ANOVA, analysis of variance; IFN-γ, interferon-γ; RFA, radiofrequency ablation.
jitc-2019-000421supp001.pdf (809KB, pdf)
Tumor antigen-specific T-cells generated by cryo-thermal therapy were more effective against tumor rechallenge than RFA
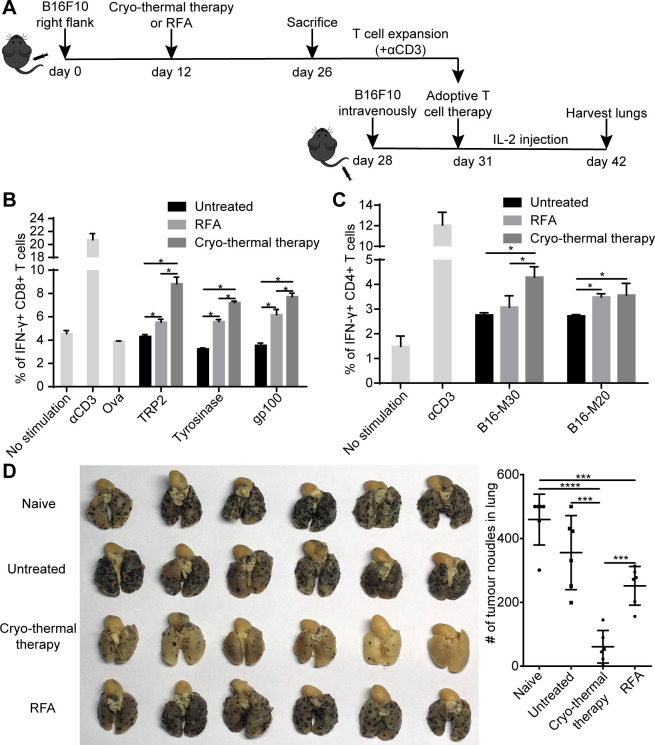
Thus far, our previously published and current results documented that cryotherapy can treat well-established tumor and generated systemic T-cell-based antitumor immunity. To further demonstrated the antitumor efficacy of T-cells induced by cryo-thermal therapy, we performed adoptive T-cell therapy experiments. To obtain enough effector T-cells, splenocytes from naïve mice, untreated and treated mice were first stimulated with anti-CD3 mAb for 2 days and then expanded with IL-2 for another 3 days. The majority of naïve T-cells differentiated into effective memory T-cells after stimulation with anti-CD3 mAb.30 The percentages of antigen-specific CD4+ and CD8+ T-cells after expansion were identified via coculturing MHC-I-restricted and MHC-II-restricted peptides. As shown in figure 3B, C, the basic levels of antigen-specific T-cells cocultured with MHC-I-restricted and MHC-II-restricted peptides were increased in all groups after expansion compared with that before expansion, indicating the maturation of T-cells during expansion. Splenocytes from mice receiving RFA and cryo-thermal therapy after stimulation yielded many more antigen-specific CD8+ T-cells than those from untreated mice. Moreover, the frequency of antigen-specific CD8+ T-cells expanded from mice receiving cryo-thermal therapy was much higher than that from mice receiving RFA (figure 3B). The results indicated that cryo-thermal therapy endowed antigen-specific CD8+ T-cells with more differential ability than did RFA. In addition, the frequency of B16-M20-specific CD4+ T-cells in spleen from mice receiving RFA and cryo-thermal therapy was increased and was higher than that from the untreated mice (figure 3C and online supplemental figure S4A). However, the frequency of B16-M30-specific CD4+ T-cells in spleen from mice receiving cryo-thermal therapy was much higher than that from mice receiving RFA and untreated mice (figure 3C). The data revealed that neoantigen-specific CD4+ T-cells were weakly activated after RFA but were strongly activated after cryo-thermal therapy. Then, 4×107 expanded T-cells were adoptively transferred into mice received 1×105 B16F10 tumor cells via tail vein injection 3 days before T-cell transfer. After T-cell transfer, 90 000 UI rhIL-2 was given three times per day for three continuous days. On day 14 after adoptive T-cell therapy, mice were sacrificed, and the number of lung tumor nodules was enumerated. The number of lung tumor nodules in mice receiving expanded T-cells from mice treated with RFA and cryo-thermal therapy was significantly less than that in naïve mice, clearly demonstrated that the antitumor immunity