Abstract
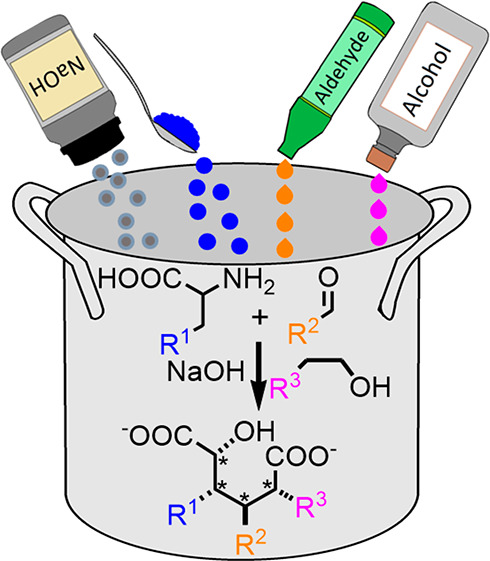
Herein we report a unique method for preparing diaryl hydroxyl dicarboxylic acids in a diastereospecific manner. The three-component reaction occurs between amino acid, aromatic aldehyde, and primary alcohol in alkaline solutions under microwave-assisted conditions. The dicarboxylic acids are isolated as sodium salts in high yields (up to 77%) by direct precipitation from the reaction solution. The experimental results suggest that the diastereospecificity originates from a [3,3]-sigmatropic rearrangement followed by a sodium-assisted hydride transfer. As further shown, the previously unreported dicarboxylic acids are easily turned into corresponding δ-lactones.
Introduction
The development of biomass-based economy is creating an abundant amount of protein waste which can be processed into amino acids.1,2 In advanced biorefinery concepts, amino acids are further transformed into nitrogen-containing platform chemicals, such as caprolactam.2−4 Amino acids have been extensively studied during the last 200 years, yet continue to be a source of new reactivity. Regardless of their well-established chemistry, to better utilize the available amino acids as synthetic precursors for value-added chemicals, new types of reactions are sought-after.4,5 In this respect, we report here a straightforward method for preparing previously unreported aryl-substituted hydroxy dicarboxylic acids (from hereon diacids) from various amino acids. These diacids are formed with up to four stereogenic centers in a diastereospecific manner. These diacids can be turned into the corresponding δ-lactones by a simple treatment with Brønsted acid. While diacids are valuable monomers as such in polymer synthesis, δ-lactones have uses as antibiotics,6−8 anticancer drugs,9−11 and food flavorings.12
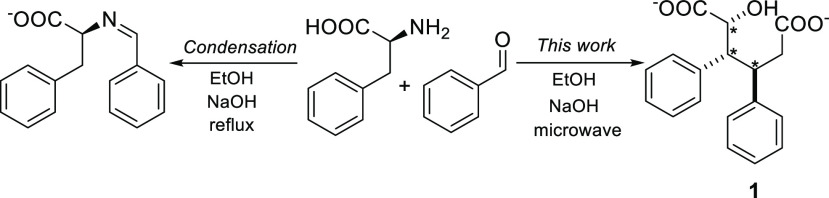
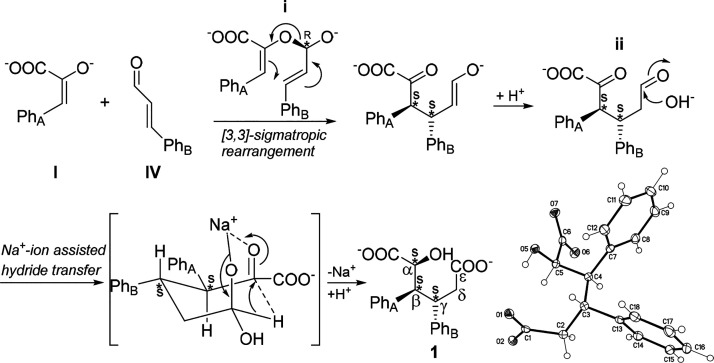
We initiated our studies by focusing on the classical condensation reaction between l-phenylalanine and benzaldehyde. Unexpectedly, in alkaline ethanol solutions under microwave-assisted conditions the reaction yielded a new, white crystalline product. Based on 1H NMR, 13C NMR, FT-IR spectroscopy, HRMS analysis, and X-ray crystallography, the substance is the unprecedented sodium salt of 2-hydroxy-3,4-diphenylhexanedioic acid 1 (Figure 1). The 1H NMR spectrum of 1 exhibits a signal in the aromatic region (δ 7.2–6.9 ppm, m, 10H, ArH) corresponding to two phenyls, while the signal at 4.61 ppm (d, J = 2.8 Hz, 1H) is characteristic for a proton attached to a carbon bearing both hydroxyl and carboxylate groups. Further on, the protons attached to the same carbons as the phenyl groups have a large coupling constant to each other (11.4 Hz) suggesting that these protons have a trans-geometry and all aliphatic protons are part of the same spin system (see the Experimental Section for details). The 13C NMR spectrum of 1 shows 14 nonequivalent carbons; shifts of 182 and 180 ppms are fingerprints of two different carboxylate groups while a signal at 72 ppm arises from the α-carbon containing both carboxylate and alcohol groups (Figure 1). Based on X-ray crystallography, the stereocenters in 1 are RRR or its enantiomer SSS due to the centrosymmetry in the crystal structure.13 As there is no amino functionality in the structure of 1, the result was intriguing and counterintuitive. Therefore, we took an initiative to carry out further studies and reveal mechanistic details of diacid synthesis.
Figure 1.
(Left) A classical imine condensation product between l-phenylalanine and benzaldehyde (phenylalanine, N-(phenylmethylene)). (Right) Diacid salt 1 (this work), which forms as an enantiomeric pair of RRR and SSS.
Results and Discussion
The optimized isolated yield of 1 was achieved from ethanol solutions of 2 equiv of benzaldehyde and 1.5 equiv of NaOH at 135 °C and in 90 min using a microwave synthesizer (see Supporting Information S3, Table S1, entry 1, Method 1). Notably, when CsOH, KOH, and LiOH were used instead of NaOH, the yields dropped to 2%, 4%, and 5%, respectively. Interestingly, when ethanol/tert-butanol solvent mixture was used as the reaction medium with 5 equiv of NaOH and benzaldehyde, isolated yield of 1 was improved drastically to 77% (Table 1, entry 1).
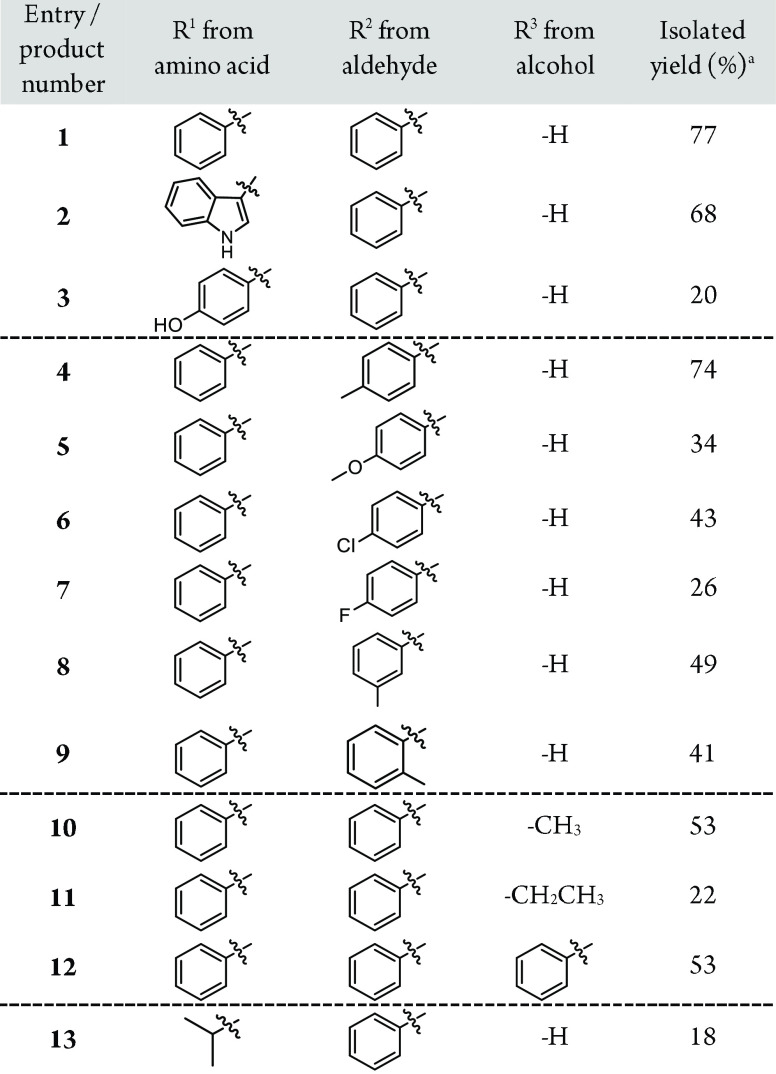
Table 1. Formation of Diacid Salts from Amino Acids and Aromatic Aldehydes in Alkaline Alcohol Solutionsb.
