Abstract
Whether and to which extent the effects of chemicals in the environment interact with other factors remains a scientific challenge. Here we assess the combined effects of temperature (16 vs. 20°C), light conditions (darkness vs. 400 lx), dissolved organic matter (DOM; 0 vs. 6 mg/L) and the model insecticide thiacloprid (0 vs. 3 µg/L) in a full-factorial experiment on molting and leaf consumption of Gammarus fossarum. Thiacloprid was the only factor significantly affecting gammarids’ molting. While DOM had low effects on leaf consumption, temperature, light and thiacloprid significantly affected this response variable. The various interactions among these factors were not significant suggesting additivity. Only the interaction of the factors temperature and thiacloprid suggested a tendency for antagonism. As most stressors interacted additively, their joint effects may be predictable with available models. However, synergistic interactions are difficult to capture while being central for securing ecosystem integrity.
Keywords: Amphipod, Feeding rate, Multiple stress, Leaf litter decomposition
The impact of chemical stressors in aquatic ecosystems is increasingly assessed against the background of multiple stress (e.g. Bracewell et al. 2019). Thereby research goes beyond the traditional focus on single substances and their impact on different levels of ecological complexity, that is from genes to ecosystems or even meta-ecosystems. Research is available, amongst others, on the interactions of effects induced by chemical stressors in combination with nutrients (Fernandez et al. 2016; Nuttens et al. 2016), food scarcity (Pereira and Goncalves 2007), salinity (Szöcs et al. 2012), suspended soil particles (Magbanua et al. 2013a, b), UV irradiation (Pelletier et al. 2006), temperature changes (Janssens et al. 2014) or daily temperature fluctuations (Verheyen et al. 2019). Most of the named additional factors can be related to changes in land use as well as climate change.
The direction and magnitude of the joint effects of multiple stressors in aquatic ecosystems is not clear cut with additive, synergistic and antagonistic interactions being reported (Jackson et al. 2016). For instance, Janssens et al. (2014) documented stronger effects of chlorpyrifos on the damselfly Ischnura elegans at 30°C relative to 20°C. For the same species, Op de Beeck et al. (2018) documented a higher impact of the same stressor at 20°C relative to 24°C. Op de Beeck et al. explained their findings with a slower degradation of chlorpyriphos at 20°C relative to 24°C leading to stronger effects. This interpretation contradicts, however, Janssens et al. (2014) showing a higher effect at 30°C despite an anticipated much faster degradation. This discrepancy may, however, be explained by the heat stress overriding the potentially positive effect of a faster chlorpyriphos degradation at 30°C. These two studies provide an excellent example for the complexity of interactions that are likely to play a significant role when assessing for effects of chemical stressors in a multiple stressor environment.
Besides these anthropogenically induced changes in environmental variables, the presence of dissolved organic matter (DOM) can have a substantial impact in the bioavailability of chemical stressors and their subsequent effects (Kukkonen and Oikari 1991). Moreover, long-term monitoring data suggest that DOM levels in surface waters are increasing (brownification) in regions with reduced sulfur deposition and wetter climate (de Wit et al. 2016). At the same time, DOM is hardly amended during ecotoxicological assessments in general making its impact on chemical stressor induced effects a substantial knowledge gap – particularly in a multiple stressor context. Similarly, light can influence organisms, particularly those with a negative phototaxis (Franke 1977), which in turn can be modified by stressors such as parasites (Benesh et al. 2005).