was induced by T-cells after RFA and cryo-thermal therapy (figure 3D). However, the number of lung tumor nodules in mice receiving expanded T-cells from mice treated with RFA was not significantly different from that in untreated mice, while the number of lung tumor nodules in mice receiving expanded T-cells from mice treated with cryo-thermal therapy was fewer than that in RFA-treated and untreated mice (figure 3D and online supplemental figure S4B). In general, mice receiving expanded T-cells from mice treated with cryo-thermal therapy developed rare lung tumor nodules, which represented a stronger antitumor immunity induced by T-cells after cryo-thermal therapy. The results showed that the antigen-specific CD4+ and CD8+ T-cells induced by cryo-thermal therapy were much more activated than those induced by RFA, and these activated cells are robust effector cells to exert a stronger antitumor immunity to inhibit metastasis.
Figure 3.
Adoptive T-cells therapy using expanded T-cells from mice treated with cryo-thermal therapy and RFA. (A) Schematic of experimental design. (B, C) Frequency of IFN-γ+ cells in CD8+ T-cells and CD4+ T-cells stimulated with αCD3 and then expanded with IL-2. Mean percentages of IFN-γ+ CD8+ T-cells (B) and IFN-γ+ CD4+ T-cells (C) in each group were shown (data for bar graphs were calculated using two-way ANOVA, *p<0.05), n=4 for each group. (D) Tumor nodules in lung after adoptive T-cells therapy. Left: photographic images of lungs from mice receiving expanded T-cells from naïve mice, untreated, cryo-thermal-treated and RFA-treated mice, respectively. Right: quantitative statistics of tumor nodules in lung. All data were shown as mean±SD n=6 per group. ***P< 0.001, ****p<0.0001. Data for graphs were calculated using one-way ANOVA. ANOVA, analysis of variance; IFN-γ, interferon-γ; RFA, radiofrequency ablation.
Neoantigen-specific CD4+ T-cells were the major contributors in the cryo-thermal therapy-induced strong antitumor immune response
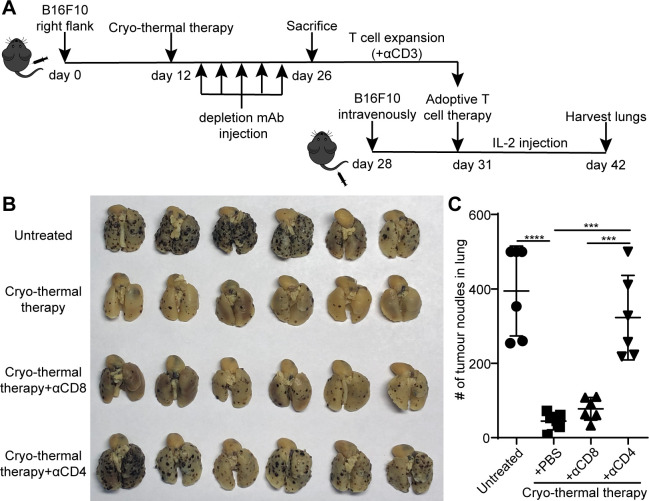
Since the antigen-specific CD4+ and CD8+ T-cell responses induced by cryo-thermal therapy after expansion were stronger than those induced by RFA, we further examined the independent role of CD4+ and CD8+ T-cells in cryo-thermal therapy-induced antitumor immunity in adoptive transfer experiments with CD4 or CD8 effector T-cells. Mice were injected with 300 µg anti-CD4 or anti-CD8 mAb on days 1, 4, 7, 10 and 13 after cryo-thermal therapy, and on day 14, mice were sacrificed to obtain splenocytes as the source of CD4+ or CD8+ T-cells (figure 4A). The depletion of CD4+ or CD8+ T-cells was verified by flow cytometry (online supplemental figure S5A‒C). Adoptive T-cell therapy was performed as described in figure 3A. As shown in figure 4B, C, the number of lung tumor nodules in mice receiving expanded T-cells from mice treated with cryo-thermal therapy was lower than that in untreated mice.
