Abstract
The nonanthocyanin phenolic compounds in juice and wine produced from fruits of white bilberry, a nonpigmented mutant of Vaccinium myrtillus, and blue bilberry (pigmented variety) were analyzed using liquid chromatography with a diode array detector (LC-DAD) and LC-DAD–electrospray ionization-quadrapole/time of flight hybrid mass spectrometry (ESI-QTOF-MS). On the basis of elution order, UV–vis spectra, accurate mass data, and fragmentation pattern and standards, 42 compounds including 22 phenolic acids, 15 flavonols, and 5 flavan-3-ols, were identified in juices and wines prepared from the two bilberry varieties. The levels of most individual nonanthocyanin phenolic compounds in white bilberry products were significantly lower than those in pigmented ones. In bilberry juices, phenolic acids were the most predominant, accounting for approximately 80% of total phenolic content, with p-coumaroyl monotropeins and caffeic acid hexoside being the major phenolic acids. After fermentation, the total contents of phenolic acids, flavonols, and nonanthocyanin phenolic compounds significantly increased, while the content of total flavan-3-ols decreased significantly. p-Coumaroyl monotropeins still dominated in the wine products, while caffeic acid content showed dramatic elevation with the significant drop of caffeic acid hexoside.
Keywords: bilberry, mutant, nonanthocyanin phenolic compounds, UHPLC-DAD−ESI-QTOF-MS, juice, wine
Introduction
Winemaking is one of the most ancient food processing technologies and is closely linked to the evolution of human civilization. Throughout the millennia, the winemaking industry has developed to be one of the most prosperous global industries, being valued at approximately USD 357 billion in 2017 reported by the Food and Agriculture Organization of the United Nations (FAO). A large number of varieties constitute the family of Vitis vinifera wines, of which white wines form a large proportion. The white wines made from Sauvignon Blanc, Chardonnay, Riesling, Semillon, and Pinot Gris are among the most common in the wine market. Over the past few years, the growing demand for novel and unique wine products from consumers and the increasing awareness of the nutritional and health-related values of berries (nongrape), as well as the simultaneously persistent exploration of new berry-based products, have promoted the development and consumption of berry wines.1,2 However, most of the berry wines have been produced from pigmented berries, such as blueberry, blackberry, elderberry, and blackcurrant.3,4 The color of pigmented berry wines is caused by the anthocyanins extracted from the cellular vacuoles of skin and from the pulps in some berries.5,6 The biosynthesis of anthocyanins in berries occurs throughout the berry ripening process via the phenylpropanoid/flavonoid pathway (Figure S1), facilitating seed dispersal by attracting herbivores.7 The berry mutants with white skin and/or pulp, as variants resulting from the mutation in structural genes and/or regulatory genes of the anthocyanin synthesis pathway, are rarely found in the nature.8,9 These facts may consequently lead to the scarcity of white berry wines in the market and thus result in less varieties for consumers, especially in comparison with berry wines against V. vinifera wines.
In some Nordic countries with rich berry resources, such as Finland, Norway, and Sweden, the forest economical preference has progressively changed from the style based on tree value only to the model considering berry yields as well.10,11 Wild bilberry (Vaccinium myrtillus, later termed pigmented or blue bilberry (BB)), also known as the European blueberry, is among the most economically valuable wild berries in Northern Europe and is well-known for its richness of antioxidants with health promoting effects, in particular of anthocyanins.12 In comparison with several of its closely related small berry species, for example blueberry (V. corymbosum) and bog bilberry (V. uliginosum), higher levels of anthocyanins exist not only in the skin but also in the pulp of BB variety.13 Hence, pigmented bilberry is considered as one of the richest natural sources of anthocyanins.14 BB is mainly derived from forest ecosystems and thus is difficult to be harvested. Along with the development of high-value-added bilberry products, like BB wine, breeders have begun to cultivate BB on arable land through a series of practices, such as fertilization, irrigation, weed control, and modulation of soil pH, to extend the output of this berry.10,15,16 In comparison with the variety of pigmented bilberry, the anthocyanin-free white bilberry (WB) is a natural variation with significantly lower expression of structural genes, particularly chalcone synthase (CHS), flavanone 3-hydroxylase (F3H), dihydroflavonol 4-reductase (DFR), anthocyanidin synthase (ANS), and flavonoid 3-O-glycosyltransferase (FGT) (Figure S1), and the strongly down-regulated expression of VuMYBPA1 and VuMYBC2 transcription factors.8,17 Beside white bilberry, mutants of white bog bilberry and white and green currants (Ribes spp.) have also received significant scrutiny from researchers.18,19 These nonpigmented nongrape berries could be considered as candidates for the production of white berry wines and may thus contribute to the diversity of the berry wine market.
Taste and mouthfeel are two extremely important elements in determining the quality of wines, of which bitterness and astringency are mainly elicited by nonanthocyanin phenolic compounds; for example, flavan-3-ols have been reported to contribute to the perceptions of bitterness and astringency and flavonols to bitterness or astringency.20,21 The contribution of phenolic acids to organoleptic properties of wines is controversial, and they may contribute more to astringency than bitterness.20,22 Flavan-3-ols and hydroxycinnamic acids have also been revealed to impact white wine browning.23 The relationship between phenolic composition and in vivo antioxidant activity has been demonstrated as well.24,25 Therefore, research on nonanthocyanin phenolic profile is an essential step when characterizing and evaluating wine products.
White mutants of bilberry have been discovered in the forests of Finland and Slovenia, and the gene expression involving the biosynthesis of anthocyanins in these mutants has recently been studied.8,17 However, to the best of our knowledge, there are no reports utilizing the albino variant of bilberry for the production of wines or other products. In analogy to the BB variety, unraveling the phenolic composition of the wine produced from WB might be a first step on the valorization of the albino bilberry mutant and may partly stimulate research on the domestication of this variant. The previous studies on BB wines have often focused on anthocyanin-related compounds,26,27 whereas the studies on the taste- and mouthfeel-active nonanthocyanin phenolic compounds are still lacking. In this work, qualitative and quantitative analyses of nonanthocyanin phenolic compounds, including phenolic acids, flavonols, and flavan-3-ols, in white and blue bilberry wines were carried out using ultra-high-performance liquid chromatography coupled to diode array and electrospray ionization-quadrupole time-of-flight mass spectrometry (UHPLC-DAD–ESI-QTOF-MS) and UHPLC-DAD. The impact of yeast fermentation on the change in phenolic composition was also studied through the comparison between bilberry juices and their fermented products.
Materials and Methods
Materials and Chemicals
Wild white bilberries were collected from several locations in Nagu, Finland in 2016. The commercial blue bilberries were harvested throughout Finland and pooled by Arctic International Oy (Sotkamo, Finland) in 2017. Both types of berries were picked when optimally ripe as defined by experienced horticulturists. The berries were immediately frozen and stored at −20 °C until posterior process.
Authentic phenolic standards of quercetin 3-O-glucoside, syringetin 3-O-glucoside, and cyanidin 3-O-glucoside were purchased from Extrasynthese (Genay, France). (+)-Catechin, (−)-epicatechin, 5-caffeoylquinic acid, 3-caffeoylquinic acid, caffeic acid, and p-coumaric acid were purchased from Sigma–Aldrich Co. (St. Louis, MO). LC and LC–MS grade chemicals were purchased from VWR International (Fontenay-sous-Bois, France). Sodium hydroxide was obtained from Mallinckrodt Baker (Deventer, The Netherlands) and food grade sucrose from Kesko Oyj (Kirkkonummi, Finland). Yeast extract peptone–dextrose (YPD) medium (1% yeast extract, 2% peptone, and 2% dextrose) and YPD agar (1% yeast extract, 2% peptone, 2% dextrose, and 2% agar) were purchased from Lab M Limited (Lancashire, UK).
Bilberry Juice Processing and Fermentation
The same technological process was used for preparing WB and BB juices. Intact fruits from the frozen samples were thawed in a microwave for 5 min. Subsequently, the thawed berries were squeezed in a hydraulic juice press (Hafico, Germany). Juices were pooled and diluted with ultra-pure water at the ratio of 1:1 (v/v). Afterward, the diluted juices were subdivided into 50 mL glass tubes with sealed caps and pasteurized in a water bath at 95 °C for 5 min. The juices were then cooled down to room temperature in a cold-water bath. The initial pH values of the obtained WB and BB juices were 3.08 and 3.04, respectively, and °Brix values were 5.8 and 5.1, respectively. The pH and °Brix were subsequently adjusted to 3.5 and 14.0 using sodium hydroxide and sucrose, respectively. Each juice processing was carried out in duplicate.
