Abstract
Many synthetic and supramolecular chiral polymeric systems are known to exhibit the “majority rules effect” (MRE), a positive nonlinear response in which a small enantiomeric excess (ee) of the chiral building blocks leads to unproportionally large chiroptical signals near zero ee. In contrast, the opposite “racemate rules effect” (RRE), a negative nonlinear response in which the chiroptical signals are “at near zero ee, while giving large nonlinear chiroptical responses to ee at high values, has only been occasionally observed. The origin of this unusual ee dependence remains elusive largely because few systems have been established that exhibit this effect. Herein, we present a design approach that enables the development of chiral supramolecular polymers with a pronounced negative nonlinear response akin to RRE. This is achieved by in situ generating a bidentate inducer for supramolecular polymerization that exists in both meso- and homochiral forms upon reacting with chiral guests. The presence of the meso-inducer creates an aggregate structure that has a little response in the circular dichroism (CD) spectra as a function of ee at a particular wavelength, but a homochiral inducer gives large changes in response to ee at this wavelength. This allowed for an RRE-like response to be observed when the CD intensity of the supramolecular polymers was plotted against the ee of the chiral guests that generate the meso- and homochiral inducers without the necessity of the racemic guest preferentially being incorporated into the polymer.
Graphical Abstract

INTRODUCTION
Chiral supramolecular polymeric systems have been the subject of intensive research efforts due to their biomimetic nature and potential applications in developing novel functional materials for sensing and catalysis.1 The chirality is often manifested in the helical conformation of the polymer backbone. The preferred handedness is usually dictated by the molecular chirality of either the monomeric building blocks or the external guest molecules that interact with monomers in the polymers via noncovalent or dynamic covalent interactions.2 Of particular interest is the existence of a nonlinear relationship between chiroptical properties of the supramolecular polymers and the enantiomeric excess (ee) of the chiral building blocks or guests.3 The “majority rules effect” (MRE)4 is one such nonlinear relationship. When the intensity of the chiroptical signal (commonly circular dichroism, CD) is plotted against ee, MRE gives rise to a steep slope around 0% ee and “at responses toward the two extreme ends of ee (i.e., 100 and −100%). Such a positive nonlinear CD–ee relationship effect is attributed to a higher energetic penalty for a helix reversal than that for accommodating a “mismatched” enantiomer into the helix.4a The effect has attracted much attention because it is proposed to be relevant to the origin of biological homochirality.3
While a linear relationship of CD versus ee or the MRE is commonly observed,5 a “at CD response around 0% ee and large slopes at the extreme ends of ee, that is, a negative nonlinear CD–ee relationship, have rarely been reported. We recently reported a serendipitous discovery of such an effect.6 In that system, the achiral perylenebisimide (PBI) monomer 1 (Figure 1a), upon binding chiral malate guests, aggregated to form optically active nanostructures. While the exact cause of these observed “antimajority rules” remains elusive, we proposed that preferential binding of malate racemates by the PBI monomers is responsible, and hence, we coined the term “racemate rules effect” (RRE).7 The hypothesis was that incorporating opposite enantiomers into the nanostructures was thermodynamically preferred, but at the extreme ends of the CD versus ee plot, the solution is depleted in racemate, and thus, the same enantiomer must be incorporated, giving rise to a CD-active helical twist of the aggregate. This led to a highly sensitive response of the CD signal to small changes in ee at the high ends of ee, which is favorable for accurate detection of high ee values chiral organic compounds for optimization of asymmetric catalysts.7
Figure 1.

(a) Previously reported two-component chirality induction system that consists of a boronic acid-functionalized PBI host 1 and malate guests themselves as the chirality inducers. The figure on the right end shows a negative nonlinear profile when the induced CD intensity of 1 is plotted against ee of malate. (b) The current three-component chirality induction system that consists of a guanidinium-functionalized PBI host 2, a bidentate linker (m-PA), and chiral leucine guests. Imine condensation of m-PA with a mixture of leucine enantiomers generates homochiral bisimines (l,l-3 and d,d-3) and a meso-bisimine (l,d-3) as inducers of aggregation of 2.
The rational design of supramolecular polymers that exhibit RRE is not straightforward because of the necessity to enforce a thermodynamic preference for binding the opposite enantiomers of the guest (racemates). Inspired by the 1-malate system, we sought to create an alternative approach in which the guest racemate binding influences the supramolecular polymerization process differently from the pure enantiomers but does not necessarily rely on a preference for racemate binding.
