Abstract
Background: Panax notoginseng, a Chinese herbal medicine, has been widely used to treat vascular diseases. Diabetic retinopathy (DR) is one of the complications of diabetic microangiopathy. According to recent studies, the application of Panax notoginseng extract and related Chinese patent medicine preparations can significantly improve DR. However, the pharmacological mechanisms remain unclear. Therefore, the purpose of this study was to decipher the potential mechanism of Panax notoginseng treatment of DR using network pharmacology.
Method: We evaluated and screened the active compounds of Panax notoginseng using the Traditional Chinese Medicine Systems Pharmacology database and collected potential targets of the compounds by target fishing. A multi-source database was also used to organize targets of DR. The potential targets as the treatment of DR with Panax notoginseng were then obtained by matching the compound targets with the DR targets. Using protein-protein interaction networks and topological analysis, interactions between potential targets were identified. In addition, we also performed gene ontology-biological process and pathway enrichment analysis for the potential targets by using the Biological Information Annotation Database.
Results: Eight active ingredients of Panax notoginseng and 31 potential targets for the treatment of DR were identified. The screening and enrichment analysis revealed that the treatment of DR using Panax notoginseng primarily involved 28 biological processes and 10 related pathways. Further analyses indicated that angiogenesis, inflammatory reactions, and apoptosis may be the main processes involved in the treatment of DR with Panax notoginseng. In addition, we determined that the mechanism of intervention of Panax notoginseng in treating DR may involve five core targets, VEGFA, MMP-9, MMP-2, FGF2, and COX-2.
Conclusion: Panax notoginseng may treat diabetic retinopathy through the mechanism of network pharmacological analysis. The underlying molecular mechanisms were closely related to the intervention of angiogenesis, inflammation, and apoptosis with VEGFA, MMP-9, MMP-2, FGF2, and COX-2 being possible targets.
Keywords: Panax notoginseng, molecular mechanism, network pharmacology, diabetic retinopathy, treatment, vascular disease
1. INTRODUCTION
Diabetic retinopathy (DR) is a serious complication of diabetes and is primarily caused by increased permeability of retinal vessels (diabetic macular edema) or the proliferation of new retina vessels following tissue hyperglycemia and hypoxemia [1]. As the disease progresses, the retinal neovasculature ruptures, resulting in blood accumulation in the vitreous with further visual impairment or even blindness, which seriously effects a patient's life and leads to a huge economic burden [2, 3]. An estimated 93 million people worldwide are affected by DR [4], being the leading cause of blindness in adults in developed countries [5, 6]. Almost all patients with type 1 diabetes and disease duration more than 20 years develop DR, while 60% of patients with type 2 diabetes develop this disease [7, 8]. Although a variety of pharmaceutical preparations have been developed based on the pathogenesis of DR, their adverse side effects and relatively poor efficacy have contributed to make DR a challenge for the medical community.
For thousands of years, traditional Chinese medicine has been the main approach used by Chinese people to prevent and treat diseases. Different from modern medicine, traditional Chinese medicine includes a unique philosophical system with characteristics of reducing side effects and improving symptoms when treating diseases and has been widely studied by scholars. Traditional Chinese medicine has a long history of treating diabetes and its complications. With the advancement of pharmacological research, the study of individual herbs has achieved good results in the treatment of diabetes and its complications. Experimental studies have shown that Panax notoginseng may exert its protective effect on retinal capillary endothelial cells and nerves by regulating the cell redox state, activating mitochondrial autophagy, and inhibiting endoplasmic reticulum stress [9-12]. However, Chinese herbal medicines have multiple components and often multi-target characteristics. Therefore, further exploration of the regulatory mechanisms of Panax notoginseng may prove conducive to identifying and developing novel therapeutic methods for improving the treatment of DR.
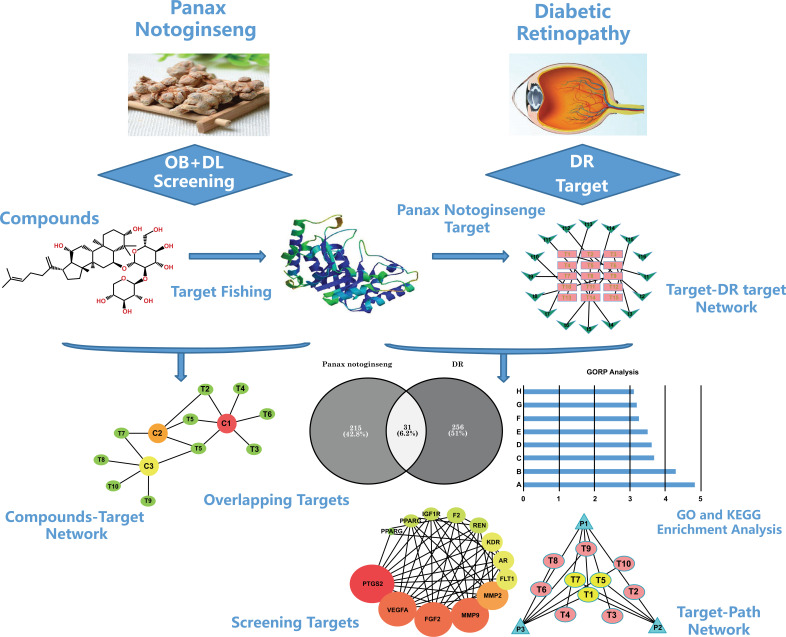
Network pharmacology is a new discipline that involves network association analysis of “drug-target-disease.” It provides a systematic and convenient method of analyzing drugs for their complex mechanisms of action and potential intervention of diseases by identifying core targets shared by the drugs and diseases. Network pharmacology can also be used to integrate and extract possible pathways for the drug-based intervention of diseases. Therefore, we chose to use network pharmacology as a tool to further analyze the possible targets, biological processes, and pathways involved in the treatment of DR with Chinese herbal medicine Panax notoginseng. Our aim was to identify a possible molecular mechanism of Panax notoginseng or prescriptions containing Panax notoginseng for the treatment of DR and potentially provide new possibilities and directions for treating DR. The technical roadmap we used is shown in Fig. (1).
