We read with interest the leading article by Gerbes et al 1 published in Gut. This roundtable meeting article proposed that hepatocellular carcinoma (HCC) staging linked to first-line treatment indication can help clinicians guide patients through treatment decision-making process, patients and researchers need reliable ways to stage disease and predict prognosis. Controversies always exist during multidisciplinary team (MDT) decision-making for HCC patients within the Milan criteria (MC) due to the lack of evidence-based studies of composite multiparametric evaluations among the three potential curative therapies: liver transplantation (LT), liver resection (LR) and local ablation (LA).2–4 Herein, we retrospectively evaluated the efficacy of LT, LR and LA for HCC patients within the MC and explored an individualised assessment prediction model to assist with MDT decision-making.
Institutional ethics committees approved the retrospective analyses of consecutive HCC patients admitted to two medical centres of Nankai University (Tianjin, China) between November 2011 and March 2016. A total of 283 HCC patients within the MC were finally enrolled and classified into LT (n=100), LR (n=89) and LA (n=94) groups based on the first-line treatments. Under the three treatment groups, subgroups were divided according to solitary tumor ≤3 cm, 2–3 tumours and each ≤3 cm (multiple tumours ≤3 cm), and solitary tumour 3–5 cm. Inverse probability of treatment weighting (IPTW) was used to overcome treatment selection bias. After IPTW adjustment, the three treatment groups were no longer different for most baseline parameters including those deemed to potentially influence treatment selection, such as tumour size and number, α-fetoprotein (AFP) and severity of underlying liver disease (baseline characteristics before and after IPTW adjustment are in the online supplementary materials).
gutjnl-2019-320073supp001.pdf (194KB, pdf)
After a median follow-up of 41.2 months, 114 patients died, 103 had an active tumour and 11 were tumour free. One hundred and fifty-three patients recurred. The 3-year and 5-year recurrence-free survival (RFS) rates were 82.5%, 79.4% in the LT group, 43.8%, 30.1% in the LR group and 21.2%, 14.5% in the LA group, respectively (p<0.001). The 3-year and 5-year overall survival (OS) rates were 85.0%, 79.4% in the LT group, 69.7%, 49.4% in the LR group and 57.4%, 31.3% in the LA group, respectively (p<0.001). The OS of LA was significantly higher than LR in the subgroup of multiple tumours ≤3 cm, but lower than LR in other two subgroups with solitary tumour. Comparison results of RFS and OS among the three treatment groups before and after IPTW adjustment were found similar (in the online supplementary materials).
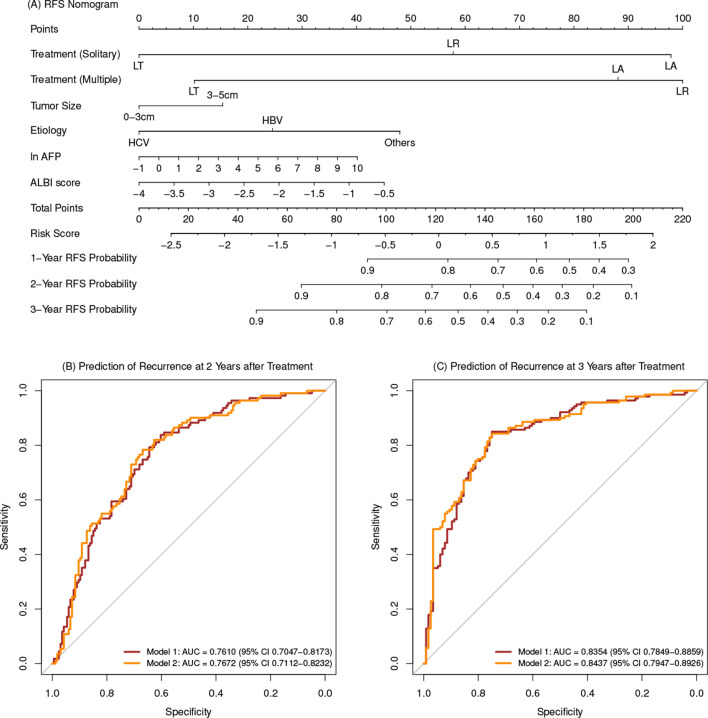
Multivariate analyses of all cohort before and after IPTW adjustment were detailed in table 1. A nomogram for RFS was derived from the final multivariate model (model 1) composing of tumour number, tumour size, treatment allocation, aetiology, AFP and albumin-bilirubin score (figure 1A). Another nomogram for RFS was derived from the final multivariate model after IPTW adjustment (model 2). Cross-validation was performed and the discrimination ability of 2-year and 3-year RFS prediction for the two models was obtained (figure 1B and C). Recurrence risk stratifications based on the two models were classified into low-risk and high-risk probability for each treatment allocation and were differentiated well. The algorithm based on model 1 was converted as a preliminary recurrence prediction prognosis calculator in website: https://yingwu.shinyapps.io/recur-pred/. RFS nomogram for model 2 and Kaplan-Meier curves of recurrence risk stratification for each treatment are presented in the online supplementary materials.
