Abstract
Lung point-of-care ultrasound (POCUS) has been shown to be useful for identifying pulmonary pathology in adult patients with coronavirus disease 2019 (COVID-19). However, pediatric literature for POCUS in COVID-19 is limited. The objective of this case series was to describe lung POCUS findings in pediatric patients with COVID-19. Three patients with COVID-19 who had lung POCUS performed in a pediatric emergency department were included. Point-of-care ultrasound revealed bilateral abnormalities in all patients, including pleural line irregularities, scattered and coalescing B-lines, consolidations, and pleural effusions. Additional pediatric studies are necessary to gain a broader understanding of COVID-19's sonographic appearance in this age group and to determine whether POCUS may be helpful to facilitate diagnosis and expedite management decisions.
Key Words: coronavirus, COVID-19, lung, POCUS, ultrasound
The coronavirus disease 2019 (COVID-19) pandemic highlights the utility of lung point-of-care ultrasound (POCUS). POCUS is an ideal modality for rapid screening during a pandemic given its portability, direct application at the bedside, and ease of disinfection of ultrasound machines.1,2 In addition, after the initial investment in purchasing an ultrasound machine, its relatively low cost makes POCUS an optimal choice for imaging in low-resource settings worldwide.
There are several adult case series describing the sonographic pulmonary features in patients positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.3–8 In contrast, the pediatric literature is limited.9–11 Although the disease has not been as severe in children, cases of respiratory failure requiring intensive care and intubation have been reported.12 This case series describes sonographic pulmonary features in patients with severe COVID-19 across a spectrum of ages. All patients had positive SARS-CoV-2 nasopharyngeal polymerase chain reaction tests, but the results were not available until after POCUS studies were performed.
Case 1
A 9-year-old overweight girl with fragile X syndrome and intermittent asthma presented with fever, cough, increased respiratory effort, diarrhea, and posttussive vomiting. Her fever and cough developed 7 days before presentation. Her primary care provider diagnosed acute otitis media and prescribed amoxicillin 2 days before she came to the emergency department (ED). On the day of presentation to the ED, she developed respiratory distress.
Her initial vital signs were a temperature of 39.5°C, heart rate (HR) of 147 beats per minute, blood pressure (BP) of 120/74 mm Hg, respiratory rate (RR) of 32 breaths per minute, and pulse oximetry (Spo2) of 85% on room air. Physical examination findings were significant for a tired appearing girl with diminished aeration in the right lower lung field without wheezes, rales, or rhonchi. Laboratory test results are displayed in Table 1. Given her history of asthma, albuterol and ipratropium were administered without improvement.
TABLE 1.
Laboratory Results
| Laboratory Test | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| WBC count, ×103/μL | 3.3 | 11.2 | 7.2 |
| Hemoglobin, g/dL | 12.2 | 11.0 | 16.4 |
| Platelet count, ×103/μL | 227 | 128 | 184 |
| CRP, HS, mg/dL | 139.9 | 37.2 | 8.3 |
| Procalcitonin, ng/mL | 0.73 | 0.31 | 0.08 |
| Ferritin, ng/mL | 351 | 251 | 416 |
| D-dimer, μg/mL | 1.79 | NA | <0.28 |
CRP, HS indicates C-reactive protein, high sensitivity; NA, not available; WBC, white blood cell.
POCUS was used to evaluate for pulmonary pathology and revealed abnormalities in bilateral posterior lung fields. A consolidation with air bronchograms spanning 4 rib spaces was identified in the right posterior lung fields (Fig. 1 and Video 1, Supplemental Digital Content 1, http://links.lww.com/PEC/A607). Scattered B-lines and a trace pleural effusion were visualized in the left posterior lung fields (Fig. 1). Areas of normal-appearing lung were also present in the left posterior lung fields. The anterior and lateral lung fields were not scanned. These findings were interpreted as evidence of pneumonia. A portable chest radiograph was interpreted by an attending pediatric radiologist as atelectasis of the left upper, left lower, right middle, and right lower lobes without a pleural effusion, but a superimposed pneumonia could not be excluded (Fig. 1).
FIGURE 1.