Fig. (1).
The whole framework based on an integration strategy of network pharmacology. Abbreviations; DR, diabetic retinopathy; PPI, protein-protein interactions.
2. METHODS
2.1. Database Building of Chemical Ingredients Panax Notoginseng
We used the Traditional Chinese Medicine System Pharmacology (TCMSP) database (http://lsp.nwu.edu.cn/tcmsp.php, update in 2019-8-11) to identify the active ingredients of Panax notoginseng. The TCMSP database is a special platform designed for Chinese herbal medicines and contains more than 400 Chinese herbal medicines registered in the Chinese Pharmacopoeia, covering nearly 3,000 components and more than 3,000 targets of Chinese herbal medicines [13]. By searching “Panax notoginseng” in this database, we identified 120 active chemicals of Panax notoginseng.
2.2. Screening of Panax notoginseng Active Ingredients
Traditional Chinese medicines, like other medicines, work in the human body through the process of absorption, distribution, metabolism, and excretion (ADME) to the target tissues or organs. To screen for potent potential compounds, we evaluated the ADME properties of the 120 identified active components of Panax notoginseng through their oral bioavailability (OB) and drug-likeness (DL).
2.3. Evaluation of OB
OB is the percentage of the drug that enters the body's systemic blood circulation following oral administration and is one of the most commonly used pharmacokinetic parameters regarding drug properties. The computer prediction model OBioavail 1.1 was constructed based on the information regarding the metabolization of cytochrome P450 3A4 and transportation of permeability glycoprotein (P-glycoprotein) and is a powerful system for predicting OB values [14]. In the current study, we set the OB threshold to 30% and used OB ≥ 30% as the screening conditions for analysis of the active ingredients.
2.4. Evaluation of DL
DL is used to assess the similarity of substances of interest to the active components of marketed drugs. To determine the appropriate drug compositions, we applied the Tanimoto Similarity (TS) coefficient modeling to calculate the drug similarity index of the active components of Panax notoginseng. The TS index was calculated as follows:

In equation 1, A was the molecular descriptor index of the Chinese herbal medicine component to be predicted and B was the average drug-like index of all components in the DrugBank database (http://www.drugbank.ca/). We reserved compounds with DL ≥ 0.18. Ultimately, the active ingredients of Chinese herbal medicine that satisfied the thresholds set for both OB and DL were included in further analysis.
2.5. Target Fishing
A pharmaceutical ingredient may exert its biological function by combining it with a specific target. Determining the target of an active ingredient is essential for elucidating the mechanism of action of the drug. For the active components above, we found the corresponding small molecule structure information in the PubChem Cid through the TCMSP database. The targets were then fished and collated with a Swiss Target Prediction web server (http://www.swisstargetprediction.ch/index.php) using a similar set approach.
2.6. Database Building of Disease Targets
We used multiple databases as sources to collect and organize related DR targets. The databases we used included DisGeNET (http://www.disgenet.org/, update 2019-8-12), DrugBank (https://www.drugbank.ca/, update 2019-8-12), Online Mendelian Inheritance in Man (OMIM; http://www.pharmgkb.org/, update 2019-8-12), and Genetic Association Database (GAD; https://www.drugbank.ca, update 2019-8-12). We searched the databases using the keyword “diabetic retinopathy” and then further sorted the DR targets.
2.7. Protein-protein Interaction (PPI) Construction and Analysis
We matched the predicted targets of the active ingredients of Panax notoginseng with the collected DR-related targets and identified overlapping targets as potential key targets for the treatment of DR with Panax notoginseng. Next, the above-mentioned overlapping therapeutic targets were subjected to PPI analysis using the STRING database (https://string-db.org/, update 2019-8-12). The data were saved in a Tab Separated Values (TSV) format.
2.8. Network Construction
2.8.1. Network Construction Method
For the process network construction, we primarily used Cytoscape 3.6.0 (http://www.cytoscape.org/, update 2019-8-12) to generate all the visual network diagrams. These included the component-target (C-T) network, Panax notoginseng target-DR target interaction (T-T) network, target-path (T-P) network, and PPI network. The networks were screened for the relevant pathways of the therapeutic targets based on Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment results.
2.8.2. Definition of Network Topological Feature Set
Three parameters were selected to present the topological features of each node in the network, degree, betweenness centrality, and closeness centrality. Degree is referred to the number of edges linked by a node. The greater value of degree, indicates the stronger interaction with other nodes [15]. Betweenness centrality referred to the proximity of a node to other nodes. The stronger the control of a node in transferring information to other nodes, the greater the value of betweenness [16]. Closeness centrality was the average distance that a reaction transfers from one node to another. Evaluation of the three parameters provided a means to determine the importance of each node. The median value of each parameter in the network analysis reflected the threshold of the central node.
2.9. Enrichment Analysis
Gene ontology (GO) analysis and KEGG pathway analysis were performed for the therapeutic targets of Panax notoginseng using the Biological Information Annotation Database (DAVID; https://david.ncifcrf.gov/, update 2019-8-13) and the results were saved. The saved results were first screened for biological processes and pathways with significant differences. These were then sorted according to the number of treatment targets involved. Finally, Microsoft Excel 2010 software was used to map the top biological processes and pathways.
3. RESULTS
3.1. Active Compound Components of Panax notoginseng
After searching the TCMSP database for the active ingredients of Panax notoginseng, 120 related components were identified (Table S1). 120 active ingredients were screened for threshold values of OB ≥ 30% and DL ≥ 0.18 and eight components were identified, as shown in Table 1. The eight active ingredients included Mandenol (MOL001494, OB = 42.00, DL = 0.19), DFV (MOL001792, OB = 32.76, DL = 0.18), Diop (MOL002879, OB = 43.59, DL = 0.39), beta-sitosterol (MOL000358, OB = 36.91, DL = 0.75), stigmasterol (MOL000449, OB = 43.83, DL = 0.76), ginsenoside Rh2 (MOL005344, OB = 36.32, DL = 0.56), ginsenoside F2 (MOL007475, OB = 36.43, DL = 0.25), and quercetin (MOL000098, OB = 46.43, DL = 0.28). These eight ingredients were included in subsequent analyses.
