Summary
Introduction TAS-114 is a potent inhibitor of deoxyuridine triphosphatase, which is a gatekeeper protein preventing uracil and 5-fluorouracil (5-FU) misincorporation into DNA. TAS-114 has been suggested to enhance the antitumor activity of 5-FU. This randomized, phase 2 study investigated TAS-114 plus S-1 (TAS-114/S-1) vs. S-1 in non-small-cell lung cancer (NSCLC) patients. Methods Patients with advanced NSCLC, previously treated with ≥ 2 regimens, were randomized 1:1 to receive TAS-114 (400 mg)/S-1 (30 mg/m2) or S-1 (30 mg/m2). Progression-free survival (PFS, independent central review) was the primary endpoint. Secondary endpoints included disease control rate (DCR), overall survival (OS), overall response rate (ORR), and safety. Results In total, 127 patients received treatment. Median PFS was 3.65 and 4.17 months in the TAS-114/S-1 and S-1 groups, respectively (hazard ratio [HR] 1.16, 95% confidence interval [CI] 0.71–1.88; P = 0.2744). DCR was similar between groups (TAS-114/S-1 80.3%, S-1 75.9%) and median OS was 7.92 and 9.82 months for the TAS-114/S-1 and S-1 groups, respectively (HR 1.31, 95% CI 0.80–2.14; P = 0.1431). The ORR was higher in the TAS-114/S-1 group than the S-1 group (19.7% vs. 10.3%), and more patients with tumor shrinkage were observed in the TAS-114/S-1 group. Incidence rates of anemia, skin toxicities, and Grade ≥ 3 treatment-related adverse events were higher in the TAS-114/S-1 group compared with the monotherapy group. Conclusions Although the TAS-114/S-1 combination improved the response rate, this did not translate into improvements in PFS. Clinical Trial Registration No. NCT02855125 (ClinicalTrials.gov) registered on 4 August 2016.
Electronic supplementary material
The online version of this article (10.1007/s10637-020-00930-5) contains supplementary material, which is available to authorized users.
Keywords: dUTPase, Non-small-cell lung cancer, 5-fluorouracil, Progression-free survival
Introduction
Non-small-cell lung cancer (NSCLC) is the leading cause of cancer incidence and cancer-related mortality worldwide [1]. Recently, the development of immunotherapy and its combination with cytotoxic chemotherapies has garnered new options for therapeutics in advanced NSCLC [2]. However, despite recent advances, most patients still progress after treatment, and cytotoxic chemotherapies remain essential in NSCLC treatment algorithms [3, 4]. Thus, highly efficacious, well-tolerated chemotherapies are still warranted.
S-1 is an oral 5-fluorouracil (5-FU) derivative that is widely used for the treatment of various solid tumors, including NSCLC [5]. Recently, a phase 3 study of S-1 monotherapy demonstrated non-inferiority compared with docetaxel in patients with NSCLC; thus, S-1 is considered a standard chemotherapy option in East Asia [6].
TAS-114 is a potent inhibitor of deoxyuridine triphosphatase (dUTPase), which is a gatekeeper protein that prevents uracil and 5-FU misincorporation into DNA. TAS-114 inhibits the conversion of deoxyuridine triphosphate (dUTP) and 5-fluoro-dUTP (FdUTP) into their monophosphate forms. TAS-114 itself does not have antitumor activity; however, when it is combined with 5-FU derivatives, it can increase the amount of FdUTP in the tumor for selective incorporation into DNA, thus enhancing the antitumor effect [7, 8].
Two previous phase 1 studies of TAS-114 combined with S-1, conducted separately in Europe and Japan, concluded that the recommended dose was TAS-114 240 mg/m2 plus S-1 30 mg/m2. The combination of TAS-114 and S-1 demonstrated promising antitumor activity, with a manageable safety profile in heavily pretreated solid tumors. The overall response rate (ORR), including unconfirmed partial response (PR), in NSCLC was approximately 30% [9, 10]. Based on these results, we conducted a randomized phase 2 study to investigate the combination of TAS-114 with S-1 vs. S-1 monotherapy in patients with advanced NSCLC.
Materials and methods
Study design
This was an open-label, multicenter, international, randomized phase 2 study conducted at 28 sites across five countries (Japan, France, Spain, Italy, and the United States). Eligible patients were enrolled by the investigators and randomly assigned to TAS-114 plus S-1 (TAS-114/S-1) or S-1 alone in a 1:1 ratio. The random allocation sequence was generated, and treatment assignment performed centrally using a dynamic allocation method (biased coin) via an interactive voice/web response system. Patients were stratified by histological subtype (squamous vs. non-squamous) and region (Europe/United States vs. Japan).
The study was approved by independent ethics committees or institutional review boards at each site. The study conduct was in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki. All patients provided written informed consent before enrollment (ClinicalTrials.gov identifier NCT02855125).
