Abstract
Objective
Early-stage ovarian cancer might represent an ideal disease scenario for sentinel lymph node application. Nevertheless, the published experience seems to be limited. Our objective was to assess the feasibility and safety concerns of sentinel lymph node biopsy in patients with clinical stage I–II ovarian cancer.
Methods
We conducted a prospective cohort study of 20 patients with histologically confirmed ovarian cancer. 99mTc and indocyanine green were injected into both the utero-ovarian and infundibulopelvic ligament stump, if they were present, during surgical staging. An intraoperative gamma probe and near-infrared fluorescence imaging were used to detect the sentinel lymph nodes. Inclusion criteria included: >18 years of age, suspicious adnexal mass (unilateral or bilateral) at ultrasound and CT imaging or confirmed ovarian tumor after previous surgery (unilateral or bilateral salpingo-oophorectomy with or without hysterectomy). Adverse events were recorded through postoperative day 30. The primary trial end point was to report adverse events related to the technique, including the use of 99mTc and ICG intraperitoneally, as well as the feasibility of the technique.
Results
A total of 20 patients were included in the analysis. Sentinel lymph nodes were detected in 14/15 (93%) pelvic and all 20 (100%) para-aortic regions. Five patients did not have utero-ovarian injection because of prior hysterectomy. The mean time from injection to sentinel lymph node resection was 53±15 min (range; 30–80). The mean number of harvested sentinel lymph nodes was 2.2±1.5 (range; 0–5) lymph nodes in the pelvis and 3.3±1.8 (range; 1–7) lymph nodes in the para-aortic region. There were no adverse intraoperative events, nor any within the 30 days of follow-up related with the technique.
Conclusion
Sentinel lymph node mapping in early-stage ovarian cancer is feasible without major intraoperative or < 30 days safety concerns. (NCT03452982).
Trial registration number
ClinicalTrials.gov, NCT03452982.
Keywords: sentinel lymph node, ovarian cancer, surgical oncology
HIGHLIGHTS.
99mTc and indocyanine green were used to detect the sentinel lymph nodes (SLN) in patients with early ovarian cancer.
SLN were detected in 14/15 (93%) pelvic and all 20 (100%) para-aortic regions.
There were no intraoperative or postoperative adverse events within 30 days of follow-up.
Introduction
Ovarian cancer is diagnosed as an early disease that is limited to the pelvis in up to 20% of cases.1 2 Once histologically confirmed, comprehensive surgical staging is recommended to accurately determine the extent of disease and guide postoperative treatment planning.3 Lymphadenectomy is a time consuming and complex procedure that requires advanced surgical skills to achieve an appropriate extended dissection and a safe result.4 Moreover, it is associated with both intraoperative and post-operative morbidity, even when performed by an expert gynecologic oncologist.5 6 Knowledge of the lymph node status determines the prognosis and helps to tailor adjuvant systemic treatment.7 8 Nevertheless, in cases of low-grade ovarian cancer, the incidence of metastasis was recently reported to be rather low.9 10 Omission of, or inadequately performed lymphadenectomy, may lead to understaged disease or even the exclusion of adjuvant chemotherapy. Notwithstanding, there is no demonstrated benefit from lymphadenectomy in terms of survival in patients with apparent early stage ovarian cancer11
Sentinel lymph node mapping is intended to detect the first at-risk node that drains from a tumor. When performed correctly, the absence of a metastasis within the sentinel lymph node predicts that the remaining lymph nodes in a certain anatomical region are not involved. Consequently, the more extensive lymphadenectomy, and thus the associated morbidity, can be avoided. Sentinel lymph node mapping has become the standard of care in breast and vulvar cancer,12 13 and in patients with cervical and endometrial cancers is gaining significant popularity.14 15
Early-stage ovarian cancer may represent an ideal disease scenario for sentinel lymph node application. Lymphadenectomy is a demanding procedure associated with morbidity that is unnecessary in a high percentage of patients due to the low incidence of lymph node involvement. Nevertheless, the technical complexity and risk of tumor dissemination associated with the injection of tracers into the cortex16 17 have limited the published experience to few studies with a small sample of patients, most not involving malignant ovarian tumors.16–23 The objective of this study was to prospectively delineate any major safety concerns related to our proposed sentinel lymph node technique and assess its feasibility in patients with clinical stage I–II ovarian cancer.
Methods
We performed a prospective, single-center trial from March 2018 until July 2019. The study protocol was approved by the institutional review board and local ethics committee before enrollment and was registered on http://clinicaltrials.gov (NCT03452982). Patients with suspicious or confirmed adnexal masses were assessed for eligibility. Those with apparent early-stage ovarian cancer (International Federation of Gynecology and Obstetrics (FIGO) stage I–II) who met the following inclusion criteria were included: >18 years of age, suspicious adnexal mass (unilateral or bilateral) at ultrasound and CT imaging or confirmed ovarian tumor after previous surgery (unilateral or bilateral salpingo-oophorectomy with or without hysterectomy) and provided written informed consent before study enrollment.