Figure 4.
Adoptive T-cells therapy using expanded CD8+ or CD4+ T-cells obtained through depletion in vivo with mAb, respectively. (A) Schematic of experimental design. (B) Tumor nodules in lung after adoptive T-cells therapy. Left: photographic images of lungs from mice receiving expanded T-cells from untreated, cryo-thermal-treated mice, CD4+ T-cells from cryo-thermal therapy (cryo-thermal therapy+αCD8) and CD8+ T-cells from cryo-thermal therapy (cryo-thermal therapy+αCD4), respectively. Right: quantitative statistics of tumor nodules in lung. All data were shown as mean±SD. n=6 per group. ***P<0.001, ****p<0.0001. Data for graphs were calculated using one-way ANOVA. ANOVA, analysis of variance; IL2, interleukin 2; mAb, monoclonal antibody.
In most animal models, it has been well established that both CD4 and CD8 are important to the efficacy of adoptive T-cell therapy.31 32 Interestingly, the number of lung tumor nodules in mice receiving adoptive CD4+ T-cell therapy (cryo-thermal therapy+αCD8) was only slightly more than that in mice receiving normal adoptive T-cell therapy (cryo-thermal therapy). However, the number of lung tumor nodules in mice receiving adoptive CD8+ T-cell therapy (cryo-thermal therapy+αCD4) was much more than that in mice receiving adoptive CD4+ T-cell therapy and normal adoptive T-cell therapy (figure 4B, C). The results demonstrated that the strong antitumor immune response induced by cryo-thermal therapy was mainly mediated by CD4+ T-cells, which had a powerful antitumor ability to reject tumor challenge.
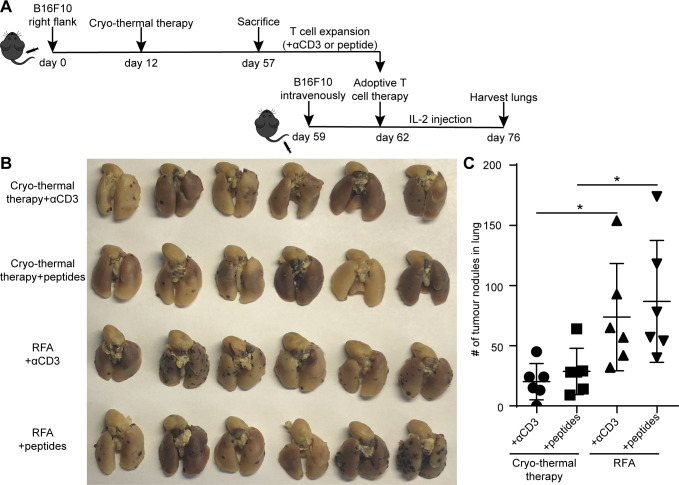
However, T-cells stimulated with anti-CD3 mAb are activated and expand, leading to a case in which many of T-cells are not antigen-specific T-cells.33 To determine whether antigen-specific CD4+ T-cells played a critical role in cryo-thermal therapy-induced antitumor immunity, MHC-II-restricted peptides were used instead of anti-CD3 mAb to stimulate T-cells to yield a monoclonal or oligoclonal response. After stimulation with MHC-II-restricted peptides (B16-M30 and B16-M20) for 2 days, splenocytes were then expanded by IL-2 for another 3 days (figure 5A). Adoptive T-cell therapy was performed, and the procedure was the same as described in figure 3A. The number of lung tumor nodules in mice receiving αCD3-stimulated T-cells from cryo-thermal therapy-treated mice was fewer than that in RFA-treated mice (figure 5B, C), which was consistent with the above results that the frequency of antigen-specific CD4+ and CD8+ T-cells expanded from mice receiving cryo-thermal therapy was much higher than that from mice receiving RFA (as shown in figure 3B, C). Moreover, the number of lung tumor nodules in mice receiving neoantigen peptide-stimulated CD4+ T-cells from cryo-thermal therapy-treated mice was also fewer than that in RFA-treated mice (figure 5B, C). This result was in agreement with much higher frequency of neoantigen-specific CD4+ T-cells after expansion from cryo-thermal therapy-treated mice than that from RFA-treated mice (figure 3C). The number of lung tumor nodules in mice receiving expanded adoptive peptide-stimulated CD4+ T-cell therapy was not significantly different from that in mice receiving adoptive αCD3-stimulated T-cell (containing both CD4+ and CD8+ T-cells) therapy, which could generate strong immune responses that protected against tumor rechallenge and lung metastases (figure 5B, C). These data demonstrated that after cryo-thermal therapy, neoantigen-specific CD4+ T-cells were likely the major contributor to the antitumor immune response in cryo-thermal therapy.
Figure 5.
Adoptive T-cells therapy using αCD3-stimulated or MHC-II-restricted peptides-stimulated CD4+ T-cells. (A) Schematic of experimental design. (B, C) Tumor nodules in lung after adoptive T-cells therapy. (B) Photographic images of lungs photographic images of lungs from mice receiving αCD3- or MHC II restricted peptides- stimulated T-cells from cryo-thermal therapy or RFA. (C) Quantitative statistics of tumor nodules in lung. All data were shown as mean±SD. n=6 per group. NS, *p≥0.05. Data for graphs were calculated using two-sided Student’s t-test. IL2, interleukin 2; NS, not significant; RFA, radiofrequency ablation.
Discussion
In this study, we documented that local cryo-thermal therapy generated a stronger systematic antitumor immune response than RFA and a long-lasting antitumor immunity that protected against tumor rechallenge. At the same time, a systemic antigen-specific T-cell immune response induced via local cryo-thermal therapy was revealed. Although the survival rate between RFA and cryo-thermal therapy was not statistically significant, the long-term immunity against tumor challenge elicited by two treatments was different. In vitro studies showed that the antigen-specific CD8+ T-cell response was induced by both cryo-thermal therapy and RFA, but the strong neoantigen-specific CD4+ T-cell response was only induced by cryo-thermal therapy. Moreover, we demonstrated that the cryo-thermal therapy-induced strong antitumor immune response was mainly mediated by CD4+ T-cells, particularly neoantigen-specific CD4+ T-cells, which are the major contributors to the cryo-thermal therapy-induced antitumor immune response.