Products were washed with EtOH, dried in air and weighed. Reaction conditions: amino acid (2.0 mmol), NaOH (10 mmol, 10 M at H2O solution), aromatic aldehyde (10 mmol), 75%/25% v/v% tert-butanol/corresponding alcohol solution (4 mL); microwave vial was heated to 135 °C over 5 min and kept at 135 °C for 90 min.
Products emerge as an enantiomeric pair of RRR(R) and SSS(S).
The substrate scope of amino acids was extended toward l-tryptophan and l-tyrosine (Table 1, entries 2 and 3), which both bear a π-activated methylene group and undergo a similar reaction as l-phenylalanine. As the aromatic groups were now distinguishable, it became evident that the second phenyl ring (R2) originated from the benzaldehyde moiety. This phenomenon was confirmed by performing experiments with substituted benzylic aldehydes: p-tolualdehyde, p-anisaldehyde, 4-chlorobenzylaldehyde, and 4-fluorobenzaldehyde (see Table 1, entries 4–7). The reaction also tolerates various sterical patterns on the benzylic aldehyde shown with ortho- and meta-substituted tolualdehyde (Table 1, entries 4, 8, and 9). It is noteworthy that the structures of these novel diacids are straightforwardly modifiable with different amino acids and aromatic aldehydes.
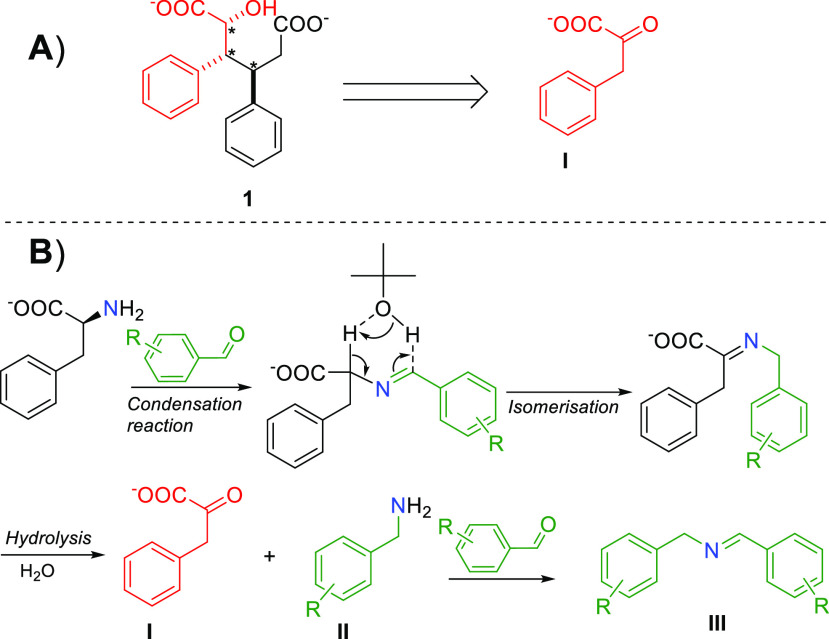
The alcohol functionality and the missing amino functionality in 1 are noteworthy, and are fingerprints of the reaction mechanism (Figure 2A). It is likely that the reaction starts, indeed, with the classical base-catalyzed imine condensation followed by a base-assisted imine isomerization and further hydrolysis to phenylpyruvic acid I and benzyl amine II (Figure 2B). Similar transaminase steps have been reported.14−16 As the ketone group in phenylpyruvic acid I is in similar position as the hydroxyl group in the final product 1, we rationalized that I could be a transient intermediate of the reaction. Next, we followed the reaction by 1H NMR spectroscopy and GC–MS analysis and observed benzylidenebenzylamine III as a major compound. This supports the generation of phenylpyruvic acid I and benzyl amine II under the reaction conditions. Analogously, when a substituted benzaldehyde is used, the corresponding substitution is found in the structure of III (see Supporting Information S9.4 for details).
Figure 2.
(A) Retrosynthetic analysis of diacid salt 1. (B) Proposed reaction mechanism for the formation of phenylpyruvic acid I. Amino acid undergoes condensation reaction with benzaldehyde followed by isomerization and hydrolysis to I.
Intriguingly, alcohol as a solvent is also an important component in the diacid synthesis. The change from ethanol to 1-propanol brings a methyl substitution to the structure of 1 (Table 1, entry 10), underlining that the side chain R3 is easily controlled by the choice of primary alcohol. Accordingly, an ethyl substituent was observed at R3 position in NMR analysis when using 1-butanol as the primary alcohol. Similarly, 2-phenylethanol gave a phenyl substituent (Table 1, entries 11 and 12). Methanol and 2-propanol gave no products, suggesting that a chain of at least two carbons is needed and the alcohol has to be primary.
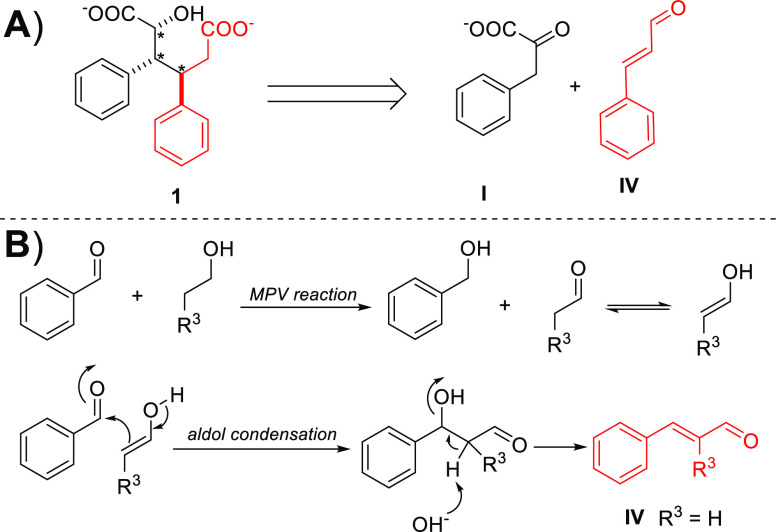
As the alcoholic solvent clearly transfers to the structure of the formed diacids, we performed a series of control experiments under microwave-assisted conditions (Figure 3A). The reaction between ethanol and benzaldehyde generated a small amount of cinnamyl alcohol (134 m/zSupporting Information S9.4). Next, we reacted benzaldehyde with 1-propanol—a longer chain alcohol—which yielded a mixture of aldehyde and alcohol analogues. In addition, the reaction between benzaldehyde and 1-octanol generated only 2-(phenylmethylene)octanal. The relation between alcohol structure and the stability of an aldehyde in the applied conditions is clear; more aldehyde is observed when a longer chain alcohol is used (Figure 3B and Supporting Information S9).
Figure 3.
(A) Retrosynthetic analysis of diacid salt 1. (B) Proposed mechanism for the formation of IV. Meerwein–Ponndorf–Verley (MPV) reaction between benzaldehyde and ethanol yields benzyl alcohol and acetaldehyde followed by aldol condensation to from cinnamaldehyde IV.
The above-described reactivity is rationalized by benzaldehyde undergoing a Meerwein–Pondorf–Verley (MPV) reaction with primary alcohol generating aliphatic aldehyde and benzyl alcohol. Then the aliphatic aldehyde transfer occurs through keto–enol tautomerization to enolate followed by aldol condensation with another benzaldehyde molecule to form corresponding cinnamaldehyde derivatives (Figure 3B). This reactivity is similar to the known formation of cinnamaldehyde IV from ethanol and benzaldehyde.17,18 The observed alcohol analogues of cinnamaldehydes are most likely generated by MPV or Cannizzaro reactions. This motivated us to further study the role of cinnamaldehyde in the reaction.
To test our hypothesis that phenylpyruvic acid I and cinnamaldehyde IV have roles as building blocks of 1, we treated pure phenylpyruvic acid I and cinnamaldehyde IV under the applied reaction conditions. By this approach, product 1 was formed in good yields (56%, see Supporting Information S9.3). The reaction is likely to proceed via the hemiacetal adduct i, which undergoes an oxy-Cope type [3,3]-sigmatropic rearrangement reaction. The intermediate undergoes H+ transfer generating ii, which bears aldehyde and pyruvic functionalities (Figure 4). Under the applied alkaline conditions, the aldehyde undergoes nucleophilic attack by OH–. The final product 1 is formed after the parallel carbonyl units undergo Na+-ion-assisted hydride transfer from the ε-carbon to α-carbon. The Na+-ion is optimal for forming an intermediate with a chairlike conformation, allowing the hydride transfer reaction to take place. The ion radius of Na+ (1.16 Å) and the C–H bond distance (1.09 Å) are similar in length. In contrast, Cs+ (1.81 Å), K+ (1.52 Å), and Li+ (0.90 Å) deviate from the C–H bond length.19 Similar hydride transfer step has been recently reported by Xiao et al.20
Figure 4.
Proposed mechanism for the formation of 1. The reaction produces enantiomers SSS (depicted) and RRR as observed in the crystal structure of 1. (Displacement parameters are drawn at 50% probability level.13) Abbreviations: PhA indicates phenyl group originated from amino acid, and PhB phenyl group originated from benzaldehyde.