Pelvic ultrasound and total body CT scan were performed to evaluate the extent of disease, including preoperative assessment of nodal involvement. Patients were excluded if they withdrew consent before surgery, had apparent stage III or IV disease on imaging, previous vascular surgery (vena cava, aorta, iliac vessels), previous lymphadenectomy (pelvic or para-aortic), history of lymphoma, radiotherapy (pelvic or para-aortic fields), a benign result after frozen section histology in the case of suspicious adnexal mass, and/or a previous allergic reaction to colloids or indocyanine green (ICG).
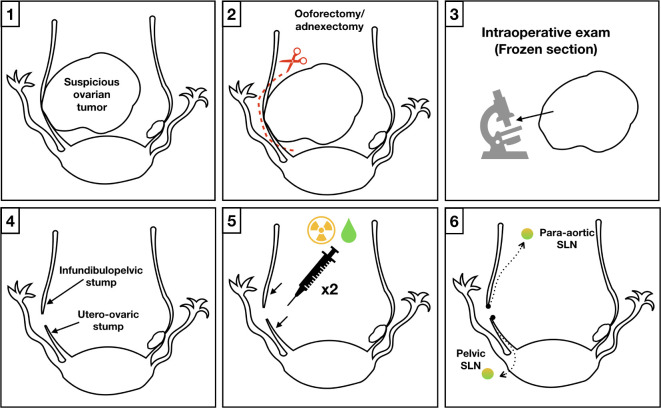
Two simultaneous methods were used for sentinel lymph node detection (Figure 1): 99mTc and ICG. After the abdominal cavity was accessed and peritoneal washings obtained, the suspicious ovarian tumor (in cases of previous unconfirmed malignant histology) was removed. We performed a unilateral or bilateral salpingo-oophorectomy (hysterectomy was performed only if it was necessary to avoid rupture of the tumor capsule) and submitted the surgical specimen for frozen sectioning.24 Sentinel lymph node mapping was performed after malignancy was confirmed. Injection points were at the ipsilateral infundibulopelvic and utero-ovarian ligament stumps for unilateral tumors having no previous hysterectomy, bilateral infundibulopelvic and utero-ovarian stumps for bilateral tumors having no previous hysterectomy, and only the infundibulopelvic stump if a hysterectomy had been previously performed. Saline solution (0.2 mL) that contained 37 mBq 99mTc nanocolloid (Albu-res, Pharmaceutical Nycomed Amersham, Braunschweig, Germany) was injected subperitoneally. At the same time, 0.5 mL ICG (1.25 mg/mL) was injected. A 27G needle was used at each injection point (Figure 2).25 26
Figure 1.
Sentinel lymph node technique in ovarian cancer. 1 and 2: In cases of previously unconfirmed malignant histology, the suspicious ovarian tumor was removed. 3: The surgical specimen was submitted for frozen sectioning. In case of malignancy, the SLN technique was performed. 4: The injection points were at the infundibulopelvic and ovarian stumps. 5: A saline solution containing 99mTC nanocoloid and ICG were injected subperitoneally at each point. 6: Guided by the acoustic signal of a gamma probe and NIR/ICG system, a minimum dissection looking for the hottest SLN dyed with indocyanine green SLN/s in the pelvic/paraaortic region was performed.
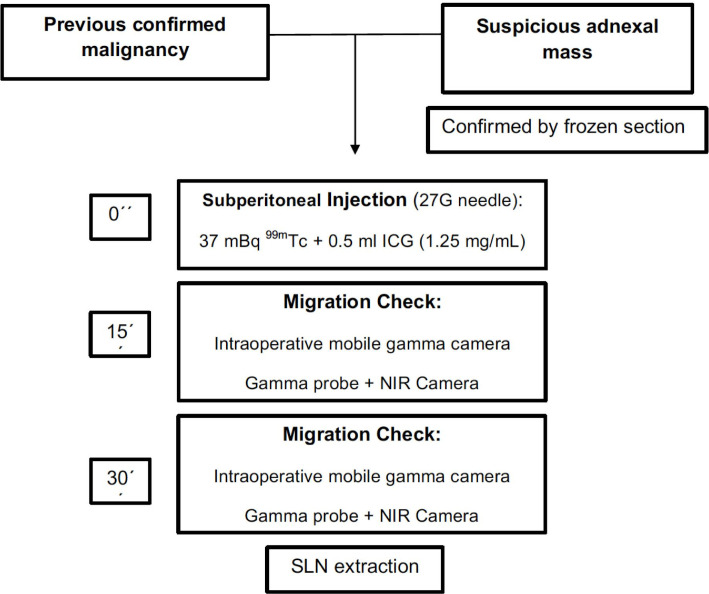
Figure 2.