To this end, we conceived a three-component approach instead of two components (i.e., host and guest). Our design includes a bidentate “linker” to interact with two equivalents of chiral guests to form a meso-bisadduct (racemate), and homochiral bisadducts, as chemical inducers of supramolecular polymerization (Figure 1b). The meso- and homochiral inducers are diasteromers and hence will interact differently with the supramolecular polymers. Provided that the inducers generate the formation of different supramolecular polymer structures whose CD spectra differ,8 deviation from linearity was expected to be found for a plot of CD versus ee of the chiral guests where different wavelengths would reflect the influence of either the meso- or homochiral inducers. Herein, we demonstrate this design principle to generate chiral supramolecular polymers exhibiting a negative nonlinear response akin to RRE.
RESULTS AND DISCUSSION
Our system is a three-component supramolecular polymer (Figure 1b) that consists of an achiral PBI-guanidinium monomer (2),9 a bidentate linker m-phthalaldehyde (m-PA), and chiral leucine guests. Imine condensation between m-PA and leucine generates bisimines 3. The meso-bisimine l,d-3 would be generated at different molar ratios with respect to the enantiomers l,l-3 and d,d-3 depending upon the ee of leucine (Figure 1b). The bisimine inducers (3) contain two carboxylate groups that bind simultaneously two molecules of the achiral monomer 2 via guanidinium-carboxylate salt bridge formation,10 thus inducing the aggregation of 2. The ability of 2 to bind 3 via the guanidinium-carboxylate interactions is verified by the downfield shift of a guanidinium −NH resonance in DMSO-d6 from 7.46 to 11.94 ppm upon the addition of 3 (Figure S1). The aggregation of 2 was investigated in MeOH. In the absence of 3, the compound 2 (50 μM) in MeOH at 298 K shows the most intense absorption maximum at 521 nm corresponding to the 0–0 vibronic band of the PBI S0 → S1 transition and a weaker 0–1 vibronic band at 486 nm (Figure 2a, black line). The ratio of these two absorption bands (A0–0/A0–1 = 1.4) is slightly lower than that of the monomeric PBIs (A0–0/A0–1 = 1.5) reported in the literature,11 suggesting that the compound 2 exists as a mixture of monomeric and oligomeric species under the described conditions. Addition of bidentate bisimine inducers 3 leads to a reversal of the relative intensities of the 0–0 and 0–1 vibronic bands and a hypochromism (Figure 2a, red and blue lines), indicating the formation of H-type aggregates of 2 induced by 3.11 The hydrodynamic diameters of the aggregates were determined to be 960 nm by dynamic light scattering (Figure S2), confirming the aggregation of 2 into high molecular weight supramolecular polymers.
Figure 2.
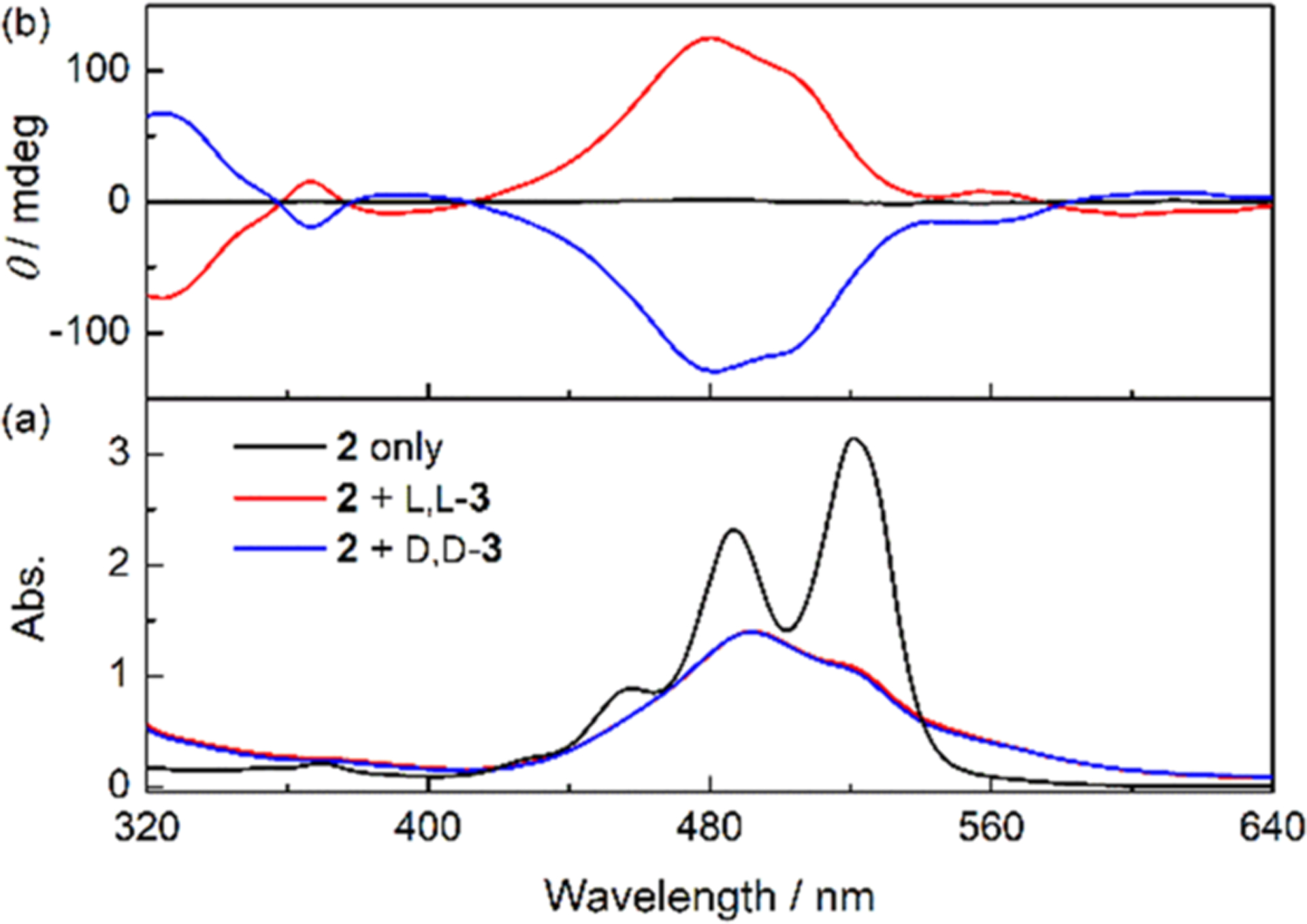
(a) Absorption and (b) CD spectra of 2 (50 μM) in MeOH at 298 K in the absence and presence of l,l-3 or d,d-3 (0.5 mM).
