Abstract
Background: Pretreatment drug resistance (PDR) poses an increasing threat to the success of antiretroviral treatment (ART) programs in China. We aimed to conduct a survey of PDR among HIV patients in an area in Southwest China with extensive drug trafficking.
Methods: Consecutive cross-sectional surveys were conducted in Liangshan Prefecture of Sichuan Province from 2009 to 2018 based on the WHO-recommended method. PDR was identified by testing pol region sequences with the Stanford HIVdb algorithm (version 7.0). PDR prevalence and related factors were assessed by multivariable logistic regression. The transmission of HIV drug resistance was analyzed using a genetic transmission network.
Results: HIV-1 pol genes from 1889 patients were successfully amplified. The distribution of HIV-1 genotypes was as follows: CRF07_BC (94.0%), CRF08_BC (2.3%), CRF01_AE (2.0%) and others (1.4%). Of the participants, 6.9% (95% CI: 4.1-8.1%) had pretreatment resistance to 12 antiretroviral drugs recommended by the WHO, and nucleoside reverse transcriptase inhibitor (NRTI), non-nucleoside reverse transcriptase inhibitor (NNRTI) and protease inhibitors (PI) resistance were identified among 1.4% (95% CI: 0.7-3.4%), 5.8% (95% CI: 1.2-8.7%) and 0.4% (95% CI: 0.1-3.0%) of the patients, respectively. In the multivariate logistic model, the prevalence of PDR was 1.52-fold higher among intravenous drug users (IDUs) than among patients infected by heterosexual transmission (95% CI: 1.07-2.38; P=0.049), and the prevalence of PDR among patients diagnosed from 2017-2018 was 2.03-fold higher than that among patients diagnosed from 2009-2016 (95% CI: 1.18-5.76; P=0.018). A total of 26 clusters containing PDR and a rapidly growing drug resistance-related cluster containing the E138Q and V179D mutations were identified by genetic transmission network analysis.
Conclusion: The results show a moderate overall level of PDR prevalence and rapidly growing drug resistance over time. Preventive intervention should be focused on controlling the HIV epidemic among drug users, and surveillance is urgently needed to monitor the trend of PDR.
Keywords: HIV, pretreatment drug resistance, transmission, genetic transmission network, cluster, prevention
1. INTRODUCTION
Acquired immunodeficiency syndrome (AIDS) is one of the most devastating pandemics worldwide; in 2017, there were, approximately 36.9 million people living with human immunodeficiency virus (HIV), 1.8 million newly infected people, and 1.0 million deaths from HIV-related causes globally [1]. Antiretroviral (ARV) therapy (ART) can effectively reduce viral load [2], restore immune function of HIV-infected people [3, 4], reduce morbidity and mortality of HIV-infected individuals [5-7], and improve their quality of life [8]. The World Health Organization (WHO) has developed the “treat all” strategy, which recommends that all people living with HIV receive ART shortly after diagnosis and that high-risk groups receive prophylactic treatments [9]. However, HIV-1 has high genetic variability and rapid viral replication [10, 11], which may result in drug resistance to the ARV drugs used in ART under drug selective pressure [12, 13]. One corollary of this fact may be that the incidence of resistance will increase in treated and ART-naïve individuals [14, 15], affecting the ability of ARV drugs to block HIV replication [16, 17]. In the 2017 WHO HIV drug resistance report, among the 11 countries reporting nationally representative survey data, the prevalence of pretreatment drug resistance (PDR) was greater than 10% in six countries, and greater than 15% in two countries [18]. In response to concerns about the increasing prevalence of PDR, the WHO developed a global HIV drug resistance (HIVDR) prevention strategy that recommends monitoring the level of PDR and the factors associated with the emergence of PDR.
The first AIDS case in China was diagnosed in 1985, and 861,042 people nationwide were reported to be living with HIV/AIDS by the end of 2018. Free ART was started in 2003 in China [19], and the population recommended to receive this treatment according to the national ART guidelines has changed over the years from patients with CD4 counts <200, to those with CD4 counts <350 in 2012, to those with CD4 counts <500 in 2014, to all HIV-diagnosed individuals under the current guidelines. Successful implementation of these recommendations will likely reduce the mortality among adult patients in China [20] and the number of infected individuals but will also increase HIVDR in China [21, 22]. Although the prevalence of HIVDR among HIV-infected individuals remains low in most areas of China, some recent reports have indicated moderate levels in specific cities [23-25]. Therefore, understanding the resistance of the virus to antiretroviral drugs is very important to further guide the selection of antiretroviral drugs in clinical settings.
Liangshan Prefecture, located in Sichuan Province, Southwest China has a population of 4.7 million and a total of 41,623 cases of HIV infection; 21,232 patients receiving ART were reported by the end of 2016. Liangshan is one of the most severely AIDS-affected areas in China [26-29]; Liangshan is a major drug transportation route linking Yunnan and Guangxi with Xinjiang [30], and previous studies have shown a high prevalence of HIV-1 infection among intravenous drug users (IDUs) [31, 32]. However, there is a shortage of data on PDR and other molecular epidemiological data regarding the HIV epidemic in Liangshan. In this study, we conducted a survey of HIV drug resistance based on the WHO HIVDR surveillance protocol to assess the level of PDR in recent years in Liangshan. To explore the trend of viral transmission, a genetic transmission network was constructed. These findings provide valuable implications for good practice in planning treatments for people living with HIV.
2. METHODS
2.1. Patients and Samples
A cross-sectional survey was conducted in Liangshan; the survey protocol was taken from the WHO-recommended cross-sectional survey on HIVDR [33]. Eligibility criteria were as follows: diagnosed between 2009 and 2018; aged ≥ 18 years; candidates for the initiation of ART at the time of enrollment; and provided signed written informed consent. Patients who met the criteria were randomly recruited into this study. Patients’ demographic information, including sex, age, marital status, ethnicity, education status, and transmission route, were collected from the National HIV/AIDS Comprehensive Response Information Management System, which is a web-based real-time database system managed by the National Center for AIDS/STD Control and Prevention (NCAIDS) of the Chinese Center for Disease Control and Prevention (CDC) [34]. Plasma samples were collected in accordance with standard procedures [35, 36] by laboratory personnel of the local Center for Disease Control and Prevention (CDC) and were delivered under constant refrigeration to a laboratory at NCAIDS, China CDC, where HIVDR genotyping was performed [36].
2.2. Amplification and Sequencing of HIV-1 pol Gene Fragments
Plasma HIV RNA was quantified with real-time NASBA (NucliSense Easy Q, BioMerieur, France) according to the manufacturer’s recommendations. A nested polymerase chain reaction (PCR) was used to amplify the HIV-1 pol gene fragments spanning the protease gene from codons 1 to 99 and part of the reverse-transcriptase gene from codons 1 to 252, as described by Liao et al., [37]. Amplified nucleic acid fragments were sequenced, and the obtained sequences were trimmed and assembled by Sequencher 4.8 analysis software (Gene Codes Corporation, USA) and aligned using Bio Edit v.7.2.
