Abstract
Background: Azodicarbonamide is a dough-enhancer used in the process of bread-making in countries like Nigeria. While there have been suggestions that it is a sensitizer of the respiratory system, there is a dearth of information on its effects on the central nervous system.
Aim: This study assessed the effects of azodicarbonamide on the central nervous system (ADA) in rats.
Objective: The effects of ADA-containing diet on neurobehaviour, brain antioxidant status, and neuromorphology of selected brain regions in rats were examined.
Methods: Forty adult rats were randomly-assigned into four groups of ten rats each, and were given standard diet or diet containing ADA at 1, 2 and 4% respectively. Rats were fed a standard diet or ADA-containing diet for a period of 28 days. Weekly body weight assessment and daily estimation of food intake were done. Behavioural tests {in the Open field, Y-maze, radial-arm maze, and Elevated Plus Maze (EPM)} were conducted on day 29. Twenty-four hours after the last behavioural test, animals were euthanised, whole brains were dissected, weighed, and either homogenised for assessment of lipid peroxidation and antioxidant status; or sectioned and processed for general histology.
Results: Consumption of ADA-containing diet was associated with a significant decrease in weight gain/food intake, and significant suppression of horizontal locomotion and rearing behaviours; however, grooming activity increased significantly. Also, there was a significant reduction of open-arm time in the EPM and a significant increase in Y-maze alternation (at the lowest concentration of ADA). ADA-containing diet was not associated with significant changes in brain oxidative status or neuromorphology.
Conclusion: The study showed that while ADA-containing diet may alter neurobehaviour in rats; this was not associated with evidence of brain oxidative stress or neuro-histomorphological alterations.
Keywords: Dough enhancer, food additive, neurobehaviour, oxidative stress, neuromorphology, central inhibition
1. INTRODUCTION
Food additives are compounds added to foods, food substances or drinks with the aim of preserving freshness, ensuring safety and enhancing texture, appearance, taste and/or flavour [1, 2]. For centuries, humans have used different kinds of chemicals or natural products like salt or vinegar to preserve meats, fish, vegetables and fruits. Herbs and spices have also been used to enhance the flavour and texture of food [1, 2]. In recent times, a rapidly enlarging human population, increased demand for food, and the need for food processing have necessitated an increased need to use food additives. However, in many countries, the unregulated or excessive use of food additives has been curbed through the enactment of legislation and laws. It is worthy to note that the world of food additives is an ever-evolving one, with newer products being introduced to the market, while scientific or health reasons had resulted in the discontinuance of some others. Increasingly, concerns about possible health risks have been associated with the use of a number of food additives [3, 4].
Azodicarbonamide (ADA) is a dough-enhancer, which in Nigeria is an approved replacement for potassium bromate (a dough-enhancer that has been banned in a number of countries due to its nephrotoxic potential). Azodicarbonamide is used in a number of other countries including the United States of America, where it is also used as a raising agent in baking products. In recent times, however, there have been public health concerns associated with the use of ADA [5]. There have been reports suggesting that ADA is a respiratory sensitizer especially in the work-place (where it is used as a blowing agent in the runner and plastic industry). This necessitated its ban in Europe [6]. While a few studies have reported some low toxicities involving the kidneys and blood following acute oral administration of ADA to rodents [7, 8], there is a dearth of scientific information regarding its effects on neurobehavioural, lipid peroxidation or antioxidant status in the brain. Meanwhile, oxidative has been implicated in the pathogenesis of neuronal injury. This study examined the possible effects of ADA on the central nervous system, testing the hypothesis that food-added ADA would alter neurobehaviour, brain oxidative status, and cerebral cortex/ hippocampal histomorphology in rats.
2. MATERIALS AND METHODS
Food-grade azodicarbonamide, Assay kits for lipid per-oxidation (Malondialdehyde), superoxide dismutase and reduced glutathione (BioVision Inc. USA). All other reagents used were of analytical grade.
2.1. Animals
Healthy male Wistar rats obtained from Empire Breeders, Osogbo, Osun State, Nigeria were used. Rats were housed singly in metal ages measuring 12 x 9 x 6 inches. General housing was located in a temperature-controlled quarter (22-25°C) with lights on at 7.00 a.m daily. All animals had access to food (commercial standard chow) and water ad-libitum. All procedures were conducted in accordance with the approved institutional protocols and within the provisions for animal care and use.
