Abstract

Herein we present the synthesis and evaluation of anion-binding properties of 12 new receptors from the unclosed cryptand family. Their core is built on the stable 26-membered tetraamidic macrocyclic scaffold, whereas various alkyl and aryl urea substituents were introduced after a yield-limiting macrocyclization step (65–98%). The receptors strongly bind anions, in particular carboxylates, even in a highly competitive solvent mixture (DMSO-d6 + H2O 95:5 v/v).
The Lehn’s concept of supramolecular chemistry1 has allowed for effective planning of diverse synthetic macrocyclic compounds, able to selectively bind neutral and ion molecules.2,3 When considering supramolecular systems, we must take into account the weak interactions, including π-stacking, hydrophobic effect, and most importantly, hydrogen bonding,4 between all molecules present in the system: host, guest, solvents, and other components of their environment. The directivity of the hydrogen bonds determines the geometry of the receptor; moreover, the geometry of the complexed object must also be taken into account.5 For a couple of years, one can observe progress in studies of anion binding receptors. This involves the designing of new specialized systems having both open-chain6 and macrocyclic structures.7 Thus, introducing efficient hydrogen bond donors, such as amide8 or especially urea groups,9 should help to form a strictly defined network of bonds, favorably affecting the receptor’s selectivity. Urea derivatives, due to their specific geometry, are capable of selectively binding the Y-shaped carboxylates.10 Urea functions can be introduced easily into the structure of most molecular receptors,11 and its importance is demonstrated by the key role they play in catalysis;6 they are also often found in pharmaceuticals12 and natural compounds.13
In an earlier paper, we presented a procedure for synthesizing macrocyclic tetralactams using sodium methoxide.14 Subsequently, we applied this procedure to synthesize “unclosed cryptands”, containing a flexible lariat arm comprising an amide function.8,15 Postfunctionalization studies regarding the said arm allowed for the introduction of large substituents within it.16 In the work reported herein, we resolved to strengthen the host–guest interactions in the said receptors, which can be obtained, as mentioned above, by introducing the urea function, characterized by a high yield and easily available and inexpensive components.
We synthesized macrocyclic receptors with a urea function in the lariat arm following the postfunctionalization procedure discussed in our previous paper.15 The tert-butyloxycarbonyl group (Boc) is used to protect the amine function, as it can be easily removed under mild conditions. This multistep procedure requires only one chromatographic purification of macrocyclic product 3 obtained with 61% yield, whereas the total yield of the procedure was 47% (Scheme 1).
Scheme 1. Synthesis of N-Boc-Protected Macrocyclic Precursor 3 and Its Postfunctionalization to 4a–l.
Through the postfunctionalization of precursor 3, carried out under one-pot conditions (successively by deprotection of the amine function, followed by a reaction with the appropriate isocyanate), we obtained 12 urea derivatives in generally excellent yields. These compounds are characterized by diverse lariat arms, containing both aliphatic units (4a, 4b) and aromatic units (4c–l). Within the group of aromatic compounds, the following derivatives should be distinguished: 4d–f (with electron-donating substituents) and 4g–l (with electron-withdrawing substituents).
We selected five urea derivatives from among the receptors so obtained and examined their complexing properties in relation to four anions (Cl–, MeCO2–, PhCO2–, and H2PO4–). This choice was dictated by the structure of the lariat arm and was limited to the following compounds: 4a with an aliphatic substituent, 4c containing a phenyl substituent, as well as 4d with an electron-donating group, and 4g and 4l with electron-withdrawing groups. The stability constants of receptor complexes with the studied anions were determined by 1H NMR titration in a DMSO-d6 mixture with the addition of 0.5% H2O (v/v). We maintained a constant receptor concentration (∼10–2 M) in each experiment. In all titration experiments, the addition of anion resulted in downfield urea moieties signals. The stability constants were calculated on the basis of chemical shift changes upon the additions of the anion. The results obtained are summarized in Table 1, where we ignore the results for H2PO4– because this anion showed a very complex stoichiometry complexation model, which precluded the determination of reliable association constants. Despite the fact that we could not match the binding model to obtained experimental data, the chemical shift values for urea group protons show a strong affinity of H2PO4– toward the used receptors (see Supporting Information).
Table 1. Stability Constants Ka (M–1) for Complexes of Hosts 4(a, c, d, g) with Anions in DMSO-d6 + 0.5% H2O at 298 Ka.
| receptor | R | Cl– | MeCO2– | PhCO2– |
|---|---|---|---|---|
| 4a | n-Bu | 90 | 5970 | 5180 |
| 4c | Ph | 140 | >104 | >104 |
| 4d | p-C6H4OMe | 140 | >104 | >104 |
| 4g | p-C6H4NO2 | 310 | b | b |
Determined by 1H NMR titration experiments using HypNMR 2008 software17 for fitting; anions added as TBA salts; estimated errors <10%.
Deprotonation of the receptor.
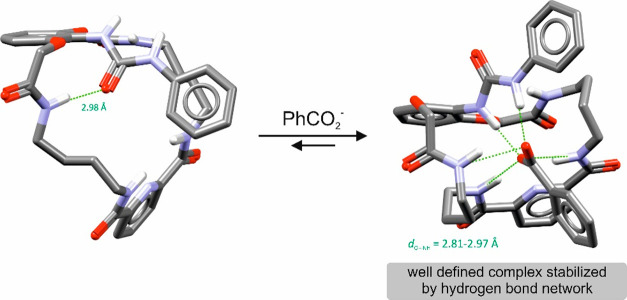
The corresponding titration curves are consistent with the 1:1 binding model for all experiments presented in Table 1.18 Receptor 4a with the n-butyl substituent showed the lowest affinity for all anions used. Its association constants for both carboxylate anions, however, are noteworthy: 5970 M–1 for MeCO2– and 5180 M–1 for PhCO2–. In the case of receptors 4c and 4d, nonlinear curve fittings indicated that these receptors form complexes with acetate and benzoate with constants more than 10000 M–1, while maintaining the 1:1 binding model, this helps to rationalize our results, as these high values indicate the strong affinity of the receptors used toward carboxylates. Strong binding of the anionic molecule and a well-defined hydrogen bonding network was corroborated by DFT calculations (Scheme 2).