Using molting and leaf consumption of a key species in the ecosystem function of leaf litter decomposition, that is Gammarus fossarum (Dangles et al. 2004), the present study assessed the individual and combined effects of four factors in a full-factorial test design. Among those factors, DOM (0 vs. 6 mg/L) and light intensity (darkness vs. 400 lx) covered environmental variables and increasing temperature (16 vs 20°C) as well as the neonicotinoid insecticide thiacloprid (0 vs. 3 µg/L) represented factors modified in aquatic ecosystems as a consequence of global climate change and agricultural land use. The tested thiacloprid concentrations are considered field relevant as similar levels have been found in agricultural streams (Morrissey et al. 2015; Süss et al. 2006). It was hypothesised that light would reduce gammarids’ leaf consumption as they would hide under the leaf material (Bundschuh et al. 2011). Similarly, thiacloprid reduces this variable (Feckler et al. 2012) with DOM having, driven by the rather low hydrophobicity of thiacloprid, a slight mitigating impact (Kukkonen and Oikari 1991). Based on the review by Holmstrup et al. (2010), who suggested mainly synergistic effects between higher temperatures and chemical stressors, and thiacloprids stability in aquatic systems (Lewis et al. 2016), it was finally assumed that the impact of thiacloprid would be elevated at higher temperatures.
Materials and Methods
The experiment followed a full-factorial design with four independent variables (temperature, light, DOM, toxicant) and two levels each. The factors temperature and light where realised in climate chambers. Temperature was set at 16 ± 1 or 20 ± 1°C to simulate a projected temperature increase under the A1B scenario (IPCC 2007). Light intensity was either approximately 400 lx – a moderate light intensity (Hubert et al. 1998) – with a light:dark cycle of 8:16 h or complete darkness. Seaweed extract (Marinure®, Glenside, Scottland) served as DOM source at a concentration of 6 mg/L, which is in the lower range of environmentally relevant levels (Evans et al. 2017). Seaweed extract was selected because of its relatively balanced composition in terms of chromophoric dissolved organic carbon, which is also representative for DOM released from waste water treatment plants. Moreover, it is recommended for chronic standard tests with Daphnids (OECD 2008). Thiacloprid was applied as commercially available formulation (Calypso® 480 SC; 480 g thiacloprid/L; Bayer CropScience, Leverkusen, Germany; containing 1,2-Benzisothiazol-3(2H)-on, 5-Chlor-2-methyl-2H-isothiazol-3-on and 2-methyl-2H-isothiazol-3-on below 0.05% of the product each), which rendered the use of further solvents unnecessary. The formulation was serially diluted in amphipod medium (SAM-5S; Borgmann 1996) to receive the respective nominal concentration of 3 µg/L, a concentration that should cause a reduction in gammarids’ leaf consumption of about 50% over 7 days (Feckler et al. 2012). To verify exposure at these nominal concentrations, at the start of the experiments triplicate 10-mL samples were taken from the thiacloprid treatments. Samples were stored at − 20°C until analysis via an ultra-high-performance liquid chromatography system (Englert et al. 2012). The analyses revealed adequate thiacloprid dosing (mean ± standard deviation: 2.5 ± 0.3 µg/L) since nominal and measured initial concentrations deviated by < 20%.
Leaf discs were prepared as described in Zubrod et al. (2010). Briefly, shortly before leaf fall in October 2015, black alder (Alnus glutinosa (L.) Gaertn.) leaves from trees near Landau, Germany (49° 11′ N; 8° 05′ E) were collected and stored at − 20°C until use. Discs of 2 cm diameter were cut from the leaves with a cork borer, while excluding the main vein. Leaf discs were subjected to microbial colonization (i.e., conditioning) for 10 days in a nutrient medium (Dang et al. 2005) using leaf material previously exposed in a near-natural stream (Rodenbach, Germany, 49° 33′ N, 8° 02′ E) as inoculum. After conditioning, leaf discs were dried to a constant weight (~ 24 h at 60°C) and weighed to the nearest 0.01 mg. Approximately 48 h prior to the start of the experiment, leaf discs were re-soaked in sterile test medium (147 mg/L CaCl2 × 2H2O, 85.5 mg/L NaHCO3, 61.5 mg/L MgSO4 × 7H20, 3.8 mg/L KCl, and 1.03/L mg NaBr, Borgmann 1996) to prevent floating during the experiments.