2.2. Diet
All animals were fed commercially-available standard rat chow (29% protein, 11% fat, 58% carbohydrate) from weaning. At the beginning of the experimental period, animals were either fed standard chow (29% protein, 11% fat, 58% carbohydrate) or standard chow into which ADA had been incorporated at 10 mg (1%), 20 mg (2%) and 40 mg (4%) respectively. Diet was administered ad libitum for a period of four weeks.
2.3. Experimental Methodology
Forty adult Wistar rats were randomly assigned into four groups of ten (10) each (animals were housed singly to allow for estimation of food intake). Rats were grouped as follows, normal control (standard diet), or a diet containing one of the three concentrations of azodicarbonamide [(ADA):1, 2 and 4%]. Animals received a standard diet or ADA diet for a period of 28 days. The dose of ADA was based on acute oral toxicity (LD50) of 6400 mg/kg in rats [9] and optimum dose of ADA used in dough conditioning which is 10-40 mg/kg of flour [10]. Weekly body weight assessment and daily estimation of food intake was done (7.00 am, before feeding) as previously described [11, 12]. Behavioural tests {in the Open field, Y-maze, radial-arm maze, and Elevated Plus Maze (EPM)} were conducted on day 29, thirty minutes after the standard diet and ADA supplemented diet. Twenty-four hours after the last behavioural test, animals were euthanized, whole brains were dissected, weighed, and either homogenised for assessment of lipid peroxidation and antioxidant status or sectioned and processed for general histology.
2.3.1. Behavioural Tests
On test days, rats were transported in their home-cages to the behavioural testing laboratory and allowed to acclimatize for 30 minutes before the behavioural tests were commenced. Animals were exposed to the elevated plus maze, Y-maze, Open field and radial arm maze respectively in this order. At the beginning of the tests, each rat was placed in the behavioural paradigm and its behaviour recorded. The animal was removed from the maze at the end of the test and returned to its home cage, following which the interior surfaces of the maze was cleaned thoroughly with 70% ethanol, and wiped dry to remove traces of con-specific odour. Behavioural parameters were later scored by two independent observers blind to the groupings.
2.3.1.1. Open Field Test
Ten minutes’ period of horizontal locomotion, rearing and grooming were observed and scored to assess behavioural changes in the rat exposed to the open-field. The open-field box is a rectangular arena made of a hard floor measuring 100 x 100 x 40 cm and made of white painted wood. Its floor was divided by permanent red marker into 16 equal squares. Each rat was placed in the centre of the field and covered by a small dome which was removed at the beginning of the recording of behavioural activities. Total horizontal locomotion (number of floor units entered with all paws), rearing frequency (number of times the rat stood on its hind legs either with its forearms against the walls of the observation cage or free in the air) and frequency of grooming (number of body-cleaning with paws, licking of the body and pubis with the mouth, and face-washing actions indicative of a stereotypic behaviour) within the 10 minute period were recorded as previously described [13].
2.3.1.2. Memory Tests (Y-maze and Radial Arm Maze)
Y-maze and the radial-arm maze were used to measure spatial working-memory as previously described [14-16]. In these paradigms, spontaneous alternation behaviour (SAB) was used to assess spatial working memory. Spontaneous alternation behaviour measures the tendency for rodents to alternate non-reinforced choices of Y-maze or radial maze arms on successive opportunities. Spontaneous alternation was assessed using a Y-maze that was made of white-painted wood with three equally-spaced arms (120°, 50 cm long, 20 cm high and 10 cm wide). The floor was also made of hard painted wood. Each rat was placed in one of the arms and allowed to move freely until its tail completely entered another arm. The sequence of arm entries was then recorded. An alternation is defined as entry into all three arms consecutively. The number of actual alternations is the number of sequential arm entries into the three arms, designated A, B and C. The percentage alternation is calculated as {(Actual alternations/Total arm entry minus two) x 100} within a 5 minute period.
The radial arm maze apparatus used in this study had eight equidistantly-spaced arms, each measuring 50 cm long, with all eight arms radiating from a small circular central platform. The radial arm maze assesses the animal’s ability to recall the arm was last visited, as it tries to enter as many different arms as possible. Each rat was placed on the central platform of the maze and allowed to move freely through each arm. Its behaviour was recorded in a single session that lasted for 5 minutes. Working memory was scored when the rat entered each arm a single time. Re-entry into an arm is regarded as a working-memory error. The sequence of arm visits is spatial working memory measured as the alternation index, which is the ratio of sequential arm entries before error as previously described [16].