Patients
Key eligibility criteria were age ≥ 18 years (≥ 20 years in Japan), histologically confirmed advanced or metastatic NSCLC; prior treatment with at least two systemic therapies (one of which must be platinum doublet and either pemetrexed, docetaxel, or immunotherapy) for advanced or metastatic NSCLC; Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1; adequate bone marrow function (absolute neutrophil count ≥ 1500/mm3, hemoglobin ≥ 10.0 g/dL, platelets ≥ 100,000/mm3); adequate liver function (total bilirubin ≤ 1.5 × upper limit of normal [ULN], aspartate aminotransferase/alanine aminotransferase ≤ 3 × ULN [in patients with existent liver metastases, ≤ 5 × ULN]); and adequate renal function (calculated creatinine clearance ≥ 50 mL/min [Cockcroft-Gault]). All eligibility criteria are listed in Online Resource Text S1.
Treatment
TAS-114 400 mg and/or S-1 30 mg/m2 were concurrently administered orally twice daily under fasting conditions in a schedule consisting of 14 consecutive days of treatment followed by a 7-day rest. TAS-114 dosing was switched from body surface area (BSA)-based to a flat-fixed dose to avoid the risk of dosing errors. TAS-114 400 mg was equivalent to 240 mg/m2, which is the recommended dose of TAS-114 for an overall mean BSA of 1.69 m2 based on the results of the two phase 1 studies [9, 10]. In these studies, there was no correlation between the oral clearance of TAS-114 and BSA. Treatment was repeated every 3 weeks until disease progression, intolerable toxicities, or withdrawal of consent. No crossover was permitted.
Endpoints and study assessments
The primary endpoint was progression-free survival (PFS) as determined by independent central review (ICR). Secondary endpoints were ORR, disease control rate (DCR), overall survival (OS), and safety. PFS was defined as the time from randomization to the date of first radiologic disease progression or death, whichever occurred first. ORR was defined as the proportion of patients with complete response (CR) or PR. DCR was defined as the proportion of patients with CR, PR, or stable disease. OS was defined as the time from randomization to death. Tumor shrinkage for each patient and its correlation with PFS by ICR was assessed as an exploratory analysis.
Tumor assessments were performed every 6 weeks from the first study drug administration until disease progression according to the RECIST version 1.1. Assessment results were evaluated by independent central radiologists (Medpace Imaging Core Lab, Ohio, USA) and by the investigators at each site.
Adverse events (AEs) were reported using the verbatim term, and the severity was graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. AEs were coded by preferred term according to the Medical Dictionary for Regulatory Activities (MedDRA®: trademark is registered by the International Federation of Pharmaceutical Manufacturers & Associations on behalf of the International Council for Harmonisation) version 19.0.
Statistical analysis
The sample size was estimated based on PFS. A total sample size of 124 patients was required to provide 60 events (progressive disease or death) by ICR for the primary analysis, which corresponds to 71 events based on investigator review under the assumption that the discrepancy in progressive disease events between ICR and investigator review is 15%. Based on the results of previous clinical trials [9–13], the median PFS in the S-1 group was estimated to be 2.2 months, and 4.2 months in the TAS-114/S-1 group, corresponding to a hazard ratio (HR) of 0.524, with 80% statistical power and a one-sided significance level of 0.05.
The PFS and OS analyses were conducted on an intent-to-treat (ITT) population, of all patients randomized in the study, regardless of whether they received any study treatment or not. The ORR was analyzed based on the tumor response-evaluable population, defined as all patients in the ITT population with measurable disease (at least one target lesion) at baseline and with at least one tumor evaluation. We performed a sensitivity analysis in which PFS, ORR, and DCR were also analyzed based on the investigator’s assessment. The safety analysis was conducted on an as-treated population, defined as all patients who received at least one dose of the study drug.
PFS and OS were estimated using the Kaplan–Meier method; HRs were estimated using a Cox proportional hazards model stratified by geographical region and histological subtypes. Medians (with 95% confidence intervals [CIs]) were calculated using the Brookmeyer-Crowley method. For between-group comparisons, the stratified log-rank test with a one-sided significance level of 5% was used for PFS and OS, and Fisher’s exact test for ORR and DCR. All statistical analyses were performed using SAS software version 9.4 in the UNIX environment (SAS Institute Inc., North Carolina, USA).
Results
Patients
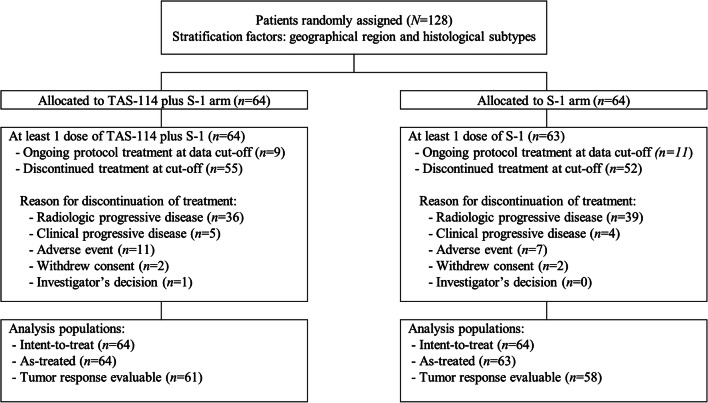
From August 2016 to July 2017, a total of 128 patients were randomly assigned to treatment: 64 patients each were assigned to the TAS-114/S-1 and S-1 groups (Fig. 1). A total of 127 patients received at least one study treatment; one patient in the S-1 group was found ineligible, and thus, discontinued before the initial study drug administration. Twenty patients were receiving treatment by the cut-off date for primary analysis (30 September 2017). Baseline characteristics were generally balanced in the two groups, except for sex, ECOG PS, and epidermal growth factor receptor (EGFR) mutation status (Table 1). More than half of the patients had received a prior systemic regimen as fourth-line or further therapy. Over 70% of patients had received prior programmed death 1/programmed death-ligand 1 antibodies.