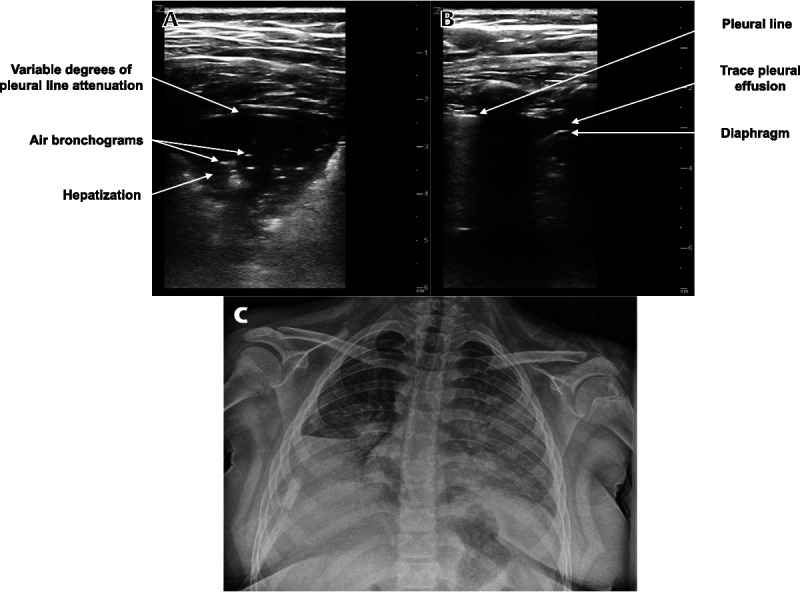
Lung POCUS and chest radiograph of a 9-year-old girl with respiratory distress. A, Transverse image of a right posterior lung field showing a consolidation with air bronchograms and variable degrees of pleural line attenuation. B, Longitudinal image of a left posterior lung field showing a trace pleural effusion. C, Portable chest radiograph showing left upper and right lower lobe atelectasis, partial left lower and right middle lobe atelectasis, and no pleural effusion, but a superimposed pneumonia could not be excluded.
The decision was made to start noninvasive ventilation with continuous positive airway pressure after POCUS was performed. This was quickly escalated in the ED to bilevel positive airway pressure because of worsening respiratory distress. The patient was admitted to the pediatric intensive care unit (PICU) and intubated the next morning for acute respiratory failure. Treatments included broad-spectrum antibiotics and hydroxychloroquine. She remained intubated for 10 days after which she was successfully extubated and discharged on the 16th day of her hospitalization.
Case 2
A 12-year-old boy with hemoglobin SC disease and a history of acute chest syndrome presented with fever, cough, and sore throat. He was evaluated in the ED 2 days before presentation for left upper quadrant abdominal pain that had been present for 3 days. During that initial visit, a complete blood count revealed a white blood cell count of 7.4 × 103/μL, hemoglobin of 10.6 g/dL (baseline), and platelet count of 162 × 103/μL. An abdominal ultrasound was performed by the radiology department and showed borderline splenomegaly. He was discharged home and his abdominal pain resolved.
Two days later, he developed a fever of 38.3°C, fatigue, sore throat, and cough. He returned to the ED for evaluation. His initial vital signs were a temperature of 39.2°C, HR of 116 beats per minute, BP of 114/74 mm Hg, RR of 36 breaths per minute, and Spo2 of 97%. Physical examination findings were significant for a tired but alert and interactive boy with decreased aeration in the right lung without wheezes, rales, or rhonchi. Laboratory test results are displayed in Table 1.
POCUS revealed abnormalities in bilateral lung fields. A consolidation with air bronchograms spanning 5 rib spaces was identified in the left posterior lung fields (Fig. 2 and Video 2, Supplemental Digital Content 2, http://links.lww.com/PEC/A608). Pleural line fragmentation and a small subpleural consolidation were seen in the left lateral lung fields. Areas of pleural line fragmentation and normal areas of lung were present in the left anterior lung fields. The right anterior, lateral, and posterior lung fields had scattered and coalescing B-lines, pleural line fragmentation, and small subpleural consolidations (Fig. 2). A trace pleural effusion was identified in the right anterior and lateral lung fields. Areas of normal lung were present in the right posterior and lateral lung fields as well. These findings were interpreted as evidence of pneumonia. A portable chest radiograph was interpreted by an attending pediatric radiologist as a retrocardiac opacity, representing atelectasis or pneumonia, without a pleural effusion (Fig. 2).