Table 1.
Multivariate analysis for RFS and OS
| Covariates | RFS | OS | ||||||
| Before IPTW-adjustment | After IPTW-adjustment | Before IPTW-adjustment | After IPTW-adjustment | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Solitary | ||||||||
| LR versus LT | 3.79 (1.93 to 7.46) | <0.001 | 3.66 (1.80 to 7.44) | <0.001 | 2.33 (1.10 to 4.91) | 0.027 | 1.62 (0.74 to 3.55) | 0.226 |
| LA versus LT | 9.56 (5.11 to 17.89) | <0.001 | 10.28 (5.22 to 20.23) | <0.001 | 6.42 (3.34 to 12.31) | <0.001 | 6.07 (3.13 to 11.75) | <0.001 |
| LA versus LR | 2.52 (1.60 to 3.98) | <0.001 | 2.81 (1.75 to 4.50) | <0.001 | 2.76 (1.55 to 4.92) | <0.001 | 3.74 (2.00 to 7.02) | <0.001 |
| Multiple | ||||||||
| LR versus LT | 7.94 (3.19 to 19.75) | <0.001 | 12.40 (4.60 to 33.44) | <0.001 | 5.56 (2.31 to 13.36) | <0.001 | 4.04 (1.78 to 9.16) | <0.001 |
| LA versus LT | 6.04 (2.36 to 15.44) | <0.001 | 7.14 (2.47 to 20.67) | <0.001 | 2.38 (0.92 to 6.18) | 0.075 | 1.79 (0.71 to 4.52) | 0.219 |
| LA versus LR | 0.76 (0.40 to 1.46) | 0.409 | 0.58 (0.29 to 1.14) | 0.115 | 0.43 (0.20 to 0.90) | 0.025 | 0.44 (0.20 to 0.98) | 0.043 |
| Operation | ||||||||
| LT: multiple versus solitary | 1.27 (0.46 to 3.49) | 0.649 | 0.75 (0.25 to 2.25) | 0.605 | 1.43 (0.56 to 3.66) | 0.461 | 1.56 (0.65 to 3.79) | 0.322 |
| LR: multiple versus solitary | 2.65 (1.48 to 4.74) | 0.001 | 2.53 (1.46 to 4.38) | <0.001 | 3.40 (1.77 to 6.56) | <0.001 | 3.90 (1.94 to 7.84) | <0.001 |
| LA: multiple versus solitary | 0.80 (0.44 to 1.45) | 0.458 | 0.52 (0.28 to 0.96) | 0.035 | 0.53 (0.27 to 1.04) | 0.065 | 0.46 (0.22 to 0.96) | 0.038 |
| Gender (male) | 1.99 (1.14 to 3.47) | 0.016 | 2.65 (1.40 to 5.03) | 0.003 | ||||
| Aetiology | ||||||||
| HCV versus HBV | 0.57 (0.26 to 1.26) | 0.164 | 0.65 (0.28 to 1.52) | 0.318 | 0.81 (0.34 to 1.95) | 0.643 | 0.93 (0.38 to 2.27) | 0.880 |
| Others versus HBV | 1.72 (1.05 to 2.80) | 0.031 | 1.80 (1.06 to 3.06) | 0.029 | 2.47 (1.43 to 4.27) | 0.001 | 2.21 (1.21 to 4.04) | 0.010 |
| Others versus HCV | 3.03 (1.25 to 7.35) | 0.014 | 2.78 (1.07 to 7.21) | 0.035 | 3.03 (1.15 to 8.02) | 0.025 | 2.37 (0.86 to 6.48) | 0.094 |
| In(AFP) (ng/mL) | 1.09 (1.00 to 1.18) | 0.039 | 1.08 (0.99 to 1.18) | 0.078 | ||||
| ALBI score | 1.35 (1.03 to 1.76) | 0.031 | 1.37 (1.04 to 1.82) | 0.027 | 1.51 (1.12 to 2.02) | 0.006 | 1.35 (1.00 to 1.83) | 0.051 |
| Tumour size (cm) (3–5 vs ≤3) | 1.43 (0.97 to 2.11) | 0.075 | ||||||
Variables that achieved significance at the 0.1 level in the univariate Cox proportional hazards (PH) model were included in the multivariate analysis. Statistical analyses were performed using R V.3.5.2, and p<0.05 was considered statistically significant.
AFP, α-fetoprotein; ALBI, albumin-bilirubin; OS, overall survival; PH, proportional hazards; RFS, recurrence-free survival.
