Purpose
18F-fluorodeoxiglucose (18F-FDG)-PET/CT has been widely used to evaluate multiple myeloma. 99mTc-sestamibi (MIBI) scintigraphy has also been proposed for assessing multiple myeloma, but its use with state-of-the-art single-photon emission computed tomography/computed tomography (SPECT/CT) technology has not been fully evaluated.This study aimed to compare these two imaging modalities in multiple myeloma staging.
Materials and methods
Sixty-two patients with recently diagnosed multiple myeloma were submitted to whole-body 18F-FDG-PET/CT and whole-body MIBI scans plus SPECT/CT of the chest and abdomen/pelvis. Number of focal lesions, contiguous soft tissue involvement (CSTI), extramedullary lesions (EMLs) and diffuse bone marrow (BM) involvement were recorded.
Results
PET/CT was positive in 59 patients (95%) and MIBI SPECT/CT in 58 (93%) (P = 0.69). MIBI detected more diffuse bone marrow involvement than PET/CT (respectively 78 vs. 58% of the patients), while PET/CT demonstrated more focal lesions than MIBI SPECT/CT (81 vs. 54% of the patients) (P = 0.002). PET/CT detected EMLs in four subjects and MIBI in one subject. CSTI was found in 28 (45%) and 23 (37%) patients on PET/CT and MIBI images, respectively (P = 0.36). Three patients with lytic lesions and no FDG uptake were MIBI positive, and two subjects with lytic lesions without MIBI uptake were FDG positive.
Conclusion
MIBI SPECT/CT performs similarly to 18F-FDG-PET/CT in identifying sites of active disease in multiple myeloma staging. MIBI is more efficient than FDG for detecting the diffuse involvement of bone marrow but less efficient for detecting focal lesions. Some patients presented a ‘mismatch’ pattern of FDG/MIBI uptake.
Keywords: 18F-fluorodeoxyglucose, multiple myeloma, PET-computed tomography, single-photon emission computed tomography, technetium-99m-sestamibi
Introduction
Multiple myeloma is a neoplasm characterized by plasma cell infiltration of the bone marrow, secretion of monoclonal immunoglobulin (paraproteins), and end-organ damage including lytic lesions in the bones [1]. About 80–90% of myeloma patients suffer from osteolytic lesions during the course of the disease [2]. Disease expression is very heterogeneous including diffuse bone marrow infiltration, focal bone lesions, and extramedullary lesions (EMLs).
PET/computed tomography (PET/CT) using 18F-fluorodeoxyglucose (FDG) is a highly sensitive imaging technique for detecting active disease in patients with multiple myeloma and has been widely used in staging and prognosis evaluation of this disease [3]. Like many other nuclear imaging exams, it has the advantages of being performed with whole-body images, absence of serious adverse reactions, the possibility of being performed in patients with renal failure and non-restriction in cases of metallic bone implants. In addition, the images can be semiquantified using the maximum standardized maximum uptake value (SUVmax). However, its routine use is still hampered by several factors, including high cost, reimbursement issues, lack of cost-effectiveness studies, and especially its limited availability in many countries.
99mTc-sestamibi (MIBI) whole-body scintigraphy (WBS) has also been proposed as a potential method for assessing multiple myeloma [3–5] and presents high availability with low costs in most countries. Patients with active multiple myeloma may have focal or diffuse bone MIBI uptake, which indicates worse prognosis with lower overall survival (OS) than patients with normal MIBI images [6–8]. There is also a linear correlation between bone biopsy results and MIBI uptake [9]. Some studies have compared MIBI to FDG PET/CT in multiple myeloma [10,11]; however, in our knowledge, none of them systematically included single-photon emission computed tomography (SPECT)/CT images for this comparation. It has been demonstrated that SPECT/CT increases the sensitivity and specificity of many scintigraphic studies [12–16]. It provides precise localization and characterization of abnormal foci, reducing false positives due to physiologic uptake, influencing the staging and clinical management as well as reader confidence [12–16]. The aim of this study was to compare MIBI SPECT/CT with FDG PET/CT at multiple myeloma staging.
Materials and methods
The local Institutional Review Board approved this prospective study (CAAE number: 24395514.7.0000.5404) of patients with multiple myeloma to undergo whole-body 18F-FDG PET/CT and MIBI WBS plus SPECT/CT images for disease staging, preferably both procedures on the same day.