Scheme 2. Energy-Minimized Structures of Free Receptor 4c and Its Complex with the Benzoate Anion.
The last of the receptors examined in this titration series, namely, 4g (with NO2 as a substituent), shows the highest affinity, among the receptors studied, toward Cl– (310 M–1): more than 2 times that of receptor 4c with a phenyl substituent. Unfortunately, our examination of this receptor’s affinity toward carboxylate anions was unsuccessful because of its rapid deprotonation process. The literature shows that this is a common phenomenon in the complexation of anions by receptors containing a nitro group in their aromatic substituent adjacent to functions possessing acidic protons.19 We confirmed the process of deprotonation by UV–vis spectroscopy tests. The results of this experiment, as well as the postulated mechanism of proton transfer, are shown in Scheme 3. The acetate anion causes deprotonation of the urea group, which results in the delocalization of the negative charge on the electron-withdrawing NO2 group. The changed color of the solution indicates the occurrence of intermolecular charge transfer (ICT). We observed a bathochromic shift of the absorption maximum by 30 nm. The absorption band at 360 nm disappeared, while a new band appeared at 390 nm. We observed the emergence of an isosbestic point at 370 nm.
Scheme 3. Carboxylate-Induced Deprotonation of the Receptor 4g and Corresponding Charge-Transfer Transition States.

In connection with the aforementioned impossibility of using the 1H NMR titration technique, we carried out titration experiments with the use of more competitive solvent systems (DMSO-d6 with 5% H2O (v/v)) (Table 2).
Table 2. Stability Constants Ka (M–1) for Complexes of Hosts 4(c, d, l) with Anions in DMSO-d6 + 5% H2O at 298 Ka.
| receptor | R | Cl– | MeCO2– | PhCO2– |
|---|---|---|---|---|
| 4c | Ph | b | 1120 | 1780 |
| 4d | p-C6H4OMe | 60 | 2400 | 4070 |
| 4l | 3,5-C6H3(CF3)2 | 120 | 7940 | 6760 |
Determined by 1H NMR titration experiments using HypNMR 2008 software17 for fitting; anions added as TBA salts; estimated errors <10%.
Not determined.
Experiments carried out in this system allowed us to determine, in the measurable range below 10000 M–1, the stability constants Ka of complexes of receptors 4c and 4d with carboxylates. Both receptors displayed a higher binding affinity for PhCO2– over the considerably more basic MeCO2– (Ka 1780 vs 1120 M–1 and 4070 M–1 vs 2400 M–1, for 4c and 4d, respectively). This unusual anti-Hofmeister type selectivity of 4c and 4d probably results from favorable π–π interactions between aromatic subunits of the host and the phenyl ring of benzoate. The literature shows that this uncommon effect occurs for the receptors containing a hydrophobic pocket in their structures.20,21 For compounds 4c and 4d, the aromatic part of the lariat arm is near flat and has a hydrophobic character with a large share of regions with a positive electrostatic surface potential (ESP). In contrast, the lariat arm of receptor 4l is deviated from the planarity due to double substitution at meta positions by CF3 groups. This substitution increases the acidity of NH protons of the urea group but also reduces the number of regions with positive ESP located on the lariat arm, thus minimizing the number of noncovalent interactions with the flat phenyl ring of benzoate (see Supporting Information for details). This diminishes the benzoate selectivity of receptor 4l, although the relative binding constants for carboxylates are highest than for receptors 4c and 4d.
Nonetheless, we decided to compare the very high association constants of receptor 4d with the constants found for the earlier-obtained amide receptor 5, with an analogous structure (Figure 1).
Figure 1.

Structures of receptors 4d and 5.
In the highly competing solvent medium of DMSO-d6 + 5% H2O, receptor 5 displayed a very weak affinity toward carboxylate anions (7 M–1 for MeCO2– and 10 M–1 for PhCO2–, respectively). The approximately 400-fold difference in bonding strength of the compared receptors indicates how well the urea system is matched geometrically to carboxylate anions. Receptor 4l with two electron-withdrawing trifluoromethyl groups showed the strongest complexing properties. The stability constants of its complexes with carboxylic anions are 7940 M–1 for MeCO2– and 6760 M–1 for PhCO2–, respectively. Moreover, we obtained a monocrystal of receptor 4l, whose structure is shown in Figure 2. It displays a high degree of preorganization; all four amide protons in the macrocycle are directed toward the center of the gap, and only the urea protons are located outside the macroring plane. The structure contains multiple water molecules in the crystal lattice, from which one is bound in the host cavity by three hydrogen bond interactions (d = 2.86–3.07 Å). Moreover, an intramolecular hydrogen bond N–O (2.90 Å) is observed between amide proton N(3) and carbonyl oxygen O(1) of the urea group. In addition, one CF3 group from the lariat arm moiety is positioned above the pyridine ring forming short intramolecular CF3-π interactions (dC–F···centroid(pyridine) = 3.25 Å).
Figure 2.
Crystal structure of receptor 4l, top (a) and side (b) views. Nonacidic protons and remaining disordered water molecules were omitted for clarity.
In conclusion, we have presented a development of the previously reported unclosed cryptand postfunctionalization method, which enabled us to obtain a broad array of 12 new macrocyclic systems with an incorporated urea group. The compounds selected for complexation studies showed a strong affinity for carboxylate anions even in a very competing solvent medium, namely, DMSO-d6 with 5% H2O (v/v).