Fig. 1.
CONSORT flow diagram
Table 1.
Baseline demographic characteristics (intent-to-treat population)
| Characteristic | TAS-114/S-1 (n = 64) |
S-1 (n = 64) |
|---|---|---|
| Age (years), median | 65.5 | 64.0 |
| Age group, n (%) | ||
| < 65 years | 28 (43.8) | 33 (51.6) |
| ≥ 65 years | 36 (56.3) | 31 (48.4) |
| Sex, n (%) | ||
| Male | 41 (64.1) | 48 (75.0) |
| Female | 23 (35.9) | 16 (25.0) |
| Region, n (%) | ||
| Western | 34 (53.1) | 34 (53.1) |
| Asian | 30 (46.9) | 30 (46.9) |
| ECOG PS, n (%) | ||
| 0 | 12 (18.8) | 17 (26.6) |
| 1 | 52 (81.3) | 47 (73.4) |
| Histology subtype, n (%) | ||
| Squamous cell carcinoma | 14 (21.9) | 14 (21.9) |
| Adenocarcinoma | 48 (75.0) | 48 (75.0) |
| Large cell carcinoma | 0 | 1 (1.6) |
| Carcinoid tumor | 1 (1.6) | 0 |
| Other | 1 (1.6) | 1 (1.6) |
| EGFR mutation status, n (%) | ||
| Positive | 7 (10.9) | 12 (18.8) |
| Negative | 46 (71.9) | 38 (59.4) |
| Unknown | 11 (17.2) | 14 (21.9) |
| ALK translocation status, n (%) | ||
| Positive | 2 (3.1) | 0 |
| Negative | 44 (68.8) | 43 (67.2) |
| Unknown | 18 (28.1) | 21 (32.8) |
| Brain metastases, n (%) | 6 (9.4) | 7 (11.0) |
| Number of prior systemic regimens, n (%) | ||
| 1 | 0 | 0 |
| 2 | 24 (37.5) | 19 (29.7) |
| 3 | 19 (29.7) | 17 (26.6) |
| 4 | 14 (21.9) | 15 (23.4) |
| ≥ 5 | 7 (10.9) | 13 (20.3) |
| Main prior systemic anti-cancer agent, n (%) | ||
| Platinum | 64 (100) | 64 (100) |
| PD-1/PD-L1 antibodies | 46 (71.9) | 49 (76.6) |
| Pemetrexed | 48 (75.0) | 47 (73.4) |
| Docetaxel | 33 (51.6) | 38 (59.4) |
| EGFR-TKI | 7 (11.0) | 11 (17.2) |
| ALK-TKI | 2 (3.1) | 0 |
ALK, anaplastic lymphoma kinase; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; PD-1, programmed death 1; PD-L1, programmed death ligand 1; TKI, tyrosine kinase inhibitor
Treatment
In the TAS-114/S-1 and S-1 groups, patients received medians of 4.0 (range, 1–14) and 3.0 (range, 1–12) treatment cycles, respectively. In the TAS-114/S-1 group, the medians for relative dose intensity (RDI) of TAS-114 and S-1 were 85.9% and 84.8%, respectively. In the S-1 group, the median RDI was 95.5%. By the data cut-off date, 107 patients had discontinued study treatment. In both groups, the main reason for discontinuation was disease progression. After study discontinuation, 54.5% and 65.4% of patients in the TAS-114/S-1 and S-1 groups, respectively, received other anticancer treatments. The median duration for survival follow-up was 9.03 months.
