Abstract
Introduction
Nintedanib, a tyrosine kinase receptor inhibitor, may be associated with increased bleeding risk. Thus, patients with an inherited predisposition to bleeding, or those receiving therapeutic doses of anticoagulants or high-dose antiplatelet therapy, have been excluded from clinical trials of nintedanib in idiopathic pulmonary fibrosis (IPF).
Objective
Our objective was to examine real-world bleeding events in patients with IPF treated with antifibrotics, including those receiving anticoagulants and/or antiplatelet therapy.
Methods
The European MultiPartner IPF Registry (EMPIRE) enrolled 2794 patients with IPF: group A (1828: no anticoagulant or antiplatelet treatment), group B (227: anticoagulant treatment), group C (659: antiplatelet treatment), and group D (80: anticoagulant and antiplatelet treatment). Overall, 673 (24.1%) received nintedanib and 933 (33.4%) received pirfenidone. Bleeding events and their relationship to antifibrotic and anticoagulation treatment were characterized.
Results
Group A patients, versus those in groups B, C, and D, were typically younger and generally had the lowest comorbidity rates. A higher proportion of patients in groups A and C, versus group B, received nintedanib. Pirfenidone, most common in group D, was more evenly balanced across groups. In patients with reported bleeding events, seven of eight received nintedanib (groups A, C, and D). Bleeding incidence was 3.0, 0, 1.3, and 18.1 per 10,000 patient-years (groups A, B, C, and D, respectively).
Conclusion
Real-world data from EMPIRE showed that patients on anticoagulant medications received nintedanib less frequently, perhaps based on its mechanism of action. Overall, bleeding incidence was low (0.29%: nintedanib 0.25%; pirfenidone 0.04%) and irrespective of anticoagulant or antiplatelet therapy received (P = 0.072).
Electronic supplementary material
The online version of this article (10.1007/s40264-020-00978-5) contains supplementary material, which is available to authorized users.
Key Points
| Nintedanib, a therapy used to treat patients with idiopathic pulmonary fibrosis (IPF), may increase the risk of bleeding, potentially because of its mechanism of action. |
| Analysis from EMPIRE, a real-world database of patients with IPF, found that nintedanib, compared with pirfenidone, was received less frequently in patients being treated with anticoagulant medications. |
| Overall, the incidence of bleeding in patients with IPF was low (0.29%). |
Introduction
Idiopathic pulmonary fibrosis (IPF) is a rare, chronic, progressive, fibrosing interstitial pneumonia of unknown cause that primarily affects older adults and has a poor prognosis [1–3]. Nintedanib and pirfenidone are both approved for the treatment of IPF [4–7]. Pirfenidone is an orally active small molecule that attenuates the progression of fibrosis and loss of lung function in IPF via its antifibrotic and anti-inflammatory properties, although the exact mechanism of action is unknown [8–11]. Nintedanib, a tyrosine kinase inhibitor, has also been shown to reduce the rate of lung function decline in patients with IPF [12] and acts to potently block vascular endothelial growth factor receptors (VEGFRs), platelet-derived growth factor receptors (PDGFRs), and fibroblast growth factor receptor kinase activity [13, 14]. Based on evidence from preclinical models, nintedanib has a role in impeding the initiation and progression of lung fibrosis, inhibiting the release of mediators of inflammation and fibrosis, as well as the proliferation and transformation of human lung fibroblasts, and reducing extracellular matrix deposition [14, 15].
Since nintedanib inhibits VEGFR and PDGFR, there is the potential for vascular dysfunction and an increased risk of bleeding [4, 5, 16, 17]. The risk of bleeding in patients with IPF treated with nintedanib and anticoagulants and/or antiplatelet drugs is currently unknown, as patients on anticoagulant or high-dose antiplatelet treatment were excluded from the two randomized, phase III INPULSIS clinical trials (nintedanib treatment in patients with IPF) (ClinicalTrials.gov identifiers: NCT01335464 and NCT01335477) [18].
As safety is of paramount importance, and to avoid refusal of nintedanib treatment for patients with IPF receiving anticoagulant treatment without evidence, the aim of our study was to assess the frequency of bleeding events in a real-world IPF setting.