Sixty-two patients, 33 (53%) male, with newly diagnosed multiple myeloma underwent both studies before treatment. Patients were diagnosed according to the International Myeloma Working Group [17] including bone marrow cytology and histology. Details of patient characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Characteristic | Value |
|---|---|
| Male | 33 (53%) |
| Agea, years | 66 (39–87) |
| Imunoglobulin typeb | |
| IgG | 22 (35%) |
| IgA | 08 (13%) |
| Light chainb | |
| Kappa | 20 (32%) |
| Lambda | 11 (18%) |
| Stage ISS | |
| I | 10 (16%) |
| II | 12 (19%) |
| III | 40 (65%) |
| Albumin, g/dLa | 3.30 (1.3–4.90) |
| Beta2 microglobuline, mg/La | 5.60 (0.26–29.99) |
| Hemoglobulin, g/dLa | 10.0 (4.9–15.2) |
| Calcium, mg/dLa | 9.5 (7.10–15.0) |
| Creatinine, mg/dLa | 1.09 (0.34–9.5) |
| Lactate dehydrogenase, mg/dLa | 198 (48–652) |
| Proteinuria, mga | 530 (50–17310) |
| Plasmocytes, %a | 26 (0–93) |
| Renal failure | 17 (27%) |
ISS, International Staging System.
Median (range).
Not all patients are excretors.
Image acquisition
Patients fasted for 6 h before PET imaging. PET/CT images were acquired 60 min after intravenous administration of 18F-FDG (0.12 mCi/Kg) in patients with glucose level less than 180 mg/dL using a Siemens Biograph True-Point mCT 40 (Siemens Medical Solutions Inc., Knoxville, Tennessee, USA). Intravenous contrast media was not used. CT was acquired with 120–140 kV, 120 mA, rotation time 0.8 s, and slice thickness 2.1 mm. PET images were acquired in three-dimensional (3D) mode using 90 s/bed position. PET images were reconstructed using a standard iterative algorithm (3D-ordered subsets expectation-maximization (OSEM) + point spread function + time-of-flight with two iterations and 21 subsets), with the CT data utilized for attenuation correction and image fusion. MIBI studies were performed by acquiring planar anterior and posterior WBS plus SPECT/CT of the chest and abdomen/pelvis (Fig. 1) 10 min after intravenous injection of 99mTc-sestamibi (0.25 mCi/Kg) by using a dual-head SPECT/CT gamma-camera (Symbia T2, Siemens Medical Solution Inc., Knoxville, Tennessee, USA), equipped with a low-energy high-resolution collimator. SPECT/CT of the head was also acquired when a suspicious lesion was observed in planar images. The projections were reconstructed using iterative OSEM (four subsets, eight interactions), gaussian filter (Full width at half maximum = 6.25 mm), and applying CT attenuation correction. CT images were acquired with 130 kV, 60 mAs, 0.8 s rotation speed, and 3 mm slice thickness.
Fig. 1.
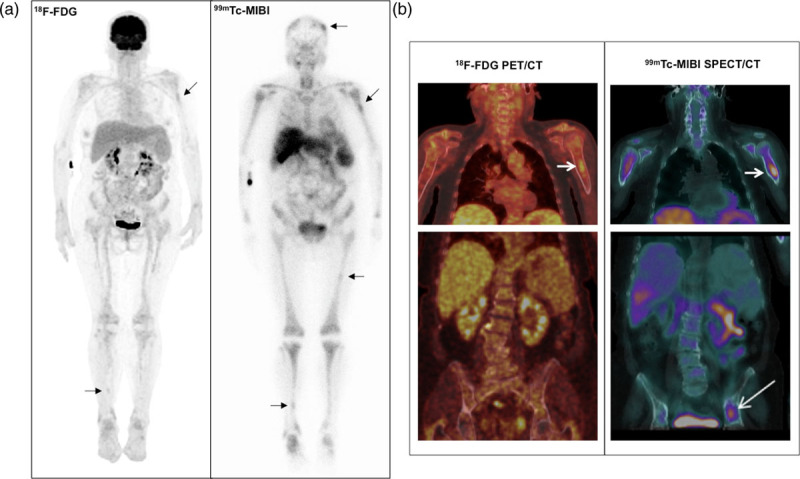
(a) 18F-FDG PET maximum intensity projection (left) and anterior view of 99mTc-MIBI whole-body scan (right) of a 65-year-old woman with multiple myeloma, IgG lambda, ISS 3. Marked diffuse bone marrow uptake is observed in MIBI but not in FDG images. Mild focal uptake is also noted in the left humerus and right tibia (in both FDG and MIBI images), and in the skull and left femur (in MIBI images only) (arrows). The sites of injection are seen on the right forearm in the two scans. (b) Coronal slices of FDG PET/CT (left) and MIBI SPECT/CT (right) fused images of the chest (upper) and abdomen/pelvis (lower) of the same patient. Note the similar aspect of the SPECT/CT and PET/CT images, both with the same ability to identify and delimit the lesion in the left humerus, which does not exceed the limits of the cortical bone (short arrows). An active focal lesion in the left iliac bone was only identified by MIBI SPECT/CT (long arrow). CT, computed tomography; 18F-FDG, 18F-fluorodeoxyglucose; ISS, International Staging System; MIBI, 99mTc-sestamibi; SPECT, single-photon emission computed tomography.
Image interpretation
Images were interpreted by visual analysis. 99mTc-MIBI SPECT/CT and the 18F-FDG PET/CT images were interpreted separately and in consensus by two nuclear medicine physicians and one radiologist. When analyzing the scans, they were aware of clinical data, but blind to the results of the other imaging study.