Efficacy
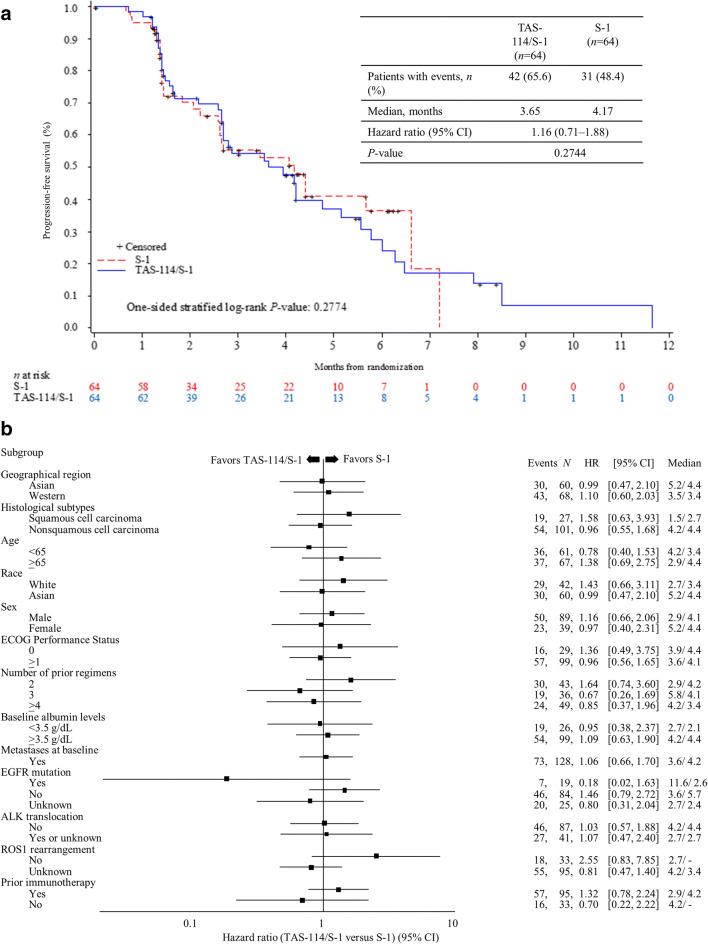
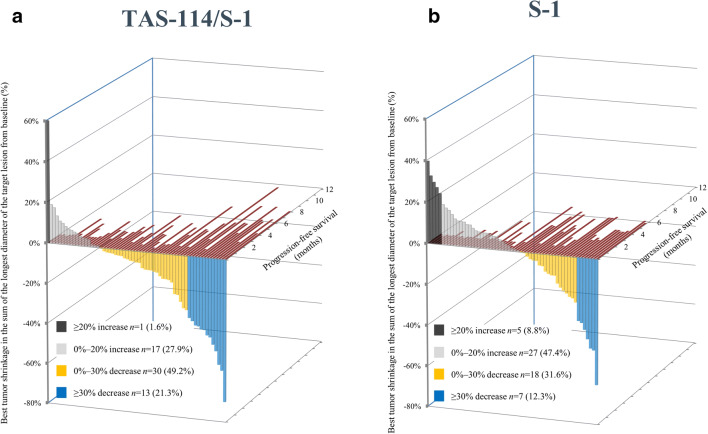
At the data cut-off date, 73 PFS events were observed by ICR. The median PFS was 3.65 months in the TAS-114/S-1 group and 4.17 months in the S-1 group (HR 1.16, 95% CI 0.71–1.88; P = 0.2744) (Fig. 2a). In the subgroup analyses, the data were generally similar to that of the overall population (Fig. 2b). The DCR was similar between TAS-114/S-1 and S-1 groups (80.3% vs. 75.9%), whereas patients in the TAS-114/S-1 group showed higher ORR than those in the S-1 group (19.7% vs. 10.3%) (Table 2). Additionally, Fig. 3a and b shows the relationship between tumor shrinkage and PFS by RECIST for each patient. Investigator-assessed results were 3.48 months vs. 2.66 months for PFS (HR 0.79, 95% CI 0.52–1.21; P = 0.1352), 78.7% vs. 59.3% for DCR, and 19.7% vs. 10.2% for ORR (Table 2; Online Resource Figures S1 and S2).
Fig. 2.
(a) Progression-free survival by ICR (intent-to-treat population), (b) forest plot of hazard ratios for treatment effect on progression-free survival (ICR). ALK, anaplastic lymphoma kinase; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; HR, hazard ratio; ICR, independent central review; ROS1, ROS proto-oncogene 1, receptor tyrosine kinase
Table 2.
Overall response rate and disease control rate by ICR and INV (tumor response evaluable population)
| ICR | INV | |||
|---|---|---|---|---|
| TAS-114/S-1 (n = 61) |
S-1 (n = 58) |
TAS-114/S-1 (n = 61) |
S-1 (n = 59) |
|
| Overall response rate, % (95% CI) |
19.7 (10.6–31.8) |
10.3 (4.0–21.5) |
19.7 (10.6–31.8) |
10.2 (3.9–21.2) |
| CR | 1 (1.6) | 0 | 0 | 0 |
| PR | 11 (18.0) | 6 (10.3) | 12 (19.7) | 6 (10.2) |
| SD | 37 (60.7) | 38 (65.5) | 36 (59.0) | 29 (49.2) |
| PD | 12 (19.7) | 13 (22.4) | 13 (21.3) | 23 (39.0) |
| Disease control rate (CR + PR + SD), % (95% CI) |
80.3 (68.2–89.4) |
75.9 (64.2–87.3) |
78.7 (66.3–88.1) |
59.3 (46.6–73.0) |
CI, confidence interval; CR, complete response; ICR, independent central review; INV, Investigator review; PR, partial response; PD, progressive disease; SD, stable disease
Fig. 3.
Individual tumor shrinkage and progression-free survival (independent central review) in the (a) TAS-114/S-1 group and (b) S-1 group
The data cut-off for OS was 30 November 2017, at which time 65 events were observed. The median OS was 7.92 in the TAS-114/S-1 group and 9.82 months in the S-1 group (HR 1.31, 95% CI 0.80–2.14; P = 0.1431) (Online Resource Figures S3 and S4).