FIGURE 2.
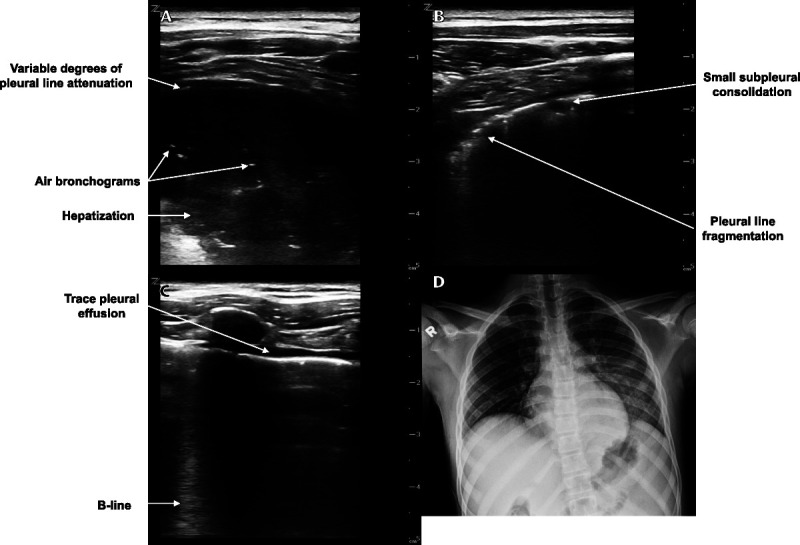
Lung POCUS and chest radiograph of a 12-year-old boy with fever and cough. A, Transverse image of a left posterior lung field showing a consolidation with air bronchograms and variable degrees of pleural line attenuation. B, Transverse image of a right posterior lung field showing pleural line irregularities including a small subpleural consolidation and pleural line fragmentation. C, Longitudinal image of a right anterior lung field showing a B-line and a trace pleural effusion. D, Portable chest radiograph showing a retrocardiac opacity and no pleural effusion.
The patient was treated with antibiotics for acute chest syndrome. He became hypoxic with Spo2 readings in the low 90s and developed respiratory distress in the ED. Bilevel positive airway pressure was initiated, and he was admitted to the PICU. The next morning, he was intubated for acute respiratory failure. He was treated with hydroxychloroquine, prophylactic enoxaparin, and an exchange transfusion. Shortly after his exchange transfusion, he became hypotensive and developed acute kidney injury. Antibiotic coverage was broadened for suspected sepsis. On the fifth day of his hospitalization, he experienced an acute hypoxic event that resulted in cardiac arrest, and he died despite attempts at cardiopulmonary resuscitation.
Case 3
A 19-year-old obese man presented with fever, chest pain, cough, shortness of breath, and diarrhea. His illness began 7 days before presentation with fever, cough, and diarrhea. He then developed chest pain that worsened the morning of presentation along with the onset of shortness of breath.
His initial vital signs in the ED were a temperature of 37.5°C, HR of 117 beats per minute, BP of 140/91 mm Hg, RR of 26 breaths per minute, and Spo2 of 97%. His Spo2 reading decreased to 94% with exertion. Physical examination findings were significant for a tired but nontoxic appearing man with diminished aeration in bilateral lung fields without wheezes, rales, or rhonchi. Laboratory test results are displayed in Table 1.
POCUS revealed abnormalities in bilateral lung fields. Multiple areas of coalescing B-lines and small subpleural consolidations were visualized in the right superior anterior lung field (Fig. 3 and Video 3, Supplemental Digital Content 3, http://links.lww.com/PEC/A609). These findings were interpreted as a focal sonographic pattern of interstitial syndrome in the right lung. In addition, bilateral trace pleural effusions were present. The remainder of the lung fields demonstrated normal findings. A portable chest radiograph was interpreted by an attending pediatric radiologist as bilateral ill-defined hazy opacities without a pleural effusion (Fig. 3).
