Abstract
In acute myeloid leukemia (AML), TP53 mutations and dysregulation of wild-type p53 is common and supports an MDM2 antagonist as a therapy. RO6839921 is an inactive pegylated prodrug of the oral MDM2 antagonist idasanutlin (active principle [AP]) that allows for IV administration. This phase 1 monotherapy study evaluated the safety, pharmacokinetics, and pharmacodynamics of RO6839921 in patients with AML. Primary objectives identified dose-limiting toxicities (DLTs) and maximum tolerated dose (MTD). Secondary objectives assessed pharmacokinetic, pharmacodynamic, and antileukemic activity. A total of 26 patients received 120–300 mg AP of idasanutlin. The MTD was 200 mg, with DLTs at 250 (2/8 patients) and 300 mg (2/5). Treatment–related adverse events in >20% of patients were diarrhea, nausea, vomiting, decreased appetite, and fatigue. Six deaths (23.1%) occurred, all unrelated to treatment. Pharmacokinetics showed rapid and near-complete conversion of the prodrug to AP and dose-proportional exposure across doses. Variability ranged from 30%–47% (22%–54% for idasanutlin). TP53 was 21 (87.5%) wild-type and 3 mutant (12.5%). The composite response rate (complete remission [CR], CR with incomplete hematologic recovery/morphological leukemia-free state [CRi/MLFS], or CR without platelet recovery [CRp]) was 7.7%. Antileukemic activity (CR, CRi/MLFS, partial response, hematologic improvement/stable disease) was observed in 11 patients (disease control rate, 42%): 10/11 were TP53 wild-type; 1 had no sample. p53 activation was demonstrated by MIC-1 induction and was associated with AP exposure. There was not sufficient differentiation or improvement in the biologic or safety profile compared with oral idasanutlin to support continued development of RO6839921. NCT02098967.
Keywords: Acute myeloid leukemia, MDM2, Idasanutlin, Safety
Introduction
The p53 protein is a growth-suppressive and pro-apoptotic protein that plays a central role in the protection of cells from tumor development. [1, 2] In normal cells, a close relationship exists between p53 and its primary regulator, murine double minute 2 (MDM2), which controls both p53 expression and degradation. MDM2 regulates p53 through a negative feedback loop. When nuclear p53 levels are elevated, they activate the transcription of the MDM2 gene; this allows MDM2 to bind to p53, blocking its transactivation domain and targeting p53 for ubiquitin-dependent degradation. [1–3]
The p53 signaling pathway is frequently inactivated in acute myeloid leukemia (AML). The TP53 mutation rate is <10% of cases of de novo AML [4]; however, inactivation of wild-type p53 occurs in many patients with AML by alternative mechanisms, including overexpression of MDM2, in order to allow proliferation and leukemogenesis. [5] Therefore, treatment with an MDM2 antagonist is a therapeutic option to restore p53 activity in these cases. [1, 6] MDM2 antagonists block p53-MDM2 binding, stabilize p53, and activate p53 signaling, thereby inducing cell cycle arrest and apoptosis. Idasanutlin, an oral MDM2 antagonist of the nutlin family of compounds, [6, 7] is being evaluated in phase 1 to 3 clinical trials in patients with solid and hematologic malignancies. [8, 9] In clinical trials in patients with AML who received MDM2 antagonists, MDM2 gene expression was related to clinical response. [10–12]
RO6839921 is an inactive pegylated prodrug of idasanutlin that allows for the solubility needed for IV administration, with the goals of improving exposure variability and pharmacokinetic parameters, reducing gastrointestinal toxicity in the absence of prophylaxis, and potentially improving efficacy compared with oral idasanutlin. The active principle (AP; idasanutlin) is released upon cleavage of its pegylated tail by esterases in the blood. IV-administered RO6839921 showed antitumor activity at nontoxic doses in established osteosarcoma and AML xenograft models in immunocompromised mice. [13] These nonclinical pharmacology results supported further evaluation of RO6839921 in clinical studies. This phase 1 study evaluated the safety, tolerability, pharmacokinetics, and pharmacodynamics of RO6839921 in patients with AML.
Methods
Patients
This phase 1 study (NCT02098967) was an open-label, first-in-human, multicenter, dose-escalation study of RO6839921 in patients with solid tumors and in patients with AML; results for solid tumors will be reported independently. Patients aged ≥18 years with relapsed/refractory AML, untreated AML with antecedent hematologic disorder, or high-risk de novo AML as defined by the 2010 European LeukemiaNet (ELN) criteria with Eastern Cooperative Oncology Group performance status of ≤2 were eligible. [14] Patients with central nervous system leukemia or any severe and/or uncontrolled medical conditions or other conditions that could affect their participation were excluded. The study was conducted in accordance with the principles of the International Conference on Harmonization Good Clinical Practice guidelines. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committees at the study sites and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all participants included in the study.
Study design
RO6839921 was administered as an IV infusion over approximately 1 h once daily for 5 days every 28 days. Dose escalation was performed using a modified rolling 6 design initiated at or below the dose at which grade ≥ 2 hematologic toxicity or projected efficacious exposure was reached in the solid tumor arm (120 mg AP). [15] Based on this design, the doses tested were 120, 200, 250, and 300 mg (in mg of AP).
The primary objectives of the study were to determine the maximum tolerated dose (MTD) and recommended phase 2 dose of RO6839921 and to characterize dose-limiting toxicities (DLTs) and the overall safety profile. The secondary objectives were to determine the pharmacokinetic parameters of RO6839921 and the AP and to assess the pharmacodynamic effects of RO6839921 and clinical responses. Treatment continued until disease progression, unacceptable toxicity, withdrawal of consent, or investigator discretion.
Assessments and analysis
Patients receiving ≥1 dose of RO6839921 were considered evaluable for safety. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03).