Patient Selection and Methods
Study Design and Participants
The European MultiPartner IPF Registry (EMPIRE) is a noninterventional, international, multicenter database of patients with IPF in Central and Eastern Europe. The main objectives of the registry are to assess IPF incidence, prevalence, and mortality in Central and Eastern Europe, and to determine the basic characteristics of patients with IPF [19, 20]. Other valuable outcomes of the registry included information on treatment patterns and patient management [19].
Patients with IPF included in EMPIRE were diagnosed according to the American Thoracic Society/European Respiratory Society (ATS/ERS) consensus classification [1].
Patients in this analysis were allocated to one of four groups according to the anticoagulant and/or antiplatelet therapy they had received during the observation period: Group A comprised patients without any anticoagulant or antiplatelet treatment; patients in groups B and C received either anticoagulant treatment only or antiplatelet drug(s) only (e.g., aspirin, clopidogrel), respectively; and patients in group D received both anticoagulant and antiplatelet therapy (Fig. 1).
Fig. 1.
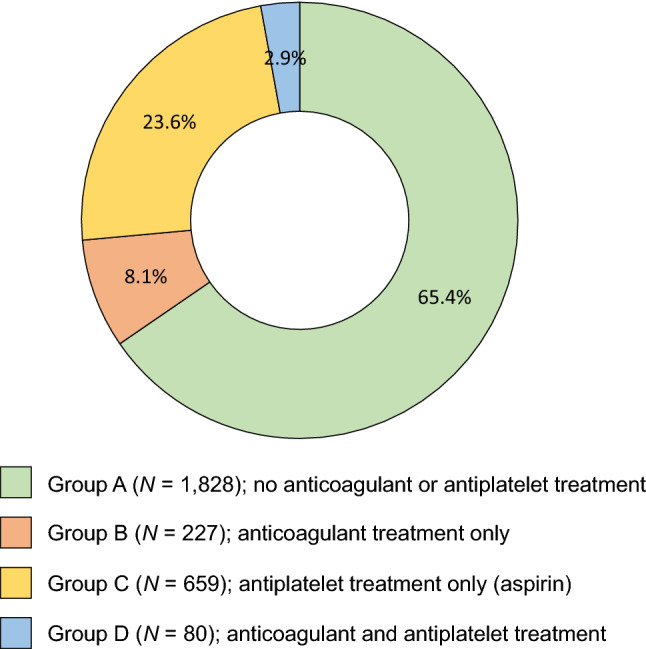
Definition of treatment group according to anticoagulant and antiplatelet treatment. Treatment was defined as at any time (time of inclusion and/or during follow-up)
Patient baseline demographics, including age, gender, and physiology (GAP) score; smoking history; symptoms; comorbidities; detailed lung function values (forced vital capacity [FVC], forced expiratory volume in 1 s [FEV1], total lung capacity, diffusing capacity of the lung for carbon monoxide [DLCO]), and high-resolution computed tomography (HRCT) pattern were analyzed. Body mass index and 6-Minute Walk Test (6MWT) results were also analyzed. Additionally, the number of patients in the respective groups were provided according to country.
All adverse events (AEs) were investigated and recorded per the Common Terminology Criteria for Adverse Events, with particular focus on bleeding events occurring in patients treated with concomitant antifibrotic therapies. The occurrence of bleeding events and the incidence of bleeding during antifibrotic treatment for A–D subgroups of patients were described.
Statistical Analysis
This study aimed to calculate the incidence of bleeding according to treatment group. For patients who were first treated with nintedanib or pirfenidone, start of follow-up was defined as start of nintedanib or pirfenidone treatment, whereas end of follow-up was defined as the end of treatment with the first drug, a bleeding event, death, or the end of follow-up in the registry. Patients not treated with either nintedanib or pirfenidone were included in the “others” group, start of follow-up was defined as date of enrolment, and end of follow-up was defined as bleeding, death, or end of follow-up in the registry. Classification of patients into groups A–D was dependent upon whether anticoagulant therapy or antiplatelet therapy was recorded at least once during the follow-up period. Bleeding events were included if they occurred during the defined follow-up period.