FIGURE 3.
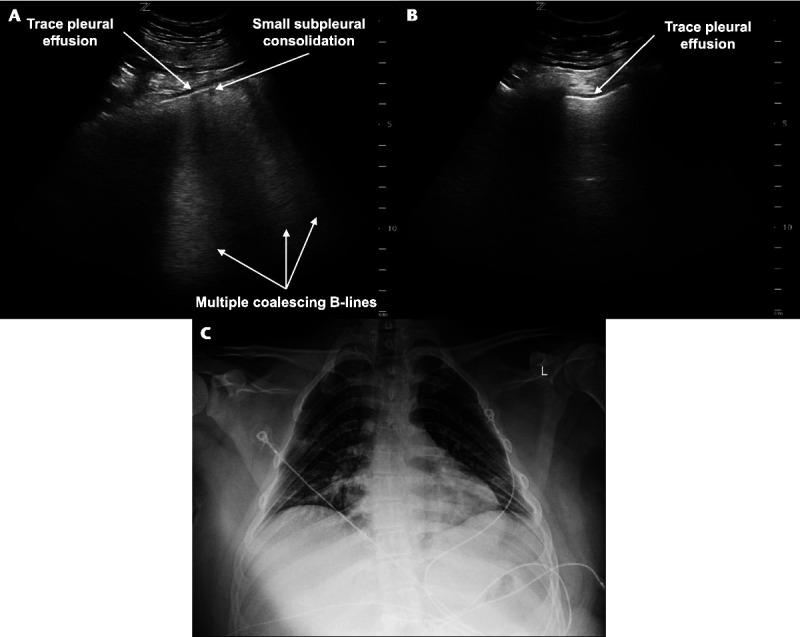
Lung POCUS and chest radiograph of a 19-year-old man with fever and shortness of breath. A, Transverse image of a right anterior lung field showing multiple coalescing B-lines, a small subpleural consolidation, and a trace pleural effusion. B, Longitudinal image of a left lateral lung field showing a trace pleural effusion. C, Portable chest radiograph showing bilateral ill-defined hazy opacities and no pleural effusion.
Supplemental oxygen via nasal cannula was administered for comfort, and the patient was admitted to the general inpatient unit for observation. The next day, he was transferred to the PICU and intubated for acute respiratory failure. He received hydroxychloroquine, remdesivir, methylprednisolone, broad-spectrum antibiotics, and prophylactic enoxaparin. His hospital course was complicated by methicillin-sensitive Staphylococcus aureus bacteremia. He was extubated on hospital day 44 and discharged home on hospital day 67.
Technique
Scans were performed using Mindray (Z.One PRO; Mindray North America, Mahwah, NJ, USA) ultrasound machines by pediatric emergency medicine attending physicians and fellows trained in lung POCUS. Physicians donned personal protective equipment before entering patients' rooms. The machines were covered in disposable, plastic drapes to minimize contamination. A small hole was cut into each drape for the transducer to pass through. Either the 5- to 10-MHz linear transducer or the 3- to 9-MHz curvilinear transducer was used, depending on body habitus, with preference given to the linear transducer as it provides better probe-to-skin contact and higher resolution of the pleura.
The hemithoraces were visualized in the anterior, lateral, and posterior regions. Each region was scanned from lung apex to base in a single sagittal or coronal plane with the transducer oriented to the body longitudinally and then transversely or obliquely by positioning it along the intercostal spaces.13 After a scan was completed, the plastic drape was discarded in the room and the machine was cleaned with disinfecting wipes. Depending on patient age, cooperation, and healthcare provider experience with lung POCUS, approximately 10 to 15 minutes is required from the time the study begins to completion of ultrasound machine disinfection.
Review of the Literature
The morbidity and mortality of SARS-CoV-2 infection in the pediatric population have been lower than those in adults.12 Dong et al. showed that among children with symptoms of COVID-19, 5.3% had dyspnea or hypoxia and 0.6% developed acute respiratory distress syndrome (ARDS) or multiorgan system dysfunction. We present a case series of 3 patients evaluated in a pediatric ED during the pandemic who had lung POCUS performed and were found to be positive for SARS-CoV-2. All cases progressed to require critical care management.