The DLT-evaluable population was defined as all patients who received ≥80% of study medication and completed the first 28-day treatment cycle. In addition, patients who had a DLT but did not meet the minimum dosing requirements were considered evaluable for DLTs. DLTs were assessed during the first treatment cycle (28 days) and included prolonged grade 4 neutropenia and prolonged grade 3/4 thrombocytopenia lasting ≥42 days from the start of the cycle in the absence of evidence of active AML as well as clinically significant grade 3 to 5 nonhematologic toxicity. The MTD was defined as the highest dose level tested with 0 to 1 DLT in a cohort of 6.
Plasma pharmacokinetic assessments of RO6839921 and AP concentrations were conducted in all patients during the first cycle of treatment on the first and fifth days of dosing immediately before dosing and at multiple postdose time points using a validated liquid chromatography–tandem mass spectrometry method, with pharmacokinetic parameters estimated using standard noncompartmental methods. Assessment of macrophage inhibitory cytokine 1 (MIC-1) protein levels was measured in serum before and after administration of RO6839921 using an enzyme-linked immunosorbent assay. TP53 mutation status was measured by next-generation sequencing at baseline.
The efficacy population was defined as all patients who received ≥80% of study medication and completed the first 28-day treatment cycle. Efficacy was evaluated on day 1 of every cycle starting with cycle 2. A composite response rate was calculated by determining the number of patients who achieved a best response (complete remission [CR], CR without platelet recovery [CRp], or CR with incomplete recovery of peripheral counts [CRi]/morphological leukemia-free state [MLFS]) divided by the total number of patients in the efficacy population. Additional outcomes were partial response with ≥50% decrease in bone marrow blasts, hematologic improvement measures, and disease progression. Hematologic improvement/stable disease (HI/SD) was defined as decreased peripheral blast percentage, decreased frequency of transfusions, and/or improvement in peripheral cell counts in the absence of CR in the marrow and were considered by Investigators and Sponsor on a case by case basis for continuation of treatment.
Results
Patient disposition and characteristics
This study was conducted at 6 sites in the United States and Canada between April 2014 and May 2018. A total of 26 patients with AML were treated at 4 doses: 120 mg AP (n = 6), 200 mg AP (n = 7), 250 mg AP (n = 8), and 300 mg AP (n = 5). Patients received a median of 5 doses (range, 4–10). The median treatment duration was 5 days (range, 4–86 days), and the median cumulative dose was 1225 mg (range, 600–3000 mg). Treatment was discontinued in 10 patients (38.5%) due to progression of disease, 9 patients (34.6%) due to physician decision (perceived lack or loss of clinical benefit), and 7 patients (26.9%) due to AEs.
The median age was 65 years (Table 1). The majority of patients had an ELN intermediate 2 [14] or adverse risk and had an Eastern Cooperative Oncology Group performance status of 0 or 1. Half the patients had prior cancer, and 81% had wild-type TP53 status.
Table 1.
Baseline demographic and clinical characteristics
| Characteristics | 120 mg AP (n = 6) |
200 mg AP (n = 7) |
250 mg AP (n = 8) |
300 mg AP (n = 5) |
Total (N = 26) |
|---|---|---|---|---|---|
| Male, n (%) | 3 (50.0) | 5 (71.4) | 3 (37.5) | 0 | 11 (42.3) |
| Median age (range), years | 50 (24–73) | 58 (47–74) | 74 (64–80) | 49 (33–71) | 65 (24–80) |
| ECOG PS, n (%) | |||||
| 0 | 4 (67) | 2 (29) | 1 (13) | 1 (20) | 8 (31) |
| 1 | 2 (33) | 5 (71) | 7 (88) | 4 (80) | 18 (69) |
| ELN risk at diagnosis, n (%) | |||||
| Favorable | 0 | 1 (14) | 1 (13) | 0 | 2 (8) |
| Intermediate 1 | 0 | 3 (43) | 3 (38) | 1 (20) | 7 (27) |
| Intermediate 2 | 1 (17) | 2 (29) | 1 (13) | 1 (20) | 5 (19) |
| Adverse | 5 (83) | 1 (14) | 3 (38) | 3 (60) | 12 (46) |
| Antecedent hematologic disorder, n (%)* | 1 (17) | 0 | 3 (38) | 2 (40) | 6 (23) |
|
Prior cancer, n (%)† |
3 (50) | 5 (71) | 2 (25) | 3 (60) | 13 (50) |
| No. of prior regimens, n (%) | |||||
| 0 | 1 (16.7) | 0 | 2 (25) | 1 (20) | 4 (15.4) |
| 1 | 1 (16.7) | 2 (28.6) | 3 (37.5) | 1 (20) | 7 (26.9) |
| 2 | 2 (33.3) | 2 (28.6) | 3 (37.5) | 2 (40) | 9 (34.6) |
| 3 | 1 (16.7) | 2 (28.6) | 0 | 1 (20) | 4 (15.4) |
| 4 | 1 (16.7) | 1 (14.3) | 0 | 0 | 2 (7.7) |
| Prior allogeneic transplant, n (%) | 1 (17) | 4 (57) | 1 (13) | 1 (20) | 7 (27) |
| Response to prior therapy‡ | |||||
| No prior therapy | 1 (17) | 0 | 2 (25) | 1 (20) | 4 (15) |
| Refractory | 2 (33) | 2 (29) | 3 (38) | 3 (60) | 10 (38) |
| CR1 < 3 months | 1 (17) | 1 (14) | 0 | 0 | 2 (8) |
| CR1 3– 12 months | 2 (33) | 4 (57) | 2 (25) | 1 (20) | 9 (35) |
| CR1 > 12 months | 0 | 0 | 1 (13) | 0 | 1 (13) |
| TP53 status | |||||
| Not evaluable | 1 (17) | 1 (14) | 0 | 0 | 2 (8) |
| Evaluable | 5 (83) | 6 (86) | 8 (100) | 5 (100) | 24 (92) |
| Wild type | 5 (100) | 5 (83) | 8 (100) | 3 (60) | 21 (81) |
| Mutant | 0 | 1 (17) | 0 | 2 (40) | 3 (12) |
AP active principle, CR1 complete remission with first treatment received, ECOG PS Eastern Cooperative Oncology Group performance status, ELN European LeukemiaNet
*Includes chronic myelomonocytic leukemia, myelodysplastic syndromes, and essential thrombocythemia
†Prior cancer includes lymphoma (n = 4), prostate cancer, and breast cancer
‡A first complete remission/complete remission without platelet recovery <12 months is associated with poor response rates in relapse
DLT and MTD determination
All 26 patients were evaluable for DLTs. Four patients (15.4%) experienced DLTs: 2 in the 300-mg cohort experienced 2 DLTs (colitis [grade 3, serious] and electrocardiogram QT interval prolonged [grade 4, serious]) and 2 in the 250-mg cohort experienced 2 DLTs (diarrhea [grade 3] and stomatitis [grade 3]). The MTD (defined as the highest dose level tested with 0–1 DLT in a cohort of 6 patients) in patients with AML was 200 mg AP (0 DLTs in 7 patients).