2.3. HIV-1 Subtyping and Drug Resistance Mutation Analysis
The edited sequences were aligned with HIV-1 reference sequences available in the Los Alamos database (http://www.hiv.lanl.gov). HIV-1 subtypes and circulating recombinant forms (CRFs) were identified based on in-house phylogenetic analysis [38], Phylogenetic trees were constructed through the neighbor-joining method based on the Kimura two-parameter model with 1000 bootstrap replicates using MEGA [39] (Molecular Evolutionary Genetic Analysis Software, Version 6.0).
All HIV-1 pol region sequences were submitted to the online analysis interface of the Stanford University HIV Drug Resistance Database online sequence analysis tool (http://hivdb.stanford.edu/), and drug resistance to nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs) was defined as the detection of at least one ARV drug in any drug class according to the WHO surveillance drug resistance guideline list [40, 41].
2.4. Genetic Transmission Network Analysis
Sequences were excluded when the sequences contained ≥5% ambiguities. The Tamura-Nei 93 (TN93) pairwise genetic distance was calculated for each pair of sequences [42]. The genetic distance threshold that could identify the maximum number of clusters in the genetic network was chosen for analysis [43]. When the genetic distance between the two sequences was below the distance threshold, it was taken to indicate a transmission partner. For visualization and analysis, the network data were processed using the Cytoscape 3.5.2 software.
2.5. Statistical Analysis
Categorical variables were described by numbers and percentages, while continuous variables were calculated as the mean ± standard deviation (SD). A univariate logistic and stepwise multivariate logistic regression model were constructed to analyze the factors that were independently associated with drug resistance. P-values < 0.05 were considered statistically significant, and all tests of significance were two-sided. All data were analyzed using Statistical Analysis System version 9.1 (SAS Institute Inc., USA).
3. RESULT
3.1. Demographic Characteristics
This survey included 1889 patients newly diagnosed in the period from 2009 to 2018. All of these individuals were treatment-naïve patients when the samples were collected. The patients ranged from 18 to 90 years old, with a mean age of 34.1±10.8 years and a male-to-female ratio of 1.3:1. The majority of the subjects were of Yi ethnicity (82.5%); 66.1% of the individuals in this study were married, 48.1% were illiterate, and 49.0% and 32.1% were infected through heterosexual intercourse and intravenous drug use, respectively (Table 1).
Table 1.
Demographic characteristics of the pretreatment HIV patients in Liangshan, China.
| Characteristic | Number | % |
|---|---|---|
| Total | 1889 | 100.0 |
| Age (years) | - | - |
| 16-29 | 705 | 37.3 |
| 30-49 | 1018 | 53.9 |
| >50 | 162 | 8.6 |
| Unknown | 4 | 0.2 |
| Sex | - | - |
| Male | 1044 | 55.3 |
| Female | 837 | 44.3 |
| Unknown | 8 | 0.4 |
| Ethnicity | - | - |
| Han | 155 | 8.2 |
| Yi | 1559 | 82.5 |
| Other or unknown | 175 | 9.3 |
| Education | - | - |
| Illiterate | 907 | 48.1 |
| Primary or Junior high school | 402 | 21.3 |
| Senior high school or higher | 130 | 6.9 |
| Unknown | 450 | 23.8 |
| Route of transmission | - | - |
| Heterosexual transmission | 925 | 49.0 |
| IDU | 606 | 32.1 |
| Homosexual transmission | 12 | 0.6 |
| Mother to children | 3 | 0.2 |
| Blood transfusion | 2 | 0.1 |
| Unknown | 341 | 18.1 |
| Marital status | - | - |
| Single | 303 | 16.1 |
| Married | 1245 | 66.1 |
| Divorced/Widowed | 95 | 5.0 |
| Unknown | 246 | 12.8 |
| Sampling year | - | - |
| 2009-2015 | 249 | 13.2 |
| 2016 | 288 | 15.3 |
| 2017 | 670 | 35.5 |
| 2018 | 682 | 36.1 |
3.2. HIV Subtype and Prevalence of PDR
Among the study subjects, CRF07_BC was found to be the most common genotype (94.0%, 1776/1889), followed by CRF08_BC (2.3%, 44/1889) and CRF01_AE (2.0%, 37/1889). Other subtypes/CRFs included subtype BC (0.5%, 9/1889), CRF07_08 (0.4%, 8/1889), CRF01_BC (0.4%, 7/1889), subtype BC (0.2%, 3/1889) and CRF01_07 (0.1%, 2/1889). In addition, CRF62_BC, CRF85_BC and CRF55_01B were found in one case each.
The overall prevalence of PDR from 2009-2018 was 6.9% (130/1889; 95% confidence interval (CI): 4.1-9.2%). NRTI, NNRTI and PI resistance were identified among 1.4% (26/1889; 95% CI: 0.7-3.4%), 5.8% (109/1889; 95% CI: 1.2-8.7%) and 0.4% (7/1889; 95% CI: 0.1-3.0%) of the patients (Table 2). The rates of PDR among patients diagnosed in 2009-12, 2013-15, 2016, 2017 and 2018 were 3.1% (3/98; 95% CI: 0.0-6.5%), 2.8% (4/144; 95% CI: 0.1-5.5%), 5.5% (15/273; 95% CI: 2.8-8.2%), 10.4% (63/607; 95% CI: 8.0-12.8%) and 7.1% (45/637; 95% CI: 5.1-9.1%), respectively. Thirty-one NRTI mutations were identified, of which the most common were M184L/V (9.1%) and K70R/E (9.1%). These mutations can cause high levels of resistance to NRTI drugs: lamivudine (3TC) and emtricitabine (FTC); intermediate levels of resistance to tenofovir (TDF), stavudine (D4T) and azidothymidine (AZT); and low levels of resistance to abacavir (ABC) and didanosine (DDI). Among the 119 NNRTI mutations identified, the most common mutation was K103N (45.5%), which can cause a high level of resistance to efavirenz (EFV) and nevirapine (NVP), the two ARV drugs that are the components of the WHO-recommended first-line regimens. In addition, 8 PI mutations were identified, of which the most common were I54V (1.5%) and V82A/I (1.5%), which can lead to resistance to darunavir (DRV), lopinavir (LPV) and atazanavir (ATV).
Table 2.