Successful chirality induction of the supramolecular polymers of 2 by the chiral bisimines l,l-3 or d,d-3 (prepared from l- or d-leucine, respectively) is demonstrated by the CD spectra showing intense CD signals in the PBI absorption region upon addition of these inducers (Figure 2b). Possible contribution from linear dichroism (LD) has been ruled out by the mirror image relationship of the CD signals induced by the enantiomers of 3 and an independent LD measurement showing no LD signals in the aggregates (Figure S3). Unlike most chiral PBI aggregates that show exciton-coupled bisignate CD signals due to through-space degenerative exciton coupling caused by a helical twisting of neighboring PBI chromophores,12 the CD spectra of 2 in the presence of l,l-3 or d,d-3 are of monosignate nature. The monosignate CD profile indicates the lack of a single-handed helical twisting between the S0 → S1 transition dipoles of PBI chromophores and arises from simple induced chirality of the PBI chromophores via distortions13 or nondegenerative coupling14 of PBI with dipoles of other chromophores, such as the π–π* transition of 3. As we will return to below, a transition from a monosignate to bisignate spectrum of 2 represents a transition from no helical twist to a helically twisted state. The sigmoidal dependence of the CD intensity on the concentration of l,l-3 suggests that both guanidinium groups in 2 likely bind to the carboxylate groups in 3 to generate CD active aggregates (Figure S4).
To explore the potential for a nonlinear relationship between the CD spectra of 2/3 supramolecular polymers and the ee of leucine used to form 3 and more importantly to examine the role of the meso-bisimine L,D-3 (Figure 1b), we prepared these aggregates with leucine of varying ee. This was done using two methods, “premix” and “postmix” (Figure 3), that differ in whether the meso-bisimine L,D-3 is created or not, respectively. In the “premix” method, m-PA was treated with a mixture of l-and d-leucines, which allows for the formation of both homochiral and meso-bisimines.15 The mixtures of homochiral and meso-bisimines were then treated with 2 in MeOH to induce chirality in the polymerization, and the CD spectra of the samples were recorded. In the “postmix” method, m-PA was allowed to react with either l- or d-leucine separately to give only the homochiral adducts l,l-3 or d,d-3. These homochiral species were then mixed in different ratios with 2, and the mixture was quickly analyzed via CD spectropolarimetry to minimize the formation of l,d-3 by imine exchange. This allowed for an investigation of chirality induction primarily, if not solely, by the homochiral bisimines. It should be noted that the UV–vis absorption titrations demonstrate no significant difference between a$nities of homochiral l,l-/d,d-3 and the meso l,d-3 for the host 2 (Figure S5).