Safety
All 26 patients received ≥1 dose of RO6839921 and were therefore considered safety evaluable. The most common AEs were nausea (57.7%), decreased appetite and febrile neutropenia (53.8% each), diarrhea and hypomagnesemia (50.0% each), hypokalemia (42.3%), constipation, fatigue, and vomiting (38.5% each), hypotension, peripheral edema, and stomatitis (34.6% each), and abdominal pain, hypophosphatemia, and hyperphosphatemia (30.8% each) (Table 2). All but 1 patient had AEs of grade ≥ 3 (25 patients [96.2%]), and the most common were febrile neutropenia (53.8% of patients), hypokalemia (23.1%), lung infection (15.4%), and hypophosphatemia and stomatitis (11.5% each). Most patients experienced a treatment-related AE (24 patients [92.3%]). The most common treatment-related AEs were diarrhea and nausea (50.0% each), vomiting (34.6%), decreased appetite (30.8%), and fatigue (26.9%) (Table 2). Serious AEs occurred in 22 patients (84.6%) (Table 3). The most common was febrile neutropenia in 13 patients (50.0%).
Table 2.
Summary of adverse events per MedDRA preferred term (≥ 30% of patients for any adverse events or ≥ 5% treatment-related adverse events)
| Patients with an AE, n (%) N = 26 | All AEs | Treatment-related AEs | ||
|---|---|---|---|---|
| Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | |
| Nausea | 15 (57.7) | 0 | 13 (50.0) | 3 (11.5) |
| Decreased appetite | 14 (53.8) | 0 | 8 (30.8) | 0 |
| Febrile neutropenia | 14 (53.8) | 14 (53.8) | 3 (11.5) | 0 |
| Hypomagnesemia | 13 (50.0) | 0 | 1 (3.8) | 0 |
| Diarrhea | 13 (50.0) | 1 (3.8) | 13 (50.0) | 1 (3.8) |
| Hypokalemia | 11 (42.3) | 6 (23.1) | 0 | 0 |
| Vomiting | 10 (38.5) | 0 | 9 (34.6) | 0 |
| Constipation | 10 (38.5) | 0 | 0 | 0 |
| Fatigue | 10 (38.5) | 1 (3.8) | 7 (26.9) | 1 (3.8) |
| Stomatitis | 9 (34.6) | 3 (11.5) | 5 (19.2) | 1 (3.8) |
| Hypotension | 9 (34.6) | 0 | 0 | 0 |
| Edema peripheral | 9 (34.6) | 0 | 1 (3.8) | 0 |
| Hypophosphatemia | 8 (30.8) | 3 (11.5) | 0 | 0 |
| Hyperphosphatemia | 8 (30.8) | 0 | 0 | 0 |
| Abdominal pain | 8 (30.8) | 1 (3.8) | 5 (19.2) | 0 |
| Epistaxis | 5 (19.2) | 2 (7.7) | 3 (11.5) | 2 (7.7) |
| Dyspepsia | 4 (15.4) | 0 | 2 (7.7) | 0 |
| Alopecia | 4 (15.4) | 0 | 4 (15.4) | 0 |
AE adverse event, MedDRA Medical Dictionary for Regulatory Activities
Table 3.
Summary of serious adverse events (per MedDRA preferred term)
| Patients with an SAE, n (%) N = 26 | All AEs | Study drug–related AEs |
|---|---|---|
| Febrile neutropenia | 13 (50.0) | 2 (7.7) |
| Lung infection | 3 (11.5) | 0 |
| Aspergillus infection | 1 (3.8) | 0 |
| Bacterial sepsis | 1 (3.8) | 0 |
| Cellulitis | 1 (3.8) | 0 |
| Enterobacter bacteremia | 1 (3.8) | 0 |
| Enterobacter infection | 1 (3.8) | 0 |
| Enterococcal sepsis | 1 (3.8) | 0 |
| Proctitis herpes | 1 (3.8) | 0 |
| Pseudomonal bacteremia | 1 (3.8) | 0 |
| Sepsis | 1 (3.8) | 0 |
| Colitis | 1 (3.8) | 1 (3.8) |
| Gastritis | 1 (3.8) | 1 (3.8) |
| Acute coronary syndrome | 1 (3.8) | 0 |
| Acute cardiac failure | 1 (3.8) | 0 |
| Myocardial ischemia | 1 (3.8) | 0 |
| Blood creatinine increased | 1 (3.8) | 1 (3.8) |
| Electrocardiogram QT interval prolonged | 1 (3.8) | 1 (3.8) |
| Dyspnea | 1 (3.8) | 0 |
| Pyrexia | 1 (3.8) | 0 |
| Menorrhagia | 1 (3.8) | 0 |
| Thrombosis | 1 (3.8) | 0 |
AE adverse event, MedDRA Medical Dictionary for Regulatory Activities, SAE serious adverse event
Seven patients (26.9%) experienced 8 AEs leading to withdrawal of study treatment (3 patients each in the 250- and 300-mg cohorts, 1 patient in the 200-mg cohort). One patient (250-mg cohort) reported 2 AEs of acute cardiac failure and sepsis; all other AEs (acute coronary syndrome, acute cardiac failure, bacterial sepsis, diarrhea, and pyrexia) were experienced by 1 patient each. Five patients (19.2%) experienced dose interruptions: 2 in the 120-mg cohort due to febrile neutropenia and aspergillus infection, respectively, both grade 3; 2 in the 200-mg cohort due to febrile neutropenia (1 patient had 1 febrile neutropenia event and 1 patient had 2 febrile neutropenia events); and 1 in the 250-mg cohort due to increased blood creatinine and decreased renal creatinine clearance, both grade 2.