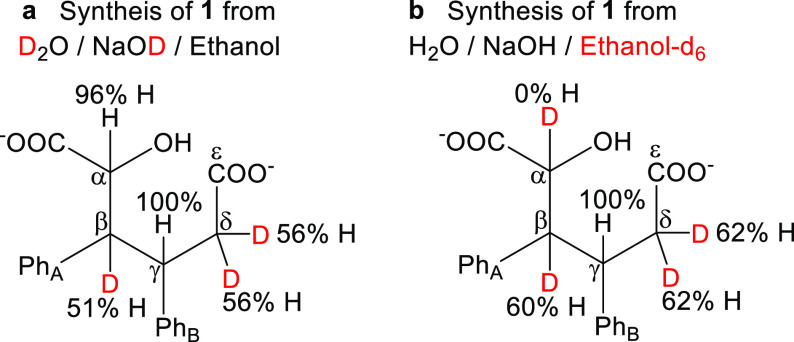
As i and ii are nondetectable intermediates, extensive 1H NMR labeling studies were carried out to further ensure the reaction pathway. When NaOD/D2O is used in the synthesis, the integrals of protons attached to β- and δ-carbons are clearly reduced due to the keto–enol tautomerization (Figure 5a, see Supporting Information S9.2). It is worth noting that the proton attached to the α-carbon was not deuterated, which strongly supports an H-transfer reaction with a nondeuterated source, namely cinnamaldehyde originated from ethanol (Figure 3B). Further studies with deuterated ethanol-d6 confirmed the hydride transfer from ethanol-d6. The proton attached to α-carbon disappeared from the 1H NMR spectrum (Figure 5b, see Supporting Information S9.2).
Figure 5.
H to D ratio of 1 when the reaction was performed (a) in D2O/NaOD/ethanol solution. The ratio of H to D was approximately 50%. (b) In H2O/NaOH/ethanol-d6 solution. The ratio of H to D was approximately 60%. The proton attached to the α-carbon is fully deuterated.
The diastereospecifity of this multicomponent reaction arises from a [3,3]-sigmatropic rearrangement, in which a new C–C bond is formed between the β- and γ-carbons and two stereocenters are generated (Figure 4, intermediate i). The steric repulsion between the phenyl groups in phenylpyruvic acid I and cinnamaldehyde IV is important for the formation of these stereocenters; the reaction proceeds only if the phenyl groups are not overlapping in the hemiacetal adduct i. The chiral hemiacetal i is formed either by re or si face attack of enolate form of I (E or Z) to the prochiral cinnamaldehyde IV. Hemiacetal i with R configuration gives ii as an SS diastereomer with E enolate or RR diastereomer with Z enolate. Similarly, hemiacetal i with S configuration gives ii as an RR diastereomer with E enolate or SS diastereomer with Z enolate. A detailed description is provided in the Suporting Information section 9.1.
The stereospecific formation of 1 continues by a Na+-ion-assisted hydride transfer. It requires a preorganized structure, wherein the carbonyl units are parallel. As shown in Figure 4, the hydride transfer is likely to occur via the energetically most favored chair conformation, placing aromatic substituents in the plane of the molecule. This conformation directs the hydride transfer and, as shown in the X-ray structure of 1 (see Supporting Information S10.1), leads to the third stereogenic center. For example, the intermediate ii with the RR stereocenters yields the RRR diastereomer, and the SS stereocenters yield the SSS diastereomer of 1 (see Supporting Information S9.1). When 1 is synthesized from racemic dl-phenylalanine, it yields the same racemic product as with l-phenylalanine. Clearly, stereospecificity originates from the intermediates instead of the starting materials.
The role of tert-butanol merits some further discussion. Since the typical impurity observed was unreacted amino acid, we rationalized that the formation of phenylpyruvic acid I is the reaction-limiting step. Even the addition of an excess of benzaldehyde did not enhance the yield of 1. Interestingly, when tert-butanol was used as an isomerization catalyst between amino acid and benzaldehyde, the yields were improved markedly (see Figure 2B). tert-Butanol is an inert solvent towards the MPV reaction (see Figure 3B) and therefore is unable to form cinnamaldehyde derivatives. This allowed us to control the cinnamaldehyde formation by simply altering the ethanol to tert-butanol ratio in the reaction. The improved control over the cinnamaldehyde formation frees benzaldehyde to react with the amino acid. As a result, an increased amount of phenylpyruvic acid I becomes available in the reaction solution. Alternatively, tert-butanol can generate tert-butoxide in the presence of OH– and serve as a powerful base for extracting the proton from the α-position. With this additional reactivity, we successfully converted a nonaromatic amino acid, l-leucine, to the corresponding diacid (Table 1, entry 13).
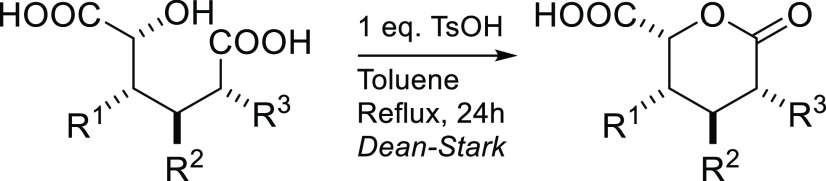
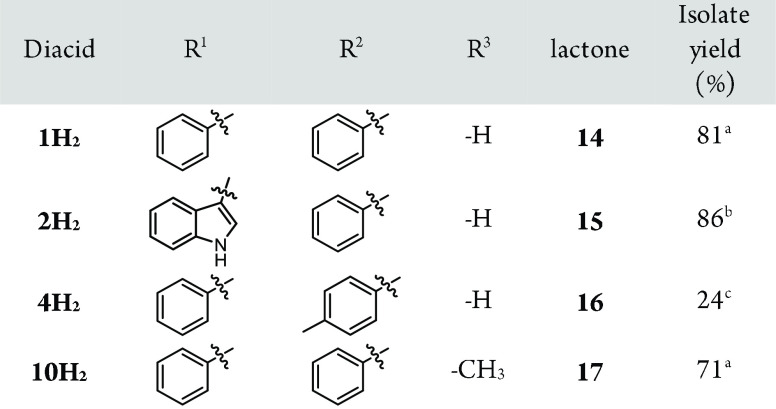
As shown with diacids 1, 2, 4, and 10, the products are transfered straightforwardly to lactones by refluxing in toluene with a stoichiometric amount of p-toluenesulfonic acid (TsOH) (Table 2). Importantly, this is a new pathway to prepare aryl-substituted δ-lactone acids which are often tedious to synthesize and, therefore, rare.21−23
Table 2. Formation of δ-Lactone Acids from Various Hydroxyl Diacids.
Reaction conditions: Diacid (2.0 mmol), TsOH (2.0 mmol), toluene (30 mL), 24 h, reflux (Dean–Stark apparatus).
Diacid (1.0 mmol) and HCl (1.0 mmol) instead of TsOH.
As in a, but TsOH (4.0 mmol), 48 h.
Conclusions
To summarize, we have successfully synthesized unprecedented, aryl-substituted dicarboxylic acids (1–13) by multicomponent, one-pot reaction from amino acids (R1), substituted benzaldehydes (R2), and primary alcohols (R3) in alkaline solutions. The products are composed of in situ generated pyruvic acid I and cinnamaldehyde IV, which react together via a [3,3]-sigmatropic oxy-Cope rearrangement, followed by Na+-ion-assisted hydride transfer. The diacids form with up to four stereogenic centers in a diastereospecific manner. Overall, this is a new and straightforward synthetic method for diaryl hydroxyl dicarboxylic acids, which enables their systematic modification. As further shown, the diacids are straightforwardly turned into novel aryl-substituted δ-lactone acids in the presence of a Brønsted acid.
Experimental Section
General Information
All chemicals were obtained from commercial sources. Amino acids, phenylpyruvic acid, cinnamaldehyde, 1-propanol, 1-butanol, tert-butanol, and substituted aromatic aldehydes were all purchased from Sigma-Aldrich and used without further purification. Ethanol was purchased from VWR chemicals (96%). Water used in the experiments was obtained from Milli-Q water purification system. Benzaldehyde was purchased from Acros organics and used as such. Labeled ethanol-d6 was purchased from Cambridge Isotope Laboratory. 1H, 13C, 19F, HSQC, and HMBC NMR spectra were recorded with an INOVA by Varian (500 MHz, 27 °C) or with an Avance Neo by Bruker (500 MHz, 25 °C). Recorded spectra were calibrated by solvent signals when applicable and processed with MestReNova software. High-resolution mass spectra were measured by Bruker microTOF-MS in both positive and negative ion modes. Sodium formate was used as a calibrant. IR spectra were measured with Alpha ATR-FTIR by Bruker. UV–vis spectra were recorded with Hewlett-Packard 8453 Spectrophotometer. A quartz cuvette (1 cm width) was used when acetone was used as solvent and a plastic cuvette when Milli-Q water was used as a solvent. The single-crystal X-ray diffraction was measured by Bruker D8 Venture diffractometer with a PhotonII CPAD detector. Measurement was done at 123 K using Cu–Kα radiation (1.541 78 Å). Syntheses were conducted with a microwave synthesizer, either Initiator by Biotage or Monowave 450 by Anton Paar. Vials of 10 mL (2–5 mL (biotage), G10 (Anton-paar)) or 30 mL (10–20 mL (biotage, G30 (Anton-paar)) in total volume were used. Initiator vials have “cap with septum” and Monowave 450 vials have “snap cap” with “teflon-coated silicon septum” as caps. The temperature during synthesis was monitored by a surface IR sensor (Monowave 450 IR eye), which was calibrated with “Ruby thermometer” internal temperature probe. GC-MS spectra were recorded with Agilent 5973 equipped with 6890N MSD using HP-5MSUI.