Sentinel lymph node technique scheme.
After a minimum of 15 min, the injection point and the area of migration were checked with an intraoperative mobile gamma camera (Sentinella, Oncovision) for descriptive purposes. Thirty minutes after injection, the sentinel lymph node procedure commenced: it was guided by the acoustic signal of a gamma probe (Wprobe wireless gamma probe STD and LAP, Oncovision) by performing minimum dissection while searching for the hottest sentinel lymph node(s) in the pelvic and para-aortic region. We simultaneously used the Imagen1 HUB- OPAL1 (NIR/ICG system; Karl Storz Endoscopy, GmbH, Mittelstrasse, Tuttlingen, Germany) or the SPY-PHI fluorescence imaging platform (Novadaq/Stryker Corp., Kalamazoo, MI, USA) to detect the sentinel lymph node(s): any lymph node dyed with ICG and with a markedly higher count 10-times higher than the background was considered a sentinel lymph node and it was harvested separately. All retrieved sentinel lymph nodes were classified according to the anatomical region where they were located.
Next, surgical staging was performed, including hysterectomy, contralateral adnexectomy (if needed), pelvic and para-aortic lymphadenectomy, omentectomy, peritoneal biopsies, and appendectomy in the case of mucinous tumors. Both sentinel lymph nodes and non-sentinel lymph nodes were processed according to a standard protocol for lymph node examination: they were cut into single sections or, when the diameter was >1 cm, into 2–3 sections and stained with haematoxylin and eosin before microscopy. No ultrastaging was performed in this study due to a limitation in funding.
A customized case report form (CRF) was created for data collection and management. A CRF was completed for each patient who provided informed consent. Study data were collected prospectively and monitored by an external technician from the Medical Research Institute La Fe (IISLAFE). The primary trial end point was to report any adverse event related to the technique, including the use of 99mTc and ICG intraperitoneally, as well as the feasibility of the technique. The second exploratory objectives were to report sensitivity, specificity, and the positive and negative predictive values of the technique, the detection rate and location of sentinel lymph nodes
Descriptive results are reported as absolute frequency (percentage) for nominal variables and as mean/SD and range for each continuous variable. Inference values were calculated for the main variables of the study. The primary objective was to demonstrate the safety of the sentinel lymph node technique as measured by the evaluation of major surgical adverse events (eg, embolism due to injection, vascular injuries) through 1-month post-surgery. Using a success rate of 90%, a non-inferiority margin of 25%, >85% power, and one-sided alpha=0.05, a sample size of n=20 patients was needed to demonstrate that sentinel lymph node technique is as safe as current standard-of-care through 1-month post-surgery.
Results
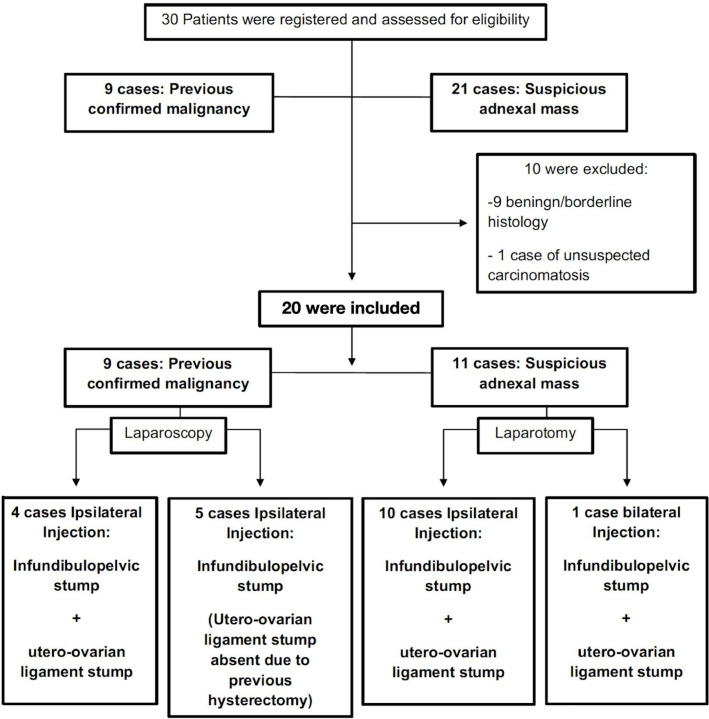
The sentinel lymph node technique was performed in 20 of 30 patients considered for inclusion (Figure 3). The mean age at diagnosis was 50±24.4 years. Mean body mass index was 24.5±4.8 kg/m2. Nine patients (45%) who had undergone previous surgery (that confirmed malignancy) were staged laparoscopically: the other 11 (55%) patients were newly presenting with a suspicious adnexal mass that underwent laparotomy (Table 1). Mean tumor size was 10.9±7.3 cm. The primary tumor occurred on the right in nine patients (45%), on the left in 10 patients (50%), and was bilateral in one patient (5%). The mean operating time was 275±29 min (from skin incision to closure). Two patients (10%) experienced intraoperative complications. Both consisted of venous vascular injuries during lymphadenectomy and were not related to the sentinel lymph node procedure. Those injuries were repaired intraoperatively without any need for blood transfusion or subsequent surgery.