Lung POCUS identified bilateral disease, commonly seen in adults, in each patient.14,15 The characteristic feature of COVID-19 pneumonia on computed tomography scan reported in the adult literature is the presence of ground-glass opacities with a peripheral distribution.16 Sonographically, ground-glass opacities are seen as coalescing B-lines, which were identified in 2 of our patients.6,7 Notably, B-lines are a nonspecific reverberation artifact that may be caused by multiple lung pathologies including pneumonia, pneumonitis, atelectasis, pulmonary contusion or infarction, pleural disease, and neoplasia.17
Areas of multiple B-lines interspersed with areas of spared, normal lung is commonly encountered in adult patients with COVID-19.5 Two patients in this series had this pattern. Unlike the homogeneous distribution of B-lines that is seen with cardiogenic pulmonary edema, acute lung injury (ALI)/ARDS presents with spared areas of lung. In addition, while cardiogenic pulmonary edema normally has no associated pleural line irregularities, ALI/ARDS almost always manifests with pleural line abnormalities such as fragmentation or small subpleural consolidations, as seen in our patients.17
Consolidations spanning multiple rib spaces were identified by POCUS in 2 of the patients. Characteristic sonographic features of consolidations seen in our patients include hepatization, air bronchograms, and variable degrees of pleural line attenuation.13 The latter is a decrease in echogenicity of the pleural line associated with consolidations. Lung POCUS has been shown to have excellent sensitivity and specificity, with better sensitivity than chest radiograph, for the diagnosis of pneumonia in children.18,19 Considering these test characteristics, lung POCUS may reduce the need for radiographs in patients under investigation for COVID-19 and thereby decrease the number of radiologic technologists exposed to SARS-CoV-2. However, an increased exposure time is necessary for the healthcare provider performing the POCUS study.
There is currently no pathognomonic feature identified by lung POCUS for COVID-19. However, the pathology identified can be used to assess the burden of pulmonary disease and may help direct management. As there have been reported cases of COVID-19 myocarditis, recognizing a pattern of areas of B-lines mixed with areas of spared, normal lung and the presence of pleural line irregularities can help healthcare providers distinguish ALI/ARDS from cardiogenic pulmonary edema.20,21 In addition, pleural effusions caused by COVID-19 have been reported in adult and pediatric patients, which POCUS can reliably identify.22–25
As SARS-CoV-2 is highly infectious and can be transmitted by respiratory droplets as well as by contact with infected surfaces, it is important to take precautions when evaluating patients under investigation for COVID-19 with POCUS. To minimize the use of personal protective equipment and the number of times healthcare staff enter a patient's room, lung POCUS can be combined with standard clinical care by bringing the ultrasound machine into the examination room for use during the initial assessment of the patient. It may be helpful to have the most trained person perform the lung POCUS in an expeditious manner. Lastly, disinfection of the ultrasound machine is critical, and use of handheld ultrasound machines may help as they are smaller and easier to disinfect.
CONCLUSIONS
We report several cases of patients with COVID-19 who had lung POCUS performed in a pediatric ED for clinical care. Sonographic pulmonary features in these patients were similar to those described in the adult literature. Bilateral findings including pleural line irregularities such as fragmentation and small subpleural consolidations, scattered and coalescing B-lines, consolidations with air bronchograms, and pleural effusions were identified. Additional pediatric studies are necessary to gain a broader understanding of COVID-19's sonographic appearance in this age group and to determine whether POCUS may be helpful to facilitate diagnosis and expedite management decisions.
Footnotes
Disclosure: The authors declare no conflict of interest.
Informed consent was obtained from all parents/guardians and is available upon request.