The laboratory-scale fermentations were carried out in duplicate in 100 mL Duran bottles containing aliquots of 50 mL of pasteurized bilberry juice. The inoculated microorganism was Saccharomyces cerevisiae Lalvin V1116 (Lallemand Inc., Montreal, Canada) with a cell count of approximately 107 CFU/mL. Prior to inoculation, the yeast strain was proliferated in Erlenmeyer flasks with autoclaved YPD medium at 25 °C for 48 h with 150 rpm shaking. All the flasks and bottles used for incubation and fermentation, respectively, were autoclaved at 121 °C for 15 min before use. The fermentation temperature was controlled at 25 °C in darkness in an incubator (Memmert GmbH, Schwabach, Germany). The bottle caps were unscrewed every 24 h under an aseptic condition to release CO2 produced from the yeast growth. A regular monitoring of °Brix values and bottle weight loss was performed throughout the process until the end of fermentation (defined as a constant weight and °Brix value for two consecutive monitoring time points). After fermentation, all the samples were centrifuged at 4500g for 10 min at room temperature to remove yeast pellets and precipitates from bilberry wines. The supernatants were collected and stored at −80 °C for posterior analysis.
UHPLC-DAD for Separation and Quantification of Nonanthocyanin Phenolic Compounds
The separation and quantification of nonanthocyanin phenolic compounds (phenolic acids, flavonols, and flavan-3-ols) in bilberry samples were performed in triplicate on a Shimadzu UHPLC system consisting of an SPD-M20A diode array detector (DAD, Shimadzu Corp., Kyoto, Japan), two LC-30AD pumps, a SIL-30AC autosampler, a CTO-20AC column oven, a DGU-20A degassing unit, and a CBM-20A system controller. After being filtered through 0.45 μm PTFE membranes, 10 μL samples were injected into a Phenomenex Aeris peptide XB-C18 column (150 mm × 4.60 mm, 3.6 μm, Torrance, CA) maintained at 40 °C. The mobile phases A and B were water and acetonitrile, respectively, both containing 5% formic acid. The gradient elution program was performed according to our published method as 0 to 5 min, 5 to 8% B; 5 to 10 min 8% B; 10 to 15 min, 8 to 12% B; 15 to 20 min, 12 to 18% B; 20 to 25 min, 18 to 30% B; 25 to 30 min, 30 to 60% B; 30 to 35 min, 60 to 5% B; 35 to 40 min, 5% B.27 The flow rate was set at 1.0 mL/min. The UV data were recorded in a range of 190–800 nm in steps of 1.2 nm, while phenolic acids, flavonols, and flavan-3-ols were detected at 320, 350, and 280 nm, respectively. Concentrations of phenolic acids, flavonols, and flavan-3-ols in bilberry samples were quantified as equivalents of caffeic acid, quercetin 3-O-glucoside, and epicatechin, respectively. The calibration data, including determination coefficient (R2), limits of detection (LOD) and quantification (LOQ), and linearity range, are reported in the Supporting Information, Table S1.
UHPLC-DAD–ESI-QTOF-MS for Identification of Nonanthocyanin Phenolic Compounds
The qualitative analysis of phenolic compounds in the bilberry samples was conducted using the Bruker Elute UHPLC systems, consisting of a HPG1300 pump, an Elute DAD, a column oven, and an autosampler, coupled with an Ultra-High Resolution Impact II Qq-Time-of-Flight (TOF) mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany) and an electrospray ionization (ESI) source in both positive and negative ionization modes in the range of m/z 20–1000. The column, mobile phase, elution program, and the chromatographic condition were the same as reported in the above LC separation. A flow of approximately 0.4 mL/min of eluent was directed to the TOF spectrometer via a distributary T-shape splitter. The ESI parameters of end plate offset and drying gas (N2) flow were set at 500 V and 12.0 L/min, respectively, for both positive and negative ionization, while capillary voltage, nebulizer gas (N2) pressure, and drying gas temperature were set at 4.5 kV, 4.8 bar, and 350 °C, respectively, for positive ion mode and 3.5 kV, 4.0 bar, and 300 °C, respectively, for negative ion mode. Before each set of injection, sodium formate (10 mM) was continually introduced to the six-port valve from a direct infusion syringe pump at a flow rate of 180 μL/min in high-precision calibration (HPC) mode for high-accuracy mass calibration. The mass spectra data were processed with the Compass DataAnalysis software 4.4 (Bruker Daltonik). The mass error (ppm) was calculated as the difference between the measured mass and the theoretical mass of a given molecular formula, expressed as
Certain nonanthocyanin phenolic compounds were identified by comparing retention time and UV–vis and mass spectroscopic information to their corresponding authentic standards, when available. Otherwise, tentative identification was performed by matching UV spectra and fragmentation patterns of compounds to the data reported in the literature (Table 1).
Table 1. Identification of Nonanthocyanin Phenolic Compounds in White and Blue Bilberry Juices and Winesa.
| DAD | measured mass (m/z) | theoretical mass (m/z) | mass error (ppm) | fragment ion (m/z) | |||||
|---|---|---|---|---|---|---|---|---|---|
| peak | molecular formula | identification | retention time (min) | λmax (nm) | [M – H]−/[M + H]+ | [M – H]−/[M + H]+ | [M – H]−/[M + H]+ | [A – H]−/[A + H]+ | identification method |
| phenolic acids | |||||||||
| 1 | C16H18O9 | 5-caffeoylquinic acid | 3.1 | 324 | 353.0884/355.1033 | 353.0877/355.1025 | 2.01/2.33 | 191.0577/– | UV, MS, standard |
| 2 | C13H16O9 | protocatechuic acid hexoside | 3.4 | 317 | 315.0732/– | 315.0720/– | 3.68/– | 153.0200/– | UV, MS, literature47 |
| 3 | p-coumaric acid derivative-1 | 4.1 | 309 | 371.0985/– | – | – | 163.0408, 119.0509/– | UV, MS, literature29 | |
| 4 | p-coumaric acid derivative-2 | 4.5 | 324 | 371.0984/– | – | – | 163.0604, 119.0507/– | UV, MS, literature29 | |
| 5 | C15H18O9 | caffeic acid hexoside-1 | 5.2 | 314 | 341.0878/343.1039 | 341.0877/343.1025 | 0.32/4.16 | 179.0354, 135.0453/181.0502, 163.0494 | UV, MS, literature30 |
| 6 | C16H18O9 | 3-caffeoylquinic acid | 5.5 | 325 | 353.0873/– | 353.0877/– | –1.11/– | 191.0561/163.0409, 135.0455 | UV, MS, standard |
| 7 | C16H18O9 | 4-caffeoylquinic acid | 5.7 | 324 | 353.0873/– | 353.0877/– | –1.11/– | 191.0559/– | UV, MS, literature28 |
| 8 | C9H8O4 | caffeic acid | 6.1 | 321 | 179.0345/181.0497 | 179.0349/181.0495 | –2.05/0.91 | 161.0451/163.0408, 135.0447 | UV, MS, standard29 |
| 9 | C15H18O9 | caffeic acid hexoside-2 | 7.1 | 302 | 341.0881/– | 341.0877/– | 1.20/– | 179.0354, 135.0455/– | UV, MS, literature30 |
| 10 | C16H18O9 | caffeoylquinic acid isomer | 8.2 | 317 | 353.0882/– | 353.0877/– | 1.44/– | 191.0564/– | UV, MS, literature29 |
| 11 | C16H18O8 | p-coumaroylquinic acid | 8.6 | 310 | 337.0917/– | 337.0928/– | –3.19/– | 191.0550, 173.0446, 163.0388/– | UV, MS, literature29 |
| 12 | C9H8O3 | p-coumaric acid | 10.1 | 309 | 163.0407/165.0546 | 163.0400/165.0546 | 4.58/–0.13 | 119.0502, 93.0346/147.0443, 91.0543, 119.0491 | UV, MS, standard29 |
| 13 | C25H24O12 | dicaffeoylquinic acid-1 | 12.0 | 309 | 515.1176/517.1326 | 515.1194/517.1342 | –3.47/–3.03 | 353.0871/355.1027 | UV, MS, literature28 |