General Procedure for Diacid Salt Synthesis
Amino acid (2 mmol), NaOH, (10 mmol, 1.0 mL, 10M), and tert-butanol/corresponding alcohol mixture (4.0 mL, 75/25 v/v%) were added together with aromatic aldehyde (10 mmol) to a 10 mL microwave vial. The reaction mixture often becomes solid after all reagents are added. Pressure fluctuation may occur during the heat up phase. Therefore, the heating program was set to heat the microwave vial to 135 °C over 5 min, and then temperature was maintained for 90 min. Stirring speed was set to 600 rpm. Next, the microwave vial was cooled to 50 °C by compressed air. Afterwards, isopropyl alcohol (2 mL) was added into the microwave vial to initiate the crystallization overnight. The formed products were collected by filtration, washed with EtOH (with the exception of products 3, 7, and 11, see their own sections), and dried in air.
General Procedure for Protonated Diacids
The corresponding diacid salts were dissolved into water, and 1 M HCl was added until all the products had precipitated from the solution. The powder was filtered, washed with water, and dried in vacuum. The reaction occurs instantaneously with near quantitative yields (>95%).
General Procedure for δ-Lactone Acid Synthesis
A solution of diacid (2 mmol), p-tolusulfonic acid monohydrate (TsOH·H2O, 2 mmol) in toluene (30 mL) in a 50 mL flask was refluxed for 24 h using a Dean–Stark apparatus. Afterwards, the reaction solution was left to cool to room temperature for 2 h, followed by removal of TsOH by filtration. The δ-lactone acid product crystallized from the remaining toluene solution within 1–3 days. The solid products were collected by filtration, washed with toluene, and dried in vacuum.
2-Hydroxy-3,4-diphenylhexanedioic Acid Salt (Enantiomeric Pair of RRR and SSS) (1)
A microwave vial (10 mL) containing l-phenylalanine (2 mmol, 330.4 mg), benzaldehyde (10 mmol, 1015 μL), NaOH (10 mmol, 1 mL, 10 M), and tert-butanol/ethanol mixture (4 mL, 75/25 v/v%) was heated to 135 °C over 5 min at a microwave reactor. Then the reaction temperature was maintained for 90 min. The vial was cooled to 50 °C using compressed air. Isopropyl alcohol (2 mL) was added to the vial to initiate crystallization. The next day the formed powder was collected by filtration, washed with ethanol, and dried in air. The product was isolated as 554 mg of a white powder (77% yield calculated from amino acid). 1H NMR (500 MHz, D2O): δ 7.21–7.00 (m, 10H), 4.57 (d, J = 2.8 Hz, 1H), 3.71 (ddd, J = 11.4, 4.5, 11.4 Hz, 1H), 3.41 (dd, J = 11.6, 2.8 Hz, 1H), 3.05 (dd, J = 13.5, 4.4 Hz, 1H), 2.54 (dd, J = 13.4, 11.3 Hz, 1H). 13C{1H}NMR (125 MHz, D2O): δ 181.5, 179.9, 143.6, 139.2, 130.0, 128.8, 128.1, 127.8, 126.5, 126.1, 72.4, 53.8, 44.2, 43.4. IR (atr): 3000–3500 (−OH, broad), 1573 (R–COONa, s), 1395 (R–OH, s), 1074 (R–C(OH)–R, m), 698 (Ph, s). UV–vis (Milli-Q water): 224 nm (shoulder), 258 nm (max), and 265 nm (shoulder). HRMS (ESI-TOF) m/z: [1+H2+Na]+ Calcd for C18H18O5Na 337.1046; Found 337.1046. See X-ray diffraction section for solved crystal structure of 1 (see Supporting Information S4.1 and S10.1). Cambridge Crystallographic Data Center number (CCDC): 1948055
2-Hydroxy-3-(1H-indol-3-yl)-4-phenylhexanedioic Acid Salt (Enantiomeric Pair of RRR and SSS) (2)
The product was synthesized according to the general procedure using l-tryptophan (2 mmol, 408.5 mg) instead of l-phenylalanine. Product was isolated as 542 mg of a white powder (68% yield). 1H NMR (500 MHz, D2O): δ 7.66 (m, 1H), 7.30–6.90 (m, 9H), 4.59 (d, J = 2.4 Hz, 1H), 3.81 (dd, J = 11.4, 2.4 Hz, 1H), 3.73 (ddd, J = 11.2, 4.3, 11.3 Hz, 1H), 3.08 (dd, J = 13.5, 4.3 Hz, 1H), 2.59 (dd, J = 13.4, 11.1 Hz, 1H). 13C{1H} NMR (125 MHz, D2O): δ 181.7, 180.3, 144.2, 135.5, 128.5, 127.9, 127.7, 125.9, 124.8, 121.1, 120.0, 118.6, 112.7, 111.3, 72.4, 45.6, 45.3, 43.5. IR (atr): 3000–3800 (−OH, −N–H, broad), 1670 (N–H, m), 1574 (R–COONa, s), 1384 (R–OH, s), 1274 (C–N, m), 1083 (R–C(OH)–R, s), 704 (Ph, s). UV–vis (Milli-Q water): 229 nm (shoulder), 282 nm (max), and 291 nm (shoulder). HRMS (ESI-TOF) m/z: [2+H2+Na]+ Calcd for C20H19NO5Na 376.1155; Found 376.1147. See X-ray diffraction section for solved crystal structure of 2 (see Supporting Information S4.1 and S10.2). CCDC: 1948056
2-Hydroxy-3-(4-hydroxyphenyl)-4-phenylhexanedioic Acid Salt (Enantiomeric Pair of RRR and SSS) (3)
The product was synthesized according to the general procedure using l-tyrosine (2 mmol, 362.4 mg) instead of l-phenylalanine. Purification: The reaction solution was evaporated, and the crude solid was dissolved in MeOH and filtered. Acetone was added to the MeOH solution, precipitating the crude product. Recrystallization from water/acetone mixtures yielded pure 3 (15 mg). According to 1H NMR measurements, a 20% yield was obtained from the original reaction solution. 1H NMR (500 MHz, D2O): δ 7.21–7.12 (m, 4H), 7.09–6.99 (m, 3H), 6.60 (m, 2H), 4.53 (d, J = 2.7 Hz, 1H), 3.63 (ddd, J = 11.5, 11.4, 4.4 Hz, 1H), 3.34 (dd, J = 11.6, 2.8 Hz, 1H), 3.02 (dd, J = 13.4, 4.4 Hz, 1H), 2.53 (dd, J = 13.4, 11.4 Hz, 1H). 13C{1H} NMR (125 MHz, D2O): δ 181.4, 179.7, 153.7, 143.5, 131.0, 130.9, 128.6, 127.9, 125.8, 114.4, 72.3, 52.7, 44.1, 43.1. IR (atr): 2800–3500 (−OH, broad), 1556 (−COONa, s), 1371 (s), 1248 (s), 700 (s) (Ph–OH), 1078 (R–HCOH–R (m)), 759 (m), 733 (m) (R–Ph 5H adjacent), 522 (w), 465 (s) (R–Ph–R (2H adjacent)). UV–vis (Milli-Q water): 298 nm (max) and 331 nm (shoulder). HRMS (ESI-TOF) m/z: [3+H]− Calcd for C18H17O6 329.1020; Found 329.1026.
2-Hydroxy-3-phenyl-4-(4-methylphenyl)hexanedioic Acid Salt (Enantiomeric Pair of RRR and SSS) (4)
The product was synthesized according to the general procedure using p-tolualdehyde (10 mmol, 1180 μL) instead of benzaldehyde. Product was isolated as 552 mg of a white powder (74% yield). 1H NMR (500 MHz, D2O): δ 7.21–7.06 (m, 7H), 7.00–6.95 (m, 2H), 4.55 (d, J = 2.7 Hz, 1H), 3.66 (ddd, J = 11.4, 4.3, 11.4 Hz, 1H), 3.37 (dd, J = 11.5, 2.6 Hz, 1H), 3.03 (dd, J = 13.4, 4.3 Hz, 1H), 2.52 (dd, J = 13.2, 11.5 Hz, 1H), 2.14 (s, 3H). 13C{1H} NMR (125 MHz, D2O): δ 184.2, 182.5, 143.1, 141.9, 138.4, 132.6, 131.3, 131.2, 130.4, 129.0, 75.0, 56.4, 46.3, 45.9, 22.7. IR (atr): 2800–3200 (−OH, broad), 1612, 1543 (R–COONa, s), 1370 (R–OH, s, R–CH3, s), 1093 (R–CH(OH)–R, m), 825 (2 adjacent H, Ph, w), 731 (m), 703 (s) (5 adjacent H, Ph). UV–vis (Milli-Q water): 258 nm (max), 265 nm (max), 274 nm (max), 321 nm (shoulder), and 334 nm (shoulder). HRMS (ESI-TOF) m/z: [4+H2+Na]+ Calcd for C19H20O5Na 351.1203; Found 351.1203.