Criteria for defining the positivity of PET/CT images included: the presence of focal areas of increased tracer uptake in bones or soft tissues (i.e. more intense than background uptake) with or without an underlying lesion identified by CT, and excluding nonpathologic fractures, articular and known infectious processes; diffuse FDG uptake in the bone marrow (≥liver), and osteolytic areas on CT images. MIBI was considered positive if there were: focal areas of radiotracer uptake in bones or soft tissues (i.e. more intense than background uptake) on planar or SPECT images; diffuse uptake in the bone marrow; and osteolytic areas on CT images.
A visual degree of diffuse bone marrow uptake was defined for both radiopharmaceuticals. For diffuse FDG uptake in the bone marrow, the liver was used as reference: similar to or above liver uptake was classified as moderate or marked, respectively (below liver uptake was considered within normal limits). Diffuse bone marrow uptake of MIBI was compared to myocardium: bellow, similar to or above myocardium uptake was classified as mild, moderate, or marked, respectively.
For both imaging modalities, the number of focal lesions, presence of diffuse bone marrow involvement, contiguous soft tissue involvement (CSTI), and EMLs were recorded. Patients with positive studies were grouped as follows: those with focal lesions only, diffuse bone marrow involvement only, or both. The number of focal lesions was classified into three groups: A (1–3 lesions); B (4–10 lesions); C (more than 10 lesions). The SUVmax of the most FDG-avid lesion was recorded for each patient. Baseline clinical data including albumin, beta2 microglobulin, hemoglobin, calcium, creatinine, lactate dehydrogenase, proteinuria, renal failure, and the International Staging System (ISS; I, II or III) were recorded. Renal failure was defined as creatinine >1.3 mg/dL for men and >1.1 mg/dL for women. Proteinuria was established as >500 mg/day of protein in the urine.
Statistical analysis
To compare FDG PET/CT and MIBI SPECT/CT in multiple myeloma staging, the chi-square test was applied for categorical variables, the t-test to compare two groups of continuous variables, and analysis of variance for three or more groups using Bonferroni post-test. The value of P < 0.05 was considered significant, and the SPSS software (IBM Corp., version 24.0, Armonk, New York, USA) was used.
Results
Most patients had both procedures performed on the same day, starting with MIBI SPECT/CT. Therefore, the median time interval between the two imaging modalities was 0 days (0–21).
Detection of active disease
Whole-body FDG PET/CT detected sites of active disease in 59/62 patients (95%) and MIBI SPECT/CT in 58/62 subjects (93%), P = 0.69 (Table 2).
Table 2.
Detection of disease involvement in multiple myeloma using 99mTc-sestamibi planar plus single-photon emission computed tomography/computed tomography and 18F-fluorodeoxyglucose PET/computed tomography
| FDG-PET/CT | MIBI planar + SPECT/CT | |
|---|---|---|
| Detection of any disease involvement (n) | 59 (95%) | 58 (93%) |
| CT lytic lesions without radiotracer uptake (n) | 4 (6%) | 3 (5%) |
| Focal lesions with radiotracer uptake (n) | 19 (31%) | 7 (12%) |
| Diffuse bone marrow involvement (n) | 5 (8%) | 22 (36%) |
| Focal lesions + diffuse bone marrow involvement (n) | 31 (50%) | 26 (42%) |
| Number of focal lesions (n) | ||
| Group A (1–3 lesions) | 11 (17.5%) | 17 (28.5%) |
| Group B (4–10 lesions) | 16 (25.5%) | 6 (9.5%) |
| Group C (>10 lesions) | 23 (38%) | 10 (16%) |
| Contiguous soft tissue involvement (n) | 28 (45%) | 23 (37%) |
| Extra osseous lesions (n) | 4 (6%) | 1 (1.5%) |
| Median SUVmax (range) | 5.3 (1.9–34.7) |
FDG, 18F-fluorodeoxiglucose; MIBI, 99mTc-sestamibi; n = number of patients.
Diffuse bone marrow involvement and focal lesions
Among all PET/CT studies, 5 patients (8%) had only diffused bone marrow involvement, 31 (50%) both focal lesions and diffuse bone marrow involvement, and 19 (31%) had only focal lesions. Considering all MIBI SPECT/CT studies, 22 patients (36%) presented only diffuse bone marrow involvement, 26 (42%) both focal lesions and diffuse bone marrow involvement, and 7 (12%) had only focal lesions, P = 0.002 (Table 2). Therefore, MIBI detected more diffuse bone marrow involvement than PET/CT (respectively 78 vs. 58% of the patients, including those with associated focal lesions), while PET/CT demonstrated more focal lesions than MIBI SPECT/CT (81 vs. 54%, including those with associated diffuse bone marrow involvement). SPET/CT images were not essential to identify diffuse bone marrow MIBI uptake, but they allowed correct identification and localization of focal lesions detected by MIBI (Fig. 1).