HIVDR mutations among pretreatment HIV patients with drug resistance.
| Antiretroviral Drug | Number (%) | HIV Drug Resistance Mutations, N (%) |
|---|---|---|
| Total | 130 (6.9) | - |
| NRTIsa | 26 (1.4) | - |
| ABC* | 11 (0.6) | K65R,7 (0.4);M184L/V,9 (0.5); D67N,6 (0.3);K70E/N/R,10 (0.5); K219E/R/Q,4 (0.2);L210W,1 (0.1); T215D/A/I/N,4 (0.2);V75I,1 (0.1); M41L,1 (0.1) |
| AZT* | 12 (0.6) | |
| D4T* | 20 (1.1) | |
| DDI* | 10 (0.5) | |
| FTC | 10 (0.5) | |
| 3TC* | 10 (0.5) | |
| TDF* | 10 (0.5) | |
| NNRTIsb | 109 (5.8) | - |
| EFV* | 100 (5.3) | A98G,1 (0.1);E138G/Q,8 (0.4);F227L,4 (0.2);G190A/E/R,8 (0.4); H221Y,8 (0.4);K101E/P,7 (0.4);K103N,65 (3.4);K238T,1 (0.1); L100I,1 (0.1);M230L/I,2 (0.1);P225H,2 (0.1);V106M,9 (0.5); V108I,2 (0.1);V179D/E/F,16 (0.8);Y181C/F,7 (0.4);Y188C/L/N,5 (0.3); |
| NVP* | 109 (5.8) | |
| PIsc | 7 (0.4) | - |
| ATV | 5 (0.2) | I54M/V,3 (0.2);L10F,1 (0.1);I47V,1 (0.1); L90M,1 (0.1);Q58E,1 (0.1); M46I,1 (0.1);V82A/I/S,3 (0.2) |
| LPV* | 5 (0.2) | |
| DRV | 1 (0.1) |
a Nucleoside reverse transcriptase inhibitors, b Non-nucleoside reverse transcriptase inhibitors, c Protease inhibitors. *Free drugs recommended in National Free Antiretroviral Treatment Program (NFATP) in China.
3.3. Factors Associated with HIV Drug Resistance
To determine the factors associated with drug resistance, logistic regression analysis was conducted, and factors including age at diagnosis, sex, ethnicity, marital status, educational level, and route of transmission were examined. According to the univariate logistic regression model, three potential factors correlated with HIVDR (Table 3). In the multivariate model, the following two factors were independently correlated with PDR: the rate of PDR among IDUs was 1.52-fold higher than that among patients infected by heterosexual transmission (95% CI: 1.07-2.38; P=0.049); furthermore, the rate of PDR among patients diagnosed in 2017-18 was 2.03-fold higher than that among patients diagnosed in 2009-16 (95% CI: 1.18-5.76; P=0.018). In addition, an increased rate of PDR was found in other subtypes. Among the 6 PDR cases classified as other subtypes, 5 cases were 01_BC and 1 case was 07_08, and the PDR rates of 01_BC and 07_08 were 71.4% (5/7) and 12.5% (1/8), respectively; however, no significant effects from other factors were observed (P>0.05).
Table 3.
Factors associated with pretreatment HIVDR among HIV patients.
| Variable | Number | Drug Resistance, N (%) | Crude OR (95%CI) | P-value | Adjusted OR (95%CI) | P-value |
|---|---|---|---|---|---|---|
| Total | 1889 | 130 (6.9) | - | - | - | - |
| Age (years) | - | - | - | - | - | - |
| 16-29 | 705 | 47 (6.7) | 1.0 | - | - | - |
| 30-49 | 1018 | 71 (7.0) | 1.05 (0.72-1.54) | 0.804 | - | - |
| ≥50 | 162 | 12 (7.4) | 1.12 (0.58-2.16) | 0.736 | - | - |
| Sex | - | - | - | - | - | - |
| Male | 1044 | 84 (8.1) | 1.0 | - | - | - |
| Female | 837 | 46 (5.5) | 0.67 (0.46-0.96) | 0.031 | - | - |
| Ethnicity | - | - | - | - | - | - |
| Han | 155 | 12 (7.7) | 1.0 | - | - | - |
| Yi | 1559 | 104 (6.7) | 0.85 (0.46-1.59) | 0.613 | - | - |
| Others or unknown | 175 | 14 (8.0) | 1.04 (0.46-2.31) | 0.931 | - | - |
| Education | - | - | - | - | - | - |
| Illiterate | 907 | 57 (6.3) | 1.0 | - | - | - |
| Primary or Junior high school | 487 | 40 (8.2) | 1.34 (0.88-2.03) | 0.178 | - | - |
| Senior high school or higher | 45 | 4 (8.9) | 1.95 (0.50-4.21) | 0.489 | - | - |
| Unknown | 450 | 29 (6.4) | 1.03 (0.65-1.63) | 0.909 | - | - |
| Subtype | - | - | - | - | - | - |
| CRF07_BC | 1776 | 118 (6.6) | - | - | - | - |
| CRF08_BC | 44 | 6 (13.6) | 1.97 (0.76-5.13) | 0.076 | - | - |
| CRF01_AE | 37 | 0 (0.0) | - | 0.984 | - | - |
| Others | 32 | 6 (18.8) | 3.37 (1.36-8.34) | 0.001 | - | - |
| Route of transmission | - | - | - | - | - | - |
| Heterosexual transmission | 925 | 56(6.1) | 1.0 | - | - | - |
| IDU | 606 | 55 (9.1) | 1.49 (1.05-2.51) | 0.034 | 1.52 (1.07-2.38) | 0.049 |
| Homosexual transmission | 12 | 1 (8.3) | 1.36 (0.22-12.75) | 0.595 | - | - |
| Others or unknown | 346 | 20 (5.8) | 0.95 (0.56-1.67) | 0.384 | - | - |
| Marital status | - | - | - | - | - | - |
| Single | 303 | 14 (4.6) | 1.0 | - | - | - |
| Married | 1245 | 86 (6.9) | 1.53 (0.86-2.73) | 0.149 | - | - |
| Divorced/Widowed | 95 | 9 (9.5) | 2.16 (0.90-5.16) | 0.083 | - | - |
| Unknown | 246 | 21 (8.5) | 1.96 (0.98-3.94) | 0.059 | - | - |
| Sampling time | - | - | - | - | - | - |
| 2009-2016 | 537 | 22 (4.1) | 1.0 | - | - | - |
| 2017-2018 | 1352 | 108 (8.0) | 2.03 (1.27-3.26) | 0.003 | 2.03 (1.18-5.76) | 0.018 |
3.4. Drug Resistance-associated Genetic Transmission Networks Analysis
This study removed 12 sequences that had a pol region shorter than 900 bp and contained ≥5% ambiguities, leaving 1,877 sequences for genetic transmission network analysis. A genetic transmission network was constructed using a genetic distance threshold of 1.0% because this distance identifies the maximum number of clusters in the genetic network. In the analysis, 32.6% (611/1877) of people were identified in clusters based on <1.0% patristic distance. There were 193 clusters consisting of different sizes ranging from 2 to 59 individuals; 72.0% were in pairs (139/193), 25.4% (49/193) included 3–9 people, and 2.6% (5/193) were ≥10 people (Fig. 1). Twenty-six clusters contained at least one individual with HIVDR, including 5 (2.6%, 5/193), 19 (9.8%, 19/193) and 1 (0.5%, 1/193) for NRTIs, NNRTIs and PIs, respectively. In addition, 1 cluster contained one individual harboring dual classes of HIVDR, which conferred resistance to both NRTIs and PIs (Fig. 1). No significant difference was observed in the clustering rate between the individuals with and without HIVDR (25.6% vs. 33.1%; P=0.082). Similarly, factors such as sex, mode of transmission, education level and ethnicity were not significantly correlated with access to the genetic transmission network (P>0.05). However, individuals infected with HIV in Xichang were more likely to enter the network cluster than those in other regions (40.6% vs. 29.3%; P=0.015).