To induce a T-cell-mediated antitumor immune response for the effective killing of tumor cells, a series of stepwise events must be initiated.34 Initially, antigens and factors that elicit the innate immune response are created by tumor cell necrosis.34 Our previous studies showed that coagulative necrosis was the major mode of death in RFA, while most tumor cells underwent immunogenic necrosis after cryo-thermal therapy, which resulted in the release of a larger amount of damage-associated molecular patterns (DAMPs), such as HSP70 and HMGB1, to peripheral tumor tissues and peripheral blood after cryo-thermal therapy compared with RFA.16 At the same time, the release of the tumor antigen TRP2 the tumor stroma was significantly increased after cryo-thermal therapy compared with RFA (online supplemental figure S6). Next, antigens are internalized, processed and presented on MHC molecules by host APCs. The DAMPs released by dying tumor cells promote the activation and maturation of APCs, enhancing their antigen presentation ability.35 The flood of DAMPs released after cryo-thermal therapy elicits the activation of innate immunity, promoting DC maturation and M1 macrophage polarization.16 18 19 36 Finally, mature APCs present the peptide derived from captured antigens on MHC-I and MHC-II molecules to CD8+ and CD4+ T-cells, resulting in activation of effector/memory CD8+ and CD4+ T-cell responses against the tumor-specific antigens (TSAs).37 Our previous studies revealed that cryo-thermal therapy induced M1 macrophage polarization and modulated DC maturation, resulting in CD4+ T-cell differentiation into Th1 and cytotoxic T-cell subsets and the generation of cytotoxic CD8+ T-cells.17 19 In particular, the synergistic effect of thermal and mechanical stresses induced by cryo-thermal therapy led to vastly increased tumor cell break-down; in addition, B16F10 melanoma has a high mutation burden,21 and more neoantigens leaking out of broken tumor cells after cryo-thermal therapy were internalized, processed and presented on MHC-II molecules by APCs, which resulted in a stronger neoantigen-specific CD4+ T-cell response triggered by cryo-thermal therapy than by RFA. However, in this study, we have not examined the release of neoantigens due to the lack of antibodies of neoantigen.
CD4+ T-cells are the orchestrators of the antitumor immune response and played a central role in antitumor efficacy.25 38 Recent studies have shown that CD4+ T-cells can clear tumors completely independently of CD8+ T-cells.39 Our previous studies identified that cryo-thermal therapy markedly promoted the differentiation of CD4+ T-cells, which contributed to the induction of a durable-specific memory immune response.17 19 IFN-γ+CD4+ T-cells are one of the CD4+ T-cell subsets and are known as Th1 cells. IFN-γ secreted by CD4+ T-cells upregulates the expression of MHC-II on tumor cells; thus, CD4+ T-cells are capable of recognizing and killing MHC-II-expressing target cells directly in an MHC-II-CD4 restricted way.40 IFN-γ can also directly induce permanent growth arrest in tumors synergistically with TNF-α.41 CD4+ T-cells can help prime CD8+ T-cells and maintain the function and promote the proliferation of CD8+ T-cells.42 Moreover, CD4+ T-cells can promote the recruitment and infiltration of other immune cells, such as CD8+ T-cells, macrophages, granulocytes, eosinophils and NK cells.43 44 Therefore, recent findings highlight new opportunities for CD4+ T-cells in cancer immunotherapy.45
Since there are several advantages of tumor specific CD4+ T-cells, we explored immunotherapeutic options for metastatic tumors. After cryo-thermal therapy, more activated and cytotoxic CD4+ and CD8+ T-cells were induced (online supplemental figure S7). Moreover, the frequencies of antigen-specific CD8+ T-cells and CD4+ T-cells were increased, especially the frequency of neoantigen-specific CD4+ T-cells. Adoptive transfer of CD4+ T-cells stimulated with neoantigen peptide could effectively mediate tumor regression and protect mice against tumor rechallenge. Therefore, we suggest that adoptive cell transfer with tumor-specific T-cells induced by cryo-thermal therapy is a promising therapeutic strategy. In adoptive T-cell therapy, tumor-infiltrating or circulating autologous lymphocytes can be isolated, selected in vitro, expanded and then reinfused into the patient.46 In general, one challenge for adoptive T-cell therapy is enough to obtain enough tumor-specific T-cells. Adoptive T-cell therapy derived from tumor-infiltrating lymphocytes has been almost exclusively used to treat patients with malignant melanoma due to the difficulty in isolating and expanding pre-existing tumor-reactive T-cells from patients with other tumor types.47 However, in this study, tumor-specific T-cells were markedly activated by cryo-thermal therapy. Moreover, many other challenges are involved in adoptive T-cell therapy, such as lack of unique tTSAs, inefficient homing of T-cells to tumor sites and the immunosuppressive microenvironment of solid tumors.48 However, cryo-thermal therapy offered abundant antigens by inducing immunogenic tumor cell death, reversed the immunosuppressive microenvironment by promoting the differentiation of MDSCs, maturation of DCs and polarization of M1 macrophages and generated abundant cytotoxic T-cells.15–19 36 The CD4+ T-cell response is diverse, including Th1, Th2, Th17, Tfh cells as well as CTLs and Tregs. In cancer, Tregs are predominantly immune suppressive.49 In the presence of adoptive immunosuppressive T-cell subsets, the antitumor activity of adoptive T-cells is impaired or inhibited, which affects the efficacy of adoptive T-cell therapy.45 Moreover, cryo-thermal therapy generated Th1-biased and CTL-biased CD4+ T-cells and cytotoxic CD8+ T-cells.17 Thus, this study provides a method for the production of activated tumor-specific T-cells via cryo-thermal therapy. At present, we did not have data about neoantigen-specific T-cell responses induced by cryo-thermal therapy in human tumors, but we will study the issue in near future.