Table 1.
List of eight kinds of Panax notoginseng compounds and their OB and DL values
| MOL ID | Molecule Name | OB | DL |
|---|---|---|---|
| MOL001494 | Mandenol | 42.00 | 0.19 |
| MOL001792 | DFV | 32.76 | 0.18 |
| MOL002879 | Diop | 43.59 | 0.39 |
| MOL000358 | beta-sitosterol | 36.91 | 0.75 |
| MOL000449 | Stigmasterol | 43.83 | 0.76 |
| MOL005344 | ginsenoside rh2 | 36.32 | 0.56 |
| MOL007475 | ginsenoside f2 | 36.43 | 0.25 |
| MOL000098 | quercetin | 46.43 | 0.28 |
3.2. Target Prediction and Analysis
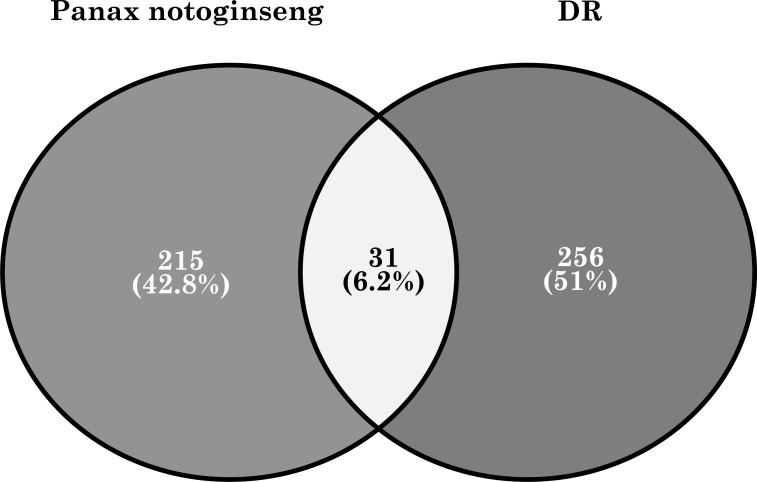
Based on the eight active ingredients of Panax notoginseng described above, we applied similar methods for target fishing and ultimately captured 246 related targets. For DR targets, we used a multi-source database integration approach that included databases DisGeNET, DrugBank, OMIM, and DAD. Ultimately, we identified 287 related DR targets and then matched the targets of the active ingredient of Panax notoginseng with the DR targets. This resulted in 31 overlapping proteins, which we considered as potential therapeutic targets of Panax notoginseng for treating DR Fig. (2).
Fig. (2).
The 31 potential therapeutic targets of Panax notoginseng for treating DR.
3.3. Network Construction and Analysis
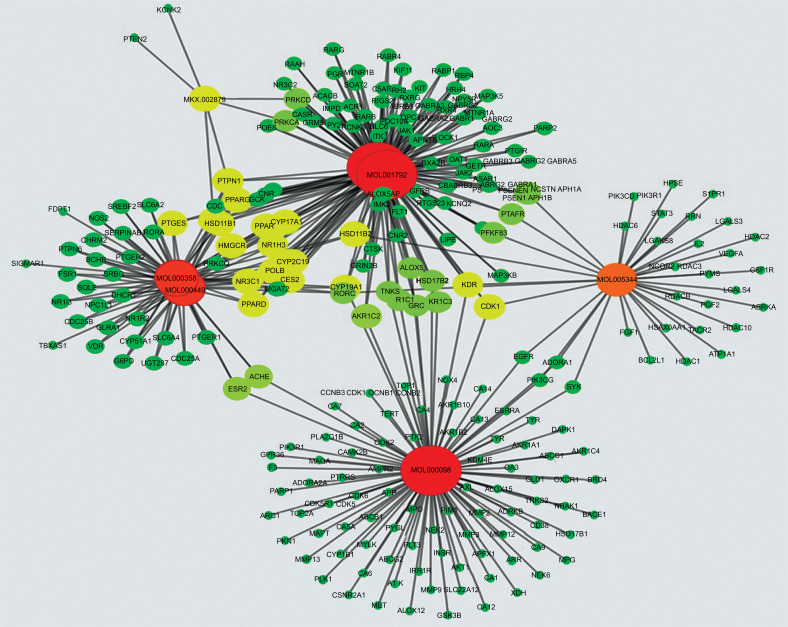
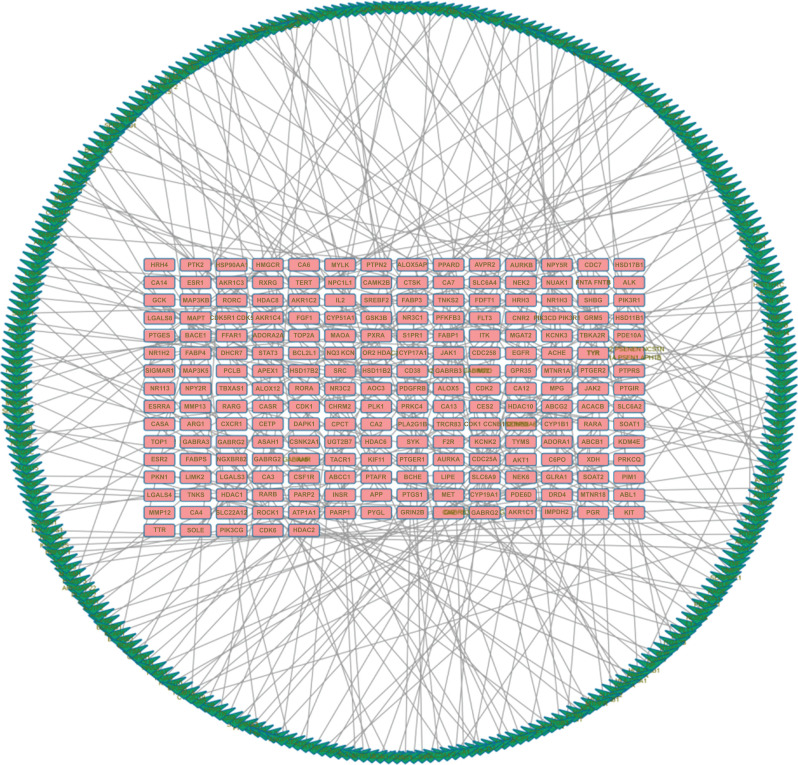
Cytoscape v3.2.1 was used to establish the C-T network for the active ingredients of Panax notoginseng and the 246 targets (Fig. 3). A total of 253 nodes and 433 edges are depicted in the figure. In addition, the figure demonstrates that Panax notoginseng has multiple targets. 246 targets of Panax notoginseng and 287 targets of DR were further analyzed by establishing a T-T network association. Fig. (4) shows a total of 502 nodes and 287 edges, which demonstrate the relevance and potential of DR treatment with Panax notoginseng. This provides good evidence for the use of Panax notoginseng for treating DR.