Number of focal lesions
FDG PET/CT detected more focal lesions than SPECT/CT. PET/CT had 11, 16, and 24 patients in groups A, B, and C, respectively. MIBI SPECT/CT had 17, 6, and 10 in the same groups, respectively (P < 0.0001). PET/CT detected extraosseous lesions in four patients and MIBI SPECT/CT in one subject (P = 0.17). CSTI was found in 28 (45%) and 23 (37%) patients on FDG PET/CT and MIBI SPECT/CT, respectively (P = 0.36) (Fig. 2).
Fig. 2.
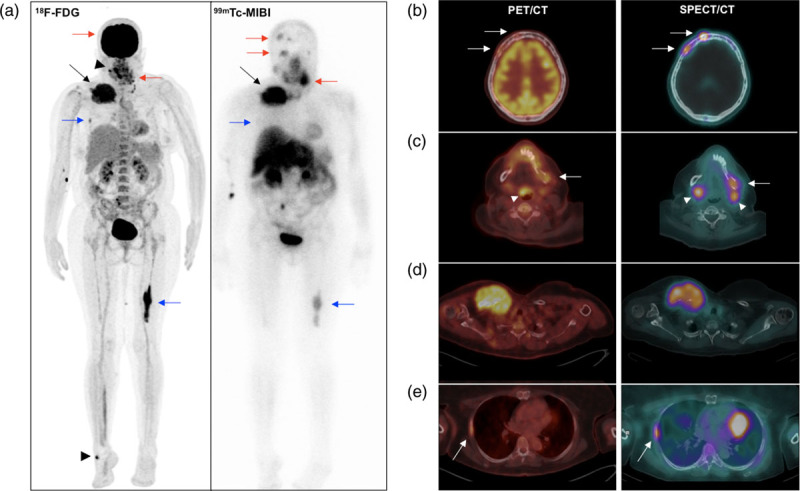
(a) 18F-FDG PET maximum intensity projection (left) and anterior view of 99mTc-MIBI whole-body scan (right) of a 54-year-old woman with multiple myeloma, IGg Kappa, ISS 3. Similar and marked uptake of FDG and MIBI is seen in an extensive mass on the right clavicle (black arrows). MIBI uptake was higher than FDG uptake in lesions of the skull and right ramus of mandible (red arrows). MIBI uptake was lower than FDG uptake in a right rib and distal left femur lesions (blue arrows). Heterogeneous uptake of FDG in the oropharynx/Waldeyer’s ring and right foot was related, respectively, to upper airway infection and skin abscess (arrowheads), both without MIBI uptake. Also note diffuse FDG uptake (without MIBI uptake) in bone marrow, which might be related to multiple myeloma infiltration or reactive bone marrow. The site of injection on the right arm is seen in both images. (b–e) Axial fused FDG PET/CT (left) and MIBI SPECT/CT (right) of the same patient. Observe the similarity of the PET/CT and SPECT/CT images, allowing the precise localization of the lesions in the skull (b), mandible (c), right clavicle with contiguous soft tissue involvement (d) and right rib (e). Also note FDG uptake in the epiglottis due to infection, and physiologic MIBI uptake in the submandibular glands (arrowheads on c). CT, computed tomography; 18F-FDG, 18F-fluorodeoxyglucose; ISS, International Staging System; MIBI, 99mTc-sestamibi; SPECT, single-photon emission computed tomography.
Intensity of diffuse bone marrow activity
Among the 38 patients with diffuse bone marrow involvement on FDG PET/CT, 20 had moderate (similar to liver) and 16 had marked (superior to liver) diffuse bone marrow uptake. Of the subjects with diffuse bone marrow involvement on MIBI, 22 had moderate uptake (similar to myocardium), 5 had marked uptake (above myocardium), and 15 with mild diffuse uptake (bellow myocardium) (Table 3).
Table 3.
Comparison of diffuse bone marrow uptake of 18F-FDG and 99mTc-sestamibi in multiple myeloma
| FDG-PET/CTa n (%) |
Sestamibi planar + SPECT/CTb n (%) | |
|---|---|---|
| Abnormal diffuse bone marrow uptake | 36 (58%) | 48 (78%) |
| Intensity of uptake | ||
| Mild | – | 21 (34%) |
| Moderate | 20 (32.2%) | 22 (36%) |
| Marked | 16 (25.8%) | 5 (8.0%) |
FDG, 18F-fluorodeoxiglucose; MIBI, 99mTc-sestamibi; –, number of patients.
18F-FDG, diffuse bone marrow uptake intensity: moderate (similar to hepatic uptake); marked (distinctly above hepatic uptake). Mild bone marrow uptake (bellow hepatic uptake) was considered normal.
99mTc-sestamibi: diffuse bone marrow uptake intensity: mild (bellow myocardium), moderate (similar to myocardium), marked (above myocardium).