Conclusions
In summary, cryo-thermal therapy induced strong long-lasting antitumor immunity through the activation of antigen-specific T-cells, especially neoantigen-specific CD4+ T-cells, to protect against tumor rechallenge and lung metastases. Thus, cryo-thermal therapy not only represents a novel local tumor therapy that leads to strong systemic antitumor immunity but also provides a method to obtain sufficient antigen-specific T-cells for tumor treatment.
Acknowledgments
We thank Weihai Yin for the donation of B16F10 mouse melanoma tumor cell line.
Footnotes
Contributors: PP performed experiments. PL coordinated the project. PL and HH designed experiments. Manuscript was written by PP and revised by PL, HH and LXX. All authors reviewed the manuscript.
Funding: This work was supported by National Natural Science Foundation of China (U1532116), National Key Research and Development Program (2016YFC0106201), and the Shanghai Science and Technology Commission of Shanghai Municipality (19DZ2280300).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: All animal experiments were approved by the Animal Welfare Committee of Shanghai Jiao Tong University, and experimental methods were performed in accordance with the guidelines of Shanghai Jiao Tong University Animal Care (approved by Shanghai Jiao Tong University Scientific Ethics Committee).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as online supplemental information.
References
- 1.Lattanzi M, Deng F-M, Chiriboga LA, et al. Durable response to anti-PD-1 immunotherapy in epithelioid angiomyolipoma: a report on the successful treatment of a rare malignancy. J Immunother Cancer 2018;6:97. 10.1186/s40425-018-0415-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma P, Wagner K, Wolchok JD, et al. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer 2011;11:805–12. 10.1038/nrc3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ott PA, Hodi FS, Kaufman HL, et al. Combination immunotherapy: a road map. J Immunother Cancer 2017;5:16. 10.1186/s40425-017-0218-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pauken KE, Dougan M, Rose NR, et al. Adverse events following cancer immunotherapy: obstacles and opportunities. Trends Immunol 2019;40:511–23. 10.1016/j.it.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haen SP, Pereira PL, Salih HR, et al. More than just tumor destruction: immunomodulation by thermal ablation of cancer. Clin Dev Immunol 2011;2011:1–19. 10.1155/2011/160250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer 2014;14:199–208. 10.1038/nrc3672 [DOI] [PubMed] [Google Scholar]
- 7.Dromi SA, Walsh MP, Herby S, et al. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology 2009;251:58–66. 10.1148/radiol.2511072175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.den Brok MHMGM, Sutmuller RPM, van der Voort R, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res 2004;64:4024–9. 10.1158/0008-5472.CAN-03-3949 [DOI] [PubMed] [Google Scholar]
- 9.Wright AS, Sampson LA, Warner TF, et al. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 2005;236:132–9. 10.1148/radiol.2361031249 [DOI] [PubMed] [Google Scholar]
- 10.den Brok MHMGM, Sutmuller RPM, Nierkens S, et al. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer 2006;95:896–905. 10.1038/sj.bjc.6603341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabel MS. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology 2009;58:1–11. 10.1016/j.cryobiol.2008.10.126 [DOI] [PubMed] [Google Scholar]
- 12.Jansen MC, van Hillegersberg R, Schoots IG, et al. Cryoablation induces greater inflammatory and coagulative responses than radiofrequency ablation or laser induced thermotherapy in a rat liver model. Surgery 2010;147:686–95. 10.1016/j.surg.2009.10.053 [DOI] [PubMed] [Google Scholar]