Figure 3.
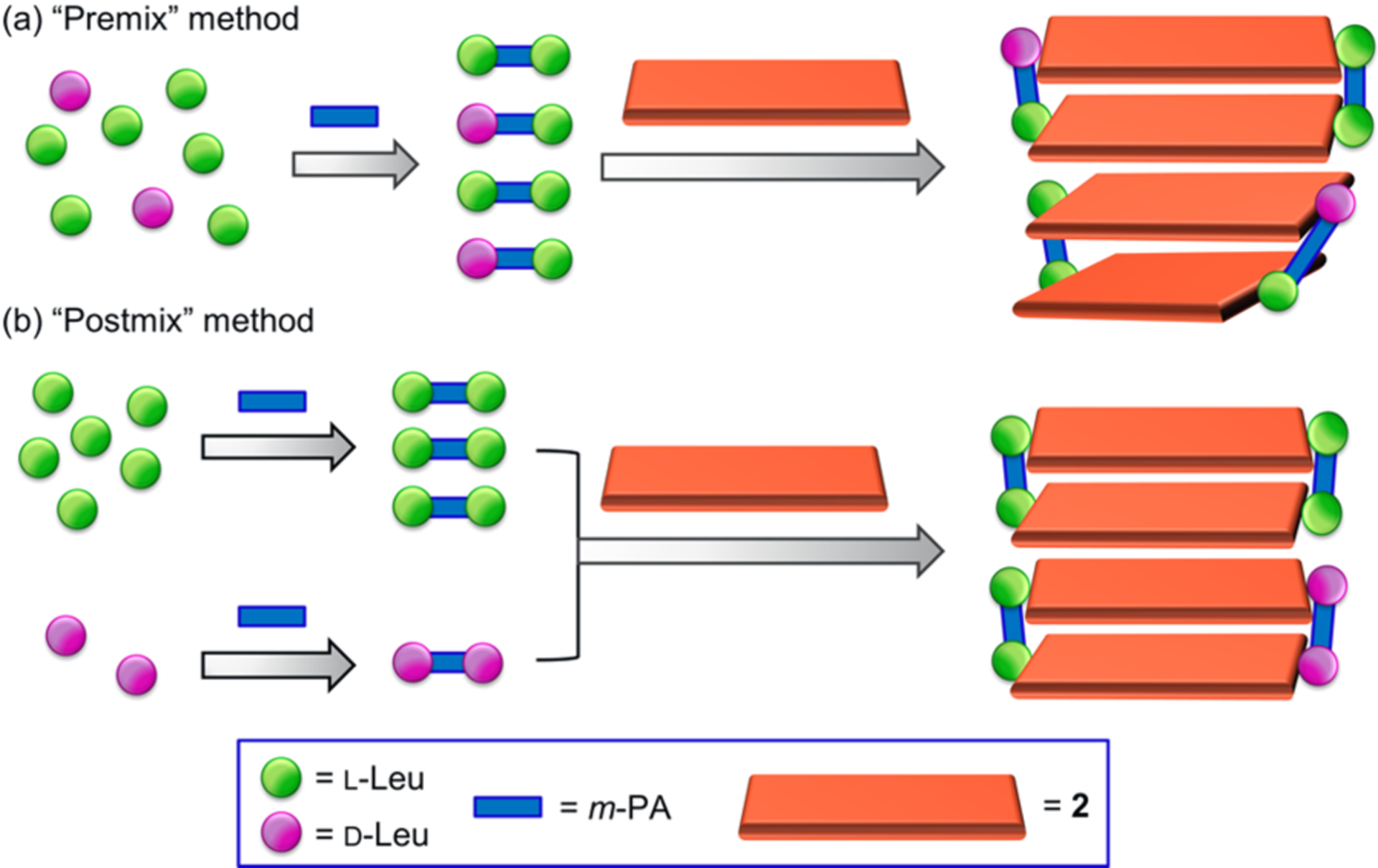
Cartoon representation of the (a) “premix” and (b) “postmix” sample preparation methods.