Fig. (3).
Component-target (C-T) network consisting of 253 nodes and 433 edges. The red, orange and yellow nodes labeled MOL denote the compounds. The remaining nodes denote the predicted targets. Nodes arranged in descending order of red, orange, yellow and green show a decrease in the strength of the association between the node and other nodes.
Fig. (4).
The Panax notoginseng Target - Disease Target (T-T) network consists of 502 nodes and 287 edges. The red node represents the predicted target of Panax notoginseng. The green node represents the target of the DR.
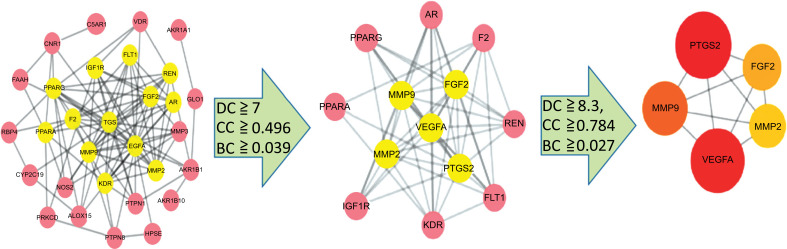
Prior to establishing a PPI network, we first analyzed the interaction of 31 target proteins using the STRING database. The results were imported into the Cytoscape software for topological analysis. The results showed that the protein interactions involved 30 nodes and 105 edges in which CA1 had no correlation with the other proteins and was therefore not included in the network. We then used degree, betweenness, and closeness as the three main parameters for critical target screening. The thresholds for screening were degree ≧ 7, closeness ≧ 0.49617257, and betweenness ≧ 0.03949096. The 13 center nodes and 54 edges greater than the median were used as the first screening result. Subsequently, we conducted a second screening of 13 key targets. The thresholds for this screening were degree ≧ 8.3, closeness ≧ 0.78406924, and betweenness ≧ 0.02797202. Finally, five large central nodes greater than the median were selected as core targets of Panax notoginseng for the treatment of DR (Fig. 5). These five targets included prostaglandin-endoperoxide synthase 2 (PTGS2; Degree = 12), vascular endothelial growth factor A (VEGFA; Degree = 11), matrix metallopeptidase 9 (MMP9; Degree = 11), fibroblast growth factor 2 (FGF2; Degree = 11), and matrix metallopeptidase 2 (MMP2; Degree = 10).
Fig. (5).
Topology screening process of PPI network.
3.4. GO Biological Process and KEGG Pathway Enrichment Analysis
We imported 31 interaction targets into DAVID v6.8 for enrichment analysis. Among the targets, the GO biological process was enriched to 97 GO processes. After screening, 28 biological processes remained. Similarly, KEGG pathway analysis identified 21 related pathways. By screening for DR-related pathways, 10 pathways ultimately remained.
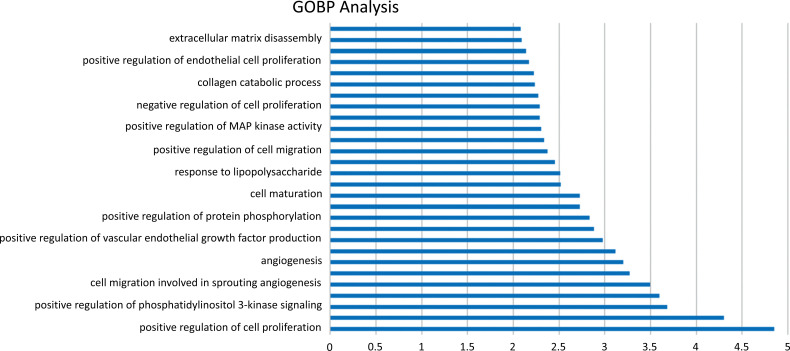
3.5. GO Biological Process Enrichment Analysis
By sorting the 28 GO processes mentioned above, the main biological processes were identified (Fig. 6). The three primary process categories included angiogenesis, inflammatory response, and apoptosis. The GO processes involved in angiogenesis included positive regulation of cell proliferation, positive regulation of angiogenesis, positive regulation of phosphatidylinositol 3-kinase (PI3K) signaling, positive regulation of the ERK1 and ERK2 cascades, angiogenesis, among others. Those involved in the inflammatory response GO process included positive regulation of (PI3K) signaling, leukocyte migration, response to lipopolysaccharides, and others. The GO processes involved in apoptosis included redox processes, negative regulation of MAP kinase activity, negative regulation of apoptotic processes, and others. Based on these findings, we speculate that Panax notoginseng mechanistically may primarily function in the treatment of DR by intervening in terms of the above three GO biological processes.