Fig. (1).
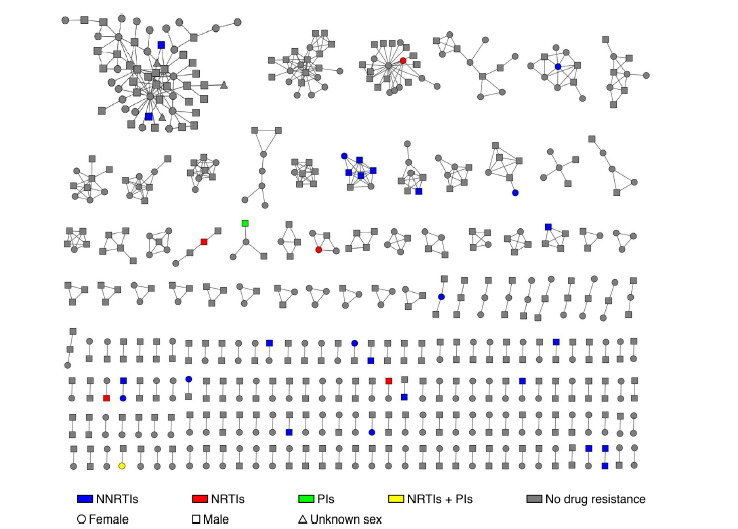
Pretreatment HIVDR and sex in the genetic transmission network. Colored nodes represent PDR, and different colors represent different categories of drugs (blue: NNRTIs, red: NRTIs, green: PIs, and yellow: NRTIs and PIs). Circles represent females, squares are males and triangles are patients whose sex was unknown.
To identify clusters that were rapidly growing, two genetic transmission networks were also constructed for HIV-infected people diagnosed from 2009-2016 and 2009-2017. In the three transmission networks (2009-2016, 2009-2017 and 2009-2018), there were 4 (9.1%, 4/44), 15 (12.9%, 15/116) and 26 (13.5%, 26/193) transmission clusters containing drug resistance mutations, respectively (Fig. 2). Among them, the most common drug resistance mutation was K103N, and a total of 12 HIV infection patients contained this drug resistance mutation and were distributed in 10 transmission clusters. From 2016 to 2018, most of the clusters containing drug resistance mutations did not show rapid growth within the cluster, but one cluster formed in 2017 contained three HIV-infected people carrying E138Q and V179D drug resistance mutations. In 2018, this cluster grew to contain 7 HIV-infected people, and five of them harbored the E138Q and V179D drug resistance mutations. These five cases contained the E138Q and V179D resistance mutations, leading to low-level resistance to EFV and NVP. The other two cases also contained the V179D locus but resulted only in potential resistance to EFV and NVP. Of those cases, five were in Xichang, one was in Meigu and one was in Butuo; four individuals were men infected by heterosexual transmission, two individuals were women infected by heterosexual transmission and one individual was a man infected with intravenous drug use.
Fig. (2).
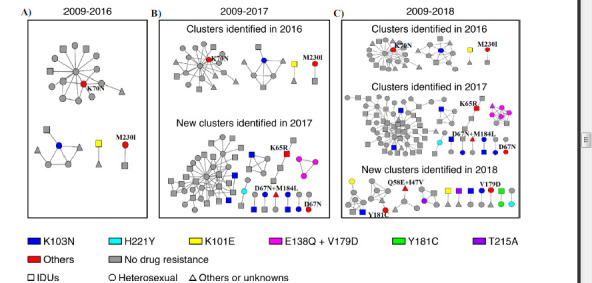
Growth of clusters with drug resistance mutations by the year of sampling. Three genetic transmission networks were constructed for HIV-infected people diagnosed from 2009-2016 (Fig. 2A), 2009-2017 (Fig. 2B) and 2009-2018 (Fig. 2C). Only transmission clusters containing drug resistance mutations are shown in the figures. Different colors represent different mutations, and red represents other mutations that appeared only once in the network; we labeled them beside the nodes in the figures. Circles represent heterosexual transmission, squares are IDUs and triangles are other methods or unknown. As shown in Fig. (2B), a cluster contained three nodes all carrying E138Q and V179D drug resistance mutations formed in 2017 and grew to contain 7 nodes (Fig. 2C); in 2018, five harbored the mutations, which are marked in pink.
4. DISCUSSION
In the present study, we analyzed the HIV-1 pol gene sequences of 1889 patients with newly diagnosed HIV-1 infection who were treatment-naïve in Liangshan from 2009-2018. The overall prevalence of PDR among the studied samples was 6.9%, and PDR in three years between 2016-2018 achieved a moderate level (5%-15%) according to the WHO categorization method [33]. The HIVDR rate was higher than that of previous studies in Liangshan [44] and other regions in China [38, 45]. However, the resistance rate was lower than those in Kenya and Mexico, where PDR rates were reported to be 21.9% and 15.5% [46, 47], respectively. Many factors, including poor compliance with medication and lack of testing for baseline resistance [48, 49], may be among the major reasons for the increase in PDR in Liangshan. Several studies have shown that HIV-1-infected individuals with drug resistance can affect treatment responses to first-line ARV therapies [35, 50-52]. PDR certainly reduced the effect of antiviral therapy in Liangshan, therefore, it is necessary to pay attention to the obstacle caused by PDR in the prevention and control of HIV epidemics.
The most common drug resistance mutations detected in the current study were against NNRTIs, and this finding is consistent with the widespread use of this drug class as part of China’s National ART Guidelines as the standard first-line ART regimen [53, 54]. All first-line regimens contain three antiviral agents, including two NRTIs and one NNRTI. When the first-line treatment fails, the second-line regimen, which consists of PIs, NRTIs and NNRTIs [55], is adopted. The more widespread and longer use of NRTIs and NNRTIs may be the major cause of the higher prevalence of resistance to NNRTI than to PI in Liangshan. EFV and NVP, which are both NNRTIs, had the highest drug resistance rate in this study which was mainly caused by K103N, V179D, V106M and E138G/Q mutations [56, 57]. NVP and EFV both have some adverse effects [58-60], but studies showed that they had an effective viral suppression effect in the treatment of HIV-infected individuals [61, 62], and the 2015 Guideline for HIV/AIDS Treatment in China recommended first-line ART drugs containing NVP or EFV. Given the high rates of resistance to these drugs, drug resistance monitoring is critical.
However, it was found that the PDR rate was higher in those infected by injectable drug use. It has been shown that high infection risk behaviors, such as sharing syringes and needles, as well as participating in unprotected sexual intercourse, are frequent among IDUs [63, 64]. Given this possibility, the epidemiological linkage of the study participants may have increased the rate of drug resistance detected in this study. In addition, the PDR rate of patients diagnosed from 2017-2018 in this study was higher than that of patients diagnosed earlier. This result is mainly due to the increasing coverage of antiviral treatment in the region and the increasing number of people receiving antiviral treatment. This result has also been confirmed in the drug resistance monitoring results carried out in other regions of China, where the drug resistance rate increased with time [21, 23, 37]. In our study, there was no association between age, sex, education and CD4 count with PDR, similar to other studies [65, 66]. It was also found that the PDR rate did not significantly differ among the three main subtypes (CRF07_BC, CRF08_BC and CRF01_AE); however, in a previous study, HIV-1 CRF07_BC showed distinctive resistance evolution pathways in which the mutations K103N, Q197K, V179D and Y188L were the major resistance mutations [15]. Interestingly, the PDR rate is higher in other subtypes, especially in subtypes 01_BC and 07_08, and these recombinant subtypes require further study and sustained attention.