2.3.1.3. Anxiety Model: Elevated Plus-Maze
The elevated plus-maze is a plus-shaped apparatus with four arms arranged at right angles to each other. The elevated plus maze is composed of two open arms measuring 50 x 10 x 0 cm lying across from each other and perpendicular to two closed arms that measure 50 x 10 x 30 cm with a central platform (10 x 10 x 1 cm). The closed arms were enclosed by 2 high walls (30 cm) while the open arms had no sidewalls. At the beginning of the experiments, rats were placed in the central platform facing the closed arm, and their behaviour was recorded for 5 minutes. The criterion for arm visit is considered when the rat decisively moved all its four limbs into an arm. Anxiety behaviours were scored as the percentage time spent in the closed arm or open arm as previously described [13, 14].
2.4. Brain Homogenization
Within 24 hours of the completion of the behavioural tests, animals in all groups were euthanized by cervical dislocation post anaesthesia with diethyl-ether. Whole brains [5] were immediately removed, weighed and homogenised. Whole brain homogenates were prepared in ice-cold phosphate buffered saline, using a Teflon-glass homogeniser. The homogenate was centrifuged at 5000 rev/min, 4°C, for 15 minutes. The supernatant obtained was then used for the estimation of lipid peroxidation levels and antioxidant status.
2.4.1. Estimation of Malondialdehyde Content (Lipid Peroxidation)
Lipid peroxidation levels measured as malondialdehyde content in whole-brain homogenate were determined as previously described [17]. Supernatant from homogenised brain tissue was transferred into a tube to which standard solution was added, followed by the addition of 2 mL of thiobarbituric acid. The tubes were then agitated following which they incubated at 95°C for 1 hour. Samples are allowed to cool to room temperature, centrifuged at 3000 r/min for 10 minutes. Colour change was measured at 532 nm. The results were expressed in nmol MDA per milligram of tissue homogenate (nmol/mg).
2.4.2. Superoxide Dismutase Activity
Superoxide dismutase activity was assessed using a commercially available assay kit, based on the principle that the enzyme inhibits phenazine methosulphate mediated reduction of nitro blue tetrazolium dye. Colour changes were measured at an absorbance of 560 nm over 5 minutes as described in previous studies [18].
2.4.3. Glutathione Activity
Glutathione activity was assayed using a glutathione assay kit (Biovision Inc., Milpitas, CA, USA) following the instruction of the manufacturer. 0.4M Tris-HCl buffer (pH 8.9) was mixed with 0.01M DTNB and colour change in the sample was measured at an absorbance of 412 nm, and expressed as ng of GSH/g as previously described [19].
2.5. Histological Preparation
Sections of the cerebral cortex and hippocampus were processed for paraffin-embedding, cut at 5 µm and stained with haematoxylin and eosin for general histological study as previously described [11, 19, 20].
2.6. Photomicrography
Slides were examined using a Sellon-Olympus trinocular microscope (XSZ-107E, China) to which a digital camera, Canon Powershot 2500) was attached to allow photomicrographs to be taken.
2.7. Statistical Analysis
Data was analyzed using Chris Rorden’s ezANOVA for Windows (version 0.98). The hypothesis was tested using analysis of variance (ANOVA). One factor ANOVA was used for analysis, while the Tukey (HSD) test was used for post-hoc analysis. Results were expressed as mean ± S.E.M, and p< 0.05 considered significant.
3. RESULTS
3.1. Effect of Azodicarbonamide on Body Weight and Food Consumption
Fig. (1) represents the effect of food-added ADA on change in body weight (upper panel) measured as the final weight subtracted from initial weight and divided by initial weight and change in food intake (lower panel) measured as a difference in final total food intake minus initial food-intake divided by initial food intake. Bodyweight decreased significantly {F (3, 36) = 286, p<0.001} in groups fed ADA at 1, 2 and 4% compared to control. Food intake decreased significantly (F (3, 36) = 432, p<0.001) with ADA at 1, 2 and 4%) compared to control.
Fig. (1).
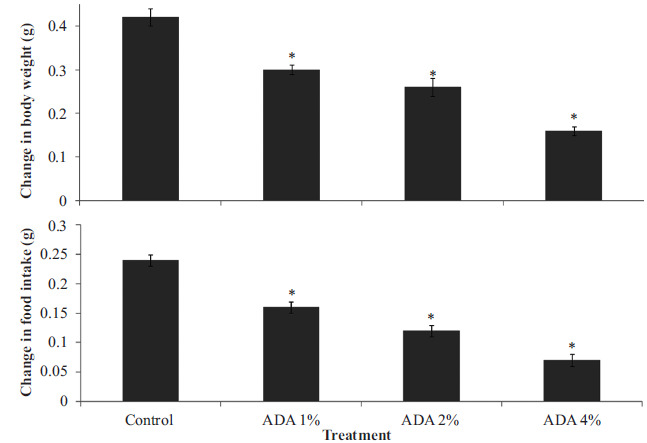
Effect of food-added ADA on change in body weight (upper panel) and change in food intake (lower panel). Each bar represents Mean ± S.E.M, *p<0.05 vs. control, number of rats per treatment group =10. ADA: Azodicarbonamide.