The test organisms, G. fossarum, were kick-sampled in the near-natural, forested stream Hainbach near Landau, Germany (49° 14′ N; 8° 03′ E; cryptic lineage B; Feckler et al. 2014) 7 days prior to the experiment. Gammarids were immediately divided into different size classes (Franke 1977) and only adults with a diameter between 1.6 and 2.0 mm being visually free of acanthocephalan parasites were used in order to reduce variability in their response (Pascoe et al. 1995). At the start of the experiment gammarid populations were in a reproductive rest. Consequently, separation between sexes was not possible and the experiment was run with both males and females. Throughout the acclimation phase, animals were kept in aerated medium in a climate-controlled chamber at 16 ± 1 or 20 ± 1°C (depending on the temperature treatment) in total darkness, while they were fed ad libitum with pre-conditioned black alder leaves and were gradually adapted to SAM-5S.
The assessment of gammarids’ leaf consumption followed Zubrod et al (2010). One G. fossarum was placed together with two preconditioned leaf discs in a 250-mL-glass beaker filled with 200 mL of SAM-5S. Each of the 16 treatments of the four-factorial test design were replicated 20 times. All beakers were aerated during the whole study duration. In addition to the replicates established to quantify gammarids’ leaf consumption, four additional replicates were set up per treatment without gammarids to account for the microbial and physical leaf mass loss. During the study, molting was monitored daily. Amphipods, the remaining leaf discs and any leaf tissue shredded off were removed after seven days of exposure, dried and weighed as described above.
Leaf consumption was expressed in consumed leaf mass and according to Maltby et al. (2000). As the leaf consumption data did not meet the requirements of parametric testing, data were rank transformed and subsequently analysed by four-way analysis of variance (ANOVA). The proportion of moulting gammarids were analysed using a binomial generalized linear model (GLM). Both models were fitted excluding four-way interactions as these are hardly interpretable and such high-level interactions were not hypothesized. ANOVA and GLM were simplified stepwise using F-tests and chi-square tests to justify simplification steps, respectively. The term “significant(ly)” is exclusively used in reference to statistical significance (p < 0.05) throughout the present study. For all statistics and figures, R version 3.3.2 for Mac was used.
Results and Discussion
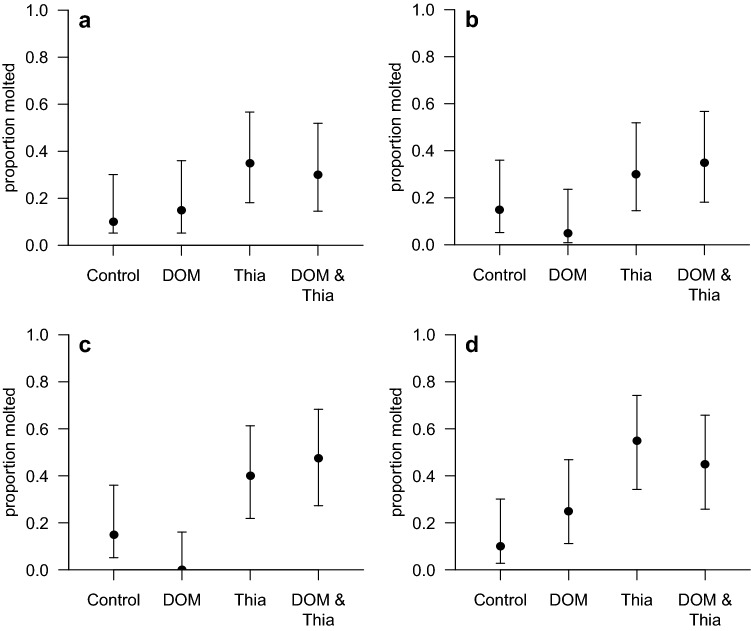
The only factor significantly affecting gammarids’ molting was the presence of thiacloprid during the experiment increasing the proportion of molted animals on average by more than three-fold compared to the thiacloprid-free treatments (Fig. 1; Table 1). This observation, may be related to detoxification, namely reducing the concentration of thiacloprid by molting – a mechanism observed for other stressors such as metals (Raessler et al. 2005). The present study, however, contrasts earlier studies reporting a reduction in molting rate, for instance, in cladoceran Daphnia magna (Qi et al. 2013) or the mayfly Deleatidium spp. (Macaulay et al. 2019) at higher concentrations of other neonicotinoids, namely guadipyr, imidacloprid, thiamethoxam and clothianidin. These contrasting effects point towards species- or neonicotinoid-specific effects in molting requiring further research to uncover the underlying mechanisms.