Safety
Treatment-related AEs (TRAEs) occurring in ≥ 10% of patients are shown in Table 3. The incidence rates of anemia, skin toxicities, and Grade ≥ 3 TRAEs were higher in the TAS-114/S-1 group compared with the monotherapy group. Serious TRAEs were reported in 17.2% of patients in the TAS-114/S-1 group, and in 6.7% in the S-1 group. In the TAS-114/S-1 group, two patients each reported treatment-related serious anemia (3.1%), diarrhea (3.1%), and maculo-papular rash (3.1%) of any grade. More patients in the TAS-114/S-1 group had dose reductions/interruptions (mainly due to skin toxicities) than patients in the S-1 group (reductions: 31.3% vs. 11.1%; interruptions: 45.3% vs. 14.3%) (Online Resource Table S1). No treatment-related death was reported.
Table 3.
Treatment-related adverse events (as-treated population)
| TAS-114/S-1 (n = 64) |
S-1 (n = 63) |
|||
|---|---|---|---|---|
| All Grades | ≥Grade 3 | All Grades | ≥Grade 3 | |
| Event | n (%) | n (%) | n (%) | n (%) |
| All | 60 (93.8) | 35 (54.7) | 57 (90.5) | 10 (15.9) |
| Hematology | ||||
| Anemia | 37 (57.8) | 14 (21.9) | 10 (15.9) | 0 |
| White blood cell count decreased | 10 (15.6) | 2 (3.1) | 5 (7.9) | 0 |
| Platelet count decreased | 8 (12.5) | 1 (1.6) | 6 (9.5) | 0 |
| Neutrophil count decreased | 6 (9.4) | 2 (3.1) | 7 (11.1) | 1 (1.6) |
| Non-hematology | ||||
| Decreased appetite | 25 (39.1) | 3 (4.7) | 22 (34.9) | 1 (1.6) |
| Nausea | 20 (31.3) | 0 | 20 (31.7) | 1 (1.6) |
| Diarrhea | 18 (28.1) | 2 (3.1) | 12 (19.0) | 1 (1.6) |
| Skin hyperpigmentation | 18 (28.1) | 0 | 11 (17.5) | 0 |
| Maculo-papular rash | 18 (28.1) | 4 (6.3) | 2 (3.2) | 0 |
| Asthenia | 17 (26.6) | 4 (6.3) | 9 (14.3) | 2 (3.2) |
| Rash | 15 (23.4) | 5 (7.8) | 3 (4.8) | 0 |
| Vomiting | 10 (15.6) | 0 | 11 (17.5) | 2 (3.2) |
| Pruritus | 10 (15.6) | 0 | 7 (11.1) | 0 |
| Stomatitis | 9 (14.1) | 1 (1.6) | 4 (6.3) | 0 |
| Malaise | 8 (12.5) | 0 | 5 (7.9) | 0 |
| Fatigue | 6 (9.4) | 1 (1.6) | 9 (14.3) | 1 (1.6) |
| Dry skin | 9 (14.1) | 0 | 5 (7.9) | 0 |
Discussion
The present randomized study is the first to evaluate the effect of a dUTPase inhibitor in combination with a 5-FU derivative. Favorable tumor response and shrinkage trends were observed in the TAS-114/S-1 group; however, they did not translate into an improved PFS. No difference in duration of response was observed, and the early progressive disease (< 3 months) rate was similar across groups. A possible reason is that more patients in the TAS-114/S-1 group experienced dose reductions/interruptions due to AEs, and these results may have affected the primary endpoint result.
According to the investigator review, the median PFS was 3.48 months for the TAS-114/S-1 group and 2.66 months for the S-1 group (95 PFS events). In the S-1 group, a significant difference in the median PFS (1.51-month difference) was observed between the ICR and investigator review, in contrast with the TAS-114/S-1 group (0.17-month difference). This may be a result of treatment bias owing to lack of blinding. In fact, the number of censored cases was higher in the S-1 group by ICR; therefore, the PFS in the S-1 group was presumably influenced by informative censoring [14].
The OS result was immature at the data cut-off, but was lower in the TAS-114/S-1 group (not statistically significant). This difference between groups might have resulted from the percentage and timing of patients receiving anticancer treatment after discontinuation, and some imbalances in patient background characteristics that are known to be prognostic factors, such as EGFR mutation [15, 16]. Based on the efficacy and safety results, the sponsor decided to terminate the TAS-114/S-1 combination, and all five patients remaining in TAS-114 treatment were switched to S-1 alone after the primary analysis.
Although the patient numbers were very limited (7 vs. 12 patients), the subgroup analysis showed that EGFR-mutant patients tended to achieve longer PFS (11.6 months vs. 2.6 months; HR 0.18; 95% CI 0.02–1.63) and OS (not reached vs. 10.6 months; HR 0.12; 95% CI 0.01–0.99) in the TAS-114/S-1 group vs. the S-1 group. It has been reported that advanced NSCLC EGFR-mutant patients present defective DNA-repair functions [17, 18]. In a preclinical model, suppression of DNA repair proteins increased its sensitivity to TAS-114 combined with the 5-FU metabolite 5-fluoro-2’-deoxyuridine [19]. Thus, it is possible that the present favorable effect in patients with EGFR mutations in the TAS-114/S-1 group resulted from increased uracil and 5-FU incorporation into DNA.