Statistical analysis was descriptive and included absolute and relative frequencies for categorical variables and medians, with 5th–95th percentile ranges calculated for quantitative variables (in plots that were completed with interquartile range). The occurrence of bleeding events was described as incidence per 10,000 patient-years and supplemented with 95% confidence intervals (CIs). The incidence of events per patient-year was calculated as the number of incident cases divided by the amount of person-time at risk (i.e., the sum of the duration of all follow-ups of all patients in each group). Significant differences among groups of patients were analyzed using the χ2 test for categorical variables and Kruskal–Wallis tests for quantitative variables. If differences were statistically significant, post hoc testing with a Bonferroni correction was used to identify homogeneous groups.
The level of statistical significance was set at α = 0.05. Analyses were performed using SPSS v25 (IBM Corporation, Armonk, NY, USA) and Stata 14.2. (StataCorp, Lakeway Drive, TX, USA).
Results
Patient Characteristics
Overall, 2942 patients with IPF were included from 38 centers in ten countries (Czech Republic, Croatia, Hungary, Poland, Serbia, Slovakia, Turkey, Austria, Bulgaria, and Israel) between 6 March 2002 and 22 January 2019. The final analysis population included 2794 patients; patients with no date of inclusion in the study (n = 62) or who had a change in diagnosis (n = 86) were excluded from the analysis (electronic supplementary material [ESM]; Supplementary Fig. 1). Patients were enrolled into groups based on whether they received anticoagulant or antiplatelet therapy at any time during the observation period (group A: N = 1828; group B: N = 227; group C: N = 659; group D: N = 80).
Information on patient characteristics is summarized in Table 1 (additional information on patient characteristics is provided in the ESM [Supplementary Table 1]). Patients in group A were typically younger than patients in groups B, C, and D (Table 1). Most patients were male, although there was a higher proportion of women in group A than in groups C and D (Table 1). Across all groups, > 50% of patients had a history of smoking, and a small proportion of patients identified themselves as current smokers (Table 1). The median body mass index of all groups indicated that many patients were classified as overweight, and statistical differences were observed between groups A and B (ESM Supplementary Table 1). Dyspnea was more frequent in group A than in group B. Crackles (a sign of IPF) were observed more frequently in group C than in group A. However, other IPF symptoms, such as cough and finger clubbing, did not differ between groups.
Table 1.
Patient characteristics
| Characteristic | Group A (N = 1828) | Group B (N = 227) | Group C (N = 659) | Group D (N = 80) |
|---|---|---|---|---|
| Median age, years (range) | ||||
| All | 67 (50–81) | 71 (54–83) | 71 (57–84) | 73 (61–85) |
| Female | 68 (50–82) | 68 (55–84) | 72 (57–84) | 75 (47–81) |
| Male | 67 (51–81) | 71 (54–83) | 70 (58–84) | 72 (61–86) |
| Sex | ||||
| Female | 604 (33.0) | 60 (26.4) | 150 (22.8) | 12 (15.0) |
| Male | 1224 (67.0) | 167 (73.6) | 509 (77.2) | 68 (85.0) |
| Smoking habit | ||||
| Never smoked | 738 (40.7) | 100 (44.1) | 223 (34.0) | 21 (26.6) |
| Current smoker | 98 (5.4) | 9 (4.0) | 26 (4.0) | 2 (2.5) |
| Ex-smoker | 978 (53.9) | 118 (52.0) | 406 (62.0) | 56 (70.9) |
| GAP score | ||||
| I | 580 (49.7) | 48 (30.0) | 153 (36.2) | 13 (23.2) |
| II | 479 (41.0) | 96 (60.0) | 210 (49.6) | 36 (64.3) |
| III | 108 (9.3) | 16 (10.0) | 60 (14.2) | 7 (12.5) |
| Comorbidities | ||||
| 0 | 228 (12.5) | 9 (4.0) | 9 (1.4) | 0 (0.0) |
| 1 | 416 (22.8) | 18 (7.9) | 55 (8.3) | 3 (3.8) |
| 2 | 394 (21.6) | 36 (15.9) | 110 (16.7) | 6 (7.5) |
| > 2 | 790 (43.2) | 164 (72.2) | 485 (73.6) | 71 (88.8) |
| Antifibrotic treatment | ||||
| Pirfenidone | 589 (32.2) | 86 (37.9) | 220 (33.4) | 38 (47.5) |
| Nintedanib | 465 (25.4) | 31 (13.7) | 161 (24.4) | 16 (20.0) |
| No antifibrotic treatment | 774 (42.3) | 110 (48.5) | 278 (42.2) | 26 (32.5) |
Data are presented as n (%) or median (5th–95th percentile) unless otherwise indicated
GAP gender, age, and physiology
More patients had GAP stage I in group A compared with patients in groups B, C, and D. The number of comorbidities was lowest in group A compared with the other three groups. HRCT lung imaging was described according to the ATS/ERS consensus classification [1] in all patients, and HRCT patterns differed between groups, with usual interstitial pneumonia (UIP) pattern the smallest in group A (ESM Supplementary Table 1). A definite UIP pattern was identified in 1969 patients, and a possible UIP pattern was identified in 730 patients and a pattern inconsistent for UIP in 88 patients.