Figure 3.
Flow chart of patients included in the study.
Table 1.
Patients' baseline and surgical characteristics
| Patient’s baseline and surgical characteristics | |
| Diagnosis; n (%) | |
| Confirmed | 9 (45) |
| Suspicious | 11(55) |
| Age [Mean±SD (Range)]; year | 50±9 (35-68) |
| BMI [Mean±SD (Range)]; kg/m2 | 24.5±4.8 |
| Previous surgery; n (%) | |
| None | 11(55) |
| Unilateral Anexectomy | 4 (20) |
| Bilateral Anexectomy | 1 (5) |
| Bilateral Anexectomy+Hysterectomy | 4 (20) |
| Ca 125 [Mean±SD (Range)]; IU/mL | 155.6±241 (6.9–818) |
| Ca 19.9 [Mean±SD (Range)]; IU/mL | 114.7±204 (2-875) |
| ASA score; n (%) | |
| I-II | 17.0 (85) |
| III | 3.0 (15) |
| Approach; n (%) | |
| Laparoscopy | 9 (45) |
| Laparotomy | 11 (55) |
| Type of surgery; n (%) | |
| Frozen section+Surgical staging | 11 (55) |
| Differed surgical staging | 9 (45) |
| Main Tumor Size [Mean±SD (Range)]; mm | 108.7±72.7 (2–250) |
| Ascites [Mean±SD (Range)]; ml | 77.5±103 (0–400) |
| Tumor location; n (%) | |
| Left | 10 (50) |
| Right | 9 (45) |
| Bilateral | 1 (5) |
| Estimated blood loss [Mean±SD (Range)]; ml | 257.5±114 (100-500) |
| Red blood transfusion; n (%) | 0 (0) |
| Surgical time [Mean±SD (Range)]; min | 275±29 (210-320) |
| Intraoperative complications; n (%) | |
| No | 18 (90) |
| Vascular injury | 2 (5) |
ASA; BMI, body mass index; FIGO, The International Federation of Gynecology and Obstetrics.
Details about the sentinel lymph node technique are described on Table 2. Fifteen patients had 99mTc + ICG injected intraoperatively in the utero-ovarian ligament stump, but in five patients, the injection was not possible because a hysterectomy was previously performed (or during the intraoperative examination to avoid tumor rupture). 99mTc + ICG was injected into the right ovarian ligament stump in six patients, into the left in eight patients and bilaterally in one patient. The injection was performed in the infundibulopelvic ligament stump in all 20 patients (right=9, left=10 and bilaterally=1). Fiveteen and thirty minutes after injection, the injection point and sentinel lymph node migration was checked with an intraoperative mobile gamma camera, a gamma probe and the NIR/ICG system (Figure 2). There were no adverse or clinically detectable pharmacologic effects in any of the 20 patients during surgery.
Table 2.
Sentinel lymph node procedure
| Point of injection; n (%) | 95% CI | |
| Utero-ovarian ligament stump | ||
| Not applicable (HT performed) | 5 (25) | – |
| Unilateral | 14 (70) | – |
| Bilateral | 1 (5) | – |
| Utero-ovarian ligament migration (n15) | ||
| No | 1 (6.7) | – |
| Unilateral | 13 (86.7) | – |
| Bilateral | 1 (6.7) | – |
| Infundibulopelvic ligament stump | ||
| Unilateral | 19 (95) | – |
| Bilateral | 1 (5) | – |
| Infundibulopelvic ligament migration (n20) | ||
| No | 0 (0) | – |
| Unilateral | 16 (80) | – |
| Bilateral | 4 (20) | – |
| SLN detection rate; n (%) | ||
| Pelvic (n15) | 14 (93.3) | 66% to 100% |
| Paraortic (n20) | 20 (100) | 80% to 100% |
| Pelvic and para-aortic | 19 (95) | 73% to 100% |
| Pelvic and/or para-aortic | 20 (100) | 80% to 100% |
| SLN detection method; n (%) | ||
| Intra-operative lymphography | 1 (5) | 0% to 27% |
| Tc 99m | 20 (100) | 80% to 100% |
| ICG | 19 (95) | 73% to 100% |
| Both (Tc 99m+ ICG) | 20 (100) | 80% to 100% |
| Time after injection [Mean±SD (Range)]; min | 53±15 (30–80) | 46.5 to 60.8 min |
HT, hysterectomy; ICG, Indocyanine green; SLN, sentinel lymph node.