The overall safety profile in the TAS-114/S-1 group was consistent with previous phase 1 studies; anemia and skin toxicities were more commonly observed in this group [9, 10]. These toxicities were considered manageable by dose modification and standard symptomatic treatment and were presumed to result from the combination of TAS-114 and S-1. In exploratory analysis, patients with anemia and/or skin toxicities in the TAS-114/S-1 group showed a tendency toward longer PFS compared with patients without these toxicities (Online Resource Text S2). Moreover, anemia was also commonly reported with DNA-damaging agents; it is possible that the frequent anemia reported with TAS-114/S-1 was attributed to increased DNA damage [20, 21].
The present study had several limitations. These include the relatively small sample size that may have prevented the identification of some group differences, the open-label design that may have introduced bias from the investigators, and the background characteristic imbalances that may have affected the primary endpoint results.
Conclusions
Although the TAS-114/S-1 combination did result in a higher response rate and tumor shrinkage in this advanced NSCLC population, it did not improve PFS or OS.
Electronic supplementary material
(PDF 2213 kb)
Acknowledgements
We thank the patients, their families, the investigators (Online Resource Table S2), and sites that participated in this study. The authors would like to thank Keyra Martinez Dunn, MD and Sarah Bubeck, PhD, of Edanz Medical Writing, for providing medical writing support which was funded by Taiho Pharmaceutical Co., Ltd.
Author Contribution
Study concepts: Karim A. Benhadji, Nital Soni, Jinhong Huang, Lukas Makris; Study design: Karim A. Benhadji, Nital Soni, Jinhong Huang, Lukas Makris; Data acquisition: Nobuyuki Yamamoto, Hidetoshi Hayashi, David Planchard, Teresa Morán, Vanesa Gregorc, Jonathan Dowell, Hiroshi Sakai, Kiyotaka Yoh, Makoto Nishio, Alexis B. Cortot, Susana Cedres; Data analysis and interpretation: Nobuyuki Yamamoto, Karim A. Benhadji, Nital Soni, Jinhong Huang, Lukas Makris; Statistical analysis: Lukas Makris; Manuscript preparation: Nobuyuki Yamamoto, Hidetoshi Hayashi, David Planchard, Teresa Morán, Vanesa Gregorc, Jonathan Dowell, Hiroshi Sakai, Kiyotaka Yoh, Makoto Nishio, Alexis B. Cortot, Susana Cedres, Karim A. Benhadji, Nital Soni, Jinhong Huang, Lukas Makris; Manuscript editing: Nobuyuki Yamamoto, Hidetoshi Hayashi, David Planchard, Teresa Morán, Vanesa Gregorc, Jonathan Dowell, Hiroshi Sakai, Kiyotaka Yoh, Makoto Nishio, Alexis B. Cortot, Susana Cedres, Karim A. Benhadji, Nital Soni, Jinhong Huang, Lukas Makris; Manuscript review: Nobuyuki Yamamoto, Hidetoshi Hayashi, David Planchard, Teresa Morán, Vanesa Gregorc, Jonathan Dowell, Hiroshi Sakai, Kiyotaka Yoh, Makoto Nishio, Alexis B. Cortot, Susana Cedres, Karim A. Benhadji, Nital Soni, Jinhong Huang, Lukas Makris.
Funding information
This work was supported by Taiho Oncology, Inc. and Taiho Pharmaceutical Co., Ltd. The funding sources played a role in the study design; collection, analysis, and interpretation of data; writing of the report; and in the decision to submit the article for publication.
Availability of data and material
Anonymized, patient-level, analyzable datasets will not be shared according to the Sponsor policy on data sharing (https://www.taiho.co.jp/en/science/policy/clinical_trial_information_disclosure_policy/index.html).