| 14 | C25H24O12 | dicaffeoylquinic acid-2 | 12.9 | 308 | 515.1173/517.1324 | 515.1194/517.1342 | –4.05/–3.42 | 353.0866/355.1026 | UV, MS, literature28 |
| 15 | caffeic acid derivative hexoside | 15.7 | 322 | –/559.1922 | –/397.1384, 181.0509 | UV, MS, literature29 | |||
| 16 | C25H28O13 | p-coumaroyl monotropein-1 | 18.2 | 307 | 535.1454/– | 535.1456/– | –0.37/– | 491.1575, 373.0942, 371.0990, 329.1035, 311.0930, 191.0357, 163.0409, 119.0509, 147.0451/– | UV, MS, literature30−32 |
| 17 | C25H28O13 | p-coumaroyl monotropein-2 | 19.2 | 311 | 535.1447/– | 535.1456/– | –1.68/– | 491.1550, 373.0924, 371.0980, 329.1023, 311.0920, 191.0348, 163.0400, 147.0451, 119.0502/– | UV, MS, literature30−32 |
| 18 | p-coumaric acid derivative | 22.6 | 311 | –/435.1664 | – | – | –/309.0954, 165.0548, 147.0427 | UV, MS, literature29 | |
| 19 | p-coumaric acid derivative-a | 24.9 | 310 | 411.1682/– | – | – | 163.0410, 145.0299, 119.0502/– | UV, MS, literature30 | |
| 20 | p-coumaric acid derivative-b | 25.0 | 312 | 411.1681/– | – | – | 163.0410, 145.0307, 119.0501/– | UV, MS, literature30 | |
| 21 | p-coumaric acid derivative-c | 25.1 | 310 | 411.1665/– | – | – | 163.0405, 145.0298, 119.0505/– | UV, MS, literature30 | |
| 22 | caffeic acid derivative | 25.3 | 310 | 445.1140/– | – | – | 411.1665, 179.0335, 135.0454/– | UV, MS, literature30 | |
| flavonols | |||||||||
| 23 | C21H20O13 | myricetin 3-O-galactoside | 13.7 | 353 | 479.0849/481.0982 | 479.0831/481.0977 | 3.73/1.11 | 317.0292/319.0452 | UV, MS, literature29,34 |
| 24 | C21H20O13 | myricetin 3-O-glucoside | 14.5 | 353 | 479.0848/481.0980 | 479.0831/481.0977 | 3.52/0.69 | 317.0295/319.0450 | UV, MS, literature29,34 |
| 25 | C21H20O12 | quercetin 3-O-galactoside | 17.8 | 351 | 463.0888/465.1043 | 463.0882/465.1028 | 1.30/3.33 | 301.0375/303.0509 | UV, MS, literature29 |
| 26 | C21H18O13 | quercetin 3-O-glucuronide | 18.6 | 352 | 477.0670/479.0836 | 477.0675/479.0820 | –0.97/3.3 | 301.0356/303.0508 | UV, MS, literature29 |
| 27 | C21H20O12 | quercetin 3-O-glucoside | 19.0 | 352 | 463.0875/465.1047 | 463.0882/465.1028 | –1.51/4.19 | 301.0330/303.0514 | UV, MS, standard29 |
| 28 | C22H22O13 | laricitrin 3-O-galactoside | 19.6 | 352 | –/495.1155 | –/495.1133 | –/4.41 | –/333.0625 | UV, MS, literature48 |
| 29 | C22H22O13 | laricitrin 3-O-glucoside | 20.1 | 352 | 493.0972/495.1153 | 493.0988/495.1133 | –3.17/4.01 | 331.0440/333.0623 | UV, MS, literature48 |
| 30 | C20H18O11 | quercetin 3-O-arabinoside | 20.4 | 353 | 433.0766/435.0925 | 433.0776/435.0922 | –2.39/0.72 | 301.0339/303.0529 | UV, MS, literature29 |
| 31 | C20H18O11 | quercetin 3-O-xyloside | 20.5 | 353 | 433.0767/435.0935 | 433.0776/435.0922 | –2.16/3.02 | 301.0344/303.0524 | UV, MS, literature29 |
| 32 | C15H10O8 | myricetin aglycone | 21.6 | 369 | 317.0289/319.0463 | 317.0303/319.0448 | –4.39/4.56 | – | UV, MS, literature48 |
| 33 | C22H22O12 | isorhamnetin 3-O-galactoside | 22.6 | 355 | 477.1024/479.1196 | 477.1039/479.1184 | –3.04/2.50 | –/317.0687 | UV, MS, literature29 |
| 34 | C23H24O13 | syringetin 3-O-galactoside | 22.7 | 353 | 507.1129/509.1293 | 507.1144/509.1290 | –2.99/0.65 | –/347.0767 | UV, MS, literature48 |
| 35 | C23H24O13 | syringetin 3-O-glucoside | 33.0 | 354 | 507.1130/509.1296 | 507.1144/509.1290 | –2.79/1.24 | –/347.0769 | UV, MS, standard48 |
| 36 | C15H10O7 | quercetin aglycone | 25.2 | 372 | 301.0360/303.0504 | 301.0354/303.0499 | 2.07/1.55 | – | UV, MS, literature48 |
| 37 | C17H14O8 | syringetin aglycone | 28.5 | 371 | 345.0627/347.0766 | 345.0616/347.0761 | 3.21/1.33 | – | UV, MS, literature48 |
| flavan-3-ols | |||||||||
| 38 | C15H14O7 | (−)-epigallocatechin | 4.4 | 279 | 305.0668/307.0820 | 305.0700/307.0812 | 0.78/2.51 | 219.0515/– | UV, MS, literature29,30 |
| 39 | C15H14O6 | (+)-catechin | 6.0 | 279 | 289.0714/291.0870 | 289.0718/291.0863 | –1.25/2.35 | 245.0824, 205.0513/– | UV, MS, standard |
| 40 | C30H26O12 | procyanidin B-type dimer | 6.1 | 280 | 577.1335/579.1514 | 577.1352/579.1497 | –2.86/2.93 | –/427.1040, 409.0923, 291.0894, 289.0739, 271.0632, 247.0629, 163.0417, 139.0393 | UV, MS, literature35 |
| 41 | C15H14O6 | (−)-epicatechin | 8.0 | 280 | 289.0709/291.0875 | 289.0718/291.0863 | –2.98/4.07 | 245.0819, 205.0509/207.0659, 165.0552, 139.0395, 123.0453 | UV, MS, standard |
| 42 | C45H38O18 | procyanidin B-type trimer | 8.9 | 279 | 865.1991/867.2172 | 865.1985/867.2131 | 0.65/4.74 | –/697.1443, 579.1522, 577.1326, 453.0842, 441.1737, 291.0875 | UV, MS, literature29,36 |
–: not recorded.
Statistical Analysis
All results were expressed as mean ± standard deviation (SD). Significant differences in the phenolic compositions between samples were analyzed using independent-samples t-test with IBM SPSS Statistics 25 (IBM Corp., Armonk, NY).
Results and Discussion
Identification of Nonanthocyanin Phenolic Compounds
In the present study, high mass accuracies were obtained (mass error < |5| ppm) for the confirmation of elemental compositions of phenolic compounds by the comparison between theoretical and measured mass (Table 1).
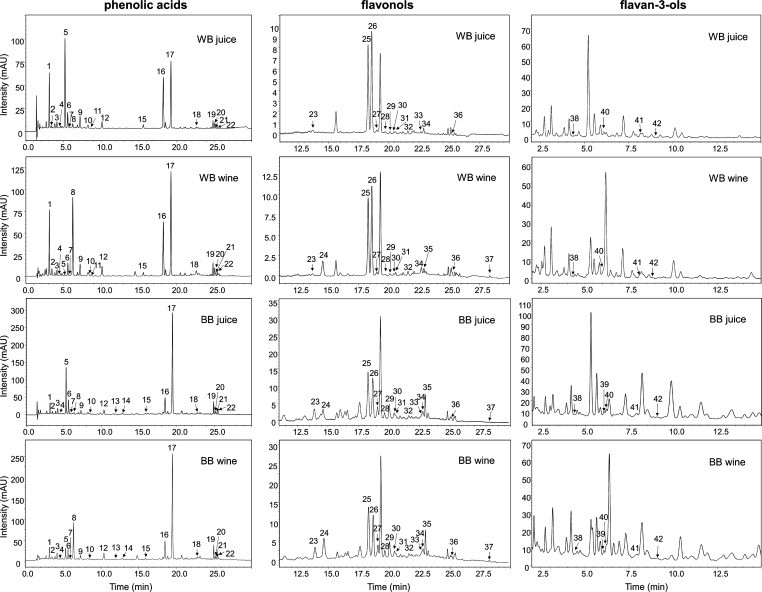
Figure 1 shows the LC-DAD chromatograms of phenolic compounds in WB and BB juices and wines. Twenty-two phenolic acids, including 9 hydroxycinnamic acids, 12 hydroxycinnamic acid derivatives, and 1 hydroxybenzoic acid derivative, were detected in the products produced from the two different types of bilberries (Figure 1A, Table 1). Among the 9 hydroxycinnamic acids, 5-caffeoylquinic acid (peak 1), 3-caffeoylquinic acid (peak 6), caffeic acid (peak 8), and p-coumaric acid (peak 12) were identified via the combination of chromatographic separation and high mass accuracy measurements and with the aid of their corresponding authentic standards. Peaks 7 and 10 had virtually the same deprotonated molecular ion ([M – H]−m/z 353.0873 and 353.0882, respectively) as 3-caffeoylquinic acid ([M – H]− at m/z 353.0873) and 5-caffeoylquinic acid ([M – H]− at m/z 353.0884). Furthermore, the identical characteristic fragment ion at m/z 191, corresponding to [quinic acid − H]−, in the MS spectra of these four compounds indicated that peaks 7 and 10 are isomers of caffeoylquinic acid. According to the reported UV spectra in a previous study, 4-caffeoylquinic acid showed a similar maximum absorption wavelength (λmax) to those of 3-caffeoylquinic acid and 5-caffeoylquinic acid.28 Therefore, peak 7 was tentatively identified as 4-caffeoylquinic acid. On the basis of the deprotonated molecular ([M – H]− at m/z 337.0917, corresponding to [C16H18O8 – H]−) and fragment ions (m/z 191.0550 to [quinic acid – H]−, 173.0446 to [quinic acid – H – H2O]−, and 163.0388 to [p-coumaric acid – H]−), peak 11 was assigned as p-coumaroylquinic acid. Peaks 13 and 14 showed almost the same deprotonated ion ([M – H]− at m/z 515, corresponding to [C25H24O12– H]−) and protonated molecular ion ([M + H]+ at m/z 517, corresponding to [C25H24O12 + H]+) as well as fragment ion pattern (m/z 353 and 355, corresponding to [caffeoylquinic acid – H]− and [caffeoylquinic acid + H]+, respectively) indicating that they are isomeric compounds of dicaffeoylquinic acid.