Experimental Section
Materials and Methods
All of the reagents were used as received. The solvents were dried by distillation over the appropriate drying agents. All solvents were obtained from common suppliers and used as received. Column chromatography was carried out using Merck Kieselgel 60 (63–100 μm mesh size), and TLC was carried out on Merck Kieselgel F254 plates. Melting points were determined using a Boëtius M HMK hot-stage apparatus and were uncorrected. The NMR spectra are recorded on a Bruker Mercury 400 instrument. Chemical shifts are reported in ppm (δ) and are set to the solvent residue peak. J coupling constants values are reported in hertz (Hz). Mass spectral analyses were performed with the ESI-TOF technique on a Mariner mass spectrometer from PerSeptive Biosystem. The lowest energy conformation of the complex of receptor 4c with PhCO2– was found after conducting a conformational search analysis, and selected conformers with lowest energies were optimized without any constrains at the DFT/M06-2X/6-31G(d)/C-PCM:DMSO level of theory using program Spartan’18 Parallel Suite (see Supporting Information for details).22−24
tert-Butyl N-{4,11,17,24-Tetraoxo-2,26-dioxa-5,10,18,23,32-pentaazatricyclo[25.3.1.112,16]dotriaconta-1(31),12,14,16(32),27,29-hexaen-31-yl}carbamate 3
The product 3 was obtained as previously described.15
General Procedure A for Obtaining Receptors 4a–l
A suspension of macrocyclic compound 3 (0.140 g, 0.23 mmol) in anhydrous DCM (3 mL) in 0 °C 4 M HCl in dioxane (0.286 mL, 1.15 mmol) was added. Then the mixture was stirred at room temperature for 1 h. Subsequently, the mixture was cooled to 0 °C, and then N,N-diisopropylethylamine (0.288 mL, 1.65 mmol) and the corresponding isocyanate (0.46 mmol) were added. The mixture was stirred for a further 30 min, the solvent was evaporated under a vacuum, and the residue was purified employing column chromatography and using a DCM/methanol mixture [99:1 → 95:5, v/v] as the eluent. The obtained colorless oil was dissolved in methanol and then sonicated in water.
3-Butyl-1-{4,11,17,24-tetraoxo-2,26-dioxa-5,10,18,23,32-pentaazatricyclo[25.3.1.112,16]dotriaconta-1(31),12,14,16(32),27,29-hexaen-31-yl}urea (4a)
Following General Procedure A and using n-butyl isocyanate (52 μL, 0.46 mmol), the product 4a (91 mg, 0.15 mmol, 65%) was obtained as a colorless solid (mp 133–134 °C). 1H NMR (400 MHz, DMSO-d6): δ 9.42 (t, J = 5.1 Hz, 2H), 8.44 (t, J = 5.4 Hz, 2H), 8.21–8.09 (m, 3H), 7.72 (s, 1H), 7.08 (t, J = 8.3 Hz, 1H), 6.65 (d, J = 8.4 Hz, 2H), 6.24 (t, J = 4.1 Hz, 1H), 4.53 (s, 4H), 3.30–3.24 (m, 4H), 3.23–3.16 (m, 4H), 2.79 (dd, J = 12.2, 6.4 Hz, 2H), 1.69–1.62 (m, 4H), 1.56–1.47 (m, 4H), 0.89–0.83 (m, 2H), 0.70 (dd, J = 14.4, 7.2 Hz, 2H), 0.48 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (100 MHz, DMSO-d6): δ 167.3, 162.8, 156.4, 152.4, 148.8, 139.1, 125.9, 123.9, 116.1, 105.0, 66.6, 39.0, 38.0, 31.4, 29.3, 26.4, 26.3, 19.0, 13.3. HRMS (ESI, MeOH): m/z calcd for C30H41N7O7Na [M + Na]+, 634.2965; found, 634.2964.
1-Cyclohexyl-3-{4,11,17,24-tetraoxo-2,26-dioxa-5,10,18,23,32-pentaazatricyclo[25.3.1.112,16]dotriaconta-1(31),12,14,16(32),27,29-hexaen-31-yl}urea (4b)
Following General Procedure A and using cyclohexyl isocyanate (59 μL, 0.46 mmol), the product (142 mg, 0.22 mmol, 97%) was obtained as a colorless solid (mp 163–164 °C). 1H NMR (400 MHz, DMSO-d6): δ 9.41 (t, J = 6.1 Hz, 2H), 8.43 (t, J = 5.6 Hz, 2H), 8.19–8.11 (m, 3H), 7.72 (s, 1H), 7.07 (t, J = 8.4 Hz, 1H), 6.65 (d, J = 8.5 Hz, 2H), 6.11 (d, J = 6.7 Hz, 1H), 4.53 (s, 4H), 3.29–3.27 (m, 2H), 3.27–3.19 (m, 8H), 1.72–1.62 (m, 6H), 1.56–1.46 (m, 7H), 1.00–0.93 (m, 2H), 0.45 (dd, J = 23.7, 11.8 Hz, 2H). 13C{1H} NMR (100 MHz, DMSO-d6): δ 167.4, 162.8, 155.5, 152.2, 148.9, 139.1, 125.7, 123.9, 116.0, 104.9, 66.6, 48.0, 37.7, 32.6, 26.4, 25.0, 24.4, 23.6. HRMS (ESI, MeOH): m/z calcd for C32H43N7O7Na [M + Na]+, 660.3122; found, 660.3126.
1-Phenyl-3-{4,11,17,24-tetraoxo-2,26-dioxa-5,10,18,23,32-pentaazatricyclo[25.3.1.112,16]dotriaconta-1(31),12,14,16(32),27,29-hexaen-31-yl}urea (4c)
Following General Procedure A and using phenyl isocyanate (50 μL, 0.46 mmol), the product 4c (141 mg, 0.22 mmol, 97%) was obtained as a colorless solid (mp 177–178 °C). 1H NMR (400 MHz, DMSO-d6): δ 9.17 (t, J = 5.9 Hz, 2H), 8.86 (s, 1H), 8.23 (t, J = 5.0 Hz, 2H), 8.18–8.06 (m, 4H), 7.30 (d, J = 7.8 Hz, 2H), 7.13 (t, J = 8.4 Hz, 1H), 6.71–6.61 (m, 4H), 6.41 (t, J = 7.2 Hz, 1H), 4.58 (s, 4H), 3.20 (s, 8H), 1.52 (s, 8H). 13C{1H} NMR (100 MHz, DMSO-d6): δ 167.4, 162.8, 153.3, 152.6, 148.8, 139.3, 138.9, 127.8, 126.3, 123.9, 121.3, 117.9, 115.4, 105.3, 66.9, 38.8, 37.9, 26.4, 26.2. HRMS (ESI, MeOH): m/z calcd for C32H37N7O7Na [M + Na]+, 654.2652; found, 654.2647.