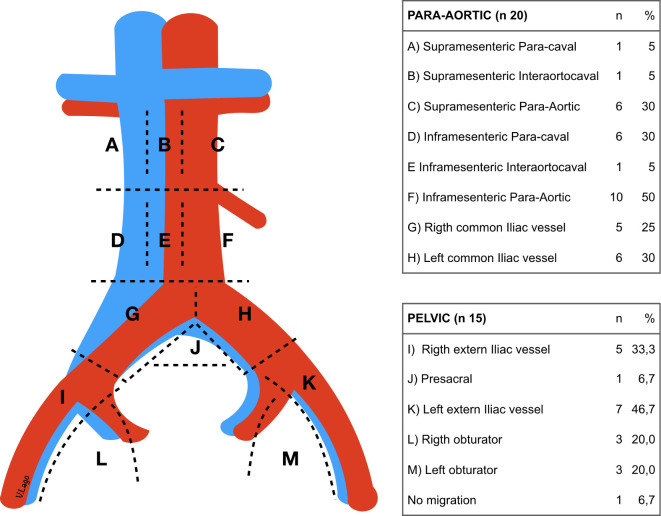
A sentinel lymph node was detected in the pelvic region using the gamma probe and ICG camera in 14 of 15 patients (93%; 95% CI=66% to 100%) and in all 20 para-aortic cases (100%; 95% CI=80% to 100%). In 19 of 20 patients (95%; 95% CI=73% to 100%) there was evidence of migration from both the pelvic and para-aortic region: from these 20 patients, 19 presented migration to the pelvic and para-aortic region and one patient presented only migration to the para-aortic region: in five patients, hysterectomy was previously performed and the utero-ovarian ligament stump was absent. Consequently, there were only 15 patients in which the utero-ovarian injection was possible (Figure 3). The sentinel lymph node migration and tracer distribution rates were 100% (95% CI=80% to 100%) for 99mTc alone and 95% (95%CI=73% to 100%) for ICG alone. The mean time from injection to sentinel lymph node resection was 53.6±14 min (range; 30–80; 95% CI=46.5 to 60.8). Mapping of the detected sentinel lymph node distribution is shown in Figure 4. Two of 20 (10%) patients had a contralateral sentinel lymph node in the para-aortic field, but none within the pelvic field after injection.
Figure 4.
Sentinel lymph node distribution.
Inpatient and histologic data are described in Table 3. The mean duration of hospitalization was 3.7±1.1 days (range; 2–6). No patient required intensive care unit admission. One patient developed intestinal pseudo-obstruction 15 days after the staging surgery that resolved within 48 hours after conservative management. One patient experienced vaginal dehiscence 2 weeks after discharge and required surgical repair. Neither complication was thought to be directly related to the sentinel node procedure. There were no other reported adverse events within 30 days of follow-up.
Table 3.
Inpatient and final histologic results
| Hospitalization time [Mean±SD (Range)]; day | 3.7±1.1 (2–6) |
| Complications (Clavien–Dindo); n(%) | |
| Grade I | 0 (0) |
| Grade II | 1 (5) |
| Grade III | 1 (5) |
| Grade IV | 0 (0) |
| Grade V | 0 (0) |
| Adverse events related with 99m TC or ICG use (<30 days); n (%) | 0 (0) |
| Histotype; n (%) | |
| Serous | 4 (20) |
| Endometrioid | 8 (40) |
| Mucinous | 2 (10) |
| Clear cells | 5 (25) |
| Other | 1 (5) |
| Grade; n (%) | |
| G1 | 8 (40) |
| G2 | 0 (0) |
| G3 | 11 (55) |
| Not applicable (disgerminoma) | 1 (5) |
| ILV; n (%) | 0 (0) |
| FIGO stage; n (%) | |
| IA | 7 (35) |
| IC | 11 (55) |
| IIA | 1 (5) |
| IIIB | 1 (5) |
| Pelvic LND [Mean±SD (Range)]; nodes | 20.1±7.6 (9–74) |
| Para-aortic LND [Mean±SD (Range)]; nodes | 19.8±10.4 (6–40) |
| Pelvic SLN removed [Mean±SD (Range)]; nodes | 2.2±1.5 (0–5) |
| Para-aortic SLN removed [Mean±SD (Range)]; nodes | 3.3±1.8 (1–7) |
FIGO, The International Federation of Gynecology and Obstetrics; ICG, Indocyanine green; LND, lymphadenectomy; LVI, lymph vascular invasion; SLN, sentinel lymph node.