Six deaths (23.1%) were reported during the study. Four deaths were due to progressive disease or relapse. Two deaths resulted from AEs with a fatal outcome (acute coronary syndrome and bacterial sepsis); both of these were judged to be unrelated to the study medication.
Pharmacokinetics
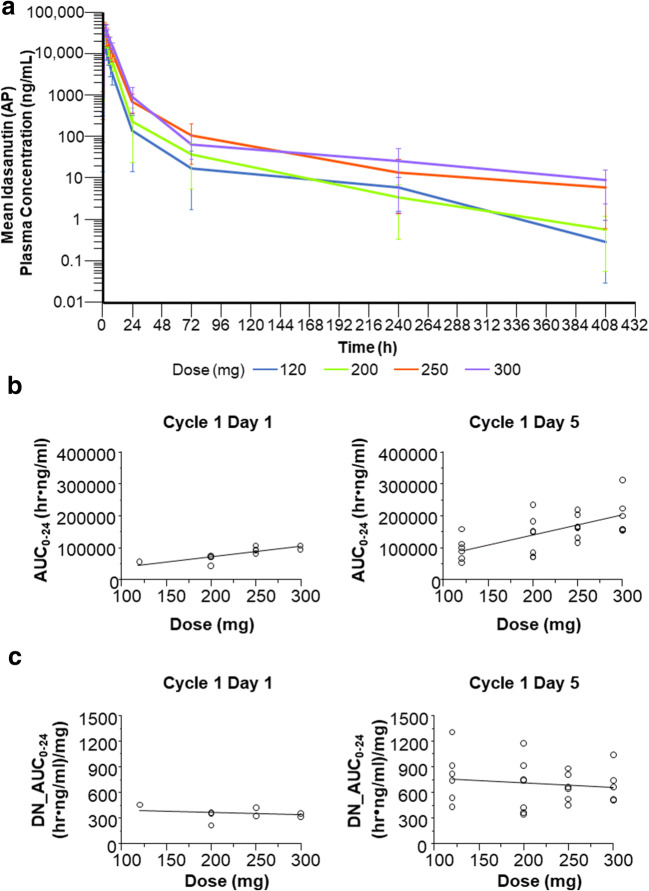
Mean plasma prodrug concentration-time profiles following RO6839921 administration of the AP are presented in Fig. 1a; pharmacokinetic parameters for RO6839921 and the AP are summarized in Tables 4 and 5. The dose-exposure relationship for the AP on days 1 and 5 was approximately linear and dose proportional (Fig. 1b and c). For the 200-, 250-, and 300-mg cohorts, the mean 24-h area under the plasma concentration-time curve on day 5 was above the target exposure of 100 h•μg/mL for RO6839921 (Table 4) and the AP (Table 5) based on preclinical studies. [13]
Fig. 1.
Pharmacokinetic analyses. a Mean plasma idasanutlin (AP) concentration-time profiles following RO6839921 (prodrug) administration; b AP dose-exposure relationship on days 1 and 5 for absolute AUC; c AP dose-exposure relationship on days 1 and 5 for dose-normalized AUC. AP, active principle; DN, dose-normalized; AUC0-24, 24-h area under the plasma concentration-time curve
Table 4.
Pharmacokinetic parameters for RO6839921 (prodrug)
| Dose, mg | Day 1 | Day 5 | Ratio (day 5/day 1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| tmax, h | Cmax, ng/mL | AUC0–24, h•ng/mL | tmax, h | Cmax, ng/mL | AUC0–24, h•ng/mL | t1/2, h* | Cmax, ng/mL | AUC0–24, h•ng/mL | ||
| 120 | N | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Mean (SD)† | 1.05 (1.00–2.03) | 19,100 (4970) | 86,200 (27,700) | 1.03 (1.00–1.18) | 18,000 (5530) | 89,600 (30,000) | 23.0 (26.0) | 0.944 (0.102) | 1.04 (0.10) | |
| CV% | 33.6 | 26.1 | 32.1 | 6.7 | 30.7 | 33.4 | 113 | 10.8 | 9.9 | |
| 200 | N | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| Mean (SD)† | 1.12 (1.00–1.98) | 27,000 (4070) | 126,000 (38,400) | 1.03 (1.00–2.08) | 32,400 (13,400) | 164,000 (40,700) | 20.8 (9.85) | 1.25 (0.724) | 1.39 (0.46) | |
| CV% | 28.7 | 15.1 | 30.5 | 33.4 | 41.4 | 24.7 | 47.5 | 57.7 | 33.1 | |
| 250 | N | 8 | 8 | 8 | 6 | 6 | 6 | 6 | 6 | 6 |
| Mean (SD)† | 1.08 (1.02–1.92) | 76,500 (84,400) | 241,000 (85,400) | 1.05 (1.00–2.00) | 43,500 (12,500) | 258,000 (79,500) | 50.0 (28.4) | 0.832 (0.368) | 1.14 (0.37) | |
| CV% | 26.2 | 110 | 35.4 | 36.1 | 28.8 | 30.9 | 56.9 | 44.3 | 32.0 | |
| 300 | N | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Mean (SD)† | 2.00 (1.03–2.08) | 66,700 (46,900) | 304,000 (89,500) | 1.98 (1.02–2.00) | 41,700 (7910) | 335,000 (93,100) | 60.3 (49.8) | 0.762 (0.252) | 1.1 (0.14) | |
| CV% | 33.1 | 70.4 | 29.4 | 28.5 | 19.0 | 27.8 | 82.5 | 33.1 | 12.3 | |
AUC0–24, 24-h area under the plasma concentration-time curve; Cmax, maximum concentration; CV, coefficient of variation; tmax, time to maximum concentration
*t½ (terminal half-life) cannot be determined for day 1 due to a limited sampling schedule
†Median (min-max) is given for tmax
Table 5.