1-(4-Methoxyphenyl)-3-{4,11,17,24-tetraoxo-2,26-dioxa-5,10,18,23,32-pentaazatricyclo[25.3.1.112,16]dotriaconta-1(31),12,14,16(32),27,29-hexaen-31-yl}urea (4d)
Following General Procedure A and using 4-methoxyphenyl isocyanate (60 μL, 0.46 mmol), the product 4d (145 mg, 0.22 mmol, 95%) was obtained as a colorless solid (mp 146–147 °C). 1H NMR (400 MHz, DMSO-d6): δ 9.19 (t, J = 6.0 Hz, 2H), 8.54 (s, 1H), 8.25 (t, J = 5.3 Hz, 2H), 8.17–8.08 (m, 3H), 8.04 (s, 1H), 7.21 (d, J = 8.9 Hz, 2H), 7.12 (t, J = 8.4 Hz, 1H), 6.67 (d, J = 8.5 Hz, 2H), 6.23 (d, J = 9.0 Hz, 2H), 4.57 (s, 4H), 3.38 (s, 3H), 3.21 (d, J = 5.6 Hz, 8H), 1.54 (dd, J = 13.7, 6.3 Hz, 8H). 13C{1H} NMR (100 MHz, DMSO-d6): δ 167.4, 162.8, 153.9, 153.5, 152.6, 148.7, 138.9, 132.3, 126.2, 123.8, 120.0, 115.5, 113.1, 105.2, 66.9, 54.6, 38.8, 37.8, 26.4, 26.2. HRMS (ESI, MeOH): m/z calcd for C33H39N7O8Na [M + Na]+, 684.2758; found, 684.2755.
1-(3-Methoxyphenyl)-3-{4,11,17,24-tetraoxo-2,26-dioxa-5,10,18,23,32-pentaazatricyclo[25.3.1.112,16]dotriaconta-1(31),12,14,16(32),27,29-hexaen-31-yl}urea (4e)
Following General Procedure A and using 3-methoxyphenyl isocyanate (60 μL, 0.46 mmol), the product 4e (143 mg, 0.22 mmol, 94%) was obtained as a colorless solid (mp 135–136 °C). 1H NMR (400 MHz, DMSO-d6): δ 9.04 (t, J = 6.1 Hz, 2H), 8.83 (s, 1H), 8.24 (t, J = 5.3 Hz, 2H), 8.14–8.08 (m, 3H), 8.06 (s, 1H), 7.12 (t, J = 8.4 Hz, 1H), 7.05 (t, J = 2.0 Hz, 1H), 6.84 (d, J = 9.1 Hz, 1H), 6.70–6.61 (m, 3H), 6.10 (dd, J = 8.2, 2.2 Hz, 1H), 4.58 (s, 4H), 3.49 (s, 3H), 3.24–3.15 (m, 8H), 1.58–1.49 (m, 8H). 13C{1H} NMR (100 MHz, DMSO-d6): δ 167.4, 162.8, 159.2, 153.3, 152.7, 148.7, 140.6, 138.8, 128.8, 126.4, 123.9, 115.3, 110.3, 106.6, 105.3, 103.7, 66.9, 54.5, 38.8, 38.0, 26.5, 26.3. HRMS (ESI, MeOH): m/z calcd for C33H39N7O8Na [M + Na]+, 684.2758; found, 684.2759.
1-(2-Methoxyphenyl)-1-{4,11,17,24-tetraoxo-2,26-dioxa-5,10,18,23,32-pentaazatricyclo[25.3.1.112,16]dotriaconta-1(31),12(32),13,15,27,29-hexaen-31-yl}urea (4f)
Following General Procedure A and using 2-methoxyphenyl isocyanate (61 μL, 0.46 mmol), the product 4f (145 mg, 0.22 mmol, 95%) was obtained as a colorless solid (mp 135–136 °C). 1H NMR (400 MHz, DMSO-d6): δ 9.12 (bs, 2H), 8.67 (s, 1H), 8.39 (s, 1H), 8.32 (bs, 2H), 8.10 (s, 3H), 7.90 (d, J = 7.8 Hz, 1H), 7.13 (t, J = 8.2 Hz, 1H), 6.73 (d, J = 8.0 Hz, 1H), 6.66 (d, J = 8.4 Hz, 2H), 6.35 (t, J = 7.5 Hz, 1H), 5.86 (t, J = 7.5 Hz, 1H), 4.58 (s, 4H), 3.82 (s, 3H), 3.20 (s, 8H), 1.52 (s, 8H). 13C{1H} NMR (100 MHz, DMSO-d6): δ 167.5, 162.8, 153.1, 152.5, 148.7, 147.3, 138.8, 128.2, 126.3, 123.8, 121.3, 119.3, 118.4, 115.3, 109.9, 105.1, 66.8, 55.5, 38.8, 38.0, 26.4, 26.1. HRMS (ESI, MeOH): m/z calcd for C33H39N7O8Na [M + Na]+, 684.2758; found, 684.2758.