Compliance with ethical standards
Conflicts of interest/Competing interests
N. Yamamoto has received grants and/or personal fees from Taiho Pharmaceutical. H. Hayashi has received grants and personal fees from AstraZeneca, Boehringer Ingelheim Japan, Bristol-Myers Squibb, Chugai Pharmaceutical, Eli Lilly Japan, MSD, Ono Pharmaceutical, Pfizer Japan, and Taiho Pharmaceutical. D. Planchard has participated in consulting, acted in an advisory role, and received lecture fees from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Merck, Novartis, Pfizer, Roche, prIME Oncology, and Peer CME, and has worked as a principal or co-investigator for clinical trial research for AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, MedImmune, Merck, Novartis, Novocure, Pfizer, Roche, Sanofi-Aventis, and Taiho Pharmaceutical. H. Sakai has received personal fees from Taiho Pharmaceutical. K. Yoh has received grants and/or personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical, Eli Lilly Japan, MSD, Novartis, Ono Pharmaceutical, Pfizer, and Taiho Pharmaceutical. M. Nishio has received grants and/or non-financial support from F. Hoffmann-La Roche and grants and/or personal fees from Astellas, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Chugai Pharmaceutical, Eli Lilly, Merck Serono, MSD, Novartis, Ono Pharmaceutical, Pfizer, Sankyo Healthcare, and Taiho Pharmaceutical. A. Cortot has received grants, personal fees, and/or non-financial support from Astra-Zeneca, Bristol Myers Squibb, Merck, MSD, Novartis, Pfizer, Roche, and Takeda. KA Benhadji and N. Soni are employees of Taiho Oncology Inc. J. Huang is an employee of Taiho Pharmaceutical and owns Taiho Pharmaceutical stock. L. Makris has received personal fees from Taiho Oncology, Inc. S. Cedres has served in a consulting or an advisory role for Bristol-Myers Squibb, F. Hoffmann-La Roche AG, Pfizer, Boehringer Ingelheim, MSD Oncology, and Amphera; and has received travel and accommodation funding from F. Hoffmann-La Roche AG, Pfizer, and Boehringer Ingelheim. T. Morán, V. Gregorc, and J. Dowell have no conflicts of interest to disclose.
Ethics approval
The study was approved by independent ethics committees or institutional review boards at each site.
Consent to participate
All patients provided written informed consent before enrollment.
Consent for publication
All patients provided written informed consent for publication before enrollment.
Code availability
All statistical analyses were performed using SAS software version 9.4 in the UNIX environment (SAS Institute Inc., North Carolina, USA).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nobuyuki Yamamoto, Email: nbyamamo@wakayama-med.ac.jp.
Hidetoshi Hayashi, Email: hidet31@med.kindai.ac.jp.
David Planchard, Email: david.planchard@gustaveroussy.fr.
Teresa Morán, Email: mmoran@iconcologia.net.
Vanesa Gregorc, Email: vanesa.gregorc@hsr.it.
Jonathan Dowell, Email: Jonathan.Dowell@UTSouthwestern.edu.
Hiroshi Sakai, Email: hiroshi_sakai@cancer-c.pref.saitama.jp.
Kiyotaka Yoh, Email: kyoh@east.ncc.go.jp.
Makoto Nishio, Email: mnishio@jfcr.or.jp.
Alexis B. Cortot, Email: alexis.cortot@chru-lille.fr
Karim A. Benhadji, Email: KBenhadji@taihooncology.com
Nital Soni, Email: nsoni@taihooncology.com.
Jinhong Huang, Email: j-huang@taiho.co.jp.
Lukas Makris, Email: lmakris@taihooncology.com.
Susana Cedres, Email: scedres@vhio.net.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Barnet MB, Cooper WA, Boyer MJ, Kao S (2018) Immunotherapy in non-small cell lung cancer: shifting prognostic paradigms. J Clin Med 7(6):151. 10.3390/jcm7060151 [DOI] [PMC free article] [PubMed]
- 3.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Non-small cell lung cancer. Version 2.2018. https://www2.tri-kobe.org/nccn/guideline/lung/english/non_small.pdf. Accessed 1 Aug 2019
- 4.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, Peters S (2019) Correction to: “Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up”. Ann Oncol 30(5):863–870. 10.1093/annonc/mdy474 [DOI] [PubMed]
- 5.Takeda K. Clinical development of S-1 for non-small cell lung cancer: a Japanese perspective. Ther Adv Med Oncol. 2013;5(5):301–311. doi: 10.1177/1758834013500702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nokihara H, Lu S, Mok TSK, Nakagawa K, Yamamoto N, Shi YK, Zhang L, Soo RA, Yang JC, Sugawara S, Nishio M, Takahashi T, Goto K, Chang J, Maemondo M, Ichinose Y, Cheng Y, Lim WT, Morita S, Tamura T. Randomized controlled trial of S-1 versus docetaxel in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy (East Asia S-1 Trial in Lung Cancer) Ann Oncol. 2017;28(11):2698–2706. doi: 10.1093/annonc/mdx419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito K, Nagashima H, Noguchi K, Yoshisue K, Yokogawa T, Matsushima E, Tahara T, Takagi S. First-in-human, phase I dose-escalation study of single and multiple doses of a first-in-class enhancer of fluoropyrimidines, a dUTPase inhibitor (TAS-114) in healthy male volunteers. Cancer Chemother Pharmacol. 2014;73(3):577–583. doi: 10.1007/s00280-014-2383-2. [DOI] [PubMed] [Google Scholar]