Fig. (6).
The GOBP enrichment analysis of 31 nodes.
3.6. KEGG Pathway Enrichment Analysis
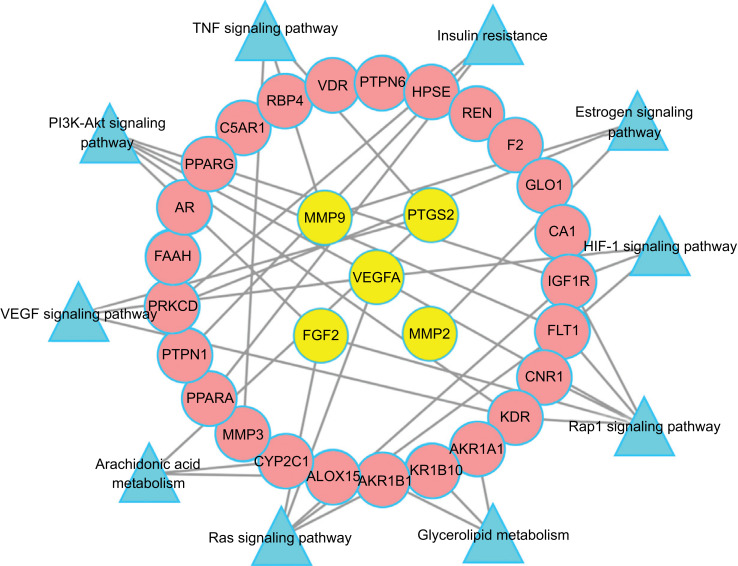
The results of KEGG pathway enrichment analysis suggested that the mechanism of action of Panax notoginseng in the treatment of DR may be closely related to multiple signaling pathways, such as the hypoxia-inducible factor 1 (HIF-1) signaling pathway (hsa04066), VEGF signaling pathway (hsa04370), arachidonic acid metabolism (hsa00590), PI3K-Akt signaling pathway (hsa04151), tumor necrosis factor (TNF) signaling pathway (hsa04668), and others. Here, we established a target-path network (Fig. 7), which clearly indicates that Panax notoginseng may achieve its purpose of treating DR through multiple targets and pathways.
Fig. (7).
Target - Path Network. The yellow nodes represent the big hub nodes, the pink round nodes represent the other nodes. The blue triangles represent the related pathways.
4. DISCUSSION
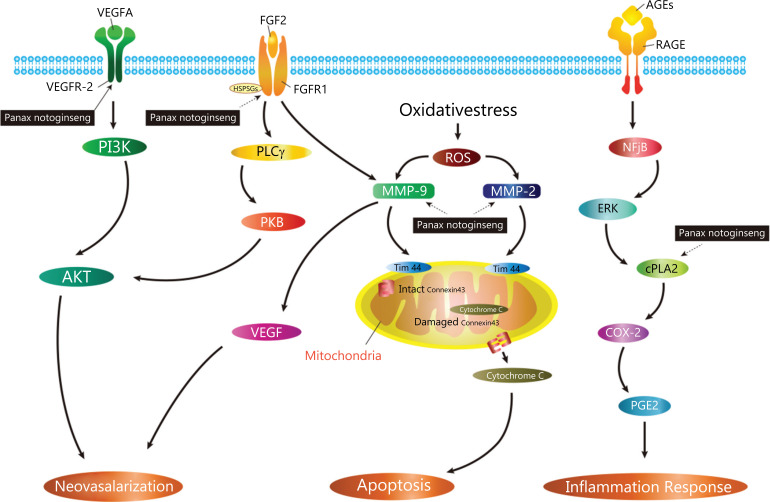
In the current work, using network pharmacology, we predicted the possible molecular mechanisms of Panax notoginseng for treating DR. The results of enrichment analysis suggested that the potential mechanism of action of Panax notoginseng may involve biological processes such as retinal neovascularization, inflammation, and apoptosis and may act through multiple closely related pathways. Among the Panax notoginseng targets identified in the current study, one is a previously known target (VEGF2) and four are putative targets (MMP-9, MMP-2, FGF2, and COX-2). These targets were recognized as active factors involved in the main biological functions of treatment, which implied that these were involved in the underlying mechanisms of Panax notoginseng on diabetic retinopathy (Fig. 8).
Fig. (8).
A graphical representation of key biological progressions caused by known core targets and putative core targets that may treat DR. Abbreviations: VEGF, vascular endothelial growth factor; VEGFA, vascular endothelial growth factor A; VEGFR2, vascular endothelial growth factor receptor 2; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; FGF2, fibroblast growth factor 2; HSPGs, heparin sulfate Proteoglycans; PLCγ, phospholipase C γ; PKB, protein kinase B; ROS, reactive oxygen species; MMP-9, Matrix Metalloproteinase 9; MMP-2, Matrix Metalloproteinase 2; TIM44, translocase of inner mitochondrial membrance 44; AGEs, advanced glycation end -products; RAGE, advanced glycation end-products receptor; NFjB, Nuclear factor jB; ERK, extracellular regulated protein kinases; cPLA2, Cytosolic phospholipase A2; COX-2, cyclooxygenase; PGE2, prostaglandin E2.