- 13.Dong J, Liu P, Xu LX. Immunologic response induced by synergistic effect of alternating cooling and heating of breast cancer. Int J Hyperthermia 2009;25:25–33. 10.1080/02656730802279534 [DOI] [PubMed] [Google Scholar]
- 14.Shen Y, Liu P, Zhang A, et al. Study on tumor microvasculature damage induced by alternate cooling and heating. Ann Biomed Eng 2008;36:1409–19. 10.1007/s10439-008-9511-2 [DOI] [PubMed] [Google Scholar]
- 15.Xue T, Liu P, Zhou Y, et al. Interleukin-6 Induced "Acute" Phenotypic Microenvironment Promotes Th1 Anti-Tumor Immunity in Cryo-Thermal Therapy Revealed By Shotgun and Parallel Reaction Monitoring Proteomics. Theranostics 2016;6:773–94. 10.7150/thno.14394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu J, Zhang Y, Zhang A, et al. Cryo-thermal therapy elicits potent anti-tumor immunity by inducing extracellular Hsp70-dependent MDSC differentiation. Sci Rep 2016;6:27136. 10.1038/srep27136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He K, Liu P, Xu LX. The cryo-thermal therapy eradicated melanoma in mice by eliciting CD4+ T-cell-mediated antitumor memory immune response. Cell Death Dis 2017;8:e2703. 10.1038/cddis.2017.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu K, He K, Xue T, et al. The cryo-thermal therapy-induced IL-6-rich acute pro-inflammatory response promoted DCs phenotypic maturation as the prerequisite to CD4+ T cell differentiation. Int J Hyperthermia 2018;34:261–72. 10.1080/02656736.2017.1332394 [DOI] [PubMed] [Google Scholar]
- 19.He K, Jia S, Lou Y, et al. Cryo-thermal therapy induces macrophage polarization for durable anti-tumor immunity. Cell Death Dis 2019;10:216. 10.1038/s41419-019-1459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017;547:217–21. 10.1038/nature22991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreiter S, Vormehr M, van de Roemer N, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 2015;520:692–6. 10.1038/nature14426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124–8. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iiizumi S, Ohtake J, Murakami N, et al. Identification of novel HLA class II-restricted neoantigens derived from driver mutations. Cancers 2019;11. 10.3390/cancers11020266. [Epub ahead of print: 24 Feb 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold PY, La Gruta NL, Miller T, et al. The majority of immunogenic epitopes generate CD4+ T cells that are dependent on MHC class II-bound peptide-flanking residues. J Immunol 2002;169:4674.2–4674. 10.4049/jimmunol.169.8.4674-a [DOI] [PubMed] [Google Scholar]
- 25.Sun Z, Chen F, Meng F, et al. Mhc class II restricted neoantigen: a promising target in tumor immunotherapy. Cancer Lett 2017;392:17–25. 10.1016/j.canlet.2016.12.039 [DOI] [PubMed] [Google Scholar]
- 26.Veatch JR, Jesernig BL, Kargl J, et al. Endogenous CD4+ T Cells Recognize Neoantigens in Lung Cancer Patients, Including Recurrent Oncogenic KRAS and ERBB2 (Her2) Driver Mutations. Cancer Immunol Res 2019;7:910–22. 10.1158/2326-6066.CIR-18-0402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linnemann C, van Buuren MM, Bies L, et al. High-Throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat Med 2015;21:81–5. 10.1038/nm.3773 [DOI] [PubMed] [Google Scholar]
- 28.Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014;344:641–5. 10.1126/science.1251102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321–30. 10.1038/nature21349 [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Wang N, Cao W, et al. Influence of various medium environment to in vitro human T cell culture. In Vitro Cell Dev Biol Anim 2018;54:559–66. 10.1007/s11626-018-0273-3 [DOI] [PubMed] [Google Scholar]