We first set out to explore any differences in the shapes of the CD spectra that arise from the incorporation of l,d-3. Figure 4a (left panel) shows the leucine ee-dependent CD spectra of the 2/3 aggregates prepared by the “premix” method, which demonstrates a dramatic transition in CD patterns from monosignate to bisignate going from 100 to 80% ee, with the rest of the CD spectra remaining bisignate down to ~0% ee. This change in the shape of the CD spectra arises from a modification of the structure of the aggregates, which could be caused by l,d-3. In contrast, the aggregates prepared by the “postmix” method (assumed to have a minimized amount of L,D-3) show predominantly the monosignate CD spectra at different ee’s of leucine (Figure 4a, right panel). These results indicate that while the homochiral bisimines l,l-/d,d-3 induce the formation of aggregates with monosignate CD, a mixture of homochiral l,l-/d,d-3 and meso l,d-3 lead to the formation of a different aggregate structure with a bisignate CD pattern. We postulate that because l,d-3 is achiral and thus incapable of chirality induction by itself, the meso 2/l,d-3 adduct intercalates into segments of 2 bound to l,l-/d,d-3, alters, and causes a twist leading to helical aggregates with the screw sense dictated by the dominant homochiral enantiomer (l,l- or d,d-3). Therefore, from approximately 80 to −80% ee, the l,d-3 formed by the leucine racemate is causing dramatic changes in the CD spectra by disrupting the original aggregates formed by 2 and l,l-/d,d-3.
Figure 4.

(a) CD spectra of 2/3 aggregates in MeOH at 298 K prepared by the premix (left) and postmix (right) methods using leucine of varying ee’s. [2] = 50 μM. [l-Leu] + [d-Leu] equals twice that of [m-PA], m-PA being 0.5 mM. (b) CD intensity at 498 nm as a function of ee of leucine. Note that for the postmix data, the deviation of linearity is due to the generation of small amounts of l,d-3 when l,l-3 and d,d-3 were mixed prior to the addition of 2.
For a confirmation of the structural transition of the polymers created by the meso-inducer, scanning electron microscopy (SEM) images of the 2/3 aggregates prepared by the “premix” method were taken. Figure S6 shows that the morphology of air-dried samples undergoes a gradual transition from curled nanoribbons to bundled nanofibers upon lowering the ee of leucine from 100% (where no l,d-3 was present) to 60% (where the fractions of l,l-3, d,d-3, and l,d-3 were calculated to be 36, 16, and 48%, respectively, based on statistical distribution of the bisimine products).15 This indicates that the meso-bisimine l,d-3 induces a structural modification of the 2/3 aggregates that leads to morphological changes of dried samples at the micrometer length scales. In contrast, SEM images of samples prepared by the postmix methods show only curled ribbons (Figure S7) similar to those observed with 100% ee, confirming that the morphological transition is induced by l,d-3.
To determine if an anticipated nonlinear CD–ee profile exists, we used the CD signal at 498 nm as the signature of the aggregates generated solely by the homochiral versions of 3 because the aggregated structures induced by mixtures of l,l-3 and d,d-3 with l,d-3 are nearly CD-silent at this wavelength. This lack of CD response at 498 nm can be seen as an isosbestic point at zero ellipticity in Figure 4a for ee values between 80 and −80%. Thus, CD at 498 nm is solely indicative of the aggregate that gives a monosignate CD, thereby reflecting l,l-3 or d,d-3. By plotting the CD intensity at 498 nm of the 2/3 aggregates prepared by the premix method against the ee of leucine, a very pronounced negative nonlinear response was found. The plot exhibits a steep slope in the ee range of 80–100% (Figure 4b, black line) and almost a “at response from 0 to 60% due to the l,d-3-induced formation of aggregates that are nearly CD-silent at this wavelength. The current system thus displays a CD–ee profile similar to the 1/malate system (Figure 1a) and yet operates via a completely different mechanism, that is, by meso-component-induced structural disruption. By using the CD signal at the wavelength that responds only to the aggregates induced by l,d-3 (580 nm), we were able to estimate the fractions of the two types of aggregates (Figures S8 and S9). It was found that the system exists as a mixture of the two types of aggregates from 90 to 60% ee of leucine and the aggregates induced by l,d-3 with bisignate CD signatures predominate at ee below 60%.