Figure 1.
UHPLC-DAD chromatograms of nonanthocyanin phenolic compounds in white (WB) and blue bilberry (BB) juices and wines. The chromatograms in the first column refer to phenolic acids, the second column to flavonols, and the third column to flavan-3-ols. The peak numbers in chromatograms refer to those in Table 1.
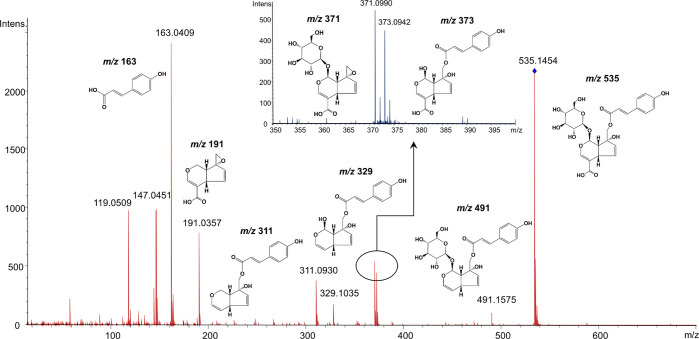
With regard to the hydroxycinnamic acid and hydroxybenzoic acid derivatives, peaks 5 and 9 both showed [M – H]− at m/z 341 (corresponding to [C15H18O9 – H]−) and yielded a fragment ion at m/z 179 ([caffeic acid – H]−) corresponding to the loss of a hexose moiety (162 amu) and 135 amu ([caffeic acid – H – CO2]−), suggesting they are isomeric caffeic acid hexoside. Similarly, for peak 2, a loss (162 amu) of hexose moiety from [M – H]− resulted in a fragment ion of m/z 153.0200 (calcd for C7H5O4), which coincided with the mass of the deprotonated protocatechuic acid. Therefore, peak 2 was assigned as a glycosylated protocatechuic acid (protocatechuic acid hexoside). The characteristic fragment ion of caffeic acid ([A – H]− at m/z 179, [A + H]+ at m/z 181) was detected in peaks 15 and 22, indicating that they both are derivatives of caffeic acid. However, a difference of 162 amu between the protonated molecular mass ([M + H]+ at m/z 559.1922) and fragmented ion ([A + H]+ at m/z 397.1384) was seen in the MS spectrum of peak 15, demonstrating that a hexoside moiety was present in its structure. Hence, peaks 15 and 22 were preliminarily identified as caffeic acid derivative hexoside and caffeic acid derivative, respectively. Analogously, peaks 3, 4, 16, 17, 18, 19, 20, and 21 were proposed to be derivatives of p-coumaric acid. Of which, peaks 3 and 4 had virtually the same molecular and fragment ion patterns, indicating that these two compounds are isomers of each other, in analogy to peaks 19, 20, and 21. Peaks 16 and 17 had identical measured mass ([M – H]− at m/z 535.1454 and 535.1447, respectively, corresponding to molecular formula C25H27O13) and fragmentation spectra. A 44 amu (CO2) loss from the molecular mass produced a fragment ion at m/z 491 (calcd for C24H27O11) indicated that the presence of a carboxylic acid in the molecule structure. A direct loss of a hexose unit (162 amu) or a coumaroyl acid (164 amu) from the parent ion produced fragment ions at m/z 373 (calcd for C19H17O8) and 371 (calcd for C16H19O10), respectively, implying that the hexose unit and the coumaroyl acid unit are directly connected to the aglycone of molecule without interfering with each other.29 The fragment ion at m/z 329 (calcd for C18H17O6) likely resulted from the loss of a hexose unit from m/z 491 or a CO2 from m/z 373. Subsequently, a further loss of H2O (18 amu) yielded a fragment ion at m/z 311 (calcd for C18H15O5). The fragment at m/z 191 may have resulted from m/z 371 by cleavage of a hexose unit and a subsequent H2O loss (C16H19O10 – 162 – 18). Figure 2 shows the speculative fragmentation pathway of these two compounds. The mass errors of molecular and fragment ions of these two compounds were less than |5| ppm (0.37–3.51 ppm). Therefore, on the basis of the obtained QTOF/MS spectra and by referencing data from the literature,30−32 these two compounds were tentatively identified as p-coumaroyl monotropeins, the iridoid glycosides acylated with p-coumaric acid. p-Coumaroyl monotropeins have also been previously detected in cranberry fruit and juice.31,33
Figure 2.
Speculative fragmentation pathway of p-coumaroyl monotropeins (p-coumaroyl monotropein-1 as an example) in negative ionization mode (ESI−).
Figure 1B shows the detected 15 flavonols in bilberry juices and wines. In the negative ionization mode, peaks 23 and 24 presented the identical [M – H]− ion at m/z 479. The fragmented ions at m/z 317 necessarily implied the loss of a hexose unit. On the basis of the QTOF/MS fragmentation patterns, elution order, and by comparison of these with published data in the literature,34 these two compounds were assigned as myricetin 3-O-galactoside and myricetin 3-O-glucoside, respectively (Table 1). Peaks 25, 26, 27, 30, and 31 with [M – H]− at m/z of 463.0888, 477.0670, 463.0875, 433.0766, and 433.0767, respectively, had the fragment ion at m/z 301 with the highest relative intensity typical for quercetin aglycone. The neutral losses of 162 amu from m/z 463, 132 from 433, and 176 from 477 corresponded to hexose, pentose, and glucuronide moieties, respectively. Therefore, compounds 25, 26, 27, 30, and 31 were preliminarily identified as quercetin 3-O-galactoside, quercetin 3-O-glucuronide, quercetin 3-O-glucoside, quercetin 3-O-arabinoside, and quercetin 3-O-xyloside, respectively. In positive ionization mode, peaks 28 and 29 showed an identical molecular ion at m/z 495 and both yielded a fragment ion at m/z 333, which is characteristic of laricitrin aglycone. On the basis of the fragmentation information and the literature data related to bilberry products,30 these two compounds were tentatively identified as laricitrin 3-O-galactoside and laricitrin 3-O-glucoside, respectively. Analogously, peaks 33, 34, and 35 were identified as isorhamnetin 3-O-galactoside, syringetin 3-O-galactoside, and syringetin 3-O-glucoside, respectively. Moreover, the identification was further confirmed by the authentic standard of syringetin 3-O-glucoside. There are no fragment ions generated from the molecular masses of peaks 32, 36, and 37 under either positive or negative ionization modes. Moreover, in comparison with the UV-absorption of flavonol 3-O-glycosides, a 15–21 nm hypsochromic shift was obtained on the UV–vis spectra of these three compounds, suggesting that they all are free flavonol aglycones. On the basis of the mass data and with the aid of comparison with a standard (myricetin aglycone), these three compounds were consequently identified as myricetin aglycone, quercetin aglycone, and syringetin aglycone, respectively.