3.2. Effects of Azodicarbonamide on Neurobehaviour
3.2.1. Effect of Azodicarbonamide on Locomotor Activity and Rearing
Fig. (2) represents the effect of food-added ADA on locomotor (upper panel) and rearing (lower panel) activity in rats. Locomotor activity decreased significantly {F (3, 28) = 18.2, p<0.001} in groups fed ADA diet at 1, 2 and 4% compared to control. Rearing decreased significantly (F (3, 28) = 15.5, p<0.004) with ADA diet at 4% compared to control.
Fig. (2).
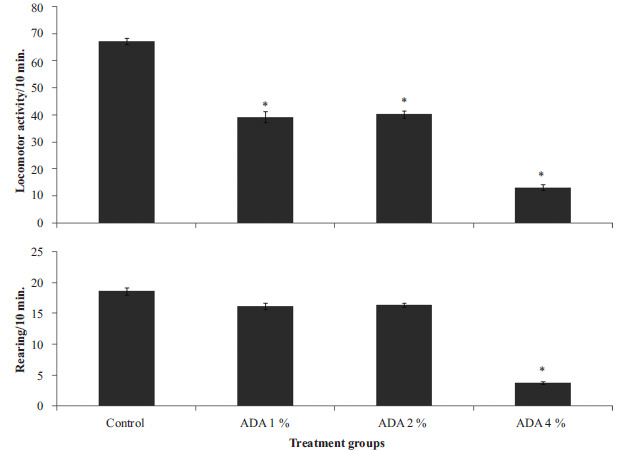
Effect of food-added ADA on locomotor (upper panel) and rearing (lower panel) activity. Each bar represents Mean ± S.E.M, *p<0.05 vs. control, number of rats per treatment group =8. ADA: Azodicarbonamide.
3.2.2. Effect of Azodicarbonamide on Grooming Behaviour and Radial Arm Memory
Fig. (3) represents the effect of food-added ADA on grooming behaviour (upper panel) and spatial working memory in the radial arm maze (lower panel). Grooming behaviour increased significantly {F (3, 28) = 6.60, p<0.02} in group fed ADA diet at 2% compared to control. Radial arm maze working memory did not differ significantly (F (3, 28) = 1.5, p<0.421) in any of the groups fed ADA diet compared to control.
Fig. (3).
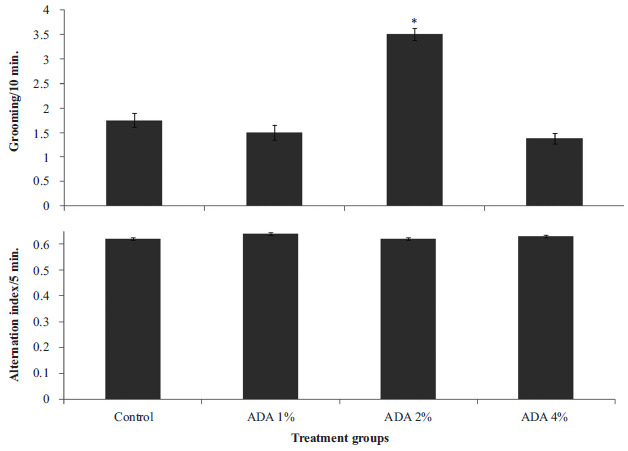
Effect of food-added ADA on grooming behaviour (upper panel) and spatial working memory in the radial arm maze (lower panel). Each bar represents Mean ± S.E.M, *p<0.05 vs. control, number of rats per treatment group =8. ADA: Azodicarbonamide.
3.2.3. Effect of Azodicarbonamide on Y-maze Spatial Working Memory and Arm Entry
Fig. (4) represents the effect of food-added ADA on Y-maze spatial working memory (upper panel) and arm entry (lower panel). Spatial working memory increased significantly {F (3, 28) = 26.10, p<0.001} in group fed ADA diet at 1% compared to control. Arm entry did not differ significantly (F (3, 28) = 0.662, p<0.710) in any of the groups fed the ADA diet compared to control.