Fig. 1.
Proportion (with 95% confidence interval) of molted gammarids exposed to DOM, thiacloprid (Thia) and their combination at 16°C (a, b) and 20°C (c, d) in presence (a, c) and absence (b, d) of light
Table 1.
Minimal adequate model resulting from simplifying four-way GLM performed on moulting data
| Estimate | Std. error | z-value | p-value | |
|---|---|---|---|---|
| Intercept | − 2.0043 | 0.2444 | − 8.202 | < 0.001 |
| Thiacloprid | 1.5831 | 0.2933 | 5.398 | < 0.001 |
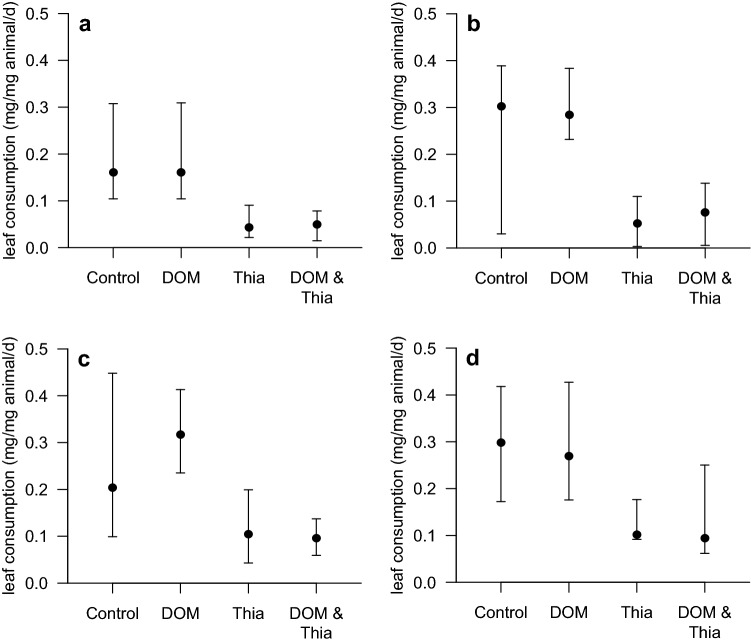
The median leaf consumption of G. fossarum was, in absence of thiacloprid and DOM, reduced by roughly 40% under light exposure relative to those tested in darkness (Fig. 2a–d; Table 2). Moreover, the effect size matched roughly with the light:dark rhythm of 8:16 h. This finding, thus, confirms one of the hypotheses, namely that light reduces gammarids leaf consumption likely driven by their negative phototaxis and tendency to hide under leaf litter instead of consuming it (see also Bundschuh et al. 2011).
Fig. 2.
Median (with 95% confidence interval) leaf consumption (mg/mg dry weight of gammarids/d) of gammarids exposed to DOM, thiacloprid (Thia) and their combination at 16°C (a, b) and 20°C (c, d) in presence (a, c) and absence (b, d) of light
Table 2.