Peaks 39 and 41 were identified as (+)-catechin and (−)-epicatechin, respectively, according to retention times and UV–vis and MS spectra of their corresponding standards. Peak 38 ([M – H]− at m/z 305.0668) yielded a fragment ion at m/z 219.0515, corresponding to (−)-epigallocatechin, which is in agreement with the previous reports.29,30 Peak 40 exhibited [M + H]+ ions at m/z 579.1514 (calcd for C30H27O12) and produced several fragment ions with high intensities, which are the characteristic fragmentations of procyanidin B-type dimers,35 as m/z 427 ([M + H – 152]+, originated from retro Diels–Alder reaction (RDA) of the heterocyclic ring), 409 ([M + H – 152 – 18]+, given from dehydration in RDA), and 291 ([M + H – 289]+ originated from direct cleavage of the interflavanoid linkage).35 It is well-known that, in nature, four isomers exist for B-type procyanidin (PC) dimer, PC B1, B2, B3, and B4. They are dimeric compounds formed from the subunits of catechin and/or epicatechin molecules. These isomers present the same elemental composition and similar UV and mass spectra, making it difficult to distinguish among these diastereomeric compounds. However, according to previous studies on the elution characteristics of procyanidins on the reverse phase HPLC with C18 column, only procyanidins B2 and B4 eluted between catechin and epicatechin.36 Therefore, according to the retention time of peak 41 in the present work, we tentatively identified this compound as procyanidins B2 or B4. The protonated molecular ion at m/z 867.2172 (calcd for C45H39O18) of peak 42 corresponded to B-type procyanidin trimer. The identification was further confirmed by the well-matched fragmentation pattern of this compound with the those reported in the previous studies.29,36
Quantification of Nonanthocyanin Phenolic Compounds
Table 2 shows the contents of individual nonanthocyanin phenolic compounds in bilberry products made from two different varieties. The contents of total phenolic acids (TA), flavonols (TFO), and flavan-3-ols (TFA) were calculated as the sum of the individual compounds in the corresponding groups. In order to study the impact of the mutation on the quantitative phenolic profile of bilberry juice, a quantitative comparison between the two juice types was carried out. The major polyphenols in both WB and BB juices were phenolic acids, being 80.5% and 76.9% of total phenolic content (TPC), respectively, followed by flavonols (14.4% and 17.8%, respectively) and flavan-3-ols (5.1% and 5.3%, respectively). BB juice possessed higher contents of TA, TFO, and TFA, being approximately 1.9, 2.4, and 2.1 times higher, respectively, than those in WB juice. This is in line with a previous study reporting a higher content of polyphenols in normal bilberry fruits than that in albino variants.17 Since the biosynthesis of flavonols and flavan-3-ols shares the same upstream pathway with that of anthocyanin over the flavonoid pathway (Figure S1), the low contents of flavonols and flavan-3-ols in WB juice may be associated with the low expression of the genes encoding enzymes. These enzymes act also on the flavonol and flavan-3-ol biosynthesis pathways. For example, the low expressions of F3H, DFR, and ANS in white bilberry may reduce the accumulations of substrates for the formations of flavonol aglycones (quercetin and myricetin from dihydroflavonols by flavonol synthase (FLS)) and monomeric flavan-3-ols (catechin and gallocatechin from leucoanthocyanidins by leucoanthocyanidin reductase (LAR), epicatechin and epigallocatechin from anthocyanidins by anthocyanidin reductase (ANR)), thus resulting in the low formations of flavonol glycosides and oligomeric flavan-3-ols (Figure S1).7,8
Table 2. Quantification of Nonanthocyanin Phenolic Compounds in White (WB) and Blue Bilberry (BB) Juices and Winesa.
| content (mg/L) |
t-testb |
|||||||
|---|---|---|---|---|---|---|---|---|
| peak | compound | WB juice | WB wine | BB juice | BB wine | WB juice vs BB juice | WB juice vs WB wine | BB juice vs BB wine |
| phenolic acids | ||||||||
| 1 | 5-caffeoylquinic acid | 8.12 ± 0.05 | 11.27 ± 0.17 | 4.34 ± 0.20 | 4.38 ± 0.14 | *** | *** | |
| 2 | protocatechuic acid hexoside | 0.38 ± 0.01 | 1.23 ± 0.31 | 0.90 ± 0.08 | 0.71 ± 0.02 | *** | * | ** |
| 3 | p-coumaric acid derivative-1 | 1.11 ± 0.02 | 1.15 ± 0.04 | 2.59 ± 0.19 | 2.65 ± 0.09 | *** | ||
| 4 | p-coumaric acid derivative-2 | 0.06 ± 0.00 | 0.04 ± 0.01 | 0.24 ± 0.08 | 0.21 ± 0.15 | * | ||
| 5 | caffeic acid hexoside-1 | 16.37 ± 0.12 | 0.16 ± 0.02 | 22.93 ± 0.64 | 5.09 ± 0.20 | *** | *** | *** |
| 6 | 3-caffeoylquinic acid | 3.39 ± 0.09 | 2.71 ± 0.08 | 6.74 ± 0.23 | 4.41 ± 0.20 | *** | *** | *** |
| 7 | 4-caffeoylquinic acid | 0.03 ± 0.03 | 0.06 ± 0.00 | 0.30 ± 0.05 | 0.43 ± 0.11 | *** | ||
| 8 | caffeic acid | 0.74 ± 0.01 | 17.21 ± 0.33 | 0.55 ± 0.13 | 17.48 ± 0.54 | *** | *** | |
| 9 | caffeic acid hexoside-2 | 2.76 ± 0.06 | 3.05 ± 0.07 | 1.95 ± 0.10 | 2.07 ± 0.26 | *** | *** | |
| 10 | caffeoylquinic acid isomer | 0.37 ± 0.02 | 0.43 ± 0.20 | 0.32 ± 0.04 | 0.24 ± 0.03 | * | * | |
| 11 | p-coumaroylquinic acid | 0.07 ± 0.01 | 0.09 ± 0.03 | – | – | |||
| 12 | p-coumaric acid | 2.28 ± 0.06 | 3.56 ± 0.13 | 4.26 ± 0.15 | 5.57 ± 0.14 | *** | *** | *** |
| 13 | dicaffeoylquinic acid-1 | – | – | 0.56 ± 0.32 | 0.52 ± 0.17 | |||
| 14 | dicaffeoylquinic acid-2 | – | – | 0.99 ± 0.24 | 1.08 ± 0.13 | |||
| 15 | caffeic acid derivative hexoside | 1.07 ± 0.02 | 1.12 ± 0.04 | 0.94 ± 0.11 | 0.93 ± 0.03 | |||
| 16 | p-coumaroyl monotropein-1 | 12.83 ± 0.13 | 14.65 ± 0.30 | 10.65 ± 0.66 | 10.91 ± 0.44 | *** | *** | |
| 17 | p-coumaroyl monotropein-2 | 14.98 ± 0.18 | 24.95 ± 0.37 | 58.82 ± 1.44 | 53.53 ± 1.57 | *** | *** | ** |
| 18 | p-coumaric acid derivative | 0.24 ± 0.01 | 1.28 ± 0.03 | 0.52 ± 0.03 | 0.55 ± 0.06 | *** | *** | |
| 19 | p-coumaric acid derivative-a | 1.21 ± 0.01 | 2.10 ± 0.04 | 4.88 ± 0.11 | 4.93 ± 0.13 | *** | *** | |
| 20 | p-coumaric acid derivative-b | 0.98 ± 0.01 | 1.54 ± 0.02 | 1.29 ± 0.14 | 1.37 ± 0.04 | * | *** | |
| 21 | p-coumaric acid derivative-c | 0.04 ± 0.01 | 0.31 ± 0.03 | 0.65 ± 0.03 | 0.75 ± 0.04 | *** | *** | * |
| 22 | caffeic acid derivative | 0.30 ± 0.01 | 0.70 ± 0.01 | 1.87 ± 0.06 | 1.71 ± 0.06 | *** | *** | * |
| total phenolic acids | 67.34 ± 0.86 | 87.62 ± 2.22 | 126.29 ± 5.03 | 119.51 ± 4.55 | *** | *** | * | |
| flavonols | ||||||||
| 23 | myricetin 3-O-galactoside | 0.29 ± 0.01 | 0.31 ± 0.04 | 2.36 ± 0.10 | 2.39 ± 0.07 | *** | ||
| 24 | myricetin 3-O-glucoside | – | 1.48 ± 0.04 | 1.94 ± 0.46 | 4.00 ± 0.69 | ** | ||
| 25 | quercetin 3-O-galactoside | 4.48 ± 0.12 | 5.01 ± 0.07 | 8.20 ± 0.34 | 8.06 ± 0.34 | *** | *** | |
| 26 | quercetin 3-O-glucuronide | 5.17 ± 0.10 | 5.76 ± 0.12 | 6.38 ± 0.18 | 5.93 ± 0.13 | *** | *** | ** |
| 27 | quercetin 3-O-glucoside | 0.25 ± 0.02 | 0.33 ± 0.01 | 1.43 ± 0.10 | 1.41 ± 0.04 | *** | *** | |
| 28 | laricitrin 3-O-galactoside | – | – | 0.22 ± 0.02 | 0.18 ± 0.00 | * | ||
| 29 | laricitrin 3-O-glucoside | 0.26 ± 0.01 | 0.31 ± 0.01 | 2.34 ± 0.05 | 2.33 ± 0.08 | *** | ** | |
| 30 | quercetin 3-O-arabinoside | 0.25 ± 0.02 | 0.26 ± 0.03 | 0.92 ± 0.06 | 0.90 ± 0.06 | *** | ||
| 31 | quercetin 3-O-xyloside | 0.27 ± 0.02 | 0.36 ± 0.01 | 0.52 ± 0.03 | 0.47 ± 0.02 | *** | ** | |
| 32 | myricetin aglycone | 0.32 ± 0.01 | 0.44 ± 0.01 | 0.60 ± 0.03 | 0.67 ± 0.03 | *** | *** | ** |
| 33 | isorhamnetin 3-O-galactoside | – | – | 0.46 ± 0.12 | 0.44 ± 0.12 | |||
| 34 | syringetin 3-O-galactoside | 0.23 ± 0.01 | 0.47 ± 0.01 | 0.90 ± 0.03 | 0.84 ± 0.04 | *** | *** | |
| 35 | syringetin 3-O-glucoside | 0.37 ± 0.00 | 0.34 ± 0.01 | 2.28 ± 0.07 | 2.40 ± 0.06 | *** | ||
| 36 | quercetin aglycone | 0.19 ± 0.00 | 0.31 ± 0.00 | 0.40 ± 0.02 | 0.42 ± 0.01 | *** | *** | |
| 37 | syringetin aglycone | – | 0.22 ± 0.00 | 0.21 ± 0.01 | 0.28 ± 0.02 | *** | ||
| total flavonols | 12.07 ± 0.15 | 15.58 ± 0.20 | 29.15 ± 0.7 | 30.72 ± 0.84 | *** | *** | * | |
| flavan-3-ols | ||||||||
| 38 | (−)-epigallocatechin | 0.21 ± 0.01 | 0.37 ± 0.25 | 0.25 ± 0.04 | 0.31 ± 0.06 | |||
| 39 | (+)-catechin | – | – | 1.48 ± 0.18 | 1.26 ± 0.02 | * | ||
| 40 | procyanidin B-type dimer | 1.86 ± 0.13 | 1.47 ± 0.36 | 3.27 ± 0.39 | 4.05 ± 0.72 | ** | ||
| 41 | (−)-epicatechin | 1.96 ± 0.14 | 0.90 ± 0.41 | 2.56 ± 0.10 | 1.63 ± 0.19 | *** | ** | *** |
| 42 | procyanidin B-type trimer | 0.21 ± 0.02 | 0.39 ± 0.20 | 1.13 ± 0.13 | 0.80 ± 0.08 | *** | ** | |
| total flavan-3-ols | 4.24 ± 0.26 | 3.14 ± 0.36 | 8.69 ± 0.35 | 8.04 ± 0.59 | *** | ** | ||
–: not detected.