Fig. (4).

Effect of food-added ADA on spatial working memory (upper panel) and total arm entry (lower panel) in the Y-maze. Each bar represents Mean ± S.E.M, *p<0.05 vs. control, number of rats per treatment group =8. ADA: Azodicarbonamide.
3.2.4. Effect of Azodicarbonamide on Anxiety-related Behaviours
Fig. (5) represents the effect of food-added ADA on time spent in the open arm (upper panel) and closed arm (lower panel) of the elevated plus maze. Open arm time decreased significantly {F (3, 28) = 18.10, p<0.001} in all groups fed ADA-containing diet compared to control. Closed arm time did not differ significantly (F (3, 28) = 0.212, p<0.10) in any of the groups fed ADA diet compared to control.
Fig. (5).
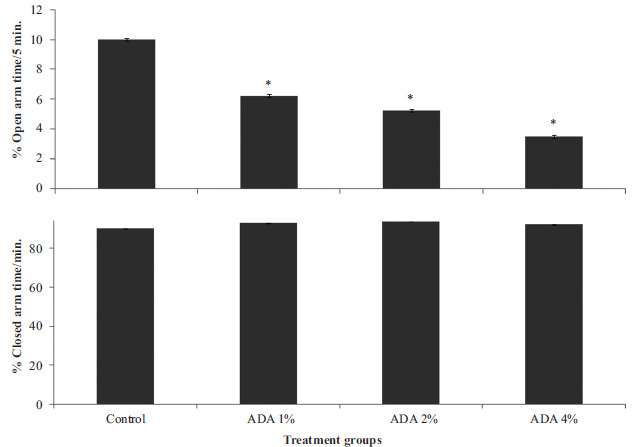
Effect of food-added ADA on time spent in the open arm (upper panel) and closed arm (lower panel) of the elevated plus maze. Each bar represents Mean ± S.E.M, *p<0.05 vs. control, number of rats per treatment group =8. ADA: Azodicarbonamide.
3.3. Effect of Azodicarbonamide on Brain Antioxidant Status and Malondialdehyde Levels
Table 1 shows the effects of food-added ADA on brain antioxidant status and lipid peroxidation levels. There was no significant difference in the activity of superoxide dismutase or brain levels of reduced glutathione, or malondialdehyde in any of the groups fed ADA diet compared to control.
Table 1.
Effect of azodicarbonamide on antioxidant activity and MDA levels.
| Groups | SOD (U/mg) | GSH (nmol/g) | MDA (nmol/mg) |
|---|---|---|---|
| Control | 5.45 ± 0.15 | 122.0± 3.10 | 13.20 ± 0.10 |
| 1% ADA | 5.40 ± 0.12 | 120.0± 3.20 | 12.90 ± 0.24 |
| 2% ADA | 5.50 ± 0.19 | 118.0± 2.15 | 13.10 ± 0.19 |
| 4% ADA | 5.39 ± 0.20 | 119.0± 1.10 | 15.09 ± 1.21 |
Values are presented as mean ± SEM, *p<0.05 significant difference from control, number of animals per group-6, ADA: azodicarbonamide.
3.4. Effect of Azodicarbonamide on Histomorphology of the Cerebral Cortex and Hippocampus
Histological examination of haematoxylin and eosin-stained sections of the rat cerebral cortex (upper panel) revealed non-distinct layers, characteristic cortical cells with varying sizes, and deeply-staining nuclei. At a magnification of x160, the regular three-layered cytoarchitecture of the rat cerebral cortex is observed. The pink-staining background known as the neuropil is interspersed with numerous multipolar shaped cells having large rounded vesicular nuclei (pyramidal cells) and round cells with large open-face nuclei, prominent nucleolus and little cytoplasm (granule cells) as well a supporting neuroglia were observed in groups of animal fed normal diet (Fig. 6A), and azodicarbonamide-containing diet at 1% (Fig. 6B), 2% (Fig. 6C) and 4% (Fig. 6D). Features are keeping with normal histology.
Fig. (6).