Minimal adequate model resulting from simplifying four-way ANOVA performed on rank-transformed leaf consumption data
| Df | Sum of squares | Mean squares | F-value | p-value | |
|---|---|---|---|---|---|
| Thiacloprid | 1 | 582,851 | 582,851 | 107.32 | < 0.001 |
| Temperature | 1 | 99,771 | 99,771 | 18.371 | < 0.001 |
| Light | 1 | 29,276 | 29,276 | 5.391 | 0.021 |
| Residuals | 300 | 1,629,282 | 5431 |
The presence of DOM was, relative to its absence, inducing no changes in leaf consumption under most light and temperature regimes applied (Table 2, Fig. 2a–d). Only at 20°C in presence of light, leaf consumption was substantially elevated, while overall DOM was a non-significant factor for explaining variability in gammarids’ feeding. This observation contradicts recent studies with Daphnia reporting in absence of UV light increasing DNA strand breaks with increasing DOM concentrations (Wolf et al. 2017). The concentrations causing these negative effects were, however, several fold above the levels applied in the present study. Moreover, DNA strand breaks are suborganismic responses that may be balanced on the level of whole organisms leading to insubstantial effects on behavioural responses as shown here.
Temperature significantly affected leaf consumption (Table 2), with the higher temperature (20°C) leading to higher leaf consumption relative to 16°C (Fig. 2a–d). A higher activity of organisms at higher temperatures (up to a individuum specific threshold) was also observed in a range of other studies (e.g. Cuco et al. 2018; Seeland et al. 2013) highlighting that the selected temperature range in the present study was still acceptable for our tested population. This difference within the same treatment between temperatures can be as high as 2.5-fold (thiacloprid in light) or close to zero (in darkness for both control and DOM). The most consistent effect of temperature was observed in presence of thiacloprid with effect sizes often around a factor of two (but not in darkness in presence of DOM and thiacloprid). The only interaction term near to significance (p = 0.058; F-test justified omission from final model; Table 2) was the interaction between temperature and thiacloprid pointing towards a lower relative reduction in gammarid feeding at 20°C relative to 16°C. Thereby, evidence against one hypothesis, namely that elevated temperatures increase the impact of pesticides, was found. This positive effect may be explained by the fact that the used temperature of 20°C is still within the range that can be accepted by G. fossarum (Peeters and Gardeniers 1998). Consequently, gammarids’ defence mechanisms may have been more efficient with a higher enzyme activity at 20°C, while this temperature did not have a direct negative impact on the test species.
Thiacloprid reduced leaf consumption by partly more than 80%. This massive effect of the neonicotinoid is supported by the four-way ANOVA highlighting this factor as significant (Table 2). The effect induced by 3 µg thiacloprid/L is in line with earlier studies reporting slightly lower but in general similar effects on gammarids (Englert et al. 2012; Feckler et al. 2012). The data additionally supports the related initial hypothesis. Any interaction with DOM and light (i.e., independent whether two or threefold interaction) was not significant suggesting additive effects of these factors with thiacloprid on gammarids’ leaf consumption (Table 2). The low impact of DOM on thiacloprid-induced effects is, as hypothesised, likely triggered by its low affinity to organic carbon and lipophilicity (Lewis et al. 2016). However, DOM is also considered as additional energy source for aquatic invertebrates such as daphnids (Bergman Filho et al. 2011) but also black fly larvae (Ciborowski et al. 1997) potentially leading to a higher tolerance to chemical stress (Seitz et al. 2015). The data of the present study suggest DOM, but also light, to play a minor role for the bioavailability of the assessed insecticide as well as for the tolerance of the test species.
All in all, the present study highlights that none of the factor combinations lead to significant interactions. Although this might be a matter of statistical power, the data suggest additive effects on the assessed variables. Consequently, the joint effects of the factors assessed in the present study should be predictable with available models. At the same time, synergistic interactions between natural factors but also those of anthropogenic origin have been reported (Holmstrup et al. 2010; Jackson et al. 2016) complicating reliable predictions. This fact warrants further research, which should consider the mechanistic framework proposed by Schäfer and Piggott (2018).