- 31.Wang L-X, Shu S, Disis ML, et al. Adoptive transfer of tumor-primed, in vitro-activated, CD4+ T effector cells (Tes) combined with CD8+ Tes provides intratumoral te proliferation and synergistic antitumor response. Blood 2007;109:4865–76. 10.1182/blood-2006-09-045245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y-Q, Li P-C, Pan N, et al. Tumor-released autophagosomes induces CD4+ T cell-mediated immunosuppression via a TLR2–IL-6 cascade. J Immunother Cancer 2019;7 10.1186/s40425-019-0646-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trickett A, Kwan YL. T cell stimulation and expansion using anti-CD3/CD28 beads. J Immunol Methods 2003;275:251–5. 10.1016/S0022-1759(03)00010-3 [DOI] [PubMed] [Google Scholar]
- 34.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1–10. 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 35.Land WG, Agostinis P, Gasser S, et al. DAMP-Induced allograft and tumor rejection: the circle is closing. Am J Transplant 2016;16:3322–37. 10.1111/ajt.14012 [DOI] [PubMed] [Google Scholar]
- 36.He K, Jia S, Lou Y, et al. Cryo-thermal therapy induces macrophage polarization for durable anti-tumor immunity. Cell Death Dis 2019;10 10.1038/s41419-019-1459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith CC, Selitsky SR, Chai S, et al. Alternative tumour-specific antigens. Nat Rev Cancer 2019;19:465–78. 10.1038/s41568-019-0162-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alspach E, Lussier DM, Miceli AP, et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature 2019;574:696–701. 10.1038/s41586-019-1671-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corthay A, Skovseth DK, Lundin KU, et al. Primary antitumor immune response mediated by CD4+ T cells. Immunity 2005;22:371–83. 10.1016/j.immuni.2005.02.003 [DOI] [PubMed] [Google Scholar]
- 40.Quezada SA, Simpson TR, Peggs KS, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 2010;207:637–50. 10.1084/jem.20091918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braumüller H, Wieder T, Brenner E, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature 2013;494:361–5. 10.1038/nature11824 [DOI] [PubMed] [Google Scholar]
- 42.Schoenberger SP, Toes RE, van der Voort EI, et al. T-Cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 1998;393:480–3. 10.1038/31002 [DOI] [PubMed] [Google Scholar]
- 43.Hung K, Hayashi R, Lafond-Walker A, et al. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med 1998;188:2357–68. 10.1084/jem.188.12.2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haabeth OAW, Lorvik KB, Hammarström C, et al. Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat Commun 2011;2:240. 10.1038/ncomms1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4(+) T cells. Curr Opin Immunol 2009;21:200–8. 10.1016/j.coi.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikeda H. T-Cell adoptive immunotherapy using tumor-infiltrating T cells and genetically engineered TCR-T cells. Int Immunol 2016;28:349–53. 10.1093/intimm/dxw022 [DOI] [PubMed] [Google Scholar]
- 47.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348:62–8. 10.1126/science.aaa4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kakarla S, Gottschalk S. Car T cells for solid tumors: armed and ready to go? Cancer J 2014;20:151–5. 10.1097/PPO.0000000000000032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakaguchi S, Yamaguchi T, Nomura T, et al. Regulatory T cells and immune tolerance. Cell 2008;133:775–87. 10.1016/j.cell.2008.05.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2019-000421supp001.pdf (809KB, pdf)