Patient Comorbidities
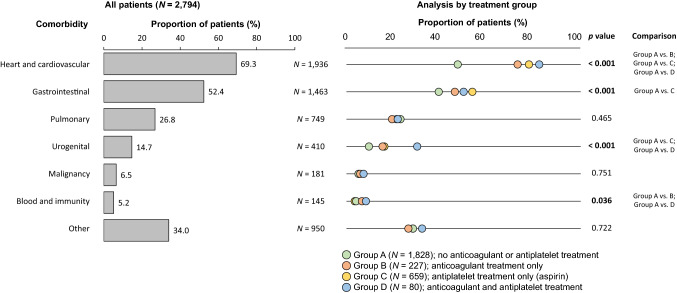
The leading comorbidity was cardiovascular issues, mainly arterial hypertension, and this was observed in more than two-thirds of patients (Fig. 2). Table 2 summarizes the distribution of cardiovascular disorders; overall, 10.6–13.7% of patients had a disease requiring anticoagulant therapy and ~ 28.2% had a disease requiring antiplatelet therapy. The next most common comorbidities were gastrointestinal and pulmonary problems. In general, patients in group A had the lowest rate of comorbidities. A detailed analysis of comorbidities is shown in Fig. 2.
Fig. 2.
Comorbidities according to treatment group. The comparisons listed had statistically significant differences between the two groups (Bonferroni correction was used)
Table 2.
Distribution and type of cardiovascular disorders
| Cardiovascular disorder | Occurrence | ||||
|---|---|---|---|---|---|
| Total N = 2794 |
Group A n = 1828 |
Group B n = 227 |
Group C n = 659 |
Group D n = 80 |
|
| Arterial hypertension | 1397 (50.0) | 777 (42.5) | 132 (58.1) | 435 (66.0) | 53 (66.3) |
| Coronary heart disease | 585 (20.9) | 170 (9.3) | 44 (19.4) | 318 (48.3) | 53 (66.3) |
| Arrhythmia – especially atrial fibrillation | 240 (8.6) | 60 (3.3) | 97 (42.7) | 55 (8.3) | 28 (35.0) |
| Pulmonary hypertension | 230 (8.2) | 153 (8.4) | 22 (9.7) | 46 (7.0) | 9 (11.3) |
| Myocardial infarction | 147 (5.3) | 27 (1.5) | 10 (4.4) | 97 (14.7) | 13 (16.3) |
| Cerebrovascular accident and transient ischemic attack | 100 (3.6) | 29 (1.6) | 41 (18.1) | 22 (3.3) | 8 (10.0) |
| Thrombosis and embolism | 99 (3.5) | 17 (0.9) | 18 (7.9) | 55 (8.3) | 9 (11.3) |
| Valvular heart defect, aneurysm | 98 (3.5) | 31 (1.7) | 21 (9.3) | 37 (5.6) | 9 (11.3) |
| Lower limb ischemia | 54 (1.9) | 11 (0.6) | 12 (5.3) | 27 (4.1) | 4 (5.0) |
| Non-ischemic cardiomyopathy | 28 (1.0) | 13 (0.7) | 6 (2.6) | 9 (1.4) | 0 (0.0) |
| Other | 203 (7.3) | 104 (5.7) | 18 (7.9) | 71 (10.8) | 10 (12.5) |
Data are presented as n (%)
Antifibrotic Treatment
A total of 673 (24.1%) patients received nintedanib therapy. A higher proportion of patients in groups A and C received nintedanib treatment compared with group B (Table 1). Pirfenidone was received by 933 (33.4%) patients. A higher proportion of patients received pirfenidone at the time of investigation in group D compared with group A (Table 1).