Acknowledgements
Open Access funding provided by Projekt DEAL. We are thankful to T. Bürgi for laboratory support and two anonymous reviewers for their insightful comments that improved this document.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Benesh DP, Duclos LM, Nickol BB. The behavioral response of amphipods harboring Corynosoma constrictum (Acanthocephala) to various components of light. J Parasitol. 2005;91:731–736. doi: 10.1645/GE-440R.1. [DOI] [PubMed] [Google Scholar]
- Bergman Filho TU, Soares AM, Loureiro S. Energy budget in Daphnia magna exposed to natural stressors. Environ Sci Pollut Res. 2011;18:655–662. doi: 10.1007/s11356-010-0413-0. [DOI] [PubMed] [Google Scholar]
- Borgmann U. Systematic analysis of aqueous ion requirements of Hyalella azteca: a standard artificial medium including the essential bromide ion. Arch Environ Contam Toxicol. 1996;30:356–363. [Google Scholar]
- Bracewell S, Verdonschot RCM, Schäfer RB, Bush A, Lapen DR, Van den Brink PJ. Qualifying the effects of single and multiple stressors on the food web structure of Dutch drainage ditches using a literature review and conceptual models. Sci Total Environ. 2019;684:727–740. doi: 10.1016/j.scitotenv.2019.03.497. [DOI] [PubMed] [Google Scholar]
- Bundschuh M, Zubrod JP, Englert D, Seitz F, Rosenfeldt RR, Schulz R. Effects of nano-TiO2 in combination with ambient UV-irradiation on a leaf shredding amphipod. Chemosphere. 2011;85:1563–1567. doi: 10.1016/j.chemosphere.2011.07.060. [DOI] [PubMed] [Google Scholar]
- Ciborowski JJH, Craig DA, Fry KM. Dissolved organic matter as food for black fly larvae (Diptera : Simuliidae) J N Am Benthol Soc. 1997;16:771–780. [Google Scholar]
- Cuco AP, Castro BB, Goncalves F, Wolinska J, Abrantes N. Temperature modulates the interaction between fungicide pollution and disease: evidence from a Daphnia-microparasitic yeast model. Parasitology. 2018;145:939–947. doi: 10.1017/S0031182017002062. [DOI] [PubMed] [Google Scholar]
- Dang CK, Chauvet E, Gessner MO. Magnitude and variability of process rates in fungal diversity-litter decomposition relationships. Ecol Lett. 2005;8:1129–1137. doi: 10.1111/j.1461-0248.2005.00815.x. [DOI] [PubMed] [Google Scholar]
- Dangles O, Gessner MO, Guerold F, Chauvet E. Impacts of stream acidification on litter breakdown: implications for assessing ecosystem functioning. J Appl Ecol. 2004;41:365–378. [Google Scholar]
- de Wit HA, et al. Current browning of surface waters will be further promoted by wetter climate. Environ Sci Technol Lett. 2016;3:430–435. [Google Scholar]
- Englert D, Bundschuh M, Schulz R. Thiacloprid affects trophic interaction between gammarids and mayflies. Environ Pollut. 2012;167:41–46. doi: 10.1016/j.envpol.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Evans CD, Futter MN, Moldan F, Valinia S, Frogbrook Z, Kothawala DN. Variability in organic carbon reactivity across lake residence time and trophic gradients. Nat Geosci. 2017;10:832. [Google Scholar]
- Feckler A, Thielsch A, Schwenk K, Schulz R, Bundschuh M. Differences in the sensitivity among cryptic lineages of the Gammarus fossarum complex. Sci Total Environ. 2012;439:158–164. doi: 10.1016/j.scitotenv.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Feckler A, Zubrod JP, Thielsch A, Schwenk K, Schulz R, Bundschuh M. Cryptic species diversity: a disregarded factor in environmental management? J Appl Ecol. 2014;51:958–967. [Google Scholar]
- Fernandez D, et al. Does nutrient enrichment compensate fungicide effects on litter decomposition and decomposer communities in streams? Aquat Toxicol. 2016;174:169–178. doi: 10.1016/j.aquatox.2016.02.019. [DOI] [PubMed] [Google Scholar]