This study used the maximum gene distance threshold to construct a transmission network. The results of a study in New York showed that the transmission relationship in the genetic transmission network established by this method was consistent with that in the actual investigation [43]. In our study, the region was the only factor that affected the clustering rate, and the clustering rate of individuals in Xichang, the capital city of Liangshan Prefecture, was increased. This result may be due to enhanced economic development and transportation level of Xichang and to the high number of migrants. As a result, individuals in Xichang were likely to link to one another in the transmission network, similar to the results of other studies [67, 68]. Interestingly, there was no significant correlation between the transmission route and the clustering rate. The main reason, in our assessment, is that the line between those infected by injectable drug use and those infected by heterosexual transmission is gradually blurring in Liangshan [69, 70]. HIV has been transmitted from IDUs to the general population through heterosexual intercourse, and the HIV strains in the two groups are closely related to each other. Therefore, two groups appeared to be interconnected in the genetic transmission network, and there is no significant difference in the clustering rate between the two groups.
However, drug resistance and clustering in the transmission network were independent of each other, which is consistent with the results of a study in Shijiazhuang [71]. The clusters containing drug-resistant individuals in the genetic transmission network accounted for 13.5% of the study population, and the proportion increased over time, which may be caused by an increase in the number of drug-resistant individuals over time. The main reason that drug resistance did not lead to an increase in the clustering rate may be that the transmission capacity of drug-resistant strains is less than that of nonresistant strains [72-74]. Second, some newly diagnosed HIV infections included in this study were not newly acquired, and the nonresistant strains became the dominant strains in their patients’ bodies [75], making it impossible to detect drug resistance mutations. In addition, the study identified a rapidly growing drug resistance-related cluster from 2017 to 2018, which contains the V179D mutation or E138Q and V179D mutations. E138Q is a nonpolymorphic accessory mutation, and in most studies, it caused low-level reductions in susceptibility to NVP, RPV, EFV and ETR [76]. Since NVP and EFV are first-line antiviral drugs in the region, attention should be paid to the antiviral treatment of patients in this cluster. In order to prevent the spread of the drug-resistant strain in the region, interventions are necessary for those individuals.
This study also provided the latest update on the molecular diversity of HIV-1 among HIV-infected patients in Liangshan. The results showed that CRF07_BC remained the major circulating recombinant form, consistent with previous studies in Liangshan [44, 77]. In 1989, the first HIV outbreak among IDUs was reported in Yunnan, a southwestern province bordering Burma (Myanmar) [78], and the CRF07_BC strains of Liangshan were introduced from Yunnan Province [44]. There was no significant change in the HIV-1 subtype distribution, suggesting that the transmission of HIV in this region is common. However, recombinant subtype strains that were not found in previous studies, such as 01_BC, also appeared. Continued studies on the source of the transmission of these new recombinant strains and their characteristics are warranted.
Our study has several limitations. First, our study did not include individuals under 18 years of age; thus, the prevalence of PDR in this population is not known. Although we selected the samples through random sampling, we obtained only a portion of samples, which had a certain impact on the representativeness of samples in our study. Second, we used serum remaining from patients’ HIV diagnostic tests for PDR surveillance; these specimens may not be representative of drug resistance at the time of treatment. In another study, there was no significant difference in the identified TDR transmitted drug resistance (TDR) between serum collected from diagnostics and baseline clinical samples [79]. However, further studies may be needed to compare these differences in PDR.
CONCLUSION
In summary, the study showed that the major circulating recombinant form was CRF07_BC and that there was a moderate PDR level among pretreatment HIV patients in Liangshan. The prevalence of PDR increased over time and was higher in IDUs than in other groups. PDR was identified in a genetic transmission network, and a rapidly growing drug resistance-related cluster was identified. These findings provide important information about the prevalence of PDR and the subtype distribution and may help guide the choice of ART regimens to ensure the effectiveness of ART in preventing the spread of HIV.
ACKNOWLEDGEMENTS
Data in this manuscript were collected by the Liangshan Center for Disease Control and Prevention and Sichuan Center for Disease Control and Prevention.
LIST OF ABBREVIATIONS
- AIDS
Acquired Immune Deficiency Syndrome
- ART
Antiretroviral Treatment
- ARV
Antiretroviral
- CDC
Center for Disease Control and Prevention
- CRFs
Circulating Recombinant Forms
- HIV
Human Immunodeficiency Virus
- HIVDR
HIV Drug Resistance
- IDUs
Intravenous Drug Users
- NCAIDS
National Center for AIDS/STD Control and Prevention
- NNRTIs
Non-Nucleoside Reverse Transcriptase Inhibitors
- NRTIs
Nucleoside Reverse Transcriptase Inhibitors
- PCR
Polymerase Chain Reaction
- PDR
Pretreatment Drug Resistance
- PIs
Protease Inhibitors
- SD
Standard Deviation
- WHO
World Health Organization
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Institutional review board approval was granted by the National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention (X140617334).
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
All the participants enrolled for the study provided signed informed consent.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and analyzed during the current study are available from the corresponding author [YR] on reasonable request.
FUNDING
This study was supported by the Ministry of Science and Technology of China (Grant No. 2017ZX10201101), the Liang-shan Prefecture AIDS Conquering Project and the National Natural Science Foundation of China (Grant No. 11971479).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
AUTHORS' CONTRIBUTIONS
Y.R., H.X., Y.F., L.L. and Y.S. conceived and designed the study. L.L. and A.D. performed the experiments and analyzed the data. L.S. performed the sample collection and the epidemiological survey. L.L., Y.R. and Y.F. interpreted the data and provided a critical review. L.L. drafted the manuscript. All authors reviewed and approved the final manuscript.