- 8.Yano W, Yokogawa T, Wakasa T, Yamamura K, Fujioka A, Yoshisue K, Matsushima E, Miyahara S, Miyakoshi H, Taguchi J, Chong KT, Takao Y, Fukuoka M, Matsuo K (2018) TAS-114, a first-in-class dual dUTPase/DPD inhibitor, demonstrates potential to improve therapeutic efficacy of fluoropyrimidine-based chemotherapy. Mol Cancer Ther 17(8):1683–1693. 10.1158/1535-7163.Mct-17-0911 [DOI] [PubMed]
- 9.Doi T, Yoh K, Shitara K, Takahashi H, Ueno M, Kobayashi S, Morimoto M, Okusaka T, Ueno H, Morizane C, Okano N, Nagashima F, Furuse J. First-in-human phase 1 study of novel dUTPase inhibitor TAS-114 in combination with S-1 in Japanese patients with advanced solid tumors. Invest New Drugs. 2019;37(3):507–518. doi: 10.1007/s10637-018-0697-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasolo A, Aoyama T, Stathis A et al. A large phase I study of TAS-114 in combination with S-1 in patients with advanced solid tumors. Proceedings: AACR Annual Meeting 2018; 14–18 April 2018; Chicago, IL. Abstract #CT014. https://cancerres.aacrjournals.org/content/78/13_Supplement/CT014
- 11.Govindan R, Morgensztern D, Kommor MD, Herbst RS, Schaefer P, Gandhi J, Saito K, Zergebel C, Schiller J. Phase II trial of S-1 as second-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2011;6(4):790–795. doi: 10.1097/JTO.0b013e3182103b51. [DOI] [PubMed] [Google Scholar]
- 12.Ono A, Naito T, Murakami H, Takahashi T, Nakamura Y, Tsuya A, Kaira K, Igawa S, Shukuya T, Tamiya A, Kaira R, Endo M, Yamamoto N. Evaluation of S-1 as third- or further-line chemotherapy in advanced non-small-cell lung cancer. Int J Clin Oncol. 2010;15(2):161–165. doi: 10.1007/s10147-010-0034-0. [DOI] [PubMed] [Google Scholar]
- 13.Miyoshi S, Ito R, Katayama H, Kadowaki T, Yano S, Watanabe A, Abe M, Hamada H, Okura T, Higaki J. Phase II trial of S-1 as third-line or further chemotherapy in patients with advanced non-small-cell lung cancer. Int J Clin Oncol. 2014;19(6):1005–1010. doi: 10.1007/s10147-014-0663-9. [DOI] [PubMed] [Google Scholar]
- 14.Dodd LE, Korn EL, Freidlin B, Jaffe CC, Rubinstein LV, Dancey J, Mooney MM. Blinded independent central review of progression-free survival in phase III clinical trials: important design element or unnecessary expense? J Clin Oncol. 2008;26(22):3791–3796. doi: 10.1200/jco.2008.16.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simmons CP, Koinis F, Fallon MT, Fearon KC, Bowden J, Solheim TS, Gronberg BH, McMillan DC, Gioulbasanis I, Laird BJ. Prognosis in advanced lung cancer–A prospective study examining key clinicopathological factors. Lung Cancer. 2015;88(3):304–309. doi: 10.1016/j.lungcan.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Mitsudomi T, Kosaka T, Endoh H, Horio Y, Hida T, Mori S, Hatooka S, Shinoda M, Takahashi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005;23(11):2513–2520. doi: 10.1200/jco.2005.00.992. [DOI] [PubMed] [Google Scholar]
- 17.Gandara DR, Grimminger P, Mack PC, Lara PN, Jr, Li T, Danenberg PV, Danenberg KD. Association of epidermal growth factor receptor activating mutations with low ERCC1 gene expression in non-small cell lung cancer. J Thorac Oncol. 2010;5(12):1933–1938. doi: 10.1097/JTO.0b013e3181fd418d. [DOI] [PubMed] [Google Scholar]
- 18.Pfaffle HN, Wang M, Gheorghiu L, Ferraiolo N, Greninger P, Borgmann K, Settleman J, Benes CH, Sequist LV, Zou L, Willers H. EGFR-activating mutations correlate with a Fanconi anemia-like cellular phenotype that includes PARP inhibitor sensitivity. Cancer Res. 2013;73(20):6254–6263. doi: 10.1158/0008-5472.Can-13-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsukioka S, Yano W, Yokogawa T et al. Expression of DNA damage repair enzymes determine the efficacy of a novel dUTPase inhibitor, TAS-114. Presented at the AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics, Boston, MA, USA, 19–23 October 2013. https://mct.aacrjournals.org/content/12/11_Supplement/B89
- 20.Zhou JX, Feng LJ, Zhang X. Risk of severe hematologic toxicities in cancer patients treated with PARP inhibitors: a meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2017;11:3009–3017. doi: 10.2147/dddt.S147726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suenaga M, Schirripa M, Cao S, Zhang W, Yang D, Murgioni S, Rossini D, Marmorino F, Mennitto A, Ning Y, Okazaki S, Berger MD, Miyamoto Y, Gopez R, Jr, Barzi A, Yamaguchi T, Loupakis F, Lenz HJ. Genetic variants of DNA repair-related genes predict efficacy of TAS-102 in patients with refractory metastatic colorectal cancer. Ann Oncol. 2017;28(5):1015–1022. doi: 10.1093/annonc/mdx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 2213 kb)
Data Availability Statement
Anonymized, patient-level, analyzable datasets will not be shared according to the Sponsor policy on data sharing (https://www.taiho.co.jp/en/science/policy/clinical_trial_information_disclosure_policy/index.html).