2-Hydroxy-3-phenyl-4-(4-methoxyphenyl)hexanedioic Acid Salt (Enantiomeric Pair of RRR and SSS) (5)
The product was synthesized according to the general procedure using p-anisaldehyde (10 mmol, 1215 μL) instead of benzaldehyde. Product was isolated as 265 mg of a white powder (34% yield). 1H NMR (500 MHz, D2O): δ 7.22–7.07 (m, 7H), 6.75–6.70 (m, 2H), 4.58 (dd, J = 2.8 Hz, 1H), 3.69 (m, 4H), 3.39 (dd, J = 11.5, 2.9 Hz, 1H), 3.06 (dd, J = 13.4, 4.4 Hz, 1H), 2.54 (dd, J = 13.4, 11.4 Hz, 1H). 13C{1H} NMR (125 MHz, D2O): δ 184.2, 182.5, 159.4, 141.9, 138.8, 132.6, 132.4, 130.4, 129.1, 116.1, 75.0, 57.9, 56.6, 46.0, 46.0. IR (atr): 2800–3500 (−OH, broad) 1612, 1555 (R–COONa, s), 1371 (−CH3, s), 1249 (−OH, s), 1027 (R–CH(OH)–R, m), 834 (2 adjacent H (Ph), m), 704 (5 adjacent H (Ph), s). UV–vis (Milli-Q water): 275 nm (max) and 296 nm (shoulder). HRMS (ESI-TOF) m/z: [5+H2+Na]+Calcd for C19H20O6Na 367.1152; Found 367.1167.
2-Hydroxy-3-phenyl-4-(4-chlorophenyl)hexanedioic Acid Salt (Enantiomeric Pair of RRR and SSS) (6)
The product was synthesized according to the general procedure using 4-chlorobenzaldehyde (10 mmol, 1240 mg) instead of benzaldehyde. Product was isolated as 334 mg of a white powder (43% yield). 1H NMR (500 MHz, D2O): δ 7.21–7.09 (m, 9H), 4.58 (d, J = 2.8 Hz, 1H), 3.72 (td, J = 11.5, 4.5, 11.5 Hz, 1H), 3.38 (dd, J = 11.6, 2.9 Hz, 1H), 3.07 (dd, J = 13.5, 4.4 Hz, 1H), 2.55 (dd, J = 13.4, 11.4 Hz, 1H). 13C{1H} NMR (125 MHz, D2O): δ 183.9, 182.4, 144.8, 141.6, 133.6, 132.8, 132.6, 130.6, 130.4, 129.1, 74.9, 56.4, 46.3, 45.8. IR (atr): 2800–3500 (−OH, broad), 1614, 1556 (R–COONa, s), 1370 (R–CH(OH)–R, s), 1093 (Ar–Cl/R–CH(OH)–R), s), 829 (2 adjacent H (Ph), m), 702 (5 adjacent H (Ph), s). UV–vis (Milli-Q water): 257 nm (max), 260 nm (max), and 278 nm (shoulder). HRMS (ESI-TOF) m/z: [6+H2+Na]+ Calcd for C18H17ClO5Na 371.0657; Found 371.0654.
2-Hydroxy-3-phenyl-4-(4-fluorophenyl)hexanedioic Acid Salt (Enantiomeric Pair of RRR and SSS) (7)
The product was synthesized according to the general procedure using 4-fluorobenzaldehyde (10 mmol, 1070 μL) instead of benzaldehyde. Characterization data is reported for crude 7, because purification was difficult. For corresponding pure acid 7H2 please see section below. (Corresponding pure 7H2 (195 mg, 26% yield) is reported later in the Experimental Section.) 1H NMR (500 MHz, D2O): δ 7.18–7.06 (m, 7H), 6.87–6.81 (m, 2H), 4.56 (d, J = 2.8 Hz, 1H), 3.69 (td, J = 11.6, 4.4 Hz, 1H), 3.36 (dd, J = 11.6, 2.9 Hz, 1H), 3.05 (dd, J = 13.4, 4.4 Hz, 1H), 2.51 (dd, J = 13.4, 11.5 Hz, 1H). 13C{1H} NMR (125 MHz, D2O): δ 181.2, 179.6, 161.7, 159.8, 139.0, 139.0, 138.9, 130.0, 129.9, 129.8, 127.6, 126.3, 114.5, 114.3, 72.1, 53.7, 43.3, 43.2. 19F NMR (470 MHz, D2O): δ −118.0. IR (atr): 2800–3500 (−OH, broad), 1605 (m), 1552 (m), 1390 (m) (−COONa) 1220 (m), 1100 (w) (R–CHOH–R), 839 (w), 791 (w) (R–Ph–R (2H adjacent)), 761 (w), 728 (w), 705 (m) (R–Ph (5H adjacent)), 663 (m), 523 (s) (C–F). UV–vis (Milli-Q water): 289 nm (max) and 298 nm (shoulder). HRMS (ESI-TOF) m/z: [7+H]− Calcd for C18H16FO5 331.0976; Found 331.0976.
2-Hydroxy-3-phenyl-4-(3-methylphenyl)hexanedioic Acid Salt (Enantiomeric Pair of RRR and SSS) (8)
The product was synthesized according to the general procedure using m-tolualdehyde (10 mmol, 1180 μL) instead of benzaldehyde. Product was isolated as 364 mg of a white powder (49% yield). 1H NMR (500 MHz, D2O): δ 7.22–7.18 (m, 2H), 7.17–7.13 (m, 2H), 7.11–6.99 (m, 4H), 6.89 (m, 1H), 4.58 (d, J = 2.7 Hz, 1H), 3.69 (ddd, J = 11.4, 4.4, 11.4 Hz, 1H), 3.43 (dd, J = 11.6, 2.8 Hz, 1H), 3.05 (dd, J = 13.5, 4.4 Hz, 1H), 2.54 (dd, J = 13.4, 11.3 Hz, 1H), 2.20 (s, 3H). 13C{1H} NMR (125 MHz, D2O): δ 181.6, 179.9, 143.7, 139.2, 138.0, 130.0, 129.5, 128.0, 127.8, 126.6, 126.5, 125.8, 72.4, 53.7, 44.0, 43.4, 20.5. IR (atr): 1610, 1572, 1402 (R-COONa, s), 1376 (−CH3, s), 1102 (R–CH(OH)–R, m), 758 (3 adjacent H (Ph), m), 694 (5 adjacent H (Ph), s). UV–vis (Milli-Q water): 258 nm (max), 265 nm (max), and 272 nm (shoulder). HRMS (ESI-TOF) m/z: [8+H2+Na]+ Calcd for C19H20O5Na 351.1203; Found 351.1209.
2-Hydroxy-3-phenyl-4-(2-methylphenyl)hexanedioic Acid Salt (Enantiomeric Pair of RRR and SSS) (9)
The product was synthesized according to the general procedure using o-tolualdehyde (10 mmol, 1160 μL) instead of benzaldehyde. Product was isolated as 312 mg of a white powder (41% yield). 1H NMR (500 MHz, D2O): δ 7.43 (d, J = 6.2 Hz, 1H), 7.21 (m, 2H), 7.16–7.05 (m, 4H), 6.98–6.91 (m, 2H), 4.60 (d, J = 2.7 Hz, 1H), 4.06 (dd, J = 11.1, 4.4 Hz, 1H), 3.43 (dd, J = 11.3, 2.0 Hz, 1H), 3.03 (dd, J = 13.0, 4.4 Hz, 1H), 2.50 (t, J = 12.0 Hz, 1H), 2.23 (s, 3H). 13C{1H} NMR (125 MHz, D2O): δ 181.4, 179.7, 142.2, 139.5, 136.6, 129.7, 129.5, 127.5, 127.1, 126.4, 125.9, 125.7, 72.3, 54.0, 43.3, 38.4, 19.2. IR (atr): 3400–2800 (−OH, broad) 1614, 1604, 1401 (R–COONa, s), 1379 (−CH3, m), 1102 (R–CH(OH)–R, m), 764 (4 adjacent H (Ph), m), 730, 702 (5 adjacent H (Ph), s). UV–vis (Milli-Q water): 259 nm (max), 265 nm (max), and 273 nm (shoulder). HRMS (ESI-TOF) m/z: [9+H2+Na]+ Calcd for C19H20O5Na 351.1203; Found 351.1208.