1-(4-Nitrophenyl)-1-{4,11,17,24-tetraoxo-2,26-dioxa-5,10,18,23,32-pentaazatricyclo[25.3.1.112,16]dotriaconta-1(31),12(32),13,15,27,29-hexaen-31-yl}urea (4g)
Following General Procedure A and using 4-nitrophenyl isocyanate (75 mg, 0.46 mmol), the product 4g (115 mg, 0.17 mmol, 74%) was obtained as a yellow solid (mp 176–177 °C). 1H NMR (400 MHz, DMSO-d6): δ 9.62 (s, 1H), 9.05 (t, J = 6.0 Hz, 2H), 8.22 (d, J = 9.1 Hz, 1H), 8.18–8.09 (m, 3H), 7.90 (d, J = 9.0 Hz, 2H), 7.72 (d, J = 9.1 Hz, 2H), 7.61 (s, 2H), 7.21 (t, J = 8.4 Hz, 1H), 6.69 (d, J = 8.5 Hz, 2H), 4.58 (s, 4H), 3.29–3.14 (m, 8H), 1.61–1.41 (m, 8H). 13C{1H} NMR (100 MHz, DMSO-d6): δ 167.3, 162.8, 153.9, 148.7, 146.1, 145.6, 141.5, 139.2, 128.6, 125.1, 124.0, 123.9, 118.0, 106.1, 102.0, 67.4, 38.0, 26.7, 26.4. HRMS (ESI, MeOH): m/z calcd for C32H36N8O9Na [M + Na]+, 699.2503; found, 699.2497.
1-(3-Nitrophenyl)-1-{4,11,17,24-tetraoxo-2,26-dioxa-5,10,18,23,32-pentaazatricyclo[25.3.1.112,16]dotriaconta-1(31),12(32),13,15,27,29-hexaen-31-yl}urea (4h)
Following General Procedure A and using 3-nitrophenyl isocyanate (75 mg, 0.46 mmol), the product 4h (140 mg, 0.21 mmol, 90%) was obtained as a yellowish solid (mp 170–171 °C). 1H NMR (400 MHz, DMSO-d6): δ 9.31 (s, 1H), 8.99 (t, J = 5.9 Hz, 2H), 8.34 (s, 1H), 8.30 (t, J = 5.1 Hz, 2H), 8.24 (s, 1H), 8.10–7.98 (m, 3H), 7.49 (d, J = 7.9 Hz, 1H), 7.17 (t, J = 8.4 Hz, 1H), 7.10 (d, J = 6.9 Hz, 1H), 6.94 (t, J = 8.1 Hz, 1H), 6.70 (d, J = 8.5 Hz, 2H), 4.61 (s, 4H), 3.25 (d, J = 5.1 Hz, 4H), 3.16 (d, J = 6.0 Hz, 4H), 1.56 (s, 8H). 13C{1H} NMR (100 MHz, DMSO-d6): δ 167.5, 162.6, 153.0, 152.5, 148.5, 147.2, 140.5, 138.7, 129.0, 126.8, 123.7, 115.7, 114.6, 111.8, 105.1, 66.8, 38.8, 38.1, 26.4, 26.2. HRMS (ESI, MeOH): m/z calcd for C32H36N8O9Na [M + Na]+, 699.2503; found, 699.2499.
1-(2-Nitrophenyl)-1-{4,11,17,24-tetraoxo-2,26-dioxa-5,10,18,23,32-pentaazatricyclo[25.3.1.112,16]dotriaconta-1(31),12(32),13,15,27,29-hexaen-31-yl}urea (4i)
Following General Procedure A and using 2-nitrophenyl isocyanate (75 mg, 0.46 mmol), the product 4i (142 mg, 0.21 mmol, 91%) was obtained as a yellowish solid (mp 156–157 °C). 1H NMR (400 MHz, DMSO-d6): δ 9.84 (s, 1H), 9.38 (s, 1H), 9.15 (t, J = 6.0 Hz, 2H), 8.28 (d, J = 8.3 Hz, 1H), 8.18 (t, J = 4.8 Hz, 2H), 8.15–8.11 (m, 3H), 7.79 (dd, J = 8.3, 1.2 Hz, 1H), 7.18 (t, J = 8.4 Hz, 1H), 6.69 (d, J = 8.5 Hz, 2H), 6.64 (t, J = 7.6 Hz, 1H), 6.57 (t, J = 7.6 Hz, 1H), 4.59 (s, 4H), 3.25–3.15 (m, 8H), 1.51 (bs, 8H). 13C{1H} NMR (100 MHz, DMSO-d6): δ 167.4, 162.7, 152.5, 152.5, 148.7, 139.0, 136.6, 134.8, 133.8, 127.0, 124.8, 124.0, 122.0, 121.5, 114.5, 105.1, 66.8, 38.7, 37.8, 26.3, 26.0. HRMS (ESI, MeOH): m/z calcd for C32H36N8O9Na [M + Na]+, 699.2503; found, 699.2498.
1-(4-Cyanophenyl)-3-{4,11,17,24-tetraoxo-2,26-dioxa-5,10,18,23,32-pentaazatricyclo[25.3.1.112,16]dotriaconta-1(31),12,14,16(32),27,29-hexaen-31-yl}urea (4j)
Following General Procedure A and using 4-cyanophenyl isocyanate (66 mg, 0.46 mmol), the product 4j (148 mg, 0.23 mmol, 98%) was obtained as a colorless solid (mp 171–172 °C). 1H NMR (400 MHz, DMSO-d6): δ 9.28 (s, 1H), 9.14 (t, J = 6.1 Hz, 2H), 8.33 (s, 1H), 8.20 (t, J = 5.3 Hz, 2H), 8.15–8.10 (m, 3H), 7.42 (d, J = 8.7 Hz, 2H), 7.16 (t, J = 8.4 Hz, 1H), 6.92 (d, J = 8.7 Hz, 2H), 6.68 (d, J = 8.5 Hz, 2H), 4.60 (s, 4H), 3.27–3.16 (m, 8H), 1.53 (s, 8H). 13C{1H} NMR (100 MHz, DMSO-d6): δ 167.4, 162.7, 152.6, 152.4, 148.6, 143.7, 139.1, 132.2, 126.8, 123.8, 118.9, 117.5, 114.6, 105.1, 102.9, 66.7, 38.8, 38.0, 26.3, 26.1. HRMS (ESI, MeOH): m/z calcd for C33H36N8O7Na [M + Na]+, 679.2605; found, 679.2605.