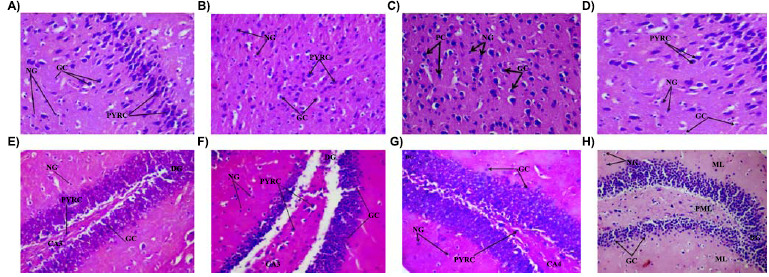
Effect of food added azodicarbonamide on the morphology of the brain [(cerebral cortex; A-D) and (dentate gyrus of the rat hippocampus, E-H)]. A and E (Normal diet control), B and F (1% Azodicarbonamide) C and G (2% Azodicarbonamide) and D and H (4% azodicarbonamide) Representative slides showing the dentate gyrus (Dg), pyramidal cells (PYRC), granule cells (Gc) and Neuroglia (Ng), DG: dentate gyrus, CA4: cornus ammonis 4 H&E x160.
Haematoxylin and eosin stained sections of rat hippocampus (lower panel) showing the dentate gyrus, the molecular layer and the cornus ammonis 4 region were observed in groups fed normal diet (Fig. 6E), and azodicarbonamide-containing diet at 1% (Fig. 6F), 2% (Fig. 6G) and 4% (Fig. 6H). The dentate gyrus is made up of distinct tightly- arranged layers of small granule cell, the molecular layer contains the processes of axons and dendrites, while the cornus ammonis 4 (CA4) region is made up of a few pyramidal cells projecting into the dentate gyrus. The CA4 region is a continuation of the tightly packed layers of pyramidal cells known as cornus ammonis 1-3. All features were in keeping normal histology.
4. DISCUSSION
In this study, the effects of food-added azodicarbonamide on body weight, food intake, open field behaviours, working-memory (in the radial arm and Y-maze), anxiety-related behaviours, brain oxidative stress and morphology of the cerebral cortex and hippocampus in rats were studied. Results showed that ADA-containing food was associated with a) a decrease in body weight and food intake; b) a decrease in horizontal locomotion, and a concentration-related decrease in rearing activity; c) an increase in grooming with ADA diet at 2%; d) an increase in Y maze-spatial working memory at the lowest concentration of ADA; e) an anxiogenic effect; f) there was no alteration in brain antioxidant status or lipid peroxidation levels; and g) No obvious changes in cerebral cortex or hippocampal histomorphology was observed.
In this study, a concentration-related decrease in weight gain and food intake was observed in rats that are fed ADA-containing food, suggesting that the inclusion of ADA in the diet was associated with a reduction in the food consumed by the rats and a corresponding decrease in body weight. While one previous study had reported no significant effect of ADA on body weight [21]; the result of another study revealed that acute (14 days) but not chronic (13 weeks) inhalational exposure to ADA resulted in a decrease in body weight at the highest dose administered ADA [22], (similar reduction in body weight was observed in this study in the second week). The duration of exposure in this study was 28 days and the route of administration was oral; the effects that we observed suggest that the effect of ADA was dependent on both route of administration and duration of exposure. Also, the asso-ciated decrease in food intake could occur possibly because ADA may have altered the flavour and palatability of the diet, as studies have reported that rodents have an aversion for bitter and sour tastes [23, 24]. Therefore, it is possible that alteration in palatability would have made the rats consume less food with a resultant reduction in weight gain as observed in this study.
Assessment of locomotor and rearing activities on the open-field behavior showed that the ADA diet was associated with a concentration-related central inhibitory response, which was most pronounced at 4% concentration. While there is a dearth of scientific information on the effects of ADA on open field behaviours, there have been reports that semi-carbazide, a by-product of ADA has been associated with alterations in exploratory behaviours [25]. It was also observed that at 2%, ADA-containing diet was associated with an increase in grooming behaviour. The results of the open-field behavioural activities show that the addition of ADA to the diet can lead to significant behavioural changes in rats; however, the factors that are responsible for these changes need to be investigated. However, we can speculate that it is possible that ADA can enhance central neurotransmitters such as gamma-aminobutyric acid that can suppress open-field exploratory behaviours, while suppressing excitatory neurotransmitters such as glutamate.
In this study, we also observed that ADA-containing diet was associated with an anxiogenic effect across the concentrations used. Anxiety response in mammals has been linked to an imbalance of neurotransmitters such as glutamate and gamma-aminobutyric acid. Also, the general systemic effect of neurotransmitters such as norepinephrine can lead to anxiety. The anxiogenic response observed in the present study suggests that ADA also has effects on the central mediators of the anxiety response. However, a dearth of previous studies in this regard does not allow for a direct comparison; despite this, it will be interesting to elucidate the mechanisms behind the possible anxiogenic effects of ADA in subsequent studies.