- Franke U. Experimentelle Untersuchungen zur respiration von Gammarus fossarum in Abhängigkeit von temperature, Sauerstoffkonzentration und Wasserbewegung. Arch für Hydrobiol Supp. 1977;3(4):369–411. [Google Scholar]
- Holmstrup M, et al. Interactions between effects of environmental chemicals and natural stressors: a review. Sci Total Environ. 2010;408(3746–3762):7. doi: 10.1016/j.scitotenv.2009.10.067. [DOI] [PubMed] [Google Scholar]
- Hubert M, Dumont M, Paquet J. Seasonal and diurnal patterns of human illumination under natural conditions. Chronobiol Int. 1998;15:59–70. doi: 10.3109/07420529808998670. [DOI] [PubMed] [Google Scholar]
- IPCC (2007) Climate change 2007: synthesis report
- Jackson MC, Loewen CJG, Vinebrooke RD, Chimimba CT. Net effects of multiple stressors in freshwater ecosystems: a meta-analysis. Glob Change Biol. 2016;22:180–189. doi: 10.1111/gcb.13028. [DOI] [PubMed] [Google Scholar]
- Janssens L, Van Dinh K, Stoks R. Extreme temperatures in the adult stage shape delayed effects of larval pesticide stress: a comparison between latitudes. Aquat Toxicol. 2014;148:74–82. doi: 10.1016/j.aquatox.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Kukkonen J, Oikari A. Bioavailability of organic pollutants in boreal waters with varying levels of dissolved organic material. Water Res. 1991;25:455–463. [Google Scholar]
- Lewis KA, Tzilivakis J, Warner D, Green A. An international database for pesticide risk assessments and management. Hum Ecol Risk Assess. 2016;22:1050–1064. doi: 10.1080/10807039.10802015.11133242. [DOI] [Google Scholar]
- Macaulay SJ, Hageman KJ, Alumbaugh RE, Lyons SM, Piggott JJ, Matthaei CD. Chronic toxicities of neonicotinoids to nymphs of the common New Zealand mayfly Deleatidium spp. Environ Toxicol Chem. 2019;38:2459–2471. doi: 10.1002/etc.4556. [DOI] [PubMed] [Google Scholar]
- Magbanua FS, Townsend CR, Hageman KJ, Lange K, Lear G, Lewis GD, Matthaei CD. Understanding the combined influence of fine sediment and glyphosate herbicide on stream periphyton communities. Water Res. 2013;47:5110–5120. doi: 10.1016/j.watres.2013.05.046. [DOI] [PubMed] [Google Scholar]
- Magbanua FS, Townsend CR, Hageman KJ, Matthaei CD. Individual and combined effects of fine sediment and the herbicide glyphosate on benthic macroinvertebrates and stream ecosystem function. Freshw Biol. 2013;58:1729–1744. [Google Scholar]
- Maltby L, Clayton SA, Yu H, McLoughlin N, Wood RM, Yin D. Using single-species toxicity tests, community-level response and toxicity identification evaluations to investigate effluent impacts. Environ Toxicol Chem. 2000;19:151–157. [Google Scholar]
- Morrissey CA, Mineau P, Devries JH, Sanchez-Bayo F, Liess M, Cavallaro MC, Liber K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: a review. Environ Int. 2015;74:291–303. doi: 10.1016/j.envint.2014.10.024. [DOI] [PubMed] [Google Scholar]
- Nuttens A, Chatellier S, Devin S, Guignard C, Lenouvel A, Gross EM. Does nitrate co-pollution affect biological responses of an aquatic plant to two common herbicides? Aquat Toxicol. 2016;177:355–364. doi: 10.1016/j.aquatox.2016.06.006. [DOI] [PubMed] [Google Scholar]
- OECD (2008) OECD 211: Daphnia magna reproduction test. Guideline for testing of chemicals
- Op de Beeck LO, Verheyen J, Stoks R. Strong differences between two congeneric species in sensitivity to pesticides in a warming world. Sci Total Environ. 2018;618:60–69. doi: 10.1016/j.scitotenv.2017.10.311. [DOI] [PubMed] [Google Scholar]