Figure 1.
Nomogram and ROC of the two models. (A) Nomogram of recurrence prediction based on model 1. (B) ROC of the 2-year recurrence prediction based on model 1 and model 2. (C) ROC of the 3-year recurrence prediction based on model 1 and model 2. AFP,α-fetoprotein; ALBI, albumin-bilirubin; AUC, area under the curve; LA, local ablation; LR, liver resection; LT, liver transplantation; RFS, recurrence-free survival; ROC, receiver operating characteristic.
In conclusion, although retrospective in nature, our study is the first to explore the recurrence prediction model which showed feasibility as an individualised assessment of therapeutic alternatives to assist with MDT decision-making for HCC patients within the MC. Future multicentre studies with larger numbers are warranted to validate the results of our study.
Acknowledgments
We acknowledge all the hospitals, departments and practitioners that participated in helping with the data collection and documentation.
Footnotes
Contributors: NZ, WJ, YZ, TS, JL, JG, YW and JQ contributed equally to this study.
Study concept: WL, ZS and NZ. Study design: WL, ZS, NZ, WJ, YZ, T-QS and JQ. Data collection: JL, JG, YQ, BY, DT, QG, LZ, JS, YX, XS, ZY, YZ and JL. Analysis and interpretation of data: YW, NZ, WJ, YZ, T-QS, JQ and ZW. Manuscript drafting: NZ, YW, JL and JG. Revising the manuscript: NZ, WL, WJ, YZ, T-QS and JQ. Acquisition and review of data, provision of critical comments or suggestions: all authors. Manuscript final version approval: all authors.
Funding: This work was supported by Key project of Science and Technology, Tianjin Municipal Science and Technology Bureau (19YFZCSY00020).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was conducted with the approval of Tianjin First Center Hospital Medical Ethics Committee and Tianjin Second People’s Hospital Medical Ethics Committee, Tianjin, China.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1. Gerbes A, Zoulim F, Tilg H, et al. Gut roundtable meeting paper: selected recent advances in hepatocellular carcinoma. Gut 2018;67:380–8. 10.1136/gutjnl-2017-315068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Galle PR, Forner A, Llovet JM, et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 3. Tateishi R, et al. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut 2005;54:419–25. 10.1136/gut.2003.035055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Toni EN, Schlesinger-Raab A, Fuchs M, et al. Age independent survival benefit for patients with hepatocellular carcinoma (HCC) without metastases at diagnosis: a population-based study. Gut 2020;69:168–76. 10.1136/gutjnl-2018-318193 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2019-320073supp001.pdf (194KB, pdf)