REFERENCES
- 1.American College of Emergency Physicians ACEP guideline on COVID-19: ultrasound machine and transducer cleaning. Available at: https://www.acep.org/globalassets/new-pdfs/guideline-on-covid-19--ultrasound-machine-and-transducer-cleaning_policy_033120.pdf. Accessed July 20, 2020. [DOI] [PMC free article] [PubMed]
- 2.Kim DJ Jelic T Woo MY, et al. Just the facts: recommendations on point-of-care ultrasound use and machine infection control during the coronavirus disease 2019 pandemic. CJEM. 22:445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Y Wang S Liu Y, et al. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non-critical novel coronavirus pneumonia (COVID-19). Available at: https://ssrn.com/abstract=3544750. Accessed May 5th, 2020.
- 4.Buonsenso D Piano A Raffaelli F, et al. Point-of-care lung ultrasound findings in novel coronavirus disease-19 pneumoniae: a case report and potential applications during COVID-19 outbreak. Eur Rev Med Pharmacol Sci. 2020;24:2776–2780. [DOI] [PubMed] [Google Scholar]
- 5.Poggiali E Dacrema A Bastoni D, et al. Can lung US help critical care clinicians in the early diagnosis of novel coronavirus (COVID-19) pneumonia? Radiology. 2020;295:E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng QY, Wang XT, Zhang LN. Chinese Critical Care Ultrasound Study Group (CCUSG). Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020;46:849–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vertrugno L Bove T Orso D, et al. Our Italian experience using lung ultrasound for identification, grading and serial follow-up of severity of lung involvement for management of patients with COVID-19. Echocardiography. 2020;37:625–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soldati G Smargiassi A Inchingolo R, et al. Proposal for international standardization of the use of lung ultrasound for patients with COVID-19; a simple, quantitative, reproducible method. J Ultrasound Med. 2020;39:1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denina M Scolfaro C Silvestro E, et al. Lung ultrasound in children with COVID-19. Pediatrics. 2020;146:e20201157. [DOI] [PubMed] [Google Scholar]
- 10.Gregorio-Hernandez R Escobar-Izquierdo AB Cobas-Pazos J, et al. Point-of-care lung ultrasound in three neonates with COVID-19. Eur J Pediatr. 2020;179:1279–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musolino AM Supino MC Buonsenso D, et al. Lung ultrasound in children with COVID-19: preliminary findings. Ultrasound Med Biol. 2020;46:2094–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong Y Mo X Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. [DOI] [PubMed] [Google Scholar]
- 13.Copetti R, Cattarossi L. Ultrasound diagnosis of pneumonia in children. Radiol Med. 2008;113:190–198. [DOI] [PubMed] [Google Scholar]
- 14.Shi H Han X Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernheim A Mei X Huang M, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295:200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye Z Zhang Y Wang Y, et al. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020;30:4381–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volpicelli G Elbarbary M Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–591. [DOI] [PubMed] [Google Scholar]
- 18.Pereda MA Chavez MA Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135:714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balk DS Lee C Schafer J, et al. Lung ultrasound compared to chest X-ray for diagnosis of pediatric pneumonia: a meta-analysis. Pediatr Pulmonol. 2018;53:1130–1139. [DOI] [PubMed] [Google Scholar]
- 20.Zeng JH Liu YX Yuan J, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paul JF Charles P Richaud C, et al. Myocarditis revealing COVID-19 infection in a young patient. Eur Heart J Cardiovasc Imaging. 2020;21:776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen HR Zou H Xue M, et al. A case of childhood COVID-19 infection with pleural effusion complicated by possible secondary Mycoplasma pneumoniae infection. Pediatr Infect Dis J. 2020;39:e135–e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salehi S Abedi A Balakrishnan S, et al. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215:87–93. [DOI] [PubMed] [Google Scholar]
- 24.Lichtenstein D Hulot JS Rabiller A, et al. Feasibility and safety of ultrasound-aided thoracentesis in mechanically ventilated patients. Intensive Care Med. 1999;25:955–958. [DOI] [PubMed] [Google Scholar]
- 25.Hajalioghli P Nemati M Dinparast Saleh L, et al. Can chest computed tomography be replaced by lung ultrasonography with or without plain chest radiography in pediatric pneumonia? J Thorac Imaging. 2016;31:247–252. [DOI] [PubMed] [Google Scholar]