3-{4,11,17,24-Tetraoxo-2,26-dioxa-5,10,18,23,32-pentaazatricyclo[25.3.1.112,16]dotriaconta-1(31),12,14,16(32),27,29-hexaen-31-yl}-1-[4-(trifluoromethyl)phenyl]urea (4k)
Following General Procedure A and using 4-(trifluoromethyl)phenyl isocyanate (66 μL, 0.46 mmol), the product 4k (148 mg, 0.21 mmol, 92%) was obtained as a colorless solid (mp 174–175 °C). 1H NMR (400 MHz, DMSO-d6): δ 9.23 (t, J = 6.1 Hz, 2H), 9.14 (s, 1H), 8.32 (s, 1H), 8.15 (t, J = 5.4 Hz, 2H), 8.13–8.07 (m, 3H), 7.51 (d, J = 8.5 Hz, 2H), 7.16 (t, J = 8.4 Hz, 1H), 6.93 (d, J = 8.7 Hz, 2H), 6.68 (d, J = 8.5 Hz, 2H), 4.59 (s, 4H), 3.21 (d, J = 3.1 Hz, 8H), 1.52 (s, 8H). 13C{1H} NMR (100 MHz, DMSO-d6): δ 167.5, 162.8, 153.1, 152.6, 148.6, 143.1, 139.0, 126.7, 125.2, 123.9, 117.8, 114.9, 105.2, 66.8, 38.7, 37.9, 26.3, 26.1. HRMS (ESI, MeOH): m/z calcd for C33H36F3N7O7Na [M + Na]+, 722.2526; found, 722.2520.
1-[3,5-Bis(trifluoromethyl)phenyl]-3-{4,11,17,24-tetraoxo-2,26-dioxa-5,10,18,23,32-pentaazatricyclo[25.3.1.112,16]dotriaconta-1(31),12,14,16(32),27,29-hexaen-31-yl}urea (4l)
Following General Procedure A and using 3,5-bis(trifluoromethyl)phenyl isocyanate (80 μL, 0.46 mmol), the product 4l (170 mg, 0.22 mmol, 96%) was obtained as a colorless solid (mp 164–165 °C). 1H NMR (400 MHz, DMSO-d6): δ 9.50 (s, 1H), 9.09 (t, J = 6.0 Hz, 2H), 8.30 (s, 1H), 8.19 (t, J = 5.3 Hz, 2H), 8.07 (s, 3H), 7.97 (s, 2H), 7.23 (s, 1H), 7.16 (t, J = 8.4 Hz, 1H), 6.69 (d, J = 8.5 Hz, 2H), 4.60 (s, 4H), 3.21 (s, 8H), 1.53 (s, 8H). 13C{1H} NMR (100 MHz, DMSO-d6): δ 167.5, 162.7, 153.1, 153.0, 148.6, 141.5, 138.7, 130.5, 127.0, 124.4, 123.8, 121.7, 121.6, 117.6, 114.6, 105.4, 67.0, 38.7, 37.9, 26.5, 26.2, 25.4. HRMS (ESI, MeOH): m/z calcd for C34H35F6N7O7Na [M + Na]+, 790.2400; found, 790.2377.
Acknowledgments
We acknowledge Poland’s National Science Centre (project 2016/21/B/ST5/03352) for financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.9b03082.
Accession Codes
CCDC 1854521 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cam-bridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: + 44 1223 336033.
Author Contributions
The authors contributed to the following: conceptualization, P.N., K.D., and J.J.; methodology, P.N., K.D., J.J., and M.M.; validation, P.N. and M.M.; formal analysis, P.N. and M.M.; investigation, P.N.; resources, J.J.; data curation, P.N. and M.M.; writing the original draft preparation, P.N., M.M., and J.J.; writing, review, and editing, P.N., M.M. and J.J.; visualization, P.N.; supervision, J.J.; project administration, J.J.; funding acquisition, J.J.
The authors declare no competing financial interest.
Supplementary Material
References
- For examples, see:; a Dietrich B.; Lehn J. M.; Sauvage J. P. Diaza-Polyoxa-Macrocycles et Macrobicycles. Tetrahedron Lett. 1969, 10, 2885–2888. 10.1016/S0040-4039(01)88299-X. [DOI] [Google Scholar]; b Dietrich B.; Lehn J. M.; Sauvage J. P. Les Cryptates. Tetrahedron Lett. 1969, 10, 2889–2892. 10.1016/S0040-4039(01)88300-3. [DOI] [Google Scholar]; c Lehn J. M. Cryptates: Inclusion Complexes of Macropolycyclic Receptor Molecules. Pure Appl. Chem. 1978, 50, 871–892. 10.1351/pac197850090871. [DOI] [Google Scholar]; d Lehn J. M. Supramolecular Chemistry - Scope and Perspectives Molecules, Supermolecules, and Molecular Devices (Nobel Lecture). Angew. Chem., Int. Ed. Engl. 1988, 27, 89–112. 10.1002/anie.198800891. [DOI] [Google Scholar]
- For examples, see:; a Armstrong C. M. The Na/K Pump, Cl Ion, and Osmotic Stabilization of Cells. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 6257–6262. 10.1073/pnas.0931278100. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Gale P. A.; Busschaert N.; Haynes C. J. E.; Karagiannidis L. E.; Kirby I. L. Anion Receptor Chemistry: Highlights from 2011 and 2012. Chem. Soc. Rev. 2014, 43, 205–241. 10.1039/C3CS60316D. [DOI] [PubMed] [Google Scholar]; c Salis A.; Ninham B. W. Models and Mechanisms of Hofmeister Effects in Electrolyte Solutions, and Colloid and Protein Systems Revisited. Chem. Soc. Rev. 2014, 43, 7358–7377. 10.1039/C4CS00144C. [DOI] [PubMed] [Google Scholar]; d Evans N. H.; Beer P. D. Advances in Anion Supramolecular Chemistry: From Recognition to Chemical Applications. Angew. Chem., Int. Ed. 2014, 53, 11716–11754. 10.1002/anie.201309937. [DOI] [PubMed] [Google Scholar]; e Busschaert N.; Caltagirone C.; Van Rossom W.; Gale P. A. Applications of Supramolecular Anion Recognition. Chem. Rev. 2015, 115, 8038–8155. 10.1021/acs.chemrev.5b00099. [DOI] [PubMed] [Google Scholar]; f Tapia L.; Pérez Y.; Bolte M.; Casas J.; Solà J.; Quesada R.; Alfonso I. pH-Dependent Chloride Transport by Pseudopeptidic Cages for the Selective Killing of Cancer Cells in Acidic Microenvironments. Angew. Chem., Int. Ed. 2019, 58, 12465–12468. 10.1002/anie.201905965. [DOI] [PubMed] [Google Scholar]