The mean number of harvested sentinel lymph nodes was 2.2±1.5 (range; 0–5; 95% CI=1.5 to 3) in the pelvis and 3.3±1.8 (range; 1–7; 95% CI=2.6 to 4.2) lymph nodes in the para-aortic region. The mean overall number of retrieved lymph nodes was 20.1±7.6 and 19.8±10.4 in the pelvic and para-aortic regions, respectively. No lymph node metastasis was found in any patient based on single sections and hematoxylin and eosin examination and therefore sensitivity, specificity, and the positive and negative predictive values of the technique could not be determined. One patient was upstaged to stage IIIB because microscopic disease was found in the omentum and peritoneal biopsies.
Discussion
We found that there were no intraoperative complications or 30-day adverse events related to the use of 99mTc or ICG. We report a novel sentinel node mapping technique with 95% detection in the pelvic and para-aortic regions. Furthermore, the approach was not a limitation and the procedure could be performed by either laparotomy or laparoscopy. The presence of severe adhesive disease was not an impediment, although this has been previously reported in our pilot experience.22 One of the advantages of the proposed scheme is that it can be performed after removal of the primary tumor and restricts the use of 99mTc and ICG only to those cases where malignancy has been proven. A disadvantage is that the surgical time is extended by approximately 1 hour.
To date, pelvic and para-aortic lymphadenectomy are the standard procedures in early ovarian cancer.3 Nevertheless, due to the lack of benefit in terms of survival11 and the low incidence of microscopic lymph node metastasis8–10 associated with morbidity related to the lymphadenectomy,4 6 this issue remains controversial. Few studies have described a feasible approach to sentinel node mapping in patients with ovarian cancer.16–21 Unlike vulvar, cervix, and endometrial cancer, where the injection site is readily accessible before surgical prepping, the ovary is much more difficult to access. Among the challenges of where, when, and what to inject, is the equally inconvenient procedural step of needing to remove the clinically suspicious ovary before confirming that it has a malignancy, prompting the need to perform sentinel node mapping. Our standardized protocol is based on our previous pilot experience,22 addresses many of these concerns, and could be adopted for further study in a more robust clinical trial.
We elected to inject both tracers in the infundibulopelvic ligament and (unless a hysterectomy had been performed) the utero-ovarian ligament stumps. Alternatively, the injection can be performed near the meso of the ovary with a reported detection rate between 67% and 100%.16–21 23 However, injection at this point is not feasible in the case of previous adnexectomy. Injection into the ovary cortex has the worst detection rate (40%–100%)16 17 and is not oncologically safe due to theoretical risk of tumor rupture.27
The physiological lymphatic drainage of the ovary is bidirectional, arising from the ovary to the para-aortic and pelvic fields through the infundibulopelvic and utero-ovarian ligaments respectively. Performing the injection after adnexal mass removal23 has been criticised.28 Authors argued that our detection rate might be artificially high due to an alteration of the lymphatic drainage. Nevertheless, the ovary resection has no influence on the tracer’s drainage: the lymphatic drainage persists from the infundibulopelvic and utero-ovarian stumps to the para-aortic and pelvic fields respectively. Therefore, the only difference is that the drainage is in a single direction. Moreover, the detection rate reported in both our pilot22 and the present study is not higher but in line with the rate of previous publications.16–21
Sentinel lymph node detection can use an assortment of tracers, such as CH40 (activated carbon particles), 99mTc albumin colloid, blue dye, or ICG, alone or in combination.16–23 99mTc has traditionally been most commonly used. Recently, the preliminary results of the SELLY clinical trial were reported,23 but this study only applies to minimally invasive surgery and the lower detection rate (67.7%) found, may be related to the use of a single agent ICG for detection. The sentinel lymph node procedure we describe does require pre-operative preparation, including the availability of a nuclear medicine department and an ICG viewing system. Furthermore, there is a learning curve to avoid tracer injection leading to inadvertent extravasation. We agree with Uccella et al23 that sentinel lymph node mapping of apparent early ovarian cancer is a more challenging procedure than for other, more directly accessible gynecologic malignancies.
We found no metastases in any sentinel or non-sentinel nodes after standard sections and hematoxylin and eosin staining and therefore could not estimate the accuracy of this technique. The histologic type of the included tumors and the absence of an ultrastaging protocol may explain the lack of detection of lymph node metastasis. The sample size may also represent a limitation for the present study. Nevertheless, our study design was not intended to demonstrate clinical utility. We have demonstrated the feasibility of our protocol and that it can be performed without major safety concerns. A subsequent collaborative clinical trial will be required to determine the negative predictive value and better define the clinical utility of sentinel node technique in early ovarian cancer.
ijgc-2020-001289supp001.pdf (63.3KB, pdf)
Acknowledgments
The authors thank Juana Roig Herrero, María Remedios Goméz, Sofía Caparros Conesa, María del Pilar Martínez Del Fresno, LOCCOmetrica S.L. and the 150 people who participated in the crowdfunding campaign due to their altruistic donations, we were able to perform this trial. The authors also thank Sara García Álvarez for her medical writing support.