Pharmacokinetic parameters for the active principle (idasanutlin)
| Dose, mg | Day 1 | Day 5 | Ratio (day 5/day 1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| tmax, h | Cmax, ng/mL | AUC0–24, h•ng/mL | tmax, h | Cmax, ng/mL | AUC0–24, h•ng/mL | t1/2, h* | Cmax, ng/mL | AUC0–24, h•ng/mL | ||
| 120 | N | 6 | 6 | 1 | 6 | 6 | 6 | 4 | 6 | 6 |
| Mean (SD)† | 6.24 (5.92–8.08) | 2840 (545) | 55,000 | 4.48 (3.02–8.00) | 4920 (1770) | 95,300 (37,100) | 29.3 (11.3) | 1.73 (0.50) | 1.76 (0.55) | |
| CV% | 12.4 | 19.2 | – | 46.7 | 36.0 | 38.9 | 38.4 | 29.0 | 31.1 | |
| 200 | N | 7 | 7 | 3 | 7 | 7 | 7 | 6 | 7 | 7 |
| Mean (SD)† | 5.78 (1.93–7.93) | 3540 (1230) | 62,100 (16,500) | 4.00 (2.98–7.82) | 6850 (3000) | 135,000 (63,100) | 31.2 (9.9) | 1.91 (0.52) | 2.02 (0.39) | |
| CV% | 39.6 | 34.9 | 26.5 | 38.5 | 43.8 | 46.8 | 31.6 | 27.1 | 19.5 | |
| 250 | N | 8 | 8 | 4 | 6 | 6 | 6 | 6 | 6 | 6 |
| Mean (SD)† | 6.77 (3.00–8.60) | 4860 (371) | 93,000 (9930) | 3.08 (2.00–5.95) | 8970 (2510) | 166,000 (41,000) | 26.8 (7.01) | 1.82 (0.42) | 1.79 (0.30) | |
| CV% | 39.4 | 7.7 | 10.7 | 38.3 | 28.0 | 24.7 | 26.6 | 23.1 | 16.8 | |
| 300 | N | 5 | 5 | 2 | 5 | 5 | 5 | 5 | 5 | 5 |
| Mean (SD)† | 6.08 (4.00–7.92) | 6120 (1030) | 101,000 (7910) | 5.92 (1.98–6.02) | 11,500 (3610) | 209,000 (64,300) | 34.3 (7.7) | 1.86 (0.33) | 1.83 (0.26) | |
| CV% | 24.9 | 16.8 | 7.86 | 42.5 | 31.4 | 30.8 | 22.5 | 17.9 | 14.4 | |
AUC0–24, 24-h area under the plasma concentration-time curve; Cmax, maximum concentration; CV, coefficient of variation; tmax, time to maximum concentration
*t½ (terminal half-life) cannot be determined for day 1 due to a limited sampling schedule
†Median (min-max) is given for tmax
Biomarkers and pharmacodynamics
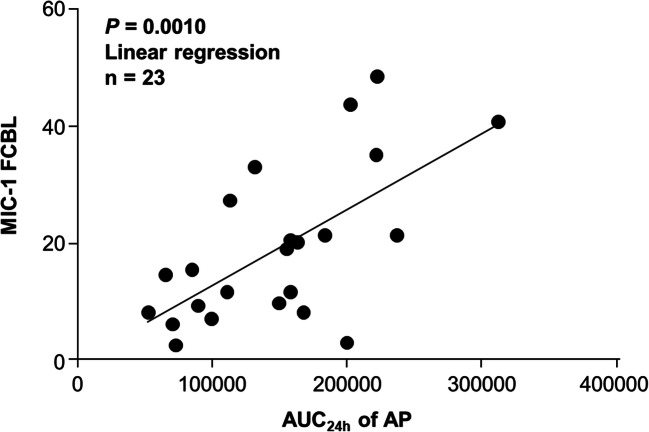
MIC-1, a secretory protein that is strongly upregulated by activated p53, can be detected in the blood of mice bearing human tumor xenografts after treatment with doxorubicin, a genotoxic p53 activator of the MDM2 antagonist nutlin-3. [16] Therefore, MIC-1 could have utility as a progressive disease biomarker for RO6839921. In previous trials that included patients with AML, concentration-related pharmacodynamic biomarker activity of the p53 pathway was demonstrated by increases in MIC-1 levels. [12] In this study, the pharmacodynamic association of change in MIC-1 levels from baseline correlated with steady-state AP exposure (Fig. 2).
Fig. 2.
Pharmacodynamic analyses. Association of MIC-1 (FCBL) levels with AUC24h. AP, active principle; AUC24h, 24-h area under the plasma concentration-time curve; FCBL, fold change from baseline; MIC-1, macrophage inhibitory cytokine 1
Bone marrow samples from 22 patients were evaluated for TP53 status, and 5 (21%) had ≥1 mutation. One patient had >1 mutation detected. The 2 patients who had a best response of CR/CRi or MLFS did not have TP53 mutations; however, 1 patient who had HI/SD as a first response had a mutation.