- Pascoe D, Kedwards TJ, Blockwell SJ, Taylor EJ. Gammarus pulex (L.) feeding bioassay—effects of parasitism. Bull Environ Contam Toxicol. 1995;55:629–632. doi: 10.1007/BF00196046. [DOI] [PubMed] [Google Scholar]
- Peeters ETHM, Gardeniers JJP. Logistic regression as a tool for defining habitat requirements of two common gammarids. Freshw Biol. 1998;39:605–615. [Google Scholar]
- Pelletier E, Sargian P, Payet J, Demers S. Ecotoxicological effects of combined UVB and organic contaminants in coastal waters: a review. Photochem Photobiol. 2006;82:981–993. doi: 10.1562/2005-09-18-ra-688.1. [DOI] [PubMed] [Google Scholar]
- Pereira JL, Goncalves F. Effects of food availability on the acute and chronic toxicity of the insecticide methomyl to Daphnia spp. Sci Total Environ. 2007;386:9–20. doi: 10.1016/j.scitotenv.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Qi S, Wang C, Chen X, Qin Z, Li X, Wang C. Toxicity assessments with Daphnia magna of Guadipyr, a new neonicotinoid insecticide and studies of its effect on acetylcholinesterase (AChE), glutathione S-transferase (GST), catalase (CAT) and chitobiase activities. Ecotoxicol Environ Saf. 2013;98:339–344. doi: 10.1016/j.ecoenv.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Raessler M, Rothe J, Hilke I. Accurate determination of Cd, Cr, Cu and Ni in woodlice and their skins—is moulting a means of detoxification? Sci Total Environ. 2005;337:83–90. doi: 10.1016/j.scitotenv.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Schäfer RB, Piggott JJ. Advancing understanding and prediction in multiple stressor research through a mechanistic basis for null models. Glob Change Biol. 2018;24:1817. doi: 10.1111/gcb.14073. [DOI] [PubMed] [Google Scholar]
- Seeland A, Albrand J, Oehlmann J, Muller R. Life stage-specific effects of the fungicide pyrimethanil and temperature on the snail Physella acuta (Draparnaud, 1805) disclose the pitfalls for the aquatic risk assessment under global climate change. Environ Pollut. 2013;174:1–9. doi: 10.1016/j.envpol.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Seitz F, Rosenfeldt RR, Lüderwald S, Schulz R, Bundschuh M. Aging of TiO2 nanoparticles transiently increases their toxicity to the pelagic microcrustacean Daphnia magna. PLoS ONE. 2015;10:e0126021. doi: 10.1371/journal.pone.0126021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Süss A, Bischoff G, Müller CW, Buhr L. Chemische-biologisches monitoring zu Pflanzenschutzmittelbelastung und Lebensgemeinschaften in Gräben des Alten Landes. Nachrichtenblatt des Deutschen Pflanzenschutzdienstes. 2006;58:28–42. [Google Scholar]
- Szöcs E, Kefford BJ, Schäfer RB. Is there an interaction of the effects of salinity and pesticides on the community structure of macroinvertebrates? Sci Total Environ. 2012;437:121–126. doi: 10.1016/j.scitotenv.2012.07.066. [DOI] [PubMed] [Google Scholar]
- Verheyen J, Delnat V, Stoks R. Increased daily temperature fluctuations overrule the ability of gradual thermal evolution to offset the increased pesticide toxicity under global warming. Environ Sci Technol. 2019;53:4600–4608. doi: 10.1021/acs.est.8b07166. [DOI] [PubMed] [Google Scholar]
- Wolf RE, Andersen TH, Hessen DO, Hylland K. The influence of dissolved organic carbon and ultraviolet radiation on the genomic integrity of Daphnia magna. Funct Ecol. 2017;31:848–855. [Google Scholar]
- Zubrod JP, Bundschuh M, Schulz R. Effects of subchronic fungicide exposure on the energy processing of Gammarus fossarum (Crustacea; Amphipoda) Ecotoxicol Environ Saf. 2010;73:1674–1680. doi: 10.1016/j.ecoenv.2010.07.046. [DOI] [PubMed] [Google Scholar]