Footnotes
Correction notice: Since the online publication of this article, the title has been updated to include the acronym 'SENTOV'.
Collaborators: Marta Gurrea Soteras and Teresa Viñas Alburquerque.
Contributors: VL: Conception and design of study, data collection, surgeon, statistical analysis, data analysis and interpretation, manuscript preparation. PB: Conception and design of study, nuclear medicine specialist, data analysis and interpretation, reviewer. BM: Conception and design of study, pathologist review, data analysis and interpretation, reviewer. LM: Surgeon, statistical analysis, data analysis and Interpretation, reviewer. PP-I: Surgeon, statistical analysis, data analysis and interpretation, reviewer. SL: Pathologist review, data analysis and interpretation, reviewer. TM: Surgeon, statistical analysis, data analysis and interpretation, reviewer. MA: Nuclear medicine specialist, statistical analysis, data analysis and interpretation, reviewer. SD: Conception and design of study, data collection, surgeon, statistical analysis, data analysis and interpretation, reviewer.
Funding: This study was funded by Precipita (Crowdfunding) and the SCReN (Spanish Clinical Research Network; SCIII-Subdirección General de Evaluación y Fomento de la Investigación: PT17/0017/0035).
Competing interests: SD has received a speaker honorarium from Ethicon.
Patient consent for publication: Not required.
Ethics approval: Obtained; EUDRACT code: 2017-003683-12
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All relevant data are included in the article, nevertheless the full data of the article will be available upon reasonable request.
References
- 1. Young RC, Walton LA, Ellenberg SS, et al. Adjuvant therapy in stage I and stage II epithelial ovarian cancer: results of two prospective randomized trials. N Engl J Med 1990;322:1021–7. 10.1056/NEJM199004123221501 [DOI] [PubMed] [Google Scholar]
- 2. Berek JS, Crum C, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet 2012;119:S118–29. 10.1016/S0020-7292(12)60025-3 [DOI] [PubMed] [Google Scholar]
- 3. Timmers PJ, Zwinderman K, Coens C, et al. Lymph node sampling and taking of blind biopsies are important elements of the surgical staging of early ovarian cancer. Int J Gynecol Cancer 2010;20:1142–7. 10.1111/IGC.0b013e3181ef8e03 [DOI] [PubMed] [Google Scholar]
- 4. Trimbos JB. Lymphadenectomy in ovarian cancer: standard of care or unnecessary risk. Curr Opin Oncol 2011;23:507–11. 10.1097/CCO.0b013e32834847e7 [DOI] [PubMed] [Google Scholar]
- 5. Maggioni A, Benedetti Panici P, Dell'Anna T, et al. Randomised study of systematic lymphadenectomy in patients with epithelial ovarian cancer macroscopically confined to the pelvis. Br J Cancer 2006;95:699–704. 10.1038/sj.bjc.6603323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Di Re F, Baiocchi G. Value of lymph node assessment in ovarian cancer: status of the art at the end of the second millennium. Int J Gynecol Cancer 2000;10:435–42. 10.1046/j.1525-1438.2000.00053.x [DOI] [PubMed] [Google Scholar]
- 7. Colombo N, Sessa C, Bois AD, et al. ESMO–ESGO ovarian cancer consensus conference Working Group. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int J Gynecol Cancer 2019;29:728–60. [DOI] [PubMed] [Google Scholar]
- 8. Kleppe M, Wang T, Van Gorp T, et al. Lymph node metastasis in stages I and II ovarian cancer: a review. Gynecol Oncol 2011;123:610–4. 10.1016/j.ygyno.2011.09.013 [DOI] [PubMed] [Google Scholar]
- 9. Lago V, Minig L, Fotopoulou C. Incidence of lymph node metastases in apparent early-stage low-grade epithelial ovarian cancer: a comprehensive review. Int J Gynecol Cancer 2016;26:1407–14. 10.1097/IGC.0000000000000787 [DOI] [PubMed] [Google Scholar]
- 10. Minig L, Heitz F, Cibula D, et al. Patterns of lymph node metastases in apparent stage I low-grade epithelial ovarian cancer: a multicenter study. Ann Surg Oncol 2017;24:2720–6. 10.1245/s10434-017-5919-y [DOI] [PubMed] [Google Scholar]