Independent-samples t-test. *, **, and ***: significant at p < 0.05, p < 0.01, and p < 0.001, respectively.
As shown in Table 2, p-coumaroyl monotropein-2 dominated among the phenolic acids in BB juice, being 46.6% of TA, followed by caffeic acid hexoside-1 (18.2%) and p-coumaroyl monotropein-1 (8.4%). While the predominant phenolic acids in WB juice were caffeic acid hexoside-1, which accounted for 24.3% of TA, p-coumaroyl monotropein-2 (22.2%), and p-coumaroyl monotropein-1 (19.1%). Interestingly, p-coumaroyl monotropeins accounted for approximately one-half of the total phenolic acid content in both types of bilberry juices. In WB juice, a high proportion (17.7%) of TA was represented by caffeoylquinic acid isomers, including 5-caffeoylquinic acid, 3-caffeoylquinic acid, 4-caffeoylquinic acid, and caffeoylquinic acid isomer, whereas these isomers together formed only 9.3% in BB juice. Although six derivatives of p-coumaric acid were detected in the two bilberry juice types, they only represented 5.4% and 8.1% of the total content of phenolic acids in WB and BB juices, respectively. p-Coumaroylquinic acid was the unique hydroxycinnamic acid in WB juice as it has not been detected in BB juice, while the dicaffeoylquinic acids were detected only in BB juice.
Among the 15 flavonols detected in BB juice, myricetin 3-O-glucoside, laricitrin 3-O-galactoside, isorhamnetin 3-O-galactoside, and syringetin aglycone were undetected in WB juice. The content of flavonols in WB juice was only 40% of the content in BB juice (Table 2). The result is in good agreement with a previous study investigating the flavonoid biosynthesis during bilberry fruit ripening, where only 50% of the TFO was observed in ripe white bilberry fruit compared to the pigmented one.8 Glycosylated quercetins were the most predominant flavonols in BB juice accounting for approximately 60% of TFO, of which quercetin 3-O-galactoside was the one with the highest content, followed by quercetin 3-O-glucuronide. Myricetin glycosides also presented at a high proportion (approximately 15%) of TFO. The high contents of these two groups of flavonol glycosides have also been reported in colored bilberry fruits, juice, and press residues.8,37,38 On the contrary, in WB juice, quercetin 3-O-glucuronide was the most abundant flavonol glycoside, followed by quercetin 3-O-galactoside. These two compounds accounted for approximately 80% of TFO in WB juice. The contents of other flavonol glycosides were found to be significantly lower (<0.4 mg/L). It is worth noting that, in previous studies on the phenolic characteristics of bilberries, myricetin was the only aglycone that could be detected in pigmented bilberry fruit but not in albino mutant.32,39 This compound was reported to be quantifiable just after the coloring period began and reached the maximum level in fully colored fruit.8 Therefore, the detection of myricetin aglycone in WB juice and quercetin and syringetin aglycones in BB juice indicated that hydrolysis likely had taken place during juice processing yielding aglycones from the corresponding glycosylated flavonols.40
Five flavan-3-ols were quantified in bilberry juices, including three monomeric flavan-3-ols ((−)-epigallocatechin, (+)-catechin, and (−)-epicatechin) and two oligomeric procyanidins (procyanidin B-type dimer and procyanidin B-type trimer) (Table 2). These two groups of compounds showed approximately equal fractions in both WB and BB juices. However, similar to phenolic acids and flavonols, significantly higher contents of most individual flavan-3-ols were detected in BB juice. For example, the concentrations of procyanidin B-type trimer and dimer in BB juice were 5.4 and 1.8 times higher than those in WB juice, and (+)-catechin was exclusively detected in BB juice. The compounds contributing most to the total flavan-3-ol content in BB juice were procyanidin B-type dimer and (−)-epicatechin, in decreasing order. Oppositely, in WB juice, (−)-epicatechin accounted for the highest proportion of flavan-3-ols, followed by procyanidin B-type dimer.
After fermentation, the contents of total phenolic acids and flavonols significantly increased (p < 0.001), being 30% and 29% higher in fermented WB sample than those in WB juice (Table 2). This may partly be due to the enhancement of extraction from the debris of bilberry pulp and skin by the increase of ethanol during fermentation.41,42 The quantitative changes of phenolic acids and flavonols led to a 27% increase in TPC. Compared to phenolic composition before fermentation, the proportions of TA (82.4%), TFO (14.7%), and TFA (2.9%) in TPC did not show remarkable changes in WB wine sample after fermentation.
Among the 20 phenolic acids quantified in WB juice and wine, only caffeic acid hexoside-1 and 3-caffeoylquinic acid showed significant decreases in content after fermentation (p < 0.001). Interestingly, the content of caffeic acid increased significantly (p < 0.001) from 0.74 to 17.21 mg/L along with the significant decrease (p < 0.001) in caffeic acid hexoside-1 content (from 16.37 to 0.16 mg/L). The consistent evolution of these two compounds was also observed in BB samples. This is probably due to the cleavage of the glycosidic bound to yield caffeic acid during fermentation. p-Coumaroyl-monotropeins still dominated in phenolic acids in bilberry wines. This is the first time that the exceptionally high concentration of p-coumaroyl monotropeins has been reported in bilberry wines.
After fermentation, the contents of all the individual flavonols in WB juice showed significant increases or remained constant. In particular, myricetin 3-O-glucoside and syringetin aglycone, which are absent in WB juice, elevated their contents to the levels accounting for 9.5% and 1.4% of TFO, respectively, in WB wine. Likewise, the significant increases (p < 0.01) in these two flavonols were detected after fermentation of BB juice.