‘Mismatch’ uptake of FDG and MIBI in focal lesions
Four (6%) FDG PET/CT and 3 (5%) MIBI SPECT/CT studies classified as positive due to bone lesions related to multiple myeloma presented only anatomic findings: lytic lesions on CT. Only one of these patients had coincident results between the two examinations, that is, no uptake of any radiopharmaceutical. Three FDG PET/CT studies with solely lytic lesions (no FDG uptake) presented MIBI uptake: one had focal lesion, one diffuse bone marrow involvement, and another focal lesion plus diffuse bone marrow involvement. Two MIBI SPECT/CTs considered positive because of anatomic findings only (no MIBI uptake), presented FDG uptake on PET/CT, respectively, with focal lesions and focal lesions plus diffuse bone marrow involvement. This image pattern of focal uptake of one tracer in the lesion and no or minimal uptake of the other radiotracer was called ‘mismatch pattern’ (Fig. 3). It was also observed that the intensities of uptake of MIBI and FDG are not parallel: some focal lesions with uptake of both tracers had higher uptake of FDG than of MIBI, while in other focal lesions the opposite occurred, in the same patient (Fig. 2).
Fig. 3.
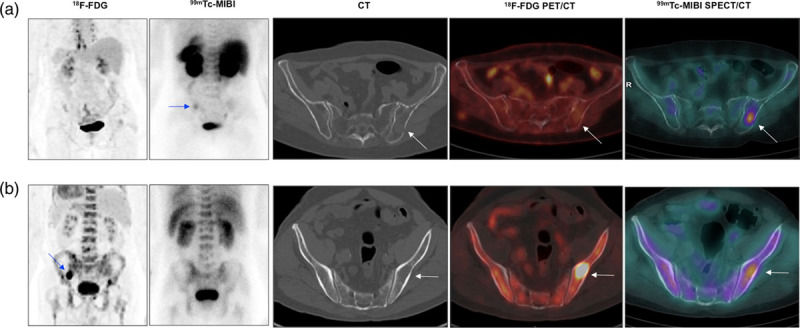
(a) A 52-year-old woman, IgG kappa, ISS 1. From left to right: abdominal/pelvic section of 18F-FDG MIP, posterior MIBI planar view, axial CT, axial fused FDG PET/CT, and axial fused MIBI SPECT/CT. Note the FDG/MIBI ‘mismatch’ in a focal lesion in the left iliac bone with moderate uptake of MIBI detected on planar and, more obviously, on SPECT/CT images, with normal FDG images (arrows). Physiological FDG uptake in brown adipose tissue of the mediastinum is observed in PET MIP and mild diffuse bone marrow uptake is seen in the MIBI planar image. (b) A 67-year-old man, IgG Lambda, ISS 3. From left to right: abdominal/pelvic section of 18F-FDG MIP, posterior MIBI planar view, axial CT, axial fused FDG PET/CT, and axial fused MIBI SPECT/CT. Observe the FDG/MIBI ‘mismatch’ in a focal lytic lesion in the left iliac bone with marked uptake of FDG seen in MIP and fused FDG PET images, with only mild uptake of MIBI visualized in the SPECT/CT image exclusively (arrows). Both 18F-FDG MIP and MIBI planar images also show diffuse uptake of the tracers in the bone marrow. CT, computed tomography; 18F-FDG, 18F-fluorodeoxyglucose; ISS, International Staging System; MIBI, 99mTc-sestamibi; SPECT, single-photon emission computed tomography.
Quantification of lesion uptake
When analyzing SUVmax and number of focal lesions, it was found that the greater the number of focal lesions, the highest the SUVmax value (P < 0.0001). SUVmax values were also higher in patients with CSTI. On the other hand, there was no correlation between the intensity of FDG uptake in bone marrow and SUVmax, nor was SUVmax higher if EMLs were detected.
Other statistic results
In the logistic regression, hemoglobin level was a single independent risk factor for positive PET/CT [Risk ratio (RR): 1.34; 95% CI, 1.19–1.51, P < 0.0001]. The logistic regression also demonstrated that SUVmax was a single independent risk factor for positive SPECT/CT (RR: 1.94; 95% CI, 1.30–2.90, P = 0.001). The other clinical parameters had no relation to any of the two imaging modalities.
Discussion
Imaging is essential for an accurate assessment of multiple myeloma, especially to evaluate the extent of the disease that may modify staging and treatment and also provide prognostic information [1,2].
The results of our study indicate that MIBI SPECT/CT performs similarly to FDG-PET/CT in detecting active disease in multiple myeloma staging. This similarity was particularly evident in the detection of skeletal involvement. There also was no statistical difference in identifying contiguous soft tissue and extraosseous disease between SPECT/CT and PET/CT, although we had only few patients with soft tissue disease. The correspondence between the two methods in the evaluation of CSTI can be explained by the fact that adding hybrid SPECT/CT images to planar MIBI scan allows a more precise lesion delineation and anatomic localization. It also provides a better differentiation between lesions and normal structures with physiologic uptake [18,19] and permits attenuation correction of SPECT data [19]. As demonstrated by several studies in different diseases, SPECT/CT images have greater sensitivity and accuracy when compared to planar images [17–21].