It is noted that a weak negative nonlinear response is also present in the CD–ee curve for the samples prepared by the postmix method (Figure 4b, red line). This is likely due to a small amount of imine exchange reaction that generates l,d-3. The hypothesis was confirmed by the restoration of the strong negative nonlinear response when the bisimine enantiomers prepared by the postmix method were stirred for 50 min to complete imine exchange before being treated with 2 (Figure S10). The occurrence of imine exchange has also been verified by 1H NMR experiments (Figures S11–S14). Despite the imine exchange reaction occurring between free isomers of 3 in solution, the aggregates induced by a mixture of l,l-3 and d,d-3 are kinetically stable once formed and do not convert to l,d-3-induced aggregates over time (Figure S15).
Optical sensors that interact with chiral organic compounds and generate ee-dependent absorption, fluorescence or CD responses, are of interest for the rapid determination of product enantiopurity in asymmetric synthesis.16 With the negative nonlinearity method introduced above, the CD intensity at 498 nm has a significantly larger dynamic range to variations of the ee of leucine from 80 to 100% (Figure 5) compared to a linear or MRE response. Thus, this response was analyzed for an improvement in the determination of ee values close to the extreme ends of 100 and −100%. For comparison, most of the chirality sensors show a linear response to ee and have absolute errors in the range of ±3 to 5%. Thus, they are incapable of distinguishing small differences in ee, which is most critical in the high ee regions when optimizing asymmetric transformations. To examine the RRE approach for the determination of ee of leucine close to 100%, four “unknown” leucine samples with ee in the range of 93–99% were prepared and subjected to ee determination using the “premix” approach and CD measurements at 498 nm. The ee values experimentally determined were compared to the actual values (Table 1). The results demonstrate a remarkably low average absolute error of ±0.5%, which is similar to or even better than chiral HPLC.17
Figure 5.

Calibration curve for ee determination (92–100%) of leucine using 2/3 aggregates prepared by the “premix” method in MeOH. [2] = 50 μM. [l-Leu] + [d-Leu] = 2 [m-PA] = 1 mM. Error bars show standard deviations from three measurements.
Table 1.
Leucine ee Determination Using the 2/3 Aggregatesa
| actual ee (%) | 93.0 | 95.0 | 97.0 | 99.0 |
| CD (498 nm) | −95.2 | −105.4 | −110.5 | −119.2 |
| calcd ee (%) | 92.6 | 95.0 | 96.2 | 98.1 |
| absolute error (%) | −0.4 | 0.0 | −0.8 | −0.9 |
| average error (%) | 0.5 |
Each “unknown” sample was subject to CD measurement once.
We also screened the aggregates of 2 with bisimines formed by m- and p-phthalaldehydes (m-PA/p-PA, respectively) and other common amino acids. Negative nonlinear CD–ee relationships were also observed for p-PA/leucine (Figure S17), p-PA/isoleucine (Figure S18), and p-PA/valine (Figure S19) aggregates. The aggregates induced by the bisimines from a few other amino acids tested exhibited approximately linear CD–ee relationships (Figures S20 and S21) because the CD spectra of the meso- and homochiral-induced polymers do not differ. These results indicate that the method to create negative nonlinear responses in the current system is not generally applicable to all amino acids. Thus, the observation of different monosignate or bisignate CD responses created by the diastereomeric inducers depends on the substituents on the amino acids. Further, structural insight to elucidate this substituent effect is needed to develop general rules to exploit our strategy for generating supramolecular polymers with negative nonlinear responses.
CONCLUSIONS
In summary, we have demonstrated a design approach that allows access to a negative nonlinear CD–ee relationship similar to previously reported racemate rules effects in a supramolecular polymer system. The key to achieve this rarely observed profile is the use of a bidentate linker to create a meso-inducer from guest racemates that intercalates into and alters the structure of the supramolecular chiral polymers whose chirality was induced by the corresponding homochiral inducers. A small amount of the meso-bisimine adduct l,d-3 was found to enable the transformation of homochiral 2/bisimine 3 aggregates from parallel to twisted arrangement of the S0 → S1 transition dipole moments of PBI chromophores, leading to a dramatic change in the CD spectral profile of 2 from monosignate to bisignate. A second key feature was to analyze at a wavelength where CD is responsive only to the aggregate induced by the homochiral species with no response to the aggregates formed in the presence of the meso-inducer. By plotting the monosignate CD signature, representative of only the aggregates created by the homochiral inducers, against the ee of leucine, a negative nonlinear CD–ee curve akin to RRE was obtained. Albeit the CD signal arises from the homochiral inducers, the meso-inducer diminishes the CD signal, keeping it low until these inducers are depleted to such an extent that the optical response corresponds to nearly enantiopure leucine. This system was successfully applied to determine the ee of leucine close to 100% with high accuracy. Unlike the previously reported 1-malate aggregates that rely on preferential binding of racemic malate, our current three-component system does not require the thermodynamic binding preference for racemic guests but instead relies on spectral differences between the polymers generated by the meso- and homochiral induces. This provides the first design principle to facilely generate systems that show the overlooked “racemate rules effect”. Learning the dependence of the phenomenon on the analyte structure, to allow generalization of our approach to other chiral analytes, is currently ongoing.