2-Hydroxy-5-methyl-3,4-diphenylhexanedioic Acid Salt (Enantiomeric Pair of RRRR and SSSS) (10)
The product was synthesized according to the general procedure using tert-butanol/1-propanol (4 mL, 75/25 v/v%) instead of tert-butanol/ethanol mixture. Product was isolated as 396 mg of a white powder (53% yield). 1H NMR (500 MHz, D2O): δ 7.25–7.21 (m, 2H), 7.18–7.10 (m, 6H), 7.07–7.02 (m, 2H), 4.54 (d, J = 2.6 Hz, 1H), 3.95 (dd, J = 12.0, 4.5 Hz, 1H), 3.73 (dd, J = 12.1, 2.7 Hz, 1H), 3.08 (dq, J = 7.1, 4.6 Hz, 1H), 1.05 (d, J = 7.0 Hz, 3H). 13C{1H} NMR (125 MHz, D2O): δ 187.2, 182.5, 142.9, 141.9, 132.8, 132.7, 130.4, 130.3, 129.1, 128.8, 75.0, 52.0, 50.4, 46.7, 13.9. IR (atr): 2800–3500 (−OH, broad), 1593, 1537 (R–COONa, m), 1450 (R–CH3, m), 1397 (R–CH(OH)–R, m), 1378 (R–CH3, m), 1281, 1076 (R–CH(OH)–R, m), 699 (5 adjacent H (Ph), s). UV–vis (Milli-Q water): 259 (max) and 264 (max). HRMS (ESI-TOF) m/z: [10+H2+Na]+ Calcd for C19H20O5Na 351.1203; Found 351.1207.
2-Hydroxy-5-ethyl-3,4-diphenylhexanedioic Acid Salt (Enantiomeric Pair of RRRR and SSSS) (11)
The product was synthesized according to the general procedure using tert-butanol/1-butanol (4 mL, 75/25 v/v%) instead of tert-butanol/ethanol mixture. Purification: The crude product was washed with isopropyl alcohol and dissolved into EtOH. The EtOH solution was added into another isopropyl alcohol solution. Crystals formed overnight at isopropyl alcohol–ethanol solution. Product was isolated as 173 mg of a white needle crystals (22% yield). 1H NMR (500 MHz, D2O): δ 7.21–7.18 (m, 2H), 7.14–7.10 (m, 6H), 7.08–7.02 (m, 2H), 4.53 (d, J = 2.6 Hz, 1H), 3.76 (dd, J = 11.4, 4.6 Hz, 1H), 3.75 (dd, J = 11.5, 2.7 Hz, 1H), 2.79 (ddd, J = 11.9, 4.7, 2.6 Hz, 1H), 1.62 (dqd, J = 14.8, 7.4, 2.6 Hz, 1H), 1.35 (m, 1H), 0.87 (t, J = 7.3 Hz, 3H). 13C{1H} NMR (125 MHz, D2O): δ 186.1, 182.5, 143.5, 141.9, 132.8, 132.6, 130.3, 130.3, 129.0, 128.7, 75.2, 55.7, 52.8, 51.0, 22.2, 15.0. IR (atr): 2800–3200 (−OH, broad), 1591 (R–COONa, s), 1449 (−CH2– or −CH3, m), 1380 (R–Me, s), 1378 (s), 1448 (s) (R–COONa), 1080 (R–CH(OH)–R, m), 701 (5 adjacent H (Ph)). UV–vis (Milli-Q water): 259 nm (max), 264 nm (shoulder), and 293 nm (shoulder). HRMS (ESI-TOF) m/z: [11+H2+Na]+ Calcd for C20H22O5Na 365.1359; Found 365.1352.
2-hHydroxy-3,4,5-triphenylhexanedioic Acid Salt (Enantiomeric Pair of 2R, 3R, 4S, 5S and 2S, 3S, 4R, 5R) (12)
The product was synthesized according to the general procedure using tert-butanol/2-phenylethanol (4 mL, 75/25 v/v%) instead of tert-butanol/ethanol mixture. Product was isolated as 458 mg of a white powder (53% yield). 1H NMR (500 MHz, D2O): δ 7.22–7.08 (m, 9H), 7.05–7.01 (m, 1H), 6.97–6.91 (m, 4H), 6.88–6.84 (m, 1H), 4.54 (d, J = 4.6 Hz, 1H), 4.21 (dd, J = 10.5, 8.2 Hz, 1H), 3.98 (d, J = 10.5 Hz, 1H), 3.60 (dd, J = 8.2, 4.6 Hz, 1H). 13C{1H} NMR (125 MHz, D2O): δ 181.7, 179.6, 141.0, 140.5, 138.7, 130.7, 129.2, 127.9, 127.3, 127.1, 126.3, 126.1, 125.5, 73.7, 59.5, 54.1, 48.6. (130.7 or 127.9 is double peak, too low resolution to tell the difference). IR (atr): 3500–3200 (−OH, broad), 1623 (s), 1599 (s), 1549 (s) (−COONa), 1374(s), 1096(w), 1071(w) (R–CH(OH)–R), 694 (5 adjacent H (Ph)). UV–vis (Milli-Q water): 299 nm (max). HRMS (ESI-TOF) m/z: [12+H]−Calcd for C24H21O5 389.1384; Found 389.1384.
2-Hydroxy-3-(isopropyl alcohol)-4-phenylhexanedioic Acid Salt (Enantiomeric Pair of 2R, 3S, 4R and 2S, 3R, 4S) (13)
The product was synthesized according to the general procedure using l-leucine (2 mmol, 262.4 mg) instead of l-phenylalanine. Product was isolated as 117 mg of a white powder (18% yield). 1H NMR (500 MHz, D2O): δ 7.40–7.36 (m, 4H), 7.27 (m, 1H), 4.03 (d, J = 1.7 Hz, 1H), 3.52 (ddd, J = 12.2, 6.6, 4.1 Hz, 1H), 2.74 (dd, J = 14.7, 4.1 Hz, 1H), 2.62 (dd, J = 14.6, 12.3 Hz, 1H), 2.11 (ddd, J = 1.7, 6.7, 6.7 Hz, 1H), 1.78 (m, 1H), 1.05 (d, J = 6.9 Hz, 3H), 0.78 (d, J = 6.9 Hz, 3H). 13C{1H} NMR (125 MHz, D2O): δ 182.4, 182.2, 144.8, 128.5, 128.3, 126.1, 71.4, 52.1, 42.5, 39.0, 26.9, 20.79, 20.77. IR (atr): 3500–3200 (−OH, broad), 1602(s), 1556(s), 1362(m) (−COONa), 1403 (s), 1319 (m), 1288 (m) (R–CH(OH)–R), 697 (s), 516 (m) (5 adjacent H (Ph)). UV–vis (Milli-Q water): 288 nm (max) and 293 nm (shoulder). HRMS (ESI-TOF) m/z: [13+H]−Calcd for C15H19O5 279.1227; Found 279.1227.
5-Carboxylic Acid-3,4-diphenyl-δ-valorelactone (Enantiomeric Pair of RRR and SSS) (14)
A toluene (30 mL) solution of 1H2 (2 mmol, 628.7 mg) and TsOH (2 mmol, 380.4 mg) was refluxed for 24 h using a Dean–Stark apparatus. Afterwards, the reaction solution was left to cool to room temperature for 2 h, followed by removal of TsOH by filtration. Product 14 precipitated out from the remaining toluene solution during the following day. Next, the product was collected by filtration, washed with toluene, and dried in vacuum. Product 14 was isolated as 484 mg of a white powder (81% yield). 1H NMR (500 MHz, acetone-d6): δ 7.35–7.32 (m, 2H), 7.28–7.14 (m, 8H), 5.32 (d, J = 5.5 Hz, 1H), 3.97 (dd, J = 10.4, 5.5 Hz, 1H), 3.81 (td, J = 10.5, 6.4 Hz, 1H), 2.92 (m, 2H). 13C{1H} NMR (125 MHz, acetone-d6): δ 170.0, 169.9, 143.3, 138.5, 129.8, 129.5, 129.1, 128.4, 128.1, 127.7, 80.0, 48.3, 41.8, 38.6. IR (atr): 3000–2500 (−COOH, broad), 1730(s), 1204(s), 1160(s), 832(s) (R–O–CO–R), 1681 (s), 1416 (w), 1087 (s), 905 (m) (R–COOH), 1493 (R–CH2–R, w) 695 (Ph, 5 adjacent H). UV–vis (acetone): 207 nm (max), 210 nm (max), 212 nm (max), 217 nm (shoulder), and 336 nm (max). HRMS (ESI-TOF) m/z: Monomer [14+Na]+ Calcd for C18H16O4Na 319.0941; Found 319.0938; Dimer [2×14+Na]+ Calcd for C36H32O8Na 615.1989; Found 615.2006.