As previously reported, FDG-PET/CT can be used to determine prognosis and risk of fractures in patients with newly diagnosed multiple myeloma [10,20–27]. Higher SUVmax are related to worse outcomes. Similarly, it has been demonstrated that the number of focal lesions detected by MIBI can predict OS and progression free survival, while diffuse bone marrow uptake is an independent predictor of disease progression [10]. In the present study, the subjects who presented the higher number of focal lesions, both on PET/CT as well as on SPECT/CT images, also had the higher values of SUVmax, suggesting that both FDG and MIBI images can be used to evaluate disease aggressiveness.
On the other hand, the pattern of image positivity was interestingly different in the two studies: FDG-PET/CT detected more focal lesions than MIBI SPECT/CT (81 vs. 54%), while MIBI demonstrated the diffuse bone marrow involvement of the disease in more patients than FDG-PET/CT did (78 vs. 58%). The good performance of PET/CT in detecting focal lesions was also noted when classifying the number of lesions detected by each method. Thirty-eight percent of the patients had more than 10 focal lesions on PET/CT, while only 16% of the subjects had the same number of lesions on MIBI. The greater number of lesions detected by PET can be partially attributed to its superior intrinsic resolution when compared to SPECT. Our findings are in line with the results obtained by Fonti et al. [10], although SPECT/CT was not performed in their study. On PET/CT, 15, 32, and 53% of their patients had diffuse bone marrow involvement, focal lesions, and both focal lesions and diffuse bone marrow involvement, respectively. On MIBI 41, 12, and 47% had diffuse bone marrow involvement, focal lesions, and both, respectively.
Our results suggest that the FDG does not perform well in assessing the diffuse involvement of the bone marrow by multiple myeloma. This is at least partially explained by the fact that bone marrow is part of the normal biodistribution of FDG [28,29]. Quantitative methods have been proposed to overcome this issue, but still with some complexity for routine use [30,31]. Bone marrow is not part of the normal biodistribution of MIBI [28] and, as suggested by the present study, it seems to be a good alternative for assessing bone marrow involvement by multiple myeloma.
The differences in the biologic behavior of multiple myeloma lesions are clearly seen in our patients. We found one subject who presented lytic lesions on CT, but no increased uptake on FDG PET or MIBI SPECT, emphasizing the additional role of CT in hybrid methods. Some subjects had radiotracer focal uptake, but no lytic lesion since metabolic changes can precede anatomic lesions. On the other hand, we observed that the intensities of uptake of MIBI and FDG are not parallel. In the same patient, it is possible to find lesions with greater uptake of FDG than of MIBI, and vice-versa.
Those differences in the image patterns of these two functional techniques are probably related to two main aspects: the distinct uptake mechanisms of the radiotracers and the marked genomic heterogeneity of multiple myeloma. FDG uptake occurs via the glucose transporter protein, GLUT-1, and reflects the increased glycolysis in tumor cells and, thus, the rapid growth and invasive characteristics of focal lesions. Further, the inflammation that can be associated with tumor proliferation may also contribute to increased FDG uptake [11]. The mechanism of MIBI uptake relates to high-negative transmembrane potentials, with increased uptake in normal and malignant cells that have increased metabolic activity and activated mitochondria. Indeed, in myeloma, MIBI uptake reflects both activated mitochondria and the density of malignant plasma cells [29].
Those striking differences in uptake mechanisms are probably enlightening the heterogeneous biologic behavior of multiple myeloma. Indeed, Rashe et al. [32] recently described an expressive inter-patient and intra-lesion heterogeneity of multiple myeloma. Cytogenetic analyses have shown that multiple myeloma is not a single disease, but it presents with unique features at the molecular level in each patient [33].
The main limitation of our study was the impossibility of performing multiple biopsies of lesions in our facilities as reported by other authors [32]. It would be very clarifying to know the different genetic aspects of lesions that uptake FDG or MIBI or both or none of them. A larger cohort including correlation with the patient’s outcome analysis would also bring important information about the state of the art FDG PET/CT and MIBI SPECT/CT used in the present study. The comparison of the two methods to assess the response to treatment would also define if they could be used interchangeably or if they would have a complementary role in evaluating multiple myeloma.
Conclusion
MIBI SPECT/CT and FDG PET/CT frequently present different imaging patterns in patients with multiple myeloma. However, both imaging modalities had similar performance in detecting active disease in the present study. Some patients present a ‘mismatch’ pattern, with lesions that concentrate one of the tracers but not the other. They might have a complementary role in newly diagnosed multiple myeloma, FDG PET/CT detecting more focal lesions and MIBI SPECT/CT more often identifying diffuse involvement of the bone marrow.