Increased brain cholinergic transmission is known to be associated with improvement in memory [26]. In this study, we observed a significant increase in Y-maze alternation (at the lowest concentration of ADA). However, a further study from our laboratory revealed that at the concentrations used in this study, ADA did not significantly alter brain acetylcholine or acetylcholinesterase activity (data not included).
Finally, in the study, the ADA-containing diet was not associated with significant changes in brain oxidative status, brain lipid peroxidation or brain cortical/hippocampal neuromorphology in rats.
CONCLUSION
In conclusion, our findings from this study suggest that while food-added ADA may be associated with significant behavioural changes in rats; it does not alter the oxidative/lipid peroxidation status of the brain, and has no deleterious effects on the morphology of the brain within the duration of the study. This shows that at the concentrations used in this study, ADA is neither injurious to the brain of rats nor does it affect its antioxidant status.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethic Committee of the Ladoke Akintola University of Technology, Nigeria.
HUMAN AND ANIMAL RIGHTS
No humans were used for studies that are the basis of this research. All the animals were used in accordance with the guidelines for animal care and USED as prescribed by the scientific procedures on living animals, European Council Directive (EU2010/63).
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data sets analyzed during the current study are available from the corresponding author, [O.J. O.] upon request.
FUNDING
None.
ACKNOWLEDGEMENTS
None declared.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.WHO World health Organisation, Food additives fact sheet. 2017.
- 2.Onaolapo A.Y., Onaolapo O.J. Food additives, food and the concept of ‘food addiction’: Is stimulation of the brain reward circuit by food sufficient to trigger addiction? Pathophysiology. 2018;25(4):263–276. doi: 10.1016/j.pathophys.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Omojokun J. Regulation and Enforcement of Legislation on Food Safety. In: Hussaini A.M., editor. In: Nigeria, Mycotoxin and Food Safety in Developing Countries. UK: Intech Open; 2013. [DOI] [Google Scholar]
- 4.Li M., Zhu K-X., Guo X-G., Brijs K., Zhou H-M. Natural additives in wheat‐based pasta and noodle products: Opportunities for enhanced nutritional and functional properties. Compr. Rev. Food Sci. Food Saf. 2014;13:347–357. doi: 10.1111/1541-4337.12066. [DOI] [PubMed] [Google Scholar]
- 5.Landau E. Subway to remove ‘dough conditioner’ chemical from bread. Available from. 2014 https://edition.cnn.com/2014/02/06/health/subway-bread-chemical/index.html
- 6.UK Occupational Health and Safety. Substances causing/worsening asthma. 2013.
- 7.Kimmerle G. Toxicological studies on Porofor ADC, Urazol and plastic foam extracts. Unpublished report, Institute of Toxicology, Bayer AG, Wuppertal-Elberfeld. 1965.
- 8.Gafford F.H., Sharry P.M., Pittman J.A., Jr Effect of azodicarbonamide (1,1′-azobisformamide) on thyroid function. J. Clin. Endocrinol. Metab. 1971;32(5):659–662. doi: 10.1210/jcem-32-5-659. [DOI] [PubMed] [Google Scholar]
- 9.Ash M., Ash I. Handbook of green chemicals. 2nd ed. 2004. [Google Scholar]
- 10.Sahi S.S. In: Bakery Products Science and Technology. 2nd ed. New Jersey, USA: Wiley Online Library; 2014. Ascorbic acid and redox agents in bakery systems. pp. 183–197. [DOI] [Google Scholar]