REFERENCES
- 1.UNAIDS. Global HIV & AIDS statistics-2018 fact sheet. https://www.unaids.org/en/resources/fact-sheet [Accessed 15 Jan 2019]
- 2.van Zyl G.U., Bedison M.A., van Rensburg A.J., Laughton B., Cotton M.F., Mellors J.W. Early Antiretroviral Therapy in South African Children Reduces HIV-1-Infected Cells and Cell-Associated HIV-1 RNA in Blood Mononuclear Cells. J. Infect. Dis. 2015;212(1):39–43. doi: 10.1093/infdis/jiu827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sempa J.B., Rossouw T.M., Lesaffre E., Nieuwoudt M. Cumulative viral load as a predictor of CD4+ T-cell response to antiretroviral therapy using Bayesian statistical models. PLoS One. 2019;14(11): e0224723. doi: 10.1371/journal.pone.0224723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pham H.V., Ishizaki A., Nguyen L.V., et al. Two-year outcome of first-line antiretroviral therapy among HIV-1 vertically-infected children in Hanoi, Vietnam. Int. J. STD AIDS. 2015;26(11):821–830. doi: 10.1177/0956462414556328. [DOI] [PubMed] [Google Scholar]
- 5.Murphy E.L., Collier A.C., Kalish L.A., et al. Viral Activation Transfusion Study Investigators. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. [J] Ann. Intern. Med. 2001;135(1):17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 6.Tersmette M., Lange J.M., de Goede R.E., et al. Association between biological properties of human immunodeficiency virus variants and risk for AIDS and AIDS mortality. Lancet. 1989;1(8645):983–985. doi: 10.1016/S0140-6736(89)92628-7. [DOI] [PubMed] [Google Scholar]
- 7.Braitstein P., Brinkhof M.W., Dabis F., et al. Antiretroviral Therapy in Lower Income Countries (ART-LINC) Collaboration. ART Cohort Collaboration (ART-CC) groups. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367(9513):817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 8.Alibhai A., Martin L.J., Kipp W., et al. Quality of life of HIV patients in a rural area of western Uganda: impact of a community-based antiretroviral treatment program. Curr. HIV Res. 2010;8(5):370–378. doi: 10.2174/157016210791330400. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization(WHO) Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. https://www.who.int/hiv/pub/arv/arv-2016/en/ [Accessed 20 May 2019]
- 10.Hemelaar J. The origin and diversity of the HIV-1 pandemic. Trends Mol. Med. 2012;18(3):182–192. doi: 10.1016/j.molmed.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Abram M.E., Ferris A.L., Shao W., Alvord W.G., Hughes S.H. Nature, position, and frequency of mutations made in a single cycle of HIV-1 replication. J. Virol. 2010;84(19):9864–9878. doi: 10.1128/JVI.00915-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perelson A.S., Ribeiro R.M. Modeling the within-host dynamics of HIV infection. BMC Biol. 2013;11:96. doi: 10.1186/1741-7007-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richman D.D., Little S.J., Smith D.M., Wrin T., Petropoulos C., Wong J.K. HIV evolution and escape. Trans. Am. Clin. Climatol. Assoc. 2004;115:289–303. [PMC free article] [PubMed] [Google Scholar]
- 14.Clutter D.S., Jordan M.R., Bertagnolio S., Shafer R.W. HIV-1 drug resistance and resistance testing. Infect. Genet. Evol. 2016;46:292–307. doi: 10.1016/j.meegid.2016.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips A.N., Cambiano V., Miners A., et al. Effectiveness and cost-effectiveness of potential responses to future high levels of transmitted HIV drug resistance in antiretroviral drug-naive populations beginning treatment: modelling study and economic analysis. Lancet HIV. 2014;1(2):e85–e93. doi: 10.1016/S2352-3018(14)70021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamers R.L., Wallis C.L., Kityo C., et al. PharmAccess African Studies to Evaluate Resistance (PASER). HIV-1 drug resistance in antiretroviral-naive individuals in sub-Saharan Africa after rollout of antiretroviral therapy: a multicentre observational study. Lancet Infect. Dis. 2011;11(10):750–759. doi: 10.1016/S1473-3099(11)70149-9. [DOI] [PubMed] [Google Scholar]
- 17.Cambiano V., Bertagnolio S., Jordan M.R., Lundgren J.D., Phillips A. Transmission of drug resistant HIV and its potential impact on mortality and treatment outcomes in resource-limited settings. J. Infect. Dis. 2013;207(Suppl. 2):S57–S62. doi: 10.1093/infdis/jit111. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization(WHO) Guidelines on the public health response to pretreatment HIV drug resistance. https://www. who.int/hiv/pub/guidelines/hivdr-guidelines-2017/en/ [Accessed 7 Dec 2018]
- 19.Ma Y., Zhang F., Zhao Y., et al. Cohort profile: the Chinese national free antiretroviral treatment cohort. Int. J. Epidemiol. 2010;39(4):973–979. doi: 10.1093/ije/dyp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang F, Dou Z, Ma Y, et al. Five-year outcomes of the China National Free Antiretroviral Treatment Program. Ann Intern Med. 2009;151(4):241–251, w-252. doi: 10.7326/0003-4819-151-4-200908180-00006. [DOI] [PubMed] [Google Scholar]
- 21.Liu H., Ma Y., Su Y., et al. Emerging trends of HIV drug resistance in Chinese HIV-infected patients receiving first-line highly active antiretroviral therapy: a systematic review and meta-analysis. Clin. Infect. Dis. 2014;59(10):1495–1502. doi: 10.1093/cid/ciu590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang F.J., Maria A., Haberer J., Zhao Y. Overview of HIV drug resistance and its implications for China. Chin. Med. J. (Engl.) 2006;119(23):1999–2004. doi: 10.1097/00029330-200612010-00010. [DOI] [PubMed] [Google Scholar]
- 23.Liao L., Xing H., Dong Y., et al. Surveys of transmitted HIV drug resistance in 7 geographic Regions in China, 2008-2009. Clin. Infect. Dis. 2012;54(Suppl. 4):S320–S323. doi: 10.1093/cid/cir1016. [DOI] [PubMed] [Google Scholar]
- 24.Zhang F., Liu L., Sun M., Sun J., Lu H. An analysis of drug resistance among people living with HIV/AIDS in Shanghai, China. PLoS One. 2017;12(2): e0165110. doi: 10.1371/journal.pone.0165110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y., Peng X., Peng X., et al. Characterization of HIV-1 subtypes and transmitted drug resistance among treatment-naive HIV-infected individuals in Zhejiang, China, 2014-2017. Arch. Virol. 2018;163(8):2233–2237. doi: 10.1007/s00705-018-3839-1. [DOI] [PubMed] [Google Scholar]