EXPERIMENTAL SECTION
General Methods.
Reagents and solvents were purchased from commercial sources and used as received. 1H NMR spectra were recorded on a Bruker AVANCE III 500 MHz or a Bruker AVANCE III 850 MHz NMR. UV–vis absorption spectra were recorded on a Varian Cary 300 UV–vis spectrophotometer. Circular dichroism (CD) spectra were obtained on a Jasco J-815 CD spectropolarimeter. Dynamic light scattering (DLS) was performed on a Malvern Zetasizer Nano ZS. All spectroscopic measurements were performed at 298 K. Scanning electron microscopic (SEM) images were taken on a HITACHI S-4800 field-emission SEM after the samples were drop casted on a silicon wafer and left to dry for 2 h. The host 2 was synthesized according to a reported procedure.9
Sample Preparation.
Solutions of 3 were prepared by stirring a mixture of leucine sodium salt (50 mM) and m-PA (25 mM) in MeOH overnight. For “premix” samples, the leucine enantiomers were mixed in MeOH and then treated with m-PA. After overnight stirring, the solutions were diluted to a bisimine concentration of 1 mM (assuming complete formation of imines), treated with a 5 mM DMSO solution of 2 (final concentration of 2 was 50 μM), and then immediately subjected to UV–vis or CD spectroscopic measurements. For “postmix” samples, separate solutions of l-leucine/m-PA and d-leucine/m-PA mixtures were prepared. After overnight stirring, the solutions were mixed in different ratios in MeOH to a final total bisimine concentration of 1 mM (assuming complete imine formation). The resultant solutions were then quickly treated with a 5 mM DMSO solution of 2 (final concentration of 2 was 50 μM) and then immediately subjected to UV–vis or CD spectroscopic measurements.
Supplementary Material
ACKNOWLEDGMENTS
We thank the National Science Foundation of China (grants 21435003, 21521004, 91856118, and 21820102006) and the Ministry of Education of China (grant IRT13036) for financial support. Further, we thank the NIH (GM077437) and the Welch Regents Chair (F-0045) to EVA and the ARC (DP180100612) to PAG.
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.joc.9b02166.
1H NMR evidence of guanidinium-carboxylate interactions, DLS data, linear dichroism study, CD titrations, UV–vis binding studies, SEM micrographs, CD spectra of aged postmix samples, 1H NMR study of imine product distribution, 1H NMR study of imine exchange, CD/UV–vis study of kinetic stability of the aggregates, and CD–ee relationships for other amino acids (PDF)
REFERENCES
- (1).(a) Yashima E; Ousaka N; Taura D; Shimomura K; Ikai T; Maeda K Supramolecular Helical Systems: Helical Assemblies of Small Molecules, Foldamers, and Polymers with Chiral Amplification and Their Functions. Chem. Rev 2016, 116, 13752–13990. [DOI] [PubMed] [Google Scholar]; (b) Liu M; Zhang L; Wang T Supramolecular Chirality in Self-Assembled Systems. Chem. Rev 2015, 115, 7304–7397. [DOI] [PubMed] [Google Scholar]
- (2).Hembury GA; Borovkov VV; Inoue Y Chirality-Sensing Supramolecular Systems. Chem. Rev 2008, 108, 1–73. [DOI] [PubMed] [Google Scholar]
- (3).Palmans AR; Meijer EE Amplification of Chirality in Dynamic Supramolecular Aggregates. Angew. Chem., Int. Ed 2007, 46, 8948–8968. [DOI] [PubMed] [Google Scholar]
- (4).(a) van Gestel J; Palmans AR; Titulaer B; Vekemans JA; Meijer EW “Majority-Rules” Operative in Chiral Columnar Stacks of C3-Symmetrical Molecules. J. Am. Chem. Soc 2005, 127, 5490–5494. [DOI] [PubMed] [Google Scholar]; (b) Green MM; Garetz BA; Munoz B; Chang H; Hoke S; Cooks RG Majority Rules in the Copolymerization of Mirror Image Isomers. J. Am. Chem. Soc 1995, 117, 4181–4182. [Google Scholar]