- 11.Onaolapo A.Y., Onaolapo O.J., Nwoha P.U. Aspartame and the hippocampus: Revealing a bi-directional, dose/time-dependent behavioural and morphological shift in mice. Neurobiol. Learn. Mem. 2017;139:76–88. doi: 10.1016/j.nlm.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Onaolapo A.Y., Onaolapo O.J., Nwoha P.U. Methyl aspartylphenylalanine, the pons and cerebellum in mice: An evaluation of motor, morphological, biochemical, immunohistochemical and apoptotic effects. J. Chem. Neuroanat. 2017;86:67–77. doi: 10.1016/j.jchemneu.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Onaolapo O.J., Adekola M.A., Azeez T.O., Salami K., Onaolapo A.Y. l-Methionine and silymarin: A comparison of prophylactic protective capabilities in acetaminophen-induced injuries of the liver, kidney and cerebral cortex. Biomed. Pharmacother. 2017;85:323–333. doi: 10.1016/j.biopha.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 14.Onaolapo A.Y., Aina O.A., Onaolapo O.J. Melatonin attenuates behavioural deficits and reduces brain oxidative stress in a rodent model of schizophrenia. Biomed. Pharmacother. 2017;92:373–383. doi: 10.1016/j.biopha.2017.05.094. [DOI] [PubMed] [Google Scholar]
- 15.Onaolapo O.J., Onaolapo A.Y., Akanmu M.A., Olayiwola G. Changes in spontaneous working-memory, memory-recall and approach-avoidance following “low dose” monosodium glutamate in mice. AIMS Neurosci. 2016a;3:317–337. doi: 10.3934/Neuroscience.2016.3.317. [DOI] [Google Scholar]
- 16.Onaolapo O.J., Ayanwale T., Agoi O., Adetimehin C., Onaolapo A.Y. Zinc tempers haloperidol-induced behavioural changes in healthy mice. Int. J. Neurosci. Behavioral Sci. 2016;4:21–31. doi: 10.13189/ijnbs.2016.040201. [DOI] [Google Scholar]
- 17.Onaolapo A.Y., Onaolapo O.J. Nevirapine mitigates monosodium glutamate induced neurotoxicity and oxidative stress changes in prepubertal mice. Ann. Med. Res. 2018;25:518–524. doi: 10.5455/annalsmedres.2018.06.118. [DOI] [Google Scholar]
- 18.Onaolapo A.Y., Adebayo A.A., Onaolapo O.J. Oral phenytoin protects against experimental cyclophosphamide-chemotherapy induced hair loss. Pathophysiology. 2018;25:31–39. doi: 10.1016/j.pathophys.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Onaolapo A.Y., Ayeni O.J., Ogundeji M.O., Ajao A., Onaolapo O.J., Owolabi A.R. Subchronic ketamine alters behaviour, metabolic indices and brain morphology in adolescent rats: Involvement of oxidative stress, glutamate toxicity and caspase-3-mediated apoptosis. J. Chem. Neuroanat. 2019;96:22–33. doi: 10.1016/j.jchemneu.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Onaolapo A.Y., Onaolapo O.J., Nwoha P.U. Alterations in behaviour, cerebral cortical morphology and cerebral oxidative stress markers following aspartame ingestion. J. Chem. Neuroanat. 2016;78:42–56. doi: 10.1016/j.jchemneu.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Oser B.L., Oser M., Morgareidge K., Sternberg S.S. Studies of the safety of azodicarbonamide as a flour maturing agent. Toxicol. Appl. Pharmacol. 1965;7:445–472. doi: 10.1016/0041-008X(65)90146-8. [DOI] [PubMed] [Google Scholar]
- 22.Medinsky M.A., Bechtold W.E., Birnbaum L.S., Bond J.A., Burt D.G., Cheng Y.S., Gillett N.A., Gulati D.K., Hobbs C.H., Pickrell J.A. Effect of inhaled azodicarbonamide on F344/N rats and B6C3F1 mice with 2-week and 13-week inhalation exposures. Fundam. Appl. Toxicol. 1990;15(2):308–319. doi: 10.1016/0272-0590(90)90057-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall W.G., Bryan T.E. The ontogeny of feeding in rats: IV. Taste development as measured by intake and behavioral responses to oral infusions of sucrose and quinine. J. Comp. Physiol. Psychol. 1981;95(2):240–251. doi: 10.1037/h0077771. [DOI] [PubMed] [Google Scholar]
- 24.Nachman M. Taste preferences for sodium salts by adrenalectomized rats. J. Comp. Physiol. Psychol. 1962;55:1124–1129. doi: 10.1037/h0041348. [DOI] [PubMed] [Google Scholar]
- 25.Maranghi F., Tassinari R., Lagatta V., Moracci G., Macrì C., Eusepi A., Di Virgilio A., Scattoni M.L., Calamandrei G. Effects of the food contaminant semicarbazide following oral administration in juvenile Sprague-Dawley rats. Food Chem. Toxicol. 2009;47(2):472–479. doi: 10.1016/j.fct.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Degroot A., Parent M.B. Increasing acetylcholine levels in the hippocampus or entorhinal cortex reverses the impairing effects of septal GABA receptor activation on spontaneous alternation. Learn. Mem. 2000;7(5):293–302. doi: 10.1101/lm.32200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets analyzed during the current study are available from the corresponding author, [O.J. O.] upon request.