Interestingly, despite the 29% and 30% increases in TFO and TA from WB juice to wine, only a 5% improvement of TFO and, conversely, a significant reduction (5.4%, p < 0.05) of TA were detected in BB samples. These observations indicated that the involvement of anthocyanins might be a crucial factor influencing the differences in TA and TFO between BB juice and WB juice during fermentation. For example, phenolic acids and flavonols react with anthocyanins to form the vertical π–π stacking complexes through intermolecular copigmentation. The formations of pyranoanthocyanins and polymeric pigments involve phenolic acids and flavonols, as well.43,44
The dramatic decrease in (−)-epicatechin content in WB juice was the main contributor to the significant reduction of TFA (p < 0.01). Similarly, fermentation of BB juice led to an approximately 36% degradation of (−)-epicatechin. The reduction may be due to the oxidation of these ortho-dihydroxyphenolic compounds through browning reaction to form quinones and subsequently to form macromolecules by polymerization.45,46
In conclusion, nonanthocyanin phenolic compounds in the juices and wines produced from berries of a white bilberry mutant and a normal bilberry were identified and quantified. The content and composition of nonanthocyanin phenolics in the products produced from white bilberry differed from those of the corresponding products prepared from pigmented bilberry. Dicaffeoylquinic acids, laricitrin 3-O-galactoside, isorhamnetin 3-O-galactoside, and (+)-catechin have only been found in blue bilberry juice or wine, while p-coumaroylquinic acid has only been detected in white bilberry products. The contents of phenolic acids, flavonols, and flavan-3-ols in white bilberry samples were significantly lower than the levels in blue ones. The excessively high amount of p-coumaroyl monotropeins was found for the first time in bilberry-based wines. Yeast fermentation significantly influenced the profiles of phenolic compounds in both pigmented and nonpigmented bilberry juices. The characterization and quantification of phenolic compounds provide a comprehensive insight into the chemical composition of bilberry products. At the same time, the study on the value-added products might stimulate the cultivation, breeding, and industrial utilization of bilberries.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jafc.0c02842.
Figure of simplified flavonoid biosynthesis pathway and table of calibration information (PDF)
This work was financially supported by the China Scholarship Council, the Finnish Foundation for Technology Promotion, the Finnish Cultural Foundation, and the Finnish Food Research Foundation.
The authors declare no competing financial interest.
Supplementary Material
References
- Jagtap U. B.; Bapat V. A. Wines from Fruits Other than Grapes: Current Status and Future Prospectus. Food Biosci. 2015, 9, 80–96. 10.1016/j.fbio.2014.12.002. [DOI] [Google Scholar]
- Vasantha Rupasinghe H. P.; Joshi V. K.; Smith A.; Parmar I.. Chemistry of Fruit Wines. Science and Technology of Fruit Wine Production; Elsevier, 2017; pp 105–176. [Google Scholar]
- Vuorinen H.; Määttä K.; Törrönen R. Content of the Flavonols Myricetin, Quercetin, and Kaempferol in Finnish Berry Wines. J. Agric. Food Chem. 2000, 48 (7), 2675–2680. 10.1021/jf991388o. [DOI] [PubMed] [Google Scholar]
- Čakar U.; Petrović A.; Janković M.; Pejin B.; Vajs V.; Čakar M.; Djordjević B. Differentiation of Wines Made from Berry and Drupe Fruits According to Their Phenolic Profiles. Eur. J. Hortic. Sci. 2018, 83 (1), 49–61. 10.17660/eJHS.2018/83.1.7. [DOI] [Google Scholar]
- Tanaka Y.; Sasaki N.; Ohmiya A. Biosynthesis of Plant Pigments: Anthocyanins, Betalains and Carotenoids. Plant J. 2008, 54 (4), 733–749. 10.1111/j.1365-313X.2008.03447.x. [DOI] [PubMed] [Google Scholar]
- Zoratti L.; Klemettilä H.; Jaakola L.. Bilberry (Vaccinium Myrtillus L.) Ecotypes. Nutritional Composition of Fruit Cultivars; Elsevier, 2016; pp 83–99. [Google Scholar]
- Chaves-Silva S.; Santos A. L. d.; Chalfun-Junior A.; Zhao J.; Peres L. E.P.; Benedito V. A. Understanding the Genetic Regulation of Anthocyanin Biosynthesis in Plants - Tools for Breeding Purple Varieties of Fruits and Vegetables. Phytochemistry 2018, 153, 11–27. 10.1016/j.phytochem.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Jaakola L.; Määttä K.; Pirttilä A. M.; Törrönen R.; Kärenlampi S.; Hohtola A. Expression of Genes Involved in Anthocyanin Biosynthesis in Relation to Anthocyanin, Proanthocyanidin, and Flavonol Levels during Bilberry Fruit Development. Plant Physiol. 2002, 130 (2), 729–739. 10.1104/pp.006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel-Shirley B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. 2001, 126 (2), 485–493. 10.1104/pp.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestby R.; Percival D.; Martinussen I.; Opstad N.; Rohloff J. The European Blueberry (Vaccinium Myrtillus L.) and the Potential for Cultivation. Eur. J. Plant Sci. Biotechnol. 2011, 5, 5–16. [Google Scholar]
- Kangas K.Wild Berry Utilisation and Markets in Finland; University of Joensuu, Faculty of Forestry, 2001. [Google Scholar]
- Smeriglio A.; Davide B.; Laganà G.; Bellocco E.; Trombetta D.. Bilberry (Vaccinium Myrtyllus L.). Nonvitamin and Nonmineral Nutritional Supplements; Elsevier, 2019; pp 159–163. [Google Scholar]
- Riihinen K.; Jaakola L.; Kärenlampi S.; Hohtola A. Organ-Specific Distribution of Phenolic Compounds in Bilberry (Vaccinium Myrtillus) and “northblue” Blueberry (Vaccinium Corymbosum x V. Angustifolium). Food Chem. 2008, 110 (1), 156–160. 10.1016/j.foodchem.2008.01.057. [DOI] [PubMed] [Google Scholar]
- Heinonen M. Antioxidant Activity and Antimicrobial Effect of Berry Phenolics - A Finnish Perspective. Mol. Nutr. Food Res. 2007, 51 (6), 684–691. 10.1002/mnfr.200700006. [DOI] [PubMed] [Google Scholar]
- Turtiainen M.; Salo K.; Saastamoinen O. Variations of Yield and Utilisation of Bilberries (Vaccinium Myrtillus L.) and Cowberries (V. Vitis-Idaea L.) in Finland. Silva Fenn. 2011, 45 (2), 237–251. 10.14214/sf.115. [DOI] [Google Scholar]
- Nestby R.; Hykkerud A. L.; Martinussen I. Review of Botanical Characterization, Growth Preferences, Climatic Adaptation and Human Health Effects of Ericaceae and Empetraceae Wild Dwarf Shrub Berries in Boreal, Alpine and Arctic Areas. J. Berry Res. 2019, 9 (3), 515–547. 10.3233/JBR-190390. [DOI] [Google Scholar]
- Zorenc Z.; Veberic R.; Slatnar A.; Koron D.; Miosic S.; Chen M. H.; Haselmair-Gosch C.; Halbwirth H.; Mikulic-Petkovsek M. A Wild ‘Albino’ Bilberry (Vaccinium Myrtillus L.) from Slovenia Shows Three Bottlenecks in the Anthocyanin Pathway and Significant Differences in the Expression of Several Regulatory Genes Compared to the Common Blue Berry Type. PLoS One 2017, 12 (12), e0190246. 10.1371/journal.pone.0190246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primetta A. K.; Karppinen K.; Riihinen K. R.; Jaakola L. Metabolic and Molecular Analyses of White Mutant Vaccinium Berries Show Down-Regulation of MYBPA1-Type R2R3MYB Regulatory Factor. Planta 2015, 242 (3), 631–643. 10.1007/s00425-015-2363-8. [DOI] [PubMed] [Google Scholar]
- Zorenc Z.; Veberic R.; Koron D.; Miosic S.; Hutabarat O. S.; Halbwirth H.; Mikulic-Petkovsek M. Polyphenol Metabolism in Differently Colored Cultivars of Red Currant (Ribes Rubrum L.) through Fruit Ripening. Planta 2017, 246 (2), 217–226. 10.1007/s00425-017-2670-3. [DOI] [PubMed] [Google Scholar]
- Cheynier V.; Sarni-Manchado P.. Wine Taste and Mouthfeel. Managing Wine Quality; Elsevier, 2010; pp 29–72. [Google Scholar]