Retinal neovascularization may be induced under conditions of persistent hyperglycemia, ischemia, and hypoxia. Degradation of extracellular matrix (ECM), migration and proliferation of endothelial cells, and synthesis of new matrix components are major processes involved in neovas-cularization [17]. VEGF has been shown to be an important mediator of neovascularization [18]. VEGFA, a pro-angiogenic factor, was the first member of the VEGF family to be discovered and is the most studied member [19]. It is considered to be one of the key factors mediating the progression of DR [20] and plays a key role in enhancing vascular permeability and stimulating angiogenesis [21]. Related studies have found that the binding of VEGFA to the receptor VEGFR-2 is able to activate the PI3K/Akt signal transduction pathway and it has been shown that this pathway may be one of the main signaling pathways of VEGFA to stimulate angiogenesis [22]. Under normal conditions, VEGFA in human serum is present at low levels and serves to maintain normal blood vessel growth and maintain the physiological stability of vascular density. However, VEGFA expression is highly detectable in patients with DR and VEGFA expression levels are significantly upregulated in patients with proliferative diabetic retinopathy (PDR) compared to VEGFA levels in the vitreous of patients with non-proliferative diabetic retinopathy (NPDR) [23]. Therefore, anti-VEGF and anti-VEGFA treatments have become research hotspots regarding treatment strategies for anti-angiogenesis. Pegaptanib (Macugen), the first VEGF inhibitor approved by the US Food and Drug Administration (FDA), is an anti-VEGF165 aptamer with a molecular weight of 50,000 and approved for the treatment of neovascular age-related macular degeneration [24]. Bevacizumab (Avastin), a recombinant humanized monoclonal anti-VEGFA antibody, is the first FDA-approved drug in the United States to inhibit tumor angiogenesis [25]. Ranibizumab (Lucentis), an antibody fragment derived from bevacizumab that binds more closely to VEGFA, is an FDA-approved drug for the treatment of neovascular age-related macular degeneration (AMD) [26, 27]. In summary, anti-VEGFA treatment has become an important direction for the treatment of neovascular eye disease.
Matrix metalloproteinases (MMPs) are important proteases involved in connective tissue remodeling and ECM degradation [28] and play important roles in various developmental processes, such as morphogenesis, angiogenesis, and vascular remodeling. Among the MMPs, those with gelatinase activity (MMP9 and MMP2) have shown a clear dual role in the development of DR. In the NPDR phase, MMP2 and MMP9 promote capillary cell apoptosis by destroying mitochondria and during the PDR phase, retinal neovascularization is promoted [29]. Under normal conditions, the expression of MMPs is relatively low and they are activated under conditions of tissue damage or remodeling. Studies have found that the expression of MMP2 and MMP9 is significantly up-regulated during the development of DR [30, 31] and they are more prominent in the serum and vitreous of patients with PDR [32]. Therefore, MMPs have been an important target for the treatment of DR. However, as the mechanism by which MMPs treat DR is not clear, clinical trials using MMPs inhibitors to treat DR have not yet yielded satisfactory results [33].
FGF2 is a member of the FGF family and can be produced by a variety of cells in the retina. When FGF2 exerts a biological effect, a cofactor such as heparan sulfate proteoglycan (HSPG) is required to promote its binding to the receptor FGFRs [34]. Studies suggest that FGF2 promotes extracellular matrix degradation by promoting endothelial cell proliferation and up-regulating the expression of urokinase plasminogen activator (uPA) and MMPs, which in turn promotes retinal neovascularization [35, 36]. Related literature reports that FGF2 is significantly up-regulated in the vitreous and serum of patients with PDR [37, 38]. Therefore, FGF2 may play an important role in neovascular retinopathy, especially during the PDR phase. Down-regulating FGF2 expression may become one approach for treating DR.
Inflammatory responses have always been one of the research hotspots regarding DR pathogenesis. Cyclooxygenase (COX)-2 is an inducible enzyme that catalyzes the production of protoxin (PGE) from arachidonic acid, mediates inflammatory responses, and is a target for non-steroidal anti-inflammatory drugs (NSAIDs) [39]. In the development of DR, the inflammatory factor COX-2 induces its downstream product PGE2 to promote increased retinal capillary permeability. Under normal circumstances, COX-2 is hardly expressed or expressed at very low levels in tissues and can induce rapid high expression of COX-2 under the action of cytokines, growth factors, and inflammatory mediators. Animal experiments have shown that COX-2 inhibitors and NSAIDs can significantly reduce rodent retinal leukocyte aggregation and vascular permeability [40]. Therefore, the application of COX-2 inhibitors has been one of the target-based approaches for the treatment of DR.
According to reports in the literature, the extract of Panax notoginseng can prevent apoptosis of retinal pigment epithelial cells by scavenging hydroxyl and superoxide radicals, suggesting that Panax notoginseng has the significant antioxidant capacity [41]. Its active ingredients can up-regulate neurotrophic factor levels and inhibit neuronal apoptosis and also demonstrate a certain advantage in the neuroprotective effects of diabetic rat retina [42]. The anti-inflammatory ability of Panax notoginseng may be achieved by down-regulating the expression of the proinflammatory mediators monocyte chemoattractant protein-1 (MCP-1) and NF-jB [43]. Other studies have suggested that its active ingredients can further attenuate NF-κB signaling by interfering with MAPKs and Akt, thereby alleviating inflammation [43]. In addition, the down-regulation of VEGF and MMP-9 expression by the active ingredients of Panax notoginseng is an effective methods for reducing the angiogenesis associated with DR in rats [44]. In conclusion, anti-angiogenesis, inhibition of inflammatory response and apoptosis may be an effective mechanism for the action of Panax notoginseng on DR.
CONCLUSION
In this study, the active ingredients of Panax notoginseng and its potential mechanism for treating DR were explored using network pharmacology methods. The mechanism of action of Panax notoginseng may be closely related to three biological processes, retinal neovascularization, inflammatory reactions, and apoptosis. We identified five important active targets in the relevant pathways of these biological processes. This work provided new clues on Panax notoginseng pharmacological targets for the treatment of DR.
ACKNOWLEDGMENTS
Declared none.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data used to support the findings of this study are available within the article.
FUNDING
This work was supported by the 2015 Traditional Chinese Medicine Scientific Research (No. 201507001-11).
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this article.