- For examples, see:; a Mahadevi A. S.; Sastry G. N. Cooperativity in Noncovalent Interactions. Chem. Rev. 2016, 116, 2775–2825. 10.1021/cr500344e. [DOI] [PubMed] [Google Scholar]; b Molina P.; Zapata F.; Caballero A. Anion Recognition Strategies Based on Combined Noncovalent Interactions. Chem. Rev. 2017, 117, 9907–9972. 10.1021/acs.chemrev.6b00814. [DOI] [PubMed] [Google Scholar]
- For examples, see:; a Dydio P.; Lichosyt D.; Jurczak J. Amide- and Urea-Functionalized Pyrroles and Benzopyrroles as Synthetic, Neutral Anion Receptors. Chem. Soc. Rev. 2011, 40, 2971–2985. 10.1039/c1cs15006e. [DOI] [PubMed] [Google Scholar]; b Jurczak J.; Dydio P.; Stępniak P.; Zieliński T. Diamidonaphthalenodipyrrole-Derived Fluorescent Sensors for Anions. Sens. Actuators, B 2016, 237, 621–627. 10.1016/j.snb.2016.06.142. [DOI] [Google Scholar]
- Bru M.; Alfonso I.; Burguete M. I.; Luis S. V. Anion-Templated Syntheses of Pseudopeptidic Macrocycles. Angew. Chem., Int. Ed. 2006, 45, 6155–6159. 10.1002/anie.200602206. [DOI] [PubMed] [Google Scholar]
- a For examples, see: Sessler J. L.; Gale P. A.; Cho W.-S.. Anion Receptor Chemistry; Royal Society of Chemistry: 2006. [Google Scholar]; c Alfonso I.; Bolte M.; Bru M.; Burguete M. I.; Luis S. V.; Rubio J. Supramolecular Control for the Modular Synthesis of Pseudopeptidic Macrocycles through an Anion-Templated Reaction. J. Am. Chem. Soc. 2008, 130, 6137–6144. 10.1021/ja710132c. [DOI] [PubMed] [Google Scholar]; d Gale P. A. Anion Receptor Chemistry. Chem. Commun. 2011, 47, 82–86. 10.1039/C0CC00656D. [DOI] [PubMed] [Google Scholar]; e Busschaert N.; Karagiannidis L. E.; Wenzel M.; Haynes C. J. E.; Wells N. J.; Young P. G.; Makuc D.; Plavec J.; Jolliffe K. A.; Gale P. A. Synthetic Transporters for Sulfate: A new Method for the Direct Detection of Lipid Bilayer Sulfate Transport. Chem. Sci. 2014, 5, 1118–1127. 10.1039/c3sc52006d. [DOI] [Google Scholar]
- For examples, see:; a Flood A. H. Creating molecular macrocycles for anion recognition. Beilstein J. Org. Chem. 2016, 12, 611–627. 10.3762/bjoc.12.60. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Pinalli R.; Pedrini A.; Dalcanale E. Biochemical sensing with macrocyclic receptors. Chem. Soc. Rev. 2018, 47, 7006–7026. 10.1039/C8CS00271A. [DOI] [PubMed] [Google Scholar]
- For examples, see:; a Dąbrowa K.; Pawlak M.; Duszewski P.; Jurczak J. Unclosed cryptands”: A Point of Departure for Developing Potent Neutral Anion Receptors. Org. Lett. 2012, 14, 6298–6301. 10.1021/ol303065k. [DOI] [PubMed] [Google Scholar]; b Dąbrowa K.; Niedbała P.; Jurczak J. Anion-Tunable Control of Thermal Z→E Isomerisation in Basic Azobenzene Receptors. Chem. Commun. 2014, 50, 15748–15751. 10.1039/C4CC07798A. [DOI] [PubMed] [Google Scholar]
- Dąbrowa K.; Niedbała P.; Jurczak J. Engineering Light-Mediated Bistable Azobenzene Switches Bearing Urea d-Aminoglucose Units for Chiral Discrimination of Carboxylates. J. Org. Chem. 2016, 81, 3576–3584. 10.1021/acs.joc.6b00200. [DOI] [PubMed] [Google Scholar]
- Amendola V.; Fabbrizzi L.; Mosca L. Anion Recognition by Hydrogen Bonding: Urea-Based Receptors. Chem. Soc. Rev. 2010, 39, 3889–3915. 10.1039/b822552b. [DOI] [PubMed] [Google Scholar]
- Hamankiewicz P.; Granda J. M.; Jurczak J. Influence of the Size and Geometry of the Anion Binding Pocket of Sugar-Urea Anion Receptors on Chiral Recognition. Tetrahedron Lett. 2013, 54, 5608–5611. 10.1016/j.tetlet.2013.08.019. [DOI] [Google Scholar]
- Belfrage A. K.; Gising J.; Svensson F.; Åkerblom E.; Sköld C.; Sandström A. Efficient and Selective Palladium-Catalysed C-3 Urea Couplings to 3,5-Dichloro-2(1H)-pyrazinones. Eur. J. Org. Chem. 2015, 2015, 978–986. 10.1002/ejoc.201403405. [DOI] [Google Scholar]