- Hufnagel J. C.; Hofmann T. Orosensory-Directed Identification of Astringent Mouthfeel and Bitter-Tasting Compounds in Red Wine. J. Agric. Food Chem. 2008, 56 (4), 1376–1386. 10.1021/jf073031n. [DOI] [PubMed] [Google Scholar]
- Ferrer-Gallego R.; Hernández-Hierro J. M.; Rivas-Gonzalo J. C.; Escribano-Bailón M. T. Sensory Evaluation of Bitterness and Astringency Sub-Qualities of Wine Phenolic Compounds: Synergistic Effect and Modulation by Aromas. Food Res. Int. 2014, 62, 1100–1107. 10.1016/j.foodres.2014.05.049. [DOI] [Google Scholar]
- Li H.; Guo A.; Wang H. Mechanisms of Oxidative Browning of Wine. Food Chem. 2008, 108 (1), 1–13. 10.1016/j.foodchem.2007.10.065. [DOI] [Google Scholar]
- Lingua M. S.; Fabani M. P.; Wunderlin D. A.; Baroni M. V. In Vivo Antioxidant Activity of Grape, Pomace and Wine from Three Red Varieties Grown in Argentina: Its Relationship to Phenolic Profile. J. Funct. Foods 2016, 20, 332–345. 10.1016/j.jff.2015.10.034. [DOI] [Google Scholar]
- George N.; Clark A. C.; Prenzler P. D.; Scollary G. R. Factors Influencing the Production and Stability of Xanthylium Cation Pigments in a Model White Wine System. Aust. J. Grape Wine Res. 2006, 12 (1), 57–68. 10.1111/j.1755-0238.2006.tb00044.x. [DOI] [Google Scholar]
- Behrends A.; Weber F. Influence of Different Fermentation Strategies on the Phenolic Profile of Bilberry Wine (Vaccinium Myrtillus L.). J. Agric. Food Chem. 2017, 65 (34), 7483–7490. 10.1021/acs.jafc.7b02268. [DOI] [PubMed] [Google Scholar]
- Liu S.; Laaksonen O.; Yang W.; Zhang B.; Yang B. Pyranoanthocyanins in Bilberry (Vaccinium Myrtillus L.) Wines Fermented with Schizosaccharomyces Pombe and Their Evolution during Aging. Food Chem. 2020, 305, 125438. 10.1016/j.foodchem.2019.125438. [DOI] [PubMed] [Google Scholar]
- Tian Y.; Liimatainen J.; Alanne A. L.; Lindstedt A.; Liu P.; Sinkkonen J.; Kallio H.; Yang B. Phenolic Compounds Extracted by Acidic Aqueous Ethanol from Berries and Leaves of Different Berry Plants. Food Chem. 2017, 220, 266–281. 10.1016/j.foodchem.2016.09.145. [DOI] [PubMed] [Google Scholar]
- Hokkanen J.; Mattila S.; Jaakola L.; Pirttilä A. M.; Tolonen A. Identification of Phenolic Compounds from Lingonberry (Vaccinium Vitis-Idaea L.), Bilberry (Vaccinium Myrtillus L.) and Hybrid Bilberry (Vaccinium x Intermedium Ruthe L.) Leaves. J. Agric. Food Chem. 2009, 57 (20), 9437–9447. 10.1021/jf9022542. [DOI] [PubMed] [Google Scholar]
- Bujor O. C.; Le Bourvellec C.; Volf I.; Popa V. I.; Dufour C. Seasonal Variations of the Phenolic Constituents in Bilberry (Vaccinium Myrtillus L.) Leaves, Stems and Fruits, and Their Antioxidant Activity. Food Chem. 2016, 213, 58–68. 10.1016/j.foodchem.2016.06.042. [DOI] [PubMed] [Google Scholar]
- Jensen H. D.; Krogfelt K. A.; Cornett C.; Hansen S. H.; Christensen S. B. Hydrophilic Carboxylic Acids and Iridoid Glycosides in the Juice of American and European Cranberries (Vaccinium Macrocarpon and V. Oxycoccos), Lingonberries (V. Vitis-Idaea), and Blueberries (V. Myrtillus). J. Agric. Food Chem. 2002, 50 (23), 6871–6874. 10.1021/jf0205110. [DOI] [PubMed] [Google Scholar]
- Mikulic-Petkovsek M.; Schmitzer V.; Slatnar A.; Stampar F.; Veberic R. A Comparison of Fruit Quality Parameters of Wild Bilberry (Vaccinium Myrtillus L.) Growing at Different Locations. J. Sci. Food Agric. 2015, 95 (4), 776–785. 10.1002/jsfa.6897. [DOI] [PubMed] [Google Scholar]
- Turner A.; Chen S. N.; Nikolic D.; Van Breemen R.; Farnsworth N. R.; Pauli G. F. Coumaroyl Iridoids and a Depside from Cranberry (Vaccinium Macrocarpori). J. Nat. Prod. 2007, 70 (2), 253–258. 10.1021/np060260f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkilä L.; Laaksonen O.; Alanne A. L.; Kortesniemi M.; Kallio H.; Yang B. Stability of Hydroxycinnamic Acid Derivatives, Flavonol Glycosides, and Anthocyanins in Black Currant Juice. J. Agric. Food Chem. 2016, 64 (22), 4584–4598. 10.1021/acs.jafc.6b01005. [DOI] [PubMed] [Google Scholar]
- Sarnoski P. J.; Johnson J. V.; Reed K. A.; Tanko J. M.; O’Keefe S. F. Separation and Characterisation of Proanthocyanidins in Virginia Type Peanut Skins by LC-MSn. Food Chem. 2012, 131 (3), 927–939. 10.1016/j.foodchem.2011.09.081. [DOI] [Google Scholar]
- Ren Q.; Wu C.; Ren Y.; Zhang J. Characterization and Identification of the Chemical Constituents from Tartary Buckwheat (Fagopyrum Tataricum Gaertn) by High Performance Liquid Chromatography/Photodiode Array Detector/Linear Ion Trap FTICR Hybrid Mass Spectrometry. Food Chem. 2013, 136 (3–4), 1377–1389. 10.1016/j.foodchem.2012.09.052. [DOI] [PubMed] [Google Scholar]
- Aaby K.; Grimmer S.; Holtung L. Extraction of Phenolic Compounds from Bilberry (Vaccinium Myrtillus L.) Press Residue: Effects on Phenolic Composition and Cell Proliferation. LWT - Food Sci. Technol. 2013, 54 (1), 257–264. 10.1016/j.lwt.2013.05.031. [DOI] [Google Scholar]
- Díaz-García M. C.; Obón J. M.; Castellar M. R.; Collado J.; Alacid M. Quantification by UHPLC of Total Individual Polyphenols in Fruit Juices. Food Chem. 2013, 138 (2–3), 938–949. 10.1016/j.foodchem.2012.11.061. [DOI] [PubMed] [Google Scholar]
- Zorenc Z.; Veberic R.; Stampar F.; Koron D.; Mikulic-Petkovsek M. White versus Blue: Does the Wild “albino” Bilberry (Vaccinium Myrtillus L.) Differ in Fruit Quality Compared to the Blue One?. Food Chem. 2016, 211, 876–882. 10.1016/j.foodchem.2016.05.142. [DOI] [PubMed] [Google Scholar]
- Marquez A.; Serratosa M. P.; Merida J. Influence of Bottle Storage Time on Colour, Phenolic Composition and Sensory Properties of Sweet Red Wines. Food Chem. 2014, 146, 507–514. 10.1016/j.foodchem.2013.09.103. [DOI] [PubMed] [Google Scholar]
- Gil-Muñoz R.; Gómez-Plaza E.; Martínez A.; López-Roca J. M. Evolution of Phenolic Compounds during Wine Fermentation and Post-Fermentation: Influence of Grape Temperature. J. Food Compos. Anal. 1999, 12 (4), 259–272. 10.1006/jfca.1999.0834. [DOI] [Google Scholar]
- Makris D. P.; Kallithraka S.; Kefalas P. Flavonols in Grapes, Grape Products and Wines: Burden, Profile and Influential Parameters. J. Food Compos. Anal. 2006, 19 (5), 396–404. 10.1016/j.jfca.2005.10.003. [DOI] [Google Scholar]
- Li S.-Y.; Duan C.-Q. Astringency, Bitterness and Color Changes in Dry Red Wines before and during Oak Barrel Aging: An Updated Phenolic Perspective Review. Crit. Rev. Food Sci. Nutr. 2019, 59 (12), 1840–1867. 10.1080/10408398.2018.1431762. [DOI] [PubMed] [Google Scholar]
- Rentzsch M.; Schwarz M.; Winterhalter P. Pyranoanthocyanins - an Overview on Structures, Occurrence, and Pathways of Formation. Trends Food Sci. Technol. 2007, 18 (10), 526–534. 10.1016/j.tifs.2007.04.014. [DOI] [Google Scholar]
- Kallithraka S.; Salacha M. I.; Tzourou I. Changes in Phenolic Composition and Antioxidant Activity of White Wine during Bottle Storage: Accelerated Browning Test versus Bottle Storage. Food Chem. 2009, 113 (2), 500–505. 10.1016/j.foodchem.2008.07.083. [DOI] [Google Scholar]
- Cheynier V.; Rigaud J.; Souquet J.-M. M.; Barillere J. M.; Moutounet M. Effect of Pomace Contact and Hyperoxidation on the Phenolic Composition and Quality of Grenache and Chardonnay Wines. Am. J. Enol. Vitic. 1989, 40 (1), 36–42. [Google Scholar]
- Stanoeva J. P.; Stefova M.; Andonovska K. B.; Vankova A.; Stafilov T. Phenolics and Mineral Content in Bilberry and Bog Bilberry from Macedonia. Int. J. Food Prop. 2017, 20 (00), S863–S883. 10.1080/10942912.2017.1315592. [DOI] [Google Scholar]
- Laaksonen O.; Sandell M.; Kallio H. Chemical Factors Contributing to Orosensory Profiles of Bilberry (Vaccinium Myrtillus) Fractions. Eur. Food Res. Technol. 2010, 231 (2), 271–285. 10.1007/s00217-010-1278-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.