- (5).Herrera BT; Pilicer SL; Anslyn EV; Joyce LA; Wolf C Optical Analysis of Reaction Yield and Enantiomeric Excess: A New Paradigm Ready for Prime Time. J. Am. Chem. Soc 2018, 140, 10385–10401. [DOI] [PubMed] [Google Scholar]
- (6).Wu X; Chen X-X; Song B-N; Huang Y-J; Li Z; Chen Z; James TD; Jiang Y-B Induced Helical Chirality of Perylenebisimide Aggregates Allows for Enantiopurity Determination and Differentiation of α-Hydroxy Carboxylates by Using Circular Dichroism. Chem. – Eur. J 2014, 20, 11793–11799. [DOI] [PubMed] [Google Scholar]
- (7).Chen X-X; Jiang Y-B; Anslyn EV A racemate-rules effect supramolecular polymer for ee determination of malic acid in the high ee region. Chem. Commun 2016, 52, 12669–12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).(a) Cao H; Zhu X; Liu M Self-Assembly of Racemic Alanine Derivatives: Unexpected Chiral Twist and Enhanced Capacity for the Discrimination of Chiral Species. Angew. Chem., Int. Ed 2013, 52, 4122–4126. [DOI] [PubMed] [Google Scholar]; (b) Samanta SK; Bhattacharya S Excellent chirality transcription in two-component photochromic organogels assembled through J-aggregation. Chem. Commun 2013, 49, 1425–1427. [DOI] [PubMed] [Google Scholar]
- (9).Roy B; Noguchi T; Yoshihara D; Tsuchiya Y; Dawn A; Shinkai S Nucleotide sensing with a perylene-based molecular receptor via amplified fluorescence quenching. Org. Biomol. Chem 2014, 12, 561–565. [DOI] [PubMed] [Google Scholar]
- (10).Schmuck C How to improve guanidinium cations for oxoanion binding in aqueous solution?: The design of artificial peptide receptors. Coord. Chem. Rev 2006, 250, 3053–3067. [Google Scholar]
- (11).Würthner F; Saha-Möller CR; Fimmel B; Ogi S; Leowanawat P; Schmidt D Perylene Bisimide Dye Assemblies as Archetype Functional Supramolecular Materials. Chem. Rev 2016, 116, 962–1052. [DOI] [PubMed] [Google Scholar]
- (12).Pescitelli G; Di Bari L; Berova N Application of electronic circular dichroism in the study of supramolecular systems. Chem. Soc. Rev 2014, 43, 5211–5233. [DOI] [PubMed] [Google Scholar]
- (13).Xie Z; Stepanenko V; Radacki K; Würthner F Chiral J-Aggregates of Atropo-Enantiomeric Perylene Bisimides and Their Self-Sorting Behavior. Chem. – Eur. J 2012, 18, 7060–7070. [DOI] [PubMed] [Google Scholar]
- (14).Mammana A; Pescitelli G; Asakawa T; Jockusch S; Petrovic AG; Monaco RR; Purrello R; Turro NJ; Nakanishi K; Ellestad GA; Balaz M; Berova N Role of Environmental Factors on the Structure and Spectroscopic Response of 5′-DNA–Porphyrin Conjugates Caused by Changes in the Porphyrin–Porphyrin Interactions. Chem. – Eur. J 2009, 15, 11853–11866. [DOI] [PubMed] [Google Scholar]
- (15). 1H NMR analyses of the bisimine adducts formed with leucine racemate (Figure S12) demonstrated a statistical distribution of the homochiral and meso-bisimines that indicates the lack of selectivity in their formation.
- (16).(a) Pu L Simultaneous Determination of Concentration and Enantiomeric Composition in Fluorescent Sensing. Acc. Chem. Res 2017, 50, 1032–1040. [DOI] [PubMed] [Google Scholar]; (b) Shcherbakova EG; Minami T; Brega V; James TD; Anzenbacher P Jr. Determination of Enantiomeric Excess in Amine Derivatives with Molecular Self-Assemblies. Angew. Chem., Int. Ed 2015, 54, 7130–7133. [DOI] [PubMed] [Google Scholar]; (c) Wolf C; Bentley KW Chirality sensing using stereodynamic probes with distinct electronic circular dichroism output. Chem. Soc. Rev 2013, 42, 5408–5424. [DOI] [PubMed] [Google Scholar]
- (17).North M, Principles and Applications of Stereochemistry. CRC Press: Cheltenham, UK, 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