REFERENCES
- 1.Yau J.W., Rogers S.L., Kawasaki R., Lamoureux E.L., Kowalski J.W., Bek T., Chen S.J., Dekker J.M., Fletcher A., Grauslund J., Haffner S., Hamman R.F., Ikram M.K., Kayama T., Klein B.E., Klein R., Krishnaiah S., Mayurasakorn K., O’Hare J.P., Orchard T.J., Porta M., Rema M., Roy M.S., Sharma T., Shaw J., Taylor H., Tielsch J.M., Varma R., Wang J.J., Wang N., West S., Xu L., Yasuda M., Zhang X., Mitchell P., Wong T.Y. Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leasher J.L., Bourne R.R., Flaxman S.R., Jonas J.B., Keeffe J., Naidoo K., Pesudovs K., Price H., White R.A., Wong T.Y., Resnikoff S., Taylor H.R. Vision Loss Expert Group of the Global Burden of Disease Study. Diabetes Care. 2016;39(9):1643–1649. doi: 10.2337/dc16-er11. [DOI] [PubMed] [Google Scholar]
- 3.Das A. Diabetic retinopathy: battling the global epidemic. Invest. Ophthalmol. Vis. Sci. 2016;57(15):6669–6682. doi: 10.1167/iovs.16-21031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Report on Diabetes. Geneva: World Health Organization; 2016. [Google Scholar]
- 5.Wong T.Y., Klein R., Islam F.M., Cotch M.F., Folsom A.R., Klein B.E., Sharrett A.R., Shea S. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am. J. Ophthalmol. 2006;141(3):446–455. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein B.E. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14(4):179–183. doi: 10.1080/09286580701396720. [DOI] [PubMed] [Google Scholar]
- 7.Klein R., Klein B.E., Moss S.E., Davis M.D., DeMets D.L. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IX. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch. Ophthalmol. 1989;107(2):237–243. doi: 10.1001/archopht.1989.01070010243030. [DOI] [PubMed] [Google Scholar]
- 8.Klein R., Klein B.E., Moss S.E., Davis M.D., DeMets D.L. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch. Ophthalmol. 1984;102(4):527–532. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 9.Fan C., Qiao Y., Tang M. Notoginsenoside R1 attenuates high glucose-induced endothelial damage in rat retinal capillary endothelial cells by modulating the intracellular redox state. Drug Des. Devel. Ther. 2017;11:3343–3354. doi: 10.2147/DDDT.S149700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan Y., Qiao Y., Huang J., Tang M. Protective effects of Panax notoginseng saponins against high glucose-induced oxidative injury in rat retinal capillary endothelial cells. Evid. Based Complement. Alternat. Med. 2016:, 5326382. doi: 10.1155/2016/5326382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D.D., Zhu H.Z., Li S.W., Yang J.M., Xiao Y., Kang Q.R., Li C.Y., Zhao Y.S., Zeng Y., Li Y., Zhang J., He Z.D., Ying Y. Crude saponins of Panax notoginseng have neuroprotective effects to inhibit palmitate-triggered endoplasmic reticulum stress-associated apoptosis and loss of postsynaptic proteins in staurosporine differentiated RGC-5 retinal ganglion cells. J. Agric. Food Chem. 2016;64(7):1528–1539. doi: 10.1021/acs.jafc.5b05864. [DOI] [PubMed] [Google Scholar]
- 12.Zhou P., Xie W., Meng X., Zhai Y., Dong X., Zhang X., Sun G., Sun X. Notoginsenoside R1 ameliorates diabetic retinopathy through PINK1-dependent activation of mitophagy. Cells. 2019;8(3):213. doi: 10.3390/cells8030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ru J., Li P., Wang J., Zhou W., Li B., Huang C., Li P., Guo Z., Tao W., Yang Y., Xu X., Li Y., Wang Y., Yang L. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X., Zhang W., Huang C., Li Y., Yu H., Wang Y., Duan J., Ling Y. A novel chemometric method for the prediction of human oral bioavailability. Int. J. Mol. Sci. 2012;13(6):6964–6982. doi: 10.3390/ijms13066964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Missiuro P.V., Liu K., Zou L., Ross B.C., Zhao G., Liu J.S., Ge H. Information flow analysis of interactome networks. PLOS Comput. Biol. 2009;5(4):, e1000350. doi: 10.1371/journal.pcbi.1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raman K., Damaraju N., Joshi G.K. The organisational structure of protein networks: revisiting the centrality-lethality hypothesis. Syst. Synth. Biol. 2014;8(1):73–81. doi: 10.1007/s11693-013-9123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe D., Suzuma K., Matsui S., Kurimoto M., Kiryu J., Kita M., Suzuma I., Ohashi H., Ojima T., Murakami T., Kobayashi T., Masuda S., Nagao M., Yoshimura N., Takagi H. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N. Engl. J. Med. 2005;353(8):782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- 18.Shen J., Choy D.F., Yoshida T., Iwase T., Hafiz G., Xie B., Hackett S.F., Arron J.R., Campochiaro P.A. Interleukin-18 has antipermeablity and antiangiogenic activities in the eye: reciprocal suppression with VEGF. J. Cell. Physiol. 2014;229(8):974–983. doi: 10.1002/jcp.24575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan Y.C., Khanna S., Roy S., Sen C.K. miR-200b targets Ets-1 and is down-regulated by hypoxia to induce angiogenic response of endothelial cells. J. Biol. Chem. 2011;286(3):2047–2056. doi: 10.1074/jbc.M110.158790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wirostko B., Wong T.Y., Simó R. Vascular endothelial growth factor and diabetic complications. Prog. Retin. Eye Res. 2008;27(6):608–621. doi: 10.1016/j.preteyeres.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Ruan G.X., Kazlauskas A. Axl is essential for VEGF-A-dependent activation of PI3K/Akt. EMBO J. 2012;31(7):1692–1703. doi: 10.1038/emboj.