5-Carboxylic Acid-4-(1H-indol-3-yl)-3-phenyl-δ-valorelactone (Enantiomeric Pair of RRR and SSS) (15)
A toluene (15 mL) solution of 2H2 (1 mmol, 353.4 mg) and HCl (1 mmol, 1 mL, 1M) was refluxed for 24 h using a Dean–Stark apparatus. Afterwards, the reaction solution was left to cool to room temperature. The product precipitated out from the toluene solution in a few hours. Next, the product was collected by filtration, washed with toluene, and dried in vacuum. Product 15 was isolated as 291 mg of a white powder (86% yield). 1H NMR (500 MHz, acetone-d6): δ 7.68 (d, J = 7.9 Hz, 1H), 7.42 (d, J = 7.5 Hz, 2H), 7.34 (d, J = 8.1 Hz, 1H), 7.28 (t, J = 7.6 Hz, 2H), 7.18 (m, 2H), 7.09 (t, J = 7.5 Hz, 1H), 7.03 (t, J = 7.4 Hz, 1H), 5.36 (d, J = 5.0 Hz, 1H), 4.27 (dd, J = 9.2, 5.0 Hz, 1H), 3.89 (td, J = 9.2, 6.9 Hz, 1H), 2.93 (m, 2H). 13C{1H} NMR (125 MHz, acetone-d6): δ 170.4, 170.0, 144.2, 137.2, 129.6, 128.2, 128.0, 127.7, 124.3, 122.4, 119.9, 119.2, 112.3, 111.7, 79.2, 41.9, 39.7, 37.9. IR (atr): 3400–3200 (NH, broad), 1735 (s), 1405 (m), 939 (m) (R–COOH), 1495 (indole, w), 1458 (R–CH2–R, m), 1213 (s), 1106 (s), 1067 (s) (R–O–CO–R), 740 (s), 694 (s) (Ph, 5 adjacent H). UV–vis (EtOH): 291 nm (max) and 299 nm (max). HRMS (ESI-TOF) m/z: Monomer [15+Na]+ Calcd for C20H17NO4Na 358.1050; Found 358.1050; Dimer [2×15+Na]+ Calcd for C40H34N2O8Na 693.2207; Found 693.2208.
5-Carboxylic Acid-3-(4-methylphenyl)-4-phenyl-δ-valorelactone (Enantiomeric Pair of RRR and SSS) (16)
The product was synthesized according to the general procedure using 4H2 (2 mmol, 656.7 mg) with additional TsOH (4 mmol, 760.9 mg) and a prolonged reaction time (48 h). The product was isolated as 149 mg of a white powder (24% yield). 1H NMR (500 MHz, acetone-d6): δ 7.28–7.25 (m, 2H), 7.24–7.19 (m, 4H), 7.18–7.14 (m, 1H), 7.08–7.04 (m, 2H), 5.30 (d, J = 5.4 Hz, 1H), 3.93 (dd, J = 5.4, 10.4 Hz, 1H), 3.76 (td, J = 10.5, 6.4 Hz, 1H), 2.91 (m, 2H), 2.22 (s, 3H). 13C{1H} NMR (125 MHz, acetone-d6): δ 170.02, 169.98, 140.4, 138.6, 137.1, 130.2, 129.8, 129.1, 128.2, 128.1, 80.0, 48.4, 41.5, 38.7, 20.9. IR (atr): 3200–2800 (−COOH, broad), 1733(s), 1049(s) (R–O–CO–R), 1686 (s), 1193 (s), 1085 (s) (R–COOH), 1415 (w, R–CH2–R), 1386 (m), 822 (m) (R–Ph–p–me), 746 (s), 695 (s) (Ph, 5 adjacent H). UV–vis (acetone): 210 nm (max), 212 nm (shoulder), 216 nm (max), and 332 nm (max). HRMS (ESI-TOF) m/z: Monomer [16+Na]+ Calcd for C19H18O4Na 333.1097; Found 333.1098; Dimer [2×16+Na]+ Calcd for C38H36O8Na 643.2302; Found 643.2312.
5-Carboxylic Acid-2-methyl-3,4-diphenyl-δ-valorelactone (Enantiomeric Pair of RRRR and SSSS) (17)
The product was synthesized according to the general procedure using 10H2 (2 mmol, 656.7 mg). The product was isolated as a white powder 71% (432 mg) yield. 1H NMR (500 MHz, acetone-d6): δ 7.35–7.26 (m, 4H) 7.22–7.15 (m, 6H), 5.49 (d, J = 5.4 Hz, 1H), 3.92 (dd, J = 8.7, 5.4 Hz, 1H), 3.24–3.13 (m, 2H), 1.06 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (125 MHz, acetone-d6): δ 173.6, 169.5, 143.0, 139.6, 129.6, 129.5, 129.1, 128.9, 128.0, 127.8, 78.7, 51.1, 50.5, 42.0, 14.5. IR (atr): 3600–3300 (−OH, broad), 3100–2800 (R-COOH, broad), 1731 (s), 1225 (s), 1187 (s), 1088 (s), 775 (m), 761 (m) (R–O–(HCO)–R), 1454 (w, R–CH3), 1412 (m), 527 (s) (R–COOH), 699 (s), 438 (s) (Ph). UV–vis (acetone): 208 nm (max), 214 nm (max), 217 nm (shoulder), and 338 nm (max). HRMS (ESI-TOF) m/z: Monomer [17–H]− Calcd for C19H17O4 309.1121; Found 309.1117; Dimer [2×17–H]−Calcd for C38H35O8 619.2326; Found 619.2323.
2-Hydroxy-3,4-diphenylhexanedioic Acid (Enantiomeric Pair of RRR and SSS) (1H2)
The product was synthesized according to the general procedure for protonated diacids from product 1. 1H NMR (500 MHz, acetone-d6): δ 7.20–7.13 (m, 4H), 7.07–6.95 (m, 6H), 4.80 (d, J = 3.4 Hz, 1H), 3.81 (td, J = 10.9, 4.1 Hz, 1H), 3.47 (dd, J = 11.0, 3.4 Hz, 1H), 3.15 (dd, J = 15.1, 4.1 Hz, 1H), 2.88 (dd, J = 15.1, 10.8 Hz, 1H). 13C{1H} NMR (125 MHz, acetone-d6): δ 175.0, 173.4, 143.5, 139.9, 130.7, 129.5, 128.5, 128.2, 127.1, 126.8, 71.3, 53.8, 45.0, 39.0. IR (atr): 3400–2500 (−OH/–COOH, broad), 1730 (−ROOH, s), 1681 (C=O, s), 1493 (−CH2–, m), 1416 (w), 1160 (s) (−COOH), 1204 (m), 1087 (s) R–(HCOH)–R, m), 695 (5 adjacent H (Ph), s). UV–vis (acetone): 208 nm (max), 211 nm (max), 214 nm (max), and 216 nm (shoulder).
2-Hydroxy-3-(1H-indol-3-yl)-4-phenylhexanedioic Acid (Enantiomeric Pair of RRR and SSS) (2H2)
The product was synthesized according to the general procedure for protonated diacids from product 2. 1H NMR (500 MHz, acetone-d6): δ 10.65 (broad, 1H), 9.91 (s, 1H), 7.60 (d, 1H), 7.32–7.28 (m, 3H), 7.22 (d, 1H), 7.10–7.06 (m, 2H), 6.99–6.94 (m, 2H), 6.92–6.88 (m, 1H), 4.71 (d, J = 2.8 Hz, 1H), 3.95 (dd, J = 9.4, 2.8 Hz, 1H), 3.87 (dt, J = 10.0, 4.3 Hz, 1H), 3.11–2.99 (m, 2H). 13C{1H} NMR (125 MHz, acetone-d6): δ 175.5, 173.7, 144.2, 136.9, 129.3, 128.9, 128.5, 126.8, 125.0, 121.6, 119.9, 119.2, 113.0, 111.8, 71.3, 45.9, 45.3, 38.4. IR (atr): 3391 (N–H, s), 1722 (−ROOH, s), 1455, 1425 (−CH2–, m), 1275, 1214 (−COOH, m), 1106 (R–HCOH–R, s), 739, 697 (5 adjacent H (Ph), s). UV–vis (acetone): 207 nm (max), 212 nm (max), 215 nm (max), and 220 nm (shoulder).
2-Hydroxy-3-phenyl-4-(4-methylphenyl)hexanedioic Acid (Enantiomeric Pair of RRR and SSS) (4H2)
The product was synthesized according to the general procedure for protonated diacids from product 4. 1H NMR (500 MHz, acetone-d6): δ 7.22–7.18 (m, 2H), 7.06–6.99 (m, 5H), 6.90–6.85 (m, 2H), 4.79 (d, J = 3.4 Hz, 1H), 3.79 (dt, J = 10.9, 4.1 Hz, 1H), 3.47 (dd, J = 11.0, 3.4 Hz, 1H), 3.12 (dd, J = 15.0, 4.1 Hz, 1H), 2.85 (dd, J = 15.0, 10.8 Hz, 1H), 2.14 (s, 3H). 13C{1H} NMR (125 MHz, acetone-d6): δ 175.0, 173.4, 140.5, 139.9, 135.9, 130.7, 129.4, 129.2, 128.2, 127.1, 71.3, 53.8, 44.5, 39.2, 20.9. IR (atr): 1729 (−ROOH, s), 1671 (C=O, s), 1493 (−CH2–, m), 1453 (−CH3, w), 1216 (m), 1158 (w), 1086 (s) (R–HCOH–R), 837, 805 (2 adjacent H (Ph), m) 741, 695 (5 adjacent H (Ph), s). UV–vis (acetone): 208 nm (max), 212 nm (max), 214 nm (max), 217 nm (max), and 222 nm (shoulder).