- 26.Yang S., Yang C., Liao Q., et al. Analysis of HIV prevalence among pregnant women in Liangshan Prefecture, China, from 2009 to 2015. PLoS One. 2017;12(9): e0183418. doi: 10.1371/journal.pone.0183418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L., Luan R., Yang W., et al. Projecting dynamic trends for HIV/AIDS in a highly endemic area of China: estimation models for Liangshan Prefecture, Sichuan Province. Curr. HIV Res. 2009;7(4):390–397. doi: 10.2174/157016209788680642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su Z., Dong C., Li P., et al. A mathematical modeling study of the HIV epidemics at two rural townships in the Liangshan Prefecture of the Sichuan Province of China. Infect. Dis. Model. 2016;1(1):3–10. doi: 10.1016/j.idm.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin Y., Yang H., Xie X., Wang H., Nie A., Chen H. Status and associated characteristics of HIV disclosure among people living with HIV/AIDS in Liangshan, China: A cross-sectional study. Medicine (Baltimore) 2019;98(31): e16681. doi: 10.1097/MD.0000000000016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beyrer C., Razak M.H., Lisam K., Chen J., Lui W., Yu X.F. Overland heroin trafficking routes and HIV-1 spread in south and south-east Asia. AIDS. 2000;14(1):75–83. doi: 10.1097/00002030-200001070-00009. [DOI] [PubMed] [Google Scholar]
- 31.Ruan Y., Qin G., Liu S., et al. HIV incidence and factors contributed to retention in a 12-month follow-up study of injection drug users in Sichuan Province, China. J. Acquir. Immune Defic. Syndr. 2005;39(4):459–463. doi: 10.1097/01.qai.0000152398.47025.0f. [DOI] [PubMed] [Google Scholar]
- 32.Ruan Y., Chen K., Hong K., et al. Community-based survey of HIV transmission modes among intravenous drug users in Sichuan, China. Sex. Transm. Dis. 2004;31(10):623–627. doi: 10.1097/01.olq.0000140018.24262.4a. [DOI] [PubMed] [Google Scholar]
- 33.Jordan M.R., Bennett D.E., Wainberg M.A., et al. Update on World Health Organization HIV drug resistance prevention and assessment strategy: 2004-2011. Clin. Infect. Dis. 2012;54(Suppl. 4):S245–S249. doi: 10.1093/cid/cis206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao Y.R., Wu Z.Y., Poundstone K., et al. Development of a unified web-based national HIV/AIDS information system in China. Int. J. Epidemiol. 2010;39(Suppl. 2):ii79–ii89. doi: 10.1093/ije/dyq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao L., Xing H., Su B., et al. Impact of HIV drug resistance on virologic and immunologic failure and mortality in a cohort of patients on antiretroviral therapy in China. AIDS. 2013;27(11):1815–1824. doi: 10.1097/QAD.0b013e3283611931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xing H., Ruan Y., Hsi J.H., et al. National HIVDR Working Group. Reductions in virological failure and drug resistance in Chinese antiretroviral-treated patients due to lamivudine-based regimens, 2003-12. J. Antimicrob. Chemother. 2015;70(7):2097–2103. doi: 10.1093/jac/dkv078. [DOI] [PubMed] [Google Scholar]
- 37.Liao L., Xing H., Shang H., et al. The prevalence of transmitted antiretroviral drug resistance in treatment-naive HIV-infected individuals in China. J. Acquir. Immune Defic. Syndr. 2010;53(Suppl. 1):S10–S14. doi: 10.1097/QAI.0b013e3181c7d363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao S., Feng Y., Hu J., et al. Prevalence of Transmitted HIV drug resistance in antiretroviral treatment naïve newly diagnosed individuals in China. Sci. Rep. 2018;8(1):12273. doi: 10.1038/s41598-018-29202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong P., Pan Q., Ning Z., et al. Genetic diversity and drug resistance of human immunodeficiency virus type 1 (HIV-1) strains circulating in Shanghai. AIDS Res. Hum. Retroviruses. 2007;23(7):847–856. doi: 10.1089/aid.2006.0196. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization(WHO) World Health Organization. Surveillance of HIV drug resistance in adults initiating antiretroviral therapy (pretreatment HIV drug resistance) Concept note Geneva. 2014 http://www.who.int/hiv/pub/drugresistance/pretreatment_drugresistance/en [Accessed 16 Jan 2019]
- 41.Geneva: World Health Organization. World Health Organization(WHO) Guidelines on the public health response to pretreatment HIV drug resistance. 2017. Licence: CC BY-NC-SA 3.0 IGO.
- 42.Tamura K., Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993;10(3):512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 43.Wertheim J.O., Kosakovsky Pond S.L., Forgione L.A., et al. Social and Genetic Networks of HIV-1 Transmission in New York City. PLoS Pathog. 2017;13(1): e1006000. doi: 10.1371/journal.ppat.1006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li L., Wei D., Hsu W.L., et al. CRF07_BC Strain Dominates the HIV-1 Epidemic in Injection Drug Users in Liangshan Prefecture of Sichuan, China. AIDS Res. Hum. Retroviruses. 2015;31(5):479–487. doi: 10.1089/aid.2014.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hua J., Lin H., Ding Y., Qiu D., Wong F., He N. HIV drug resistance in newly diagnosed adults in a rural prefecture of eastern China. Epidemiol. Infect. 2015;143(3):663–672. doi: 10.1017/S0950268814001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silverman R.A., Beck I.A., Kiptinness C., et al. Prevalence of Pre-antiretroviral-Treatment Drug Resistance by Gender, Age, and Other Factors in HIV-Infected Individuals Initiating Therapy in Kenya, 2013-2014. J. Infect. Dis. 2017;216(12):1569–1578. doi: 10.1093/infdis/jix544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ávila-Ríos S., García-Morales C., Matías-Florentino M., et al. HIVDR MexNet Group. Pretreatment HIV-drug resistance in Mexico and its impact on the effectiveness of first-line antiretroviral therapy: a nationally representative 2015 WHO survey. Lancet HIV. 2016;3(12):e579–e591. doi: 10.1016/S2352-3018(16)30119-9. [DOI] [PubMed] [Google Scholar]
- 48.Yang S., Zhai W., Pei R., et al. Factors associated with HIV infection among Yi minority residents in Liangshan Prefecture, Sichuan Province: A path analysis. Medicine (Baltimore) 2018;97(14): e0250. doi: 10.1097/MD.0000000000010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H., Chen A.C., Wan S., Chen H. Status and associated factors of self-management in people living with HIV/AIDS in Liangshan area, China: a cross-sectional study. Patient Prefer. Adherence. 2019;13:863–870. doi: 10.2147/PPA.S203799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Epstein B.J. Drug resistance among patients recently infected with HIV. N. Engl. J. Med. 2002;347(23):1889–1890. doi: 10.1056/NEJM200212053472315. [DOI] [PubMed] [Google Scholar]
- 51.Lai C.C., Hung C.C., Chen M.Y., et al. Trends of transmitted drug resistance of HIV-1 and its impact on treatment response to first-line antiretroviral therapy in Taiwan. J. Antimicrob. Chemother. 2012;67(5):1254–1260. doi: 10.1093/jac/dkr601. [DOI] [PubMed] [Google Scholar]
- 52.Li J.F., Linley L., Kline R., Ziebell R., Heneine W., Johnson J.A. Sensitive sentinel mutation screening reveals differential underestimation of transmitted HIV drug resistance among demographic groups. AIDS. 2016;30(9):1439–1445. doi: 10.1097/QAD.0000000000001099. [DOI] [PubMed] [Google Scholar]
- 53.Zhang F.J., Pan J., Yu L., Wen Y., Zhao Y. Current progress of China’s free ART program. Cell Res. 2005;15(11-12):877–882. doi: 10.1038/sj.cr.7290362. [DOI] [PubMed] [Google Scholar]
- 54.Luo L., Li T.S. Overview of antiretroviral treatment in China: advancement and challenges. Chin. Med. J. (Engl.) 2011;124(3):440–444. [PubMed] [Google Scholar]