Efficacy
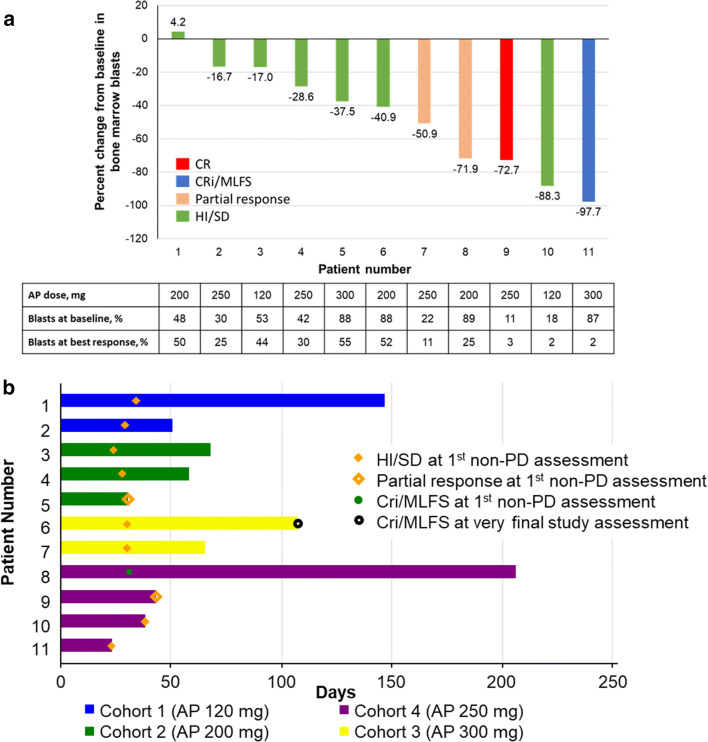
The composite response rate (CRc; CR, CRp, or CRi/MLFS) was 7.7% (2 patients): 1 patient each in the 250-mg (CR) and 300-mg (CRi/MLFS) cohorts (Table 6). Two patients (7.7%) achieved a partial response: 1 patient each in the 200- and 250-mg cohorts. Seven patients (26.9%) had HI/SD. The disease control rate (CRc, partial response, or HI/SD) was 42% (11 of 26 patients). Five patients were not evaluated: 4 due to AEs and 1 due to physician decision to administer hydroxyurea off protocol because of increasing white count. Of patients who demonstrated antileukemic activity (CR, CRi/MLFS, partial response, or HI/SD), the best change in bone marrow blasts from baseline was varied (Fig. 3a). The median overall duration of antileukemic activity (CR, CRp, CRi/MLFS, partial response, or HI/SD) was 58 days (range, 23–206 days; Fig. 3b).
Table 6.
Best overall responses
| Response, n (%) | 120 mg AP (n = 6) |
200 mg AP (n = 7) |
250 mg AP (n = 8) |
300 mg AP (n = 5) |
Total (N = 26) |
|---|---|---|---|---|---|
| CR | 0 | 0 | 1 (12.5) | 0 | 1 (3.8) |
| CRp | 0 | 0 | 0 | 0 | 0 |
| CRi/MLFS | 0 | 0 | 0 | 1 (20.0) | 1 (3.8) |
| Partial response | 0 | 1 (14.3) | 1 (12.5) | 0 | 2 (7.7) |
| HI/SD | 2 (33.3) | 2 (28.6) | 2 (25.0) | 1 (20.0) | 7 (26.9) |
| PD | 4 (66.7) | 2 (28.6) | 2 (25.0) | 2 (40.0) | 10 (38.5) |
| Not evaluable/missing | 0 | 2 (28.6) | 2 (25.0) | 1 (20.0) | 5 (19.2) |
CR complete remission, CRi complete remission with incomplete recovery of peripheral counts, CRp complete remission without platelet recovery, HI hematologic improvement, MLFS morphological leukemia-free state, PD progressive disease, SD stable disease
Fig. 3.
Antileukemic activity. a Percent change from baseline in bone marrow blasts at best response in patients with antileukemic activity (CR, CRi/MLFS, PR, or HI/SD). b Response duration from treatment start in patients with antileukemic activity (CR, CRi/MLFS, partial response, or HI/SD). Patients without a symbol at the final study assessment experienced relapse. Initial response for patient 6 in part B was CRi/MLFS; best response was CR. AP, active principle; CR, complete remission; CRi, complete remission with incomplete recovery of peripheral counts; HI, hematologic improvement; MLFS, morphological leukemia-free state; PD, progressive disease; SD, stable disease
Discussion
RO6839921 is a potent pegylated prodrug and selective new-generation antagonist of the p53-MDM2 interaction for IV administration. RO6839921 is metabolized to idasanutlin, which then binds selectively to the p53 site on the surface of the MDM2 molecule in vitro with high affinity and can effectively displace p53 from MDM2, leading to stabilization and accumulation of the p53 protein and activation of the p53 pathway. [13] In this phase 1 study in patients with AML, RO6839921 demonstrated a pharmacokinetic, pharmacodynamic, and safety profile similar to that of idasanutlin, with evidence of antileukemic activity.
RO6839921 was developed to decrease variability in exposure observed with idasanutlin and allow expansion into indications such as pediatrics or cases where patients cannot swallow or absorb the oral compound. Pediatric oral formulations of idasanutlin are in development for clinical testing (NCT04029688). The prodrug RO6839921 was cleaved rapidly to release the AP in a dose-proportional manner and demonstrated significantly improved interpatient variability compared with oral idasanutlin in solid tumors (27% vs 46%; P = 0.01). [17] In this study, RO6839921 also significantly improved interpatient variability of the AP compared with historical values for oral idasanutlin (33% vs 48%; P = 0.01), although the difference was less pronounced than in solid tumors. [18] In addition, evidence of p53 activation by RO6839921 as measured by the increase in levels of the marker MIC-1 was associated with AP exposure. [9, 16]
RO6839921 was generally well tolerated, and its safety profile was consistent with that of oral idasanutlin. [8] The most common treatment-related AEs with RO6839921 were diarrhea, nausea, vomiting, decreased appetite, and fatigue. DLTs noted at doses of 300 and 250 mg included QT interval prolongation, colitis, stomatitis, and diarrhea. By protocol definition, the MTD was 200 mg, with 0 of 7 patients having a DLT. However, in this population of patients with AML, a dose of up to 250 mg could be considered tolerable since 2 of 8 patients (25%) experienced DLT events of diarrhea and stomatitis.