Acknowledgements
We thank the Nuclear and Energy Research Institute (IPEN-CNEN) São Paulo, Brazil, for kindly supplying radiopharmaceuticals used in the present project (IPEN/UNICAMP agreement No. 01342000458/2017-15). We are grateful for financial support from FAPESP (Fundação de Amparo a Pesquisa do Estado de São Paulo), grant numbers 2009/54065-0 and 2018/00654-4. This study was also financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. CDR has a research grant from CNPq (National Council of Research) proc 311841/2018-0. ILM has a research grant from CNPq (National Council of Research) proc 305110/2018-7.
This study was funded by (1) Energy Research Institute – IPEN, São Paulo, Brazil, (IPEN/UNICAMP agreement No. 01342000458/2017-15); (2) Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (grant numbers 2009/54065-0, 2018/14132-0 and 2018/00654-4); (3) Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil – CAPES (finance code 001); and (4) Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (grant number 311841/2018-0).
Conflicts of interest
There are no conflicts of interest.
References
- 1.International Myeloma Working G. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003; 121:749–757 [PubMed] [Google Scholar]
- 2.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004; 350:1655–1664 [DOI] [PubMed] [Google Scholar]
- 3.el-Shirbiny AM, Yeung H, Imbriaco M, Michaeli J, Macapinlac H, Larson SM. Technetium-99m-MIBI versus fluorine-18-FDG in diffuse multiple myeloma. J Nucl Med. 1997; 38:1208–1210 [PubMed] [Google Scholar]
- 4.Pace L, Perrone-Filardi P, Mainenti P, Prastaro M, Vezzuto P, Varrone A, et al. Combined evaluation of rest-redistribution thallium-201 tomography and low-dose dobutamine echocardiography enhances the identification of viable myocardium in patients with chronic coronary artery disease. Eur J Nucl Med. 1998; 25:744–750 [DOI] [PubMed] [Google Scholar]
- 5.Luthra K, Bhave A, Lele RD. Tc 99m sestamibi scanning in multiple myeloma–a new look with SPECT-CT. J Assoc Physicians India. 2014; 62:801–812 [PubMed] [Google Scholar]
- 6.Bacovsky J, Myslivecek M, Scudla V, Koranda P, Buriankova E, Minarik J, et al. Tc-99m MIBI scintigraphy in multiple myeloma: prognostic value of different Tc-99m MIBI uptake patterns. Clin Nucl Med. 2010; 35:667–670 [DOI] [PubMed] [Google Scholar]
- 7.Fonti R, Del Vecchio S, Zannetti A, De Renzo A, Catalano L, Pace L, et al. Functional imaging of multidrug resistant phenotype by 99mTc-MIBI scan in patients with multiple myeloma. Cancer Biother Radiopharm. 2004; 19:165–170 [DOI] [PubMed] [Google Scholar]
- 8.Martín MG, Romero Colás MS, Dourdil Sahún MV, Olave P, Alba PR, Banzo JB. BaselinTc99-MIBI scanning predicts survival in multiple myeloma and helps to differentiate this disease from monoclonal gammopathy of unknown significance. Haematologica. 2005; 90:1141–1143 [PubMed] [Google Scholar]
- 9.Khalafallah AA, Snarski A, Heng R, Hughes R, Renu S, Arm J, et al. Assessment of whole body MRI and sestamibi technetium-99m bone marrow scan in prediction of multiple myeloma disease progression and outcome: a prospective comparative study. BMJ Open. 2013; 10:e002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fonti R, Pace L, Cerchione C, Catalano L, Salvatore B, De Luca S, et al. 18F-FDG PET/CT, 99mTc-MIBI, and MRI in the prediction of outcome of patients with multiple myeloma: a comparative study. Clin Nucl Med. 2015; 40:303–308 [DOI] [PubMed] [Google Scholar]
- 11.Fonti R, Salvatore B, Quarantelli M, Sirignano C, Segreto S, Petruzziello F, et al. 18F-FDG PET/CT, 99mTc-MIBI, and MRI in evaluation of patients with multiple myeloma. J Nucl Med. 2008; 49:195–200 [DOI] [PubMed] [Google Scholar]
- 12.Trogrlic M, Težak S. Incremental value of 99mTc-HYNIC-TOC SPECT/CT over whole-body planar scintigraphy and SPECT in patients with neuroendocrine tumours. Nuklearmedizin. 2017; 56:97–107 [DOI] [PubMed] [Google Scholar]
- 13.Siddique M, Nawaz MK, Bashir H. The usefulness of SPECT/CT in sentinel node mapping of early stage breast cancer patients showing negative or equivocal findings on planar scintigraphy. Asia Ocean J Nucl Med Biol. 2018; 6:80–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu B, Servaes S, Zhuang H. SPECT/CT MIBG imaging is crucial in the follow-up of the patients with high-risk neuroblastoma. Clin Nucl Med. 2018; 43:232–238 [DOI] [PubMed] [Google Scholar]