- 11. Sakuragi N, Yamada H, Oikawa M, et al. Prognostic significance of lymph node metastasis and clear cell histology in ovarian carcinoma limited to the pelvis (pT1M0 and pT2M0). Gynecol Oncol 2000;79:251–5. 10.1006/gyno.2000.5933 [DOI] [PubMed] [Google Scholar]
- 12. de Hullu JA, Hollema H, Piers DA, et al. Sentinel lymph node procedure is highly accurate in squamous cell carcinoma of the vulva. J Clin Oncol 2000;18:2811–6. 10.1200/JCO.2000.18.15.2811 [DOI] [PubMed] [Google Scholar]
- 13. Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC trial. J Natl Cancer Inst 2006;98:599–609. 10.1093/jnci/djj158 [DOI] [PubMed] [Google Scholar]
- 14. Colombo N, Creutzberg C, Amant F, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer 2016;26:2–30. 10.1097/IGC.0000000000000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cibula D, Pötter R, Planchamp F, et al. The ESGO/ESTRO/ESP guidelines for the management of patients with cervical cancer. Int J Gynecol Cancer 2018;28:641–55. [DOI] [PubMed] [Google Scholar]
- 16. Negishi H, Takeda M, Fujimoto T, et al. Lymphatic mapping and sentinel node identification as related to the primary sites of lymph node metastasis in early stage ovarian cancer. Gynecol Oncol 2004;94:161–6. 10.1016/j.ygyno.2004.04.023 [DOI] [PubMed] [Google Scholar]
- 17. Hassanzadeh M, Hosseini Farahabadi E, Yousefi Z, et al. Lymphatic mapping and sentinel node biopsy in ovarian tumors: a study using intra-operative Tc-99m-Phytate and lymphoscintigraphy imaging. J Ovarian Res 2016;9:55. 10.1186/s13048-016-0265-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nyberg RH, Korkola P, Mäenpää J. Ovarian sentinel node: is it feasible? Int J Gynecol Cancer 2011;21:568–72. 10.1097/IGC.0b013e318211ef75 [DOI] [PubMed] [Google Scholar]
- 19. Kleppe M, Brans B, Van Gorp T, et al. The detection of sentinel nodes in ovarian cancer: a feasibility study. J Nucl Med 2014;55:1799–804. 10.2967/jnumed.114.144329 [DOI] [PubMed] [Google Scholar]
- 20. Buda A, Passoni P, Corrado G, et al. Near-infrared fluorescence-guided sentinel node mapping of the ovary with indocyanine green in a minimally invasive setting: a feasible study. J Minim Invasive Gynecol 2017;24:165–70. 10.1016/j.jmig.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 21. Nyberg RH, Korkola P, Mäenpää JU. Sentinel node and ovarian tumors: a series of 20 patients. Int J Gynecol Cancer 2017;27:684–9. 10.1097/IGC.0000000000000948 [DOI] [PubMed] [Google Scholar]
- 22. Lago V, Bello P, Montero B, et al. Clinical application of the sentinel lymph node technique in early ovarian cancer: a pilot study. Int J Gynecol Cancer 2019;29:377–81. 10.1136/ijgc-2018-000049 [DOI] [PubMed] [Google Scholar]
- 23. Uccella S, Nero C, Vizza E, et al. Sentinel-node biopsy in early-stage ovarian cancer: preliminary results of a prospective multicentre study (SELLY). Am J Obstet Gynecol 2019;221:324.e1–324.e10. 10.1016/j.ajog.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 24. Medeiros LR, Rosa DD, Edelweiss MI, et al. Accuracy of frozen-section analysis in the diagnosis of ovarian tumors: a systematic quantitative review. Int J Gynecol Cancer 2005;15:192–202. 10.1136/ijgc-00009577-200503000-00002 [DOI] [PubMed] [Google Scholar]
- 25. Lago V, Bello P, Marina Martín MT, et al. Sentinel lymph node in apparent early ovarian cancer: open technique. Int J Gynecol Cancer 2019;29:1449. 10.1136/ijgc-2019-000732 [DOI] [PubMed] [Google Scholar]
- 26. Lago V, Bello P, Matute L, et al. Sentinel lymph node technique in apparent early ovarian cancer: laparoscopic technique. J Minim Invasive Gynecol 2019. 10.1016/j.jmig.2019.09.790. [Epub ahead of print: 16 Oct 2019]. [DOI] [PubMed] [Google Scholar]
- 27. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet 2001;357:176–82. 10.1016/S0140-6736(00)03590-X [DOI] [PubMed] [Google Scholar]
- 28. Dell'Orto F, Laven P, Delle Marchette M, et al. Feasibility of sentinel lymph node mapping of the ovary: a systematic review. Int J Gynecol Cancer 2019;29:1209–15. 10.1136/ijgc-2019-000606 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ijgc-2020-001289supp001.pdf (63.3KB, pdf)