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Im E., Kazlauskas A. Regulating angiogenesis at the level of PtdIns-4,5-P2. EMBO J. 2006;25(10):2075–2082. doi: 10.1038/sj.emboj.7601100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katsura Y., Okano T., Matsuno K., Osako M., Kure M., Watanabe T., Iwaki Y., Noritake M., Kosano H., Nishigori H., Matsuoka T. Erythropoietin is highly elevated in vitreous fluid of patients with proliferative diabetic retinopathy. Diabetes Care. 2005;28(9):2252–2254. doi: 10.2337/diacare.28.9.2252. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham E.T., Jr, Adamis A.P., Altaweel M., Aiello L.P., Bressler N.M., D’Amico D.J., Goldbaum M., Guyer D.R., Katz B., Patel M., Schwartz S.D. Macugen Diabetic Retinopathy Study Group. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005;112(10):1747–1757. doi: 10.1016/j.ophtha.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Kerbel R.S. Tumor angiogenesis. N. Engl. J. Med. 2008;358(19):2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown D.M., Kaiser P.K., Michels M., Soubrane G., Heier J.S., Kim R.Y., Sy J.P., Schneider S. ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 27.Rosenfeld P.J., Brown D.M., Heier J.S., Boyer D.S., Kaiser P.K., Chung C.Y., Kim R.Y. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 28.Singh K., Goyal P., Singh M., Deshmukh S., Upadhyay D., Kant S., Agrawal N.K., Gupta S.K., Singh K. Association of functional SNP-1562C>T in MMP9 promoter with proliferative diabetic retinopathy in north Indian type 2 diabetes mellitus patients. J. Diabetes Complications. 2017;31(12):1648–1651. doi: 10.1016/j.jdiacomp.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Kowluru R.A., Zhong Q., Santos J.M. Matrix metalloproteinases in diabetic retinopathy: potential role of MMP-9. Expert Opin. Investig. Drugs. 2012;21(6):797–805. doi: 10.1517/13543784.2012.681043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammad G., Kowluru R.A. Matrix metalloproteinase-2 in the development of diabetic retinopathy and mitochondrial dysfunction. Lab. Invest. 2010;90(9):1365–1372. doi: 10.1038/labinvest.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohammad G., Kowluru R.A. Diabetic retinopathy and signaling mechanism for activation of matrix metalloproteinase-9. J. Cell. Physiol. 2012;227(3):1052–1061. doi: 10.1002/jcp.22822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosano H., Okano T., Katsura Y., Noritake M., Kado S., Matsuoka T., Nishigori H. ProMMP-9 (92 kDa gelatinase) in vitreous fluid of patients with proliferative diabetic retinopathy. Life Sci. 1999;64(25):2307–2315. doi: 10.1016/S0024-3205(99)00184-8. [DOI] [PubMed] [Google Scholar]
- 33.Kowluru R.A., Mishra M. Regulation of matrix metalloproteinase in the pathogenesis of diabetic retinopathy. Prog. Mol. Biol. Transl. Sci. 2017;148:67–85. doi: 10.1016/bs.pmbts.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Ronca R., Giacomini A., Rusnati M., Presta M. The potential of fibroblast growth factor/fibroblast growth factor receptor signaling as a therapeutic target in tumor angiogenesis. Expert Opin. Ther. Targets. 2015;19(10):1361–1377. doi: 10.1517/14728222.2015.1062475. [DOI] [PubMed] [Google Scholar]
- 35.Qazi Y., Maddula S., Ambati B.K. Mediators of ocular angiogenesis. J. Genet. 2009;88(4):495–515. doi: 10.1007/s12041-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathew J.G., Clyne A.M. Fibroblast growth factor-2 did not restore plasminogen system activity in endothelial cells on glycated collagen. Biochem. Biophys. Rep. 2015;4:104–110. doi: 10.1016/j.bbrep.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boulton M., Gregor Z., McLeod D., Charteris D., Jarvis-Evans J., Moriarty P., Khaliq A., Foreman D., Allamby D., Bardsley B. Intravitreal growth factors in proliferative diabetic retinopathy: correlation with neovascular activity and glycaemic management. Br. J. Ophthalmol. 1997;81(3):228–233. doi: 10.1136/bjo.81.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beranek M., Kolar P., Tschoplova S., Kankova K., Vasku A. Genetic variation and plasma level of the basic fibroblast growth factor in proliferative diabetic retinopathy. Diabetes Res. Clin. Pract. 2008;79(2):362–367. doi: 10.1016/j.diabres.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Zheng L., Howell S.J., Hatala D.A., Huang K., Kern T.S. Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy. Diabetes. 2007;56(2):337–345. doi: 10.2337/db06-0789. [DOI] [PubMed] [Google Scholar]
- 40.Joussen A.M., Poulaki V., Mitsiades N., Kirchhof B., Koizumi K., Döhmen S., Adamis A.P. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J. 2002;16(3):438–440. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- 41.Han S.Y., Li H.X., Bai C.C., Wang L., Tu P.F. Component analysis and free radical-scavenging potential of Panax notoginseng and Carthamus tinctorius extracts. Chem. Biodivers. 2010;7(2):383–391. doi: 10.1002/cbdv.200800313. [DOI] [PubMed] [Google Scholar]
- 42.Ola M.S., Ahmed M.M., Shams S., Al-Rejaie S.S. Neuroprotective effects of quercetin in diabetic rat retina. Saudi J. Biol. Sci. 2017;24(6):1186–1194. doi: 10.1016/j.sjbs.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee M., Yun S., Lee H., Yang J. Quercetin mitigates inflammatory responses induced by vascular endothelial growth factor in mouse retinal photoreceptor cells through suppression of nuclear factor kappa B. Int. J. Mol. Sci. 2017;18(11) doi: 10.3390/ijms18112497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen B., He T., Xing Y., Cao T. Effects of quercetin on the expression of MCP-1, MMP-9 and VEGF in rats with diabetic retinopathy. Exp. Ther. Med. 2017;14(6):6022–6026. doi: 10.3892/etm.2017.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available within the article.