- 15.Naumann CM, Colberg C, Jüptner M, Marx M, Zhao Y, Jiang P, et al. Evaluation of the diagnostic value of preoperative sentinel lymph node (SLN) imaging in penile carcinoma patients without palpable inguinal lymph nodes via single photon emission computed tomography/computed tomography (SPECT/CT) as compared to planar scintigraphy. Urol Oncol. 2018; 36:92.e17–92.e24 [DOI] [PubMed] [Google Scholar]
- 16.Rager O, Nkoulou R, Exquis N, Garibotto V, Tabouret-Viaud C, Zaidi H, et al. Whole-body SPECT/CT versus planar bone scan with targeted SPECT/CT for metastatic workup. Biomed Res Int. 2017; 2017:7039406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landgren O, Rajkumar SV. New developments in diagnosis, prognosis, and assessment of response in multiple myeloma. Clin Cancer Res. 2016; 22:5428–5433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huellner MW, Strobel K. Clinical applications of SPECT/CT in imaging the extremities. Eur J Nucl Med Mol Imaging. 2014; 41Suppl 1S50–S58 [DOI] [PubMed] [Google Scholar]
- 19.Israel O, Pellet O, Biassoni L, De Palma D, Estrada-Lobato E, Gnanasegaran G, et al. Two decades of SPECT/CT – the coming of age of a technology: an updated review of literature evidence. Eur J Nucl Med Mol Imaging. 2019; 46:1990–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamagni E, Patriarca F, Nanni C, Zannetti B, Englaro E, Pezzi A, et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011; 118:5989–5995 [DOI] [PubMed] [Google Scholar]
- 21.Bartel TB, Haessler J, Brown TL, Shaughnessy JD, Jr, van Rhee F, Anaissie E, et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009; 114:2068–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zamagni E, Nanni C, Mancuso K, Tacchetti P, Pezzi A, Pantani L, et al. PET/CT improves the definition of complete response and allows to detect otherwise unidentifiable skeletal progression in multiple myeloma. Clin Cancer Res. 2015; 21:4384–4390 [DOI] [PubMed] [Google Scholar]
- 23.Patriarca F, Carobolante F, Zamagni E, Montefusco V, Bruno B, Englaro E, et al. The role of positron emission tomography with 18F-fluorodeoxyglucose integrated with computed tomography in the evaluation of patients with multiple myeloma undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2015; 21:1068–1073 [DOI] [PubMed] [Google Scholar]
- 24.Lapa C, Lückerath K, Malzahn U, Samnick S, Einsele H, Buck AK, et al. 18 FDG-PET/CT for prognostic stratification of patients with multiple myeloma relapse after stem cell transplantation. Oncotarget. 2014; 5:7381–7391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chantry A, Kazmi M, Barrington S, Goh V, Mulholland N, Streetly M, et al. ; British Society for Haematology Guidelines. Guidelines for the use of imaging in the management of patients with myeloma. Br J Haematol. 2017; 178:380–393 [DOI] [PubMed] [Google Scholar]
- 26.Sonmez M, Akagun T, Topbas M, Cobanoglu U, Sonmez B, Yilmaz M, et al. Effect of pathologic fractures on survival in multiple myeloma patients: a case control study. J Exp Clin Cancer Res. 2008; 27:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terpos E, Ntanasis-Stathopoulos I, Dimopoulos MA. Myeloma bone disease: from biology findings to treatment approaches. Blood. 2019; 133:1534–1539 [DOI] [PubMed] [Google Scholar]
- 28.Maffioli L, Steens J, Pauwels E, Bombardieri E. Applications of 99mTc-sestamibi in oncology. Tumori. 1996; 82:12–21 [DOI] [PubMed] [Google Scholar]
- 29.Mileshkin L, Blum R, Seymour JF, Patrikeos A, Hicks RJ, Prince HM. A comparison of fluorine-18 fluoro-deoxyglucose PET and technetium-99m sestamibi in assessing patients with multiple myeloma. Eur J Haematol. 2004; 72:32–37 [DOI] [PubMed] [Google Scholar]
- 30.Takahashi MES, Mosci C, Souza EM, Brunetto SQ, Etchebehere E, Santos AO, et al. Proposal for a quantitative 18F-FDG PET/CT metabolic parameter to assess the intensity of bone involvement in multiple myeloma. Sci Rep. 2019; 9:16429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi MES, Mosci C, Souza EM, Brunetto SQ, de Souza C, Pericole FV, et al. Computed tomography-based skeletal segmentation for quantitative PET metrics of bone involvement in multiple myeloma. Nucl Med Commun. 2020; 41:377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasche L, Kortum KM, Raab MS, Weinhold N. The impact of tumor heterogeneity on diagnostics and novel therapeutic strategies in multiple myeloma. Int J Mol Sci. 2019; 20:1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawyer JR. The prognostic significance of cytogenetics and molecular profiling in multiple myeloma. Cancer Genet. 2011; 204:3–12 [DOI] [PubMed] [Google Scholar]