- 55.Liu P., Liao L., Xu W., et al. Adherence, virological outcome, and drug resistance in Chinese HIV patients receiving first-line antiretroviral therapy from 2011 to 2015. Medicine (Baltimore) 2018;97(50): e13555. doi: 10.1097/MD.0000000000013555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mendoza Y., Castillo Mewa J., Martínez A.A., et al. HIV-1 Antiretroviral Drug Resistance Mutations in Treatment Naïve and Experienced Panamanian Subjects: Impact on National Use of EFV-Based Schemes. PLoS One. 2016;11(4): e0154317. doi: 10.1371/journal.pone.0154317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo X.L., Mo L.D., Su G.S., et al. Incidence and types of HIV-1 drug resistance mutation among patients failing first-line antiretroviral therapy. J. Pharmacol. Sci. 2019;139(4):275–279. doi: 10.1016/j.jphs.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 58.Shikuma C.M., Kohorn L., Paul R., et al. Sleep and neuropsychological performance in HIV+ subjects on efavirenz-based therapy and response to switch in therapy. HIV Clin. Trials. 2018;19(4):139–147. doi: 10.1080/15284336.2018.1511348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shubber Z., Calmy A., Andrieux-Meyer I., et al. Adverse events associated with nevirapine and efavirenz-based first-line antiretroviral therapy: a systematic review and meta-analysis. AIDS. 2013;27(9):1403–1412. doi: 10.1097/QAD.0b013e32835f1db0. [DOI] [PubMed] [Google Scholar]
- 60.Avihingsanon A., Maek-A-Nantawat W., Gatechompol S., et al. Efficacy and safety of a once-daily single-tablet regimen of tenofovir, lamivudine, and efavirenz assessed at 144 weeks among antiretroviral-naïve and experienced HIV-1-infected Thai adults. Int. J. Infect. Dis. 2017;61:89–96. doi: 10.1016/j.ijid.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 61.Larru B., Eby J., Lowenthal E.D. Antiretroviral treatment in HIV-1 infected pediatric patients: focus on efavirenz. Pediatric Health Med. Ther. 2014;5:29–42. doi: 10.2147/PHMT.S47794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kiselinova M., Geretti A.M., Malatinkova E., et al. HIV-1 RNA and HIV-1 DNA persistence during suppressive ART with PI-based or nevirapine-based regimens. J. Antimicrob. Chemother. 2015;70(12):3311–3316. doi: 10.1093/jac/dkv250. [DOI] [PubMed] [Google Scholar]
- 63.Ruan Y., Liang S., Zhu J., et al. Gender and ethnic disparities of HIV and syphilis seroconversions in a 4-year cohort of injection drug users. Southeast Asian J. Trop. Med. Public Health. 2013;44(5):842–853. [PubMed] [Google Scholar]
- 64.Qian H.Z., Schumacher J.E., Chen H.T., Ruan Y.H. Injection drug use and HIV/AIDS in China: review of current situation, prevention and policy implications. Harm Reduct. J. 2006;3:4. doi: 10.1186/1477-7517-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mungati M., Mhangara M., Gonese E., et al. Pre-treatment drug resistance among patients initiating antiretroviral therapy (ART) in Zimbabwe: 2008-2010. BMC Res. Notes. 2016;9:302. doi: 10.1186/s13104-016-2101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hunt G.M., Coovadia A., Abrams E.J., et al. HIV-1 drug resistance at antiretroviral treatment initiation in children previously exposed to single-dose nevirapine. AIDS. 2011;25(12):1461–1469. doi: 10.1097/QAD.0b013e3283492180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Su L., Liang S., Hou X., et al. Impact of worker emigration on HIV epidemics in labour export areas: a molecular epidemiology investigation in Guangyuan, China. Sci. Rep. 2018;8(1):16046. doi: 10.1038/s41598-018-33996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li X., Gao R., Zhu K., et al. Genetic transmission networks reveal the transmission patterns of HIV-1 CRF01_AE in China. Sex. Transm. Infect. 2018;94(2):111–116. doi: 10.1136/sextrans-2016-053085. [DOI] [PubMed] [Google Scholar]
- 69.Liu S., Wang Q.X., Nan L., et al. The changing trends of HIV/AIDS in an ethnic minority region of China: modeling the epidemic in Liangshan prefecture, Sichuan Province. Biomed. Environ. Sci. 2013;26(7):562–570. doi: 10.3967/0895-3988.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 70.Yang Y., Luan R.S., Liu P., Wu C.L., Zhou Y., Chen W. Casual sex and concurrent sexual partnerships among young people from an Yi community with a high prevalence of HIV in China. Asian J. Androl. 2012;14(5):758–765. doi: 10.1038/aja.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang X., Liu X., Li F., et al. Epidemiological surveillance of HIV-1 transmitted drug resistance among newly diagnosed individuals in Shijiazhuang, northern China, 2014-2015.[J]. PLoS One. 2018;13(6): e0198005. doi: 10.1371/journal.pone.0198005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pollicita M., Surdo M., Di Santo F., et al. Comparative replication capacity of raltegravir-resistant strains and antiviral activity of the new-generation integrase inhibitor dolutegravir in human primary macrophages and lymphocytes. J. Antimicrob. Chemother. 2014;69(9):2412–2419. doi: 10.1093/jac/dku144. [DOI] [PubMed] [Google Scholar]
- 73.Buckheit R.W., Jr Understanding HIV resistance, fitness, replication capacity and compensation: targeting viral fitness as a therapeutic strategy. Expert Opin. Investig. Drugs. 2004;13(8):933–958. doi: 10.1517/13543784.13.8.933. [DOI] [PubMed] [Google Scholar]
- 74.Hofstra L.M., Nijhuis M., Pingen M., et al. Evolution and viral characteristics of a long-term circulating resistant HIV-1 strain in a cluster of treatment-naive patients. J. Antimicrob. Chemother. 2013;68(6):1246–1250. doi: 10.1093/jac/dkt038. [DOI] [PubMed] [Google Scholar]
- 75.Castro H., Pillay D., Cane P., et al. UK Collaborative Group on HIV Drug Resistance. Persistence of HIV-1 transmitted drug resistance mutations. J. Infect. Dis. 2013;208(9):1459–1463. doi: 10.1093/infdis/jit345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tambuyzer L., Nijs S., Daems B., Picchio G., Vingerhoets J. Effect of mutations at position E138 in HIV-1 reverse transcriptase on phenotypic susceptibility and virologic response to etravirine. J. Acquir. Immune Defic. Syndr. 2011;58(1):18–22. doi: 10.1097/QAI.0b013e3182237f74. [DOI] [PubMed] [Google Scholar]
- 77.Meng Z., Xin R., Abubakar Y.F., et al. Five new CRF07_BC near full-length sequences isolated from Sichuan, China. AIDS Res. Hum. Retroviruses. 2013;29(1):191–197. doi: 10.1089/aid.2012.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma Ye L.Z., Zhang K.X. Identification of HIV infection among drug users in China. Zhonghua Liu Xing Bing Xue Za Zhi. 1990;11:184–185. [Google Scholar]
- 79.Ragonnet-Cronin M., Lee B.E., Plitt S.S., et al. Baseline clinical HIV genotypes are a valid measure of transmitted drug resistance within the treatment-naive population. J. Acquir. Immune Defic. Syndr. 2013;64(5):443–447. doi: 10.1097/QAI.0b013e3182a4b991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author [YR] on reasonable request.