2-Hydroxy-3-phenyl-4-(4-fluorophenyl)hexanedioic Acid (Enantiomeric Pair of RRR and SSS) (7H2)
The product was synthesized according to the general procedure for protonated diacids from product 7. 1H NMR (500 MHz, acetone-d6): δ 7.20–7.15 (m, 4H), 7.06–6.98 (m, 3H), 6.84–6.79 (m, 2H), 4.81 (d, J = 3.4 Hz, 1H), 3.83 (td, J = 11.0, 4.1 Hz, 1H), 3.45 (dd, J = 11.1, 3.4 Hz, 1H), 3.17 (dd, J = 15.2, 4.1 Hz, 1H), 2.86 (dd, J = 15.1, 11.0 Hz, 1H). 13C{1H} NMR (125 MHz, acetone-d6): δ 174.9, 173.3, 162.9, 161.0, 139.7, 139.6, 139.6, 131.3, 131.2, 130.7, 128.2, 127.2, 115.1, 115.0, 71.1, 53.8, 44.3, 39.1. 19F NMR (470 MHz, acetone-d6): δ −118.7. IR (atr): 3437 (−OH, br), 3200–2800 (−COOH, br), 1730 (m), 1680 (m), 1158 (s) (−COOH), 1218 (s), 1090 (s) (R–HCOH–R), 847 (s), 811 (m), 699 (s), 671 (w), 568 (s), 420 (m) (R–Ph–F, either 2H adjacent vibrations or C–F movements), 746 (s), 718 (w), 628 (m) (Ph-R, 5H adjacent). UV–vis (acetone): 209 nm (max) and 214 nm (shoulder).
2-Hydroxy-5-methyl-3,4-diphenylhexanedioic Acid (Enantiomeric Pair of RRRR and SSSS) (10H2)
The product was synthesized according to the general procedure for protonated diacids from product 10. 1H NMR (500 MHz, acetone-d6): δ 7.24–7.20 (m, 2H), 7.14–7.10 (m, 2H), 7.07–6.95 (m, 6H), 4.77 (d, J = 3.4 Hz, 1H), 4.15 (dd, J = 11.3, 4.7 Hz, 1H), 3.75 (dd, J = 11.3, 3.4 Hz, 1H), 3.31 (dq, J = 7.0, 4.7 Hz, 1H), 1.17 (d, J = 7.0 Hz, 3H). 13C{1H} NMR (125 MHz, acetone-d6): δ 176.1, 175.0, 139.8, 139.2, 131.0, 130.9, 128.2, 128.1, 127.1, 126.9, 71.2, 50.0, 48.2, 41.5, 11.3. IR (atr): 1704 (−ROOH, s), 1453, 1392 (−CH3, m), 1233 (−COOH, s), 1093 (R–HCOH–R, s), 697 (5 adjacent H (Ph), s). UV–vis (acetone): 209 nm (max), 214 nm (shoulder), and 216 nm (shoulder).
Acknowledgments
A.E. acknowledges the University of Helsinki and Magnus Ehrnrooth Foundation for financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.9b03320.
The authors declare no competing financial interest.
Supplementary Material
References
- van Haveren J.; Scott E. L.; Sanders J. Bulk chemicals from biomass. Biofuels, Bioprod. Biorefin. 2008, 2, 41–57. 10.1002/bbb.43. [DOI] [Google Scholar]
- Sheldon R. A. Green and sustainable manufacture of chemicals from biomass: state of the art. Green Chem. 2014, 16, 950–963. 10.1039/C3GC41935E. [DOI] [Google Scholar]
- Li S.-Y.; Ng I.-S.; Chen P. T.; Chiang C.-J.; Chao Y.-P. Biorefining of protein waste for production of sustainable fuels and chemicals. Biotechnol. Biofuels 2018, 11, 256–271. 10.1186/s13068-018-1234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuck C. O.; Pérez E.; Horvàth I. T.; Sheldon R. A.; Poliakoff M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. 10.1126/science.1218930. [DOI] [PubMed] [Google Scholar]
- Lynd L. R. The grand challenge of cellulosic biofuels. Nat. Biotechnol. 2017, 35, 912–915. 10.1038/nbt.3976. [DOI] [PubMed] [Google Scholar]
- Ohkuma H.; Naruse N.; Nishiyama Y.; Tsuno T.; Hoshino Y.; Sawada Y.; Konishi M.; Oki T. Sultriecin, a new antifungal and antitumor antibiotic from Streptomyces roseiscleroticus: production, isolation, structure and biological activity. J. Antibiot. 1992, 45, 1239–1249. 10.7164/antibiotics.45.1239. [DOI] [PubMed] [Google Scholar]
- Konno H.; Hiroya K.; Ogasawara K. A New Tactic for Diastereo- and Enantiocontrolled Synthesis of (−)-Malyngolide via Catalytic Meso-Asymmetrization. Tetrahedron Lett. 1997, 38, 6023–6026. 10.1016/S0040-4039(97)01339-7. [DOI] [Google Scholar]
- Cardona W.; Quiñones W.; Robledo S.; Vélez I. D.; Murga J.; García-Fortanet J.; Carda M.; Cardona D.; Echeverri F. Antiparasite and antimycobacterial activity of passifloricin analogues. Tetrahedron 2006, 62, 4086–4092. 10.1016/j.tet.2006.02.017. [DOI] [Google Scholar]
- McMurry J. E.; Dushin R. G. Total Synthesis of (±)-Isolobophytolide and (±)-Crassin by Titanium-Induced Carbonyl Coupling. J. Am. Chem. Soc. 1990, 112, 6942–6942. 10.1021/ja00175a031. [DOI] [Google Scholar]
- Zhang H.; Conte M. M.; Capon R. J. Franklinolides A-C from an Australian Marine Sponge Complex: Phosphodiesters Strongly Enhance Polyketide Cytotoxicity. Angew. Chem., Int. Ed. 2010, 49, 9904–9906. 10.1002/anie.201005883. [DOI] [PubMed] [Google Scholar]
- Igarashi Y.; Asano D.; Furihata K.; Oku N.; Miyanaga S.; Sakurai H.; Saiki I. Absolute configuration of pterocidin, a potent inhibitor of tumor cell invasion from a marine-derived Streptomyces. Tetrahedron Lett. 2012, 53, 654–656. 10.1016/j.tetlet.2011.11.115. [DOI] [Google Scholar]
- Dufossé L.; Latrasse A.; Spinnler H.-E. Importance des lactones dans les arômes alimentaires: Structure, distribution, propriétés sensorielles et biosynthèse. Sciences des Aliments 1994, 14, 17–50. [Google Scholar]
- CCDC 1948055 (1) and 1948056 (2) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre. For details, see the Supporting Information.
- Willems J. G. H.; de Vries J. G.; Nolte R. J. M.; Zwanenburg B. Asymmetric Imine Isomerisation in the Enantioselective Synthesis of Chiral Amines from Prochiral Ketones. Tetrahedron Lett. 1995, 36, 3917–3920. 10.1016/0040-4039(95)00641-O. [DOI] [Google Scholar]
- Yasumoto M.; Ueki H.; Soloshonok V. A. Thermal 1,3-proton shift reaction and its application for operationally convenient and improved synthesis of α-(trifluoromethyl)benzylamine. J. Fluorine Chem. 2007, 128, 736–739. 10.1016/j.jfluchem.2007.02.008. [DOI] [Google Scholar]
- Chen F.-F.; Zhang Y.-H.; Zhang Z.-J.; Liu L.; Wu J.-P.; Xu J.-H.; Zheng G.-W. An Ammonium-Formate-Driven Trienzymatic Cascade for ω-Transaminase-Catalyzed (R)-Selective Amination. J. Org. Chem. 2019, 84, 14987–14993. 10.1021/acs.joc.9b02445. [DOI] [PubMed] [Google Scholar]
- Aramendía M. A.; Borau V.; Jiménez C.; Marinas J. M.; Ruiz J. R.; Urbano F. J. Activity of Baisc Catalysts in the Meerwein-Ponndorf-Verley reaction of Benzaldehyde with Ethanol. J. Colloid Interface Sci. 2001, 238, 385–389. 10.1006/jcis.2001.7519. [DOI] [PubMed] [Google Scholar]
- Wang F.; Ta N.; Shen W. MgO nanosheets, nanodisks, and nanofibers for the Meerwein-Ponndorf-Verley reaction. Appl. Catal., A 2014, 475, 76–81. 10.1016/j.apcata.2014.01.026. [DOI] [Google Scholar]
- Shannon R. D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr. 1976, A32, 751–767. 10.1107/S0567739476001551. [DOI] [Google Scholar]
- Xiao M.; Yue X.; Xu R.; Tang W.; Xue D.; Li C.; Lei M.; Xiao J.; Wang C. Transition-Metal-Free Hydrogen Autotransfer: Diastereoselective N-Alkylation of Amines with Racemic Alcohols. Angew. Chem., Int. Ed. 2019, 58, 10528–10536. 10.1002/anie.201905870. [DOI] [PubMed] [Google Scholar]
- Collins I. Saturated and unsaturated lactones. J. Chem. Soc., Perkin Trans. 1 1998, 1, 1869–1888. 10.1039/a800740c. [DOI] [Google Scholar]
- Palanichamy K.; Kaliappan K.. Synthesis of Saturated Oxygenated Heterocycles. I. Topics in Heterocyclic Chemistry; Cossy J., Ed.; Springler-Verlag: Berlin, 2014; Vol. 35, pp 97–140. [Google Scholar]
- Marco J. A.; Carda M.. Natural Lactones and Lactams: Synthesis, Occurrence and Biological Activity; Janecki T., Ed.; Wiley-VCH: Weinheim, 2013; pp 51–100. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.