Antileukemic activity (CR, CRi/MLFS, partial response, or HI/SD) was observed with RO6839921 in 11 of 26 patients, for a disease control rate of 42% and CRc rate of 8%. Other compounds in the nutlin family have also showed efficacy in patients with AML (manuscript submitted, Lancet Haematol August 2019). RG7112 resulted in complete remissions in a phase 1 study in patients with relapsed/refractory AML. [12] Idasanutlin has also demonstrated significant clinical activity in a phase 1 study, with some CR durations lasting >12 months in patients with relapsed/refractory AML [8]; based on these results, a phase 3 study (MIRROS; NCT02545283) is ongoing in patients with relapsed or refractory AML treated with idasanutlin in combination with cytarabine vs placebo + cytarabine. [19]
Overall, this phase 1 study showed that a soluble form of an MDM2 antagonist in the form of a pegylated prodrug to oral idasanutlin could be administered IV to patients with AML. The rapid cleavage of the prodrug RO6839921 to the AP (idasanutlin) accounts for the similar safety profile. Although RO6839921 demonstrated moderately improved variability compared with idasanutlin in patients with AML, [8] the data from this study, including those from the solid tumor arm [18], did not provide sufficient differentiation or improvement in the biologic or safety profile compared with oral idasanutlin to support continued development of the IV prodrug RO6839921.
Acknowledgments
We thank the participants of the study and their families. Special thanks to Steven Middleton, Gwen Nichols, William Pierceall, Jianguo Zhi, Lori Jukofsky, Patricia Delora, David Stephen Ward, and Monica Reckner, and in memory of Zoran Filipovic.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, the data and accuracy of the data analysis, and have given their approval for this version to be published. All authors had full access to all of the data in this study. All authors contributed substantially to the study design, the data analysis and interpretation, and the writing of the manuscript.
Funding
This study was funded by F. Hoffmann-La Roche Ltd. The sponsor was responsible for the clinical operations oversight, data management, medical monitoring, drug supply, statistical analysis, drug safety process, medical writing, and journal article processing charges. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Qualified researchers may request access to individual patient level data through the ClinicalStudyDataRequest.com platform (www.clinicalstudydatarequest.com). Further details on Roche’s criteria for eligible studies are available here (https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx). For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Compliance with ethical standards
Conflict of interest
GLU has no conflicts of interest. SA received consultancy fees from Pfizer, Roche, Novartis, Janssen and AbbVie. A-MY, SB, BH and L-CC were employed by Roche at the time of the study. KY received research funding from Astex, F. Hoffmann-LaRoche, MedImmune, Merck, Millennium and Roche/Genentech; honoraria from Novartis and Pfizer; and was a member of the Board of Directors or advisory committees for Astellas, Celgene, Novartis, Pfizer and Takeda.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Support for third-party writing assistance for this manuscript, furnished by writer Denise Kenski, PhD, CMPP, of Health Interactions, Inc, was provided by F. Hoffmann-La Roche Ltd. and Genentech, Inc.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vassilev LT. MDM2 inhibitors for cancer therapy. Trends Mol Med. 2007;13(1):23–31. doi: 10.1016/j.molmed.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Ozaki T, Nakagawara A. Role of p53 in cell death and human cancers. Cancers (Basel) 2011;3(1):994–1013. doi: 10.3390/cancers3010994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nag S, Qin J, Srivenugopal KS, Wang M, Zhang R. The MDM2-p53 pathway revisited. J Biomed Res. 2013;27(4):254–271. doi: 10.7555/JBR.27.20130030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research Network, Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A, Hoadley K, Triche TJ, Laird PW, Baty JD, Fulton LL, Fulton R, Heath SE, Kalicki-Veizer J, Kandoth C, Klco JM, Koboldt DC, Kanchi KL, Kulkarni S, Lamprecht TL, Larson DE, Lin L, Lu C, MD ML, JF MM, Payton J, Schmidt H, Spencer DH, Tomasson MH, Wallis JW, Wartman LD, Watson MA, Welch J, Wendl MC, Ally A, Balasundaram M, Birol I, Butterfield Y, Chiu R, Chu A, Chuah E, Chun HJ, Corbett R, Dhalla N, Guin R, He A, Hirst C, Hirst M, Holt RA, Jones S, Karsan A, Lee D, Li HI, Marra MA, Mayo M, Moore RA, Mungall K, Parker J, Pleasance E, Plettner P, Schein J, Stoll D, Swanson L, Tam A, Thiessen N, Varhol R, Wye N, Zhao Y, Gabriel S, Getz G, Sougnez C, Zou L, Leiserson MD, Vandin F, Wu HT, Applebaum F, Baylin SB, Akbani R, Broom BM, Chen K, Motter TC, Nguyen K, Weinstein JN, Zhang N, Ferguson ML, Adams C, Black A, Bowen J, Gastier-Foster J, Grossman T, Lichtenberg T, Wise L, Davidsen T, Demchok JA, Shaw KR, Sheth M, Sofia HJ, Yang L, Downing JR, Eley G (2013) Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 368(22):2059–2074 [DOI] [PMC free article] [PubMed]