- For examples, see:; a Rogers E. W.; Molinski T. F. (+)-Zwittermicin A. Rapid Assembly of C9-C15 and a Formal Total Synthesis. J. Org. Chem. 2009, 74, 7660–7664. 10.1021/jo901007v. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Knott K. E.; Auschill S.; Jäger A.; Knölker H. J. First Total Synthesis of the Whole Series of the Antiostatins A and B. Chem. Commun. 2009, 1467–1469. 10.1039/b821039j. [DOI] [PubMed] [Google Scholar]; c Reyes J. C. P.; Romo D. Bioinspired Total Synthesis of Agelastatin A. Angew. Chem., Int. Ed. 2012, 51, 6870–6873. 10.1002/anie.201200959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryko D. T.; Gryko D.; Jurczak J. Improved Method for the Preparation of Macrocyclic Diamides. Synlett 1999, 1999, 1310–1312. 10.1055/s-1999-2798. [DOI] [Google Scholar]
- Dąbrowa K.; Niedbała P.; Majdecki M.; Duszewski P.; Jurczak J. A General Method for Synthesis of Unclosed Cryptands via H-Bond Templated Macrocyclization and Subsequent Mild Postfunctionalization. Org. Lett. 2015, 17, 4774–4777. 10.1021/acs.orglett.5b02324. [DOI] [PubMed] [Google Scholar]
- Jurczak J.; Sobczuk A.; Dąbrowa K.; Lindner M.; Niedbała P. An Indirect Synthetic Approach Toward Conformationally Constrained 20-Membered Unclosed Cryptands via Late-Stage Installation of Intraannular Substituents. J. Org. Chem. 2018, 83, 13560–13567. 10.1021/acs.joc.8b02160. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Barrientos D.; Rojas-Hernández A.; Gutiérrez A.; Moya-Hernández R.; Gómez-Balderas R.; Ramírez-Silva M. T. Determination of pKa values of tenoxicam from 1H NMR chemical shifts and of oxicams from electrophoretic mobilities (CZE) with the aid of programs SQUAD and HYPNMR. Talanta 2009, 80, 754–762. 10.1016/j.talanta.2009.07.058. [DOI] [PubMed] [Google Scholar]
- For examples, see:; a Brynn Hibbert D.; Thordarson P. The Death of the Job Plot, Transparency, Open Science and Online Tools, Uncertainty Estimation Methods and Other Developments in Supramolecular Chemistry Data Analysis. Chem. Commun. 2016, 52, 12792–12805. 10.1039/C6CC03888C. [DOI] [PubMed] [Google Scholar]; b Ulatowski F.; Dąbrowa K.; Bałakier T.; Jurczak J. Recognizing the Limited Applicability of Job Plots in Studying Host-Guest Interactions in Supramolecular Chemistry. J. Org. Chem. 2016, 81, 1746–1756. 10.1021/acs.joc.5b02909. [DOI] [PubMed] [Google Scholar]
- For examples, see:; a Boiocchi M.; Del Boca L.; Esteban-Gómez D.; Fabbrizzi L.; Licchelli M.; Monzani E. Nature of Urea-Fluoride Interaction: Incipient and Definitive Proton Transfer. J. Am. Chem. Soc. 2004, 126, 16507–16514. 10.1021/ja045936c. [DOI] [PubMed] [Google Scholar]; b Boiocchi M.; Del Boca L.; Esteban-Gómez D.; Fabbrizzi L.; Licchelli M.; Monzani E. Anion-Induced Urea Deprotonation. Chem. - Eur. J. 2005, 11, 3097–3104. 10.1002/chem.200401049. [DOI] [PubMed] [Google Scholar]
- Kadam S. A.; Martin K.; Haav K.; Toom L.; Mayeux C.; Pung A.; Gale P. A.; Hiscock J. R.; Brooks S. J.; Kirby I. L.; Busschaert N.; Leito I. Towards the Discrimination of Carboxylates by Hydrogen-Bond Donor Anion Receptors. Chem. - Eur. J. 2015, 21, 5145–5160. 10.1002/chem.201405858. [DOI] [PubMed] [Google Scholar]
- Martin K.; Nõges J.; Haav K.; Kadam S. A.; Pung A.; Leito I. Exploring Selectivity of 22 Acyclic Urea-, Carbazole- and Indolocarbazole-Based Receptors towards 11 Monocarboxylates. Eur. J. Org. Chem. 2017, 2017, 5231–5237. 10.1002/ejoc.201700931. [DOI] [Google Scholar]
- Spartan’18; Wavefunction Inc., 2018.
- Zhao Y.; Truhlar D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. 10.1007/s00214-007-0310-x. [DOI] [Google Scholar]
- For examples, see:; a Kozuch S.; Martin J. M. L. Halogen Bonds: Benchmarks and Theoretical Analysis. J. Chem. Theory Comput. 2013, 9, 1918–1931. 10.1021/ct301064t. [DOI] [PubMed] [Google Scholar]; b Bauzá A.; Alkorta I.; Frontera A.; Elguero J. On the Reliability of Pure and Hybrid DFT Methods for the Evaluation of Halogen, Chalcogen, and Pnicogen Bonds Involving Anionic and Neutral Electron Donors. J. Chem. Theory Comput. 2013, 9, 5201–5210. 10.1021/ct400818v. [DOI] [PubMed] [Google Scholar]; c Dey K. R.; Wong B. M.; Hossain M. A. Rational design of a macrocycle-based chemosensor for anions. Tetrahedron Lett. 2010, 51, 1329–1332. 10.1016/j.tetlet.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Hohenstein E. G.; Chill S. T.; Sherrill C. D. Assessment of the Performance of the M05-2X and M06-2X Exchange-Correlation Functionals for Noncovalent Interactions in Biomolecules. J. Chem. Theory Comput. 2008, 4, 1996–2000. 10.1021/ct800308k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.