- 5.Quintás-Cardama A, Hu C, Qutub A, Qiu YH, Zhang X, Post SM, Zhang N, Coombes K, Kornblau SM. p53 pathway dysfunction is highly prevalent in acute myeloid leukemia independent of TP53 mutational status. Leukemia. 2017;31(6):1296–1305. doi: 10.1038/leu.2016.350. [DOI] [PubMed] [Google Scholar]
- 6.Ding Q, Zhang Z, Liu JJ, Jiang N, Zhang J, Ross TM, Chu XJ, Bartkovitz D, Podlaski F, Janson C, Tovar C, Filipovic ZM, Higgins B, Glenn K, Packman K, Vassilev LT, Graves B. Discovery of RG7388, a potent and selective p53-MDM2 inhibitor in clinical development. J Med Chem. 2013;56(14):5979–5983. doi: 10.1021/jm400487c. [DOI] [PubMed] [Google Scholar]
- 7.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303(5659):844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 8.Yee K, Martinelli G, Vey N, Dickinson MJ, Seiter K, Assouline S, Drummond M, Yoon S-S, Kasner M, Lee J-H, Kelly KR, Blotner S, Higgins B, Middleton S, Nichols G, Chen G, Zhong H, Pierceall WE, Zhi J, Chen L-C. Phase 1/1b study of RG7388, a potent MDM2 antagonist, in acute Myelogenous leukemia (AML) patients (Pts) (abstract) Blood. 2014;124(21):116. doi: 10.1182/blood.V124.21.116.116. [DOI] [Google Scholar]
- 9.Siu LL, Italiano AI, Miller WH, Blay J-Y, Gietema JA, Bang Y-J, Mileshkin LR, Hirte HW, Reckner M, Higgins B, Jukofsky L, Blotner S, Zhi J, Middleton S, Nichols GL, Chen LC (2014) Phase 1 dose escalation, food effect, and biomarker study of RG7388, a more potent second-generation MDM2 antagonist, in patients (pts) with solid tumors (abstract 2535). J Clin Oncol 32(15_suppl):2535
- 10.Zhong H, Chen G, Jukofsky L, Geho D, Han SW, Birzele F, Bader S, Himmelein L, Cai J, Albertyn Z, Rothe M, Essioux L, Burtscher H, Middleton SA, Rueger R, Chen LC, Dangl M, Nichols G, Pierceall WE. MDM2 antagonist clinical response association with a gene expression signature in acute myeloid leukaemia. Br J Haematol. 2015;171(3):432–435. doi: 10.1111/bjh.13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reis B, Jukofsky L, Chen G, Martinelli G, Zhong H, So WV, Dickinson MJ, Drummond M, Assouline S, Hashemyan M, Theron M, Blotner S, Lee JH, Kasner M, Yoon SS, Rueger R, Seiter K, Middleton SA, Kelly KR, Vey N, Yee K, Nichols G, Chen LC, Pierceall WE. Acute myeloid leukemia patients' clinical response to idasanutlin (RG7388) is associated with pre-treatment MDM2 protein expression in leukemic blasts. Haematologica. 2016;101(5):e185–e188. doi: 10.3324/haematol.2015.139717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andreeff M, Kelly KR, Yee K, Assouline S, Strair R, Popplewell L, Bowen D, Martinelli G, Drummond MW, Vyas P, Kirschbaum M, Iyer SP, Ruvolo V, González GM, Huang X, Chen G, Graves B, Blotner S, Bridge P, Jukofsky L, Middleton S, Reckner M, Rueger R, Zhi J, Nichols G, Kojima K. Results of the phase I trial of RG7112, a small-molecule MDM2 antagonist in leukemia. Clin Cancer Res. 2016;22(4):868–876. doi: 10.1158/1078-0432.CCR-15-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins B, Tovar C, Glen K, Railkar A, Filipovic Z, Qureshi F, Vu B, Ehrlich G, Fishlock D, Chen L-C, Middleton S, Nichols G, Packman K, Vassilev L. Preclinical activity of MDM2 antagonist RO6839921, a pegylated prodrug for intravenous administration (abstract A156) Mol Cancer Ther. 2014;14(12 Supplement 2):A156. [Google Scholar]
- 14.Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz MA, Sierra J, Tallman MS, Löwenberg B, Bloomfield CD, LeukemiaNet E. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 15.Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC. Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol. 2008;26(2):190–195. doi: 10.1200/JCO.2007.12.7712. [DOI] [PubMed] [Google Scholar]
- 16.Yang H, Filipovic Z, Brown D, Breit SN, Vassilev LT. Macrophage inhibitory cytokine-1: a novel biomarker for p53 pathway activation. Mol Cancer Ther. 2003;2(10):1023–1029. [PubMed] [Google Scholar]
- 17.Razak A, Gore L, Britten CD, Miller WH, Uy GL, Nichols G, Middleton S, Blotner S, Zhi J, Jukofsky L, Pierceall W, Higgins B, Chen LC (2016) A phase I study of the MDM2 antagonist RO6839921, a pegylated prodrug of idasanutlin, for intravenous (IV) administration in patients with advanced solid tumors (abstract). Eur J Cancer 69:S21–S22
- 18.Abdul Razak AR, Miller WH, Uy GL, Blotner S, Young AM, Higgins B, Chen LC, Gore L (2019) A phase 1 study of the MDM2 antagonist RO6839921, a pegylated prodrug of idasanutlin, in patients with advanced solid tumors. Invest New Drugs. [Epub ahead of print] doi: 10.1007/s10637-019-00869-2 [DOI] [PubMed]
- 19.Montesinos P, Esteve J, Konopleva M, Martinelli G, Ottmann O, Rodriguez-Veiga R, Röllig C, Wei A, Vey N, Chiu I, Monnet A, Ott MG, Fenaux P (2019) MIRROS: An ongoing randomized phase 3 trial of idasanutlin + ARA-C in patients with relapsed or refractory acute myeloid leukemia (abstract). J Clin Oncol 37(15_suppl):TPS7063-TPS7063
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Qualified researchers may request access to individual patient level data through the ClinicalStudyDataRequest.com platform (www.clinicalstudydatarequest.com). Further details on Roche’s criteria for eligible studies are available here (https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx). For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).