Analysis of Lung Function
Information on lung function and 6MWT is summarized in Table 3 (additional information on lung function is provided in the ESM [Supplementary Table 2]). FVC % predicted and DLCO % predicted were assessed at inclusion. Information was unavailable for 886 patients for FVC %, 1187 patients for FEV1 %, and 1069 patients for DLCO %. When observing the FVC % values across the four groups, 171 patients had < 50% predicted, 913 patients had values between 50 and 80% predicted, and 824 patients had > 80% predicted; observed mean FVC % predicted values were similar between the four groups (Table 3). There were numerical differences in DLCO % predicted between groups A and C: values were < 30% predicted in 219 patients, between 30 and 80% predicted in 1403 patients, and > 80% predicted in 103 patients. 6MWT distance was numerically higher in group A compared with group B.
Table 3.
Lung function values and 6MWT according to treatment groups
| Values | Group A | Group B | Group C | Group D |
|---|---|---|---|---|
| FVC (L) | 2.47 (1.26–4.10) | 2.49 (1.26–3.95) | 2.54 (1.38–3.99) | 2.63 (1.60–3.65) |
| FVC (% predicted) | 77 (44–117) | 73 (42–105) | 77 (49–117) | 74 (49–115) |
| DLCO % | 47.8 (21.9–86.1) | 44.9 (21.4–72.9) | 44.8 (20.4–78.0) | 44.0 (22.5–72.7) |
| 6MWT | ||||
| Distance, m | 398 (175–559) | 360 (63–540) | 386 (167–543) | 350 (142–500) |
Data are presented as mean (5th–95th percentile)
6MWT six-minute walk test, DLCO diffusing lung capacity for carbon monoxide, FVC forced vital capacity
Bleeding as an Adverse Event
Bleeding was observed in eight patients across groups A, C, and D (8/2794 patients [0.29%]; nintedanib = 0.25%; pirfenidone = 0.04%); details of these events are shown in Table 4. Overall, 147 patients were identified where anticoagulant and/or antiplatelet treatment was recorded after the initiation of antifibrotic therapy (mean 9.6 months; 5th–95th percentile 0.5–49.0 months); bleeding events were not observed in any of these patients. The number of patients who received anticoagulant and/or antiplatelet treatment following antifibrotic therapy was relatively low (5.3% of all patients and 10.3% of patients treated with antifibrotics), and their distribution within groups B–D was approximately equal (B, 17.2%; C, 14.1%; D, 18.8%). The incidence of bleeding was 8.9 and 136.9 per 10,000 patient-years in patients treated with pirfenidone and nintedanib, respectively (Table 5). Overall, the relative incidence ratio for nintedanib to pirfenidone treatment was 15.4 (95% CI 2.0–695.3; P < 0.001) (Table 5). The incidence of bleeding was 3.0, 0, 1.3, and 18.1 per 10,000 patient-years in groups A, B, C, and D, respectively, and group D was significantly different from the other groups (Table 6). The absolute occurrence of bleeding was highest in group D, although there was no significant difference in bleeding between groups (P = 0.072; Table 6). Bleeding events were observed in groups A, C, and D, and the majority of events were mild or moderate in severity (seven of eight cases; Table 6). Except for one patient who received treatment with pirfenidone, all patients with bleeding events had received nintedanib (seven of eight cases; Table 7).
Table 4.
Bleeding events across all patients
| Patient | Treatment group | Antifibrotic treatment | Severity | Description | Associated CVD | Time from anticoagulant/antiplatelet treatment to event (months)a | Duration of antifibrotic treatment to event (months) |
|---|---|---|---|---|---|---|---|
| 1 | Group D | Pirfenidone | Mild | Ear bleeding | Coronary heart disease | 31.4 | 27.9 |
| 2 | Group A | Nintedanib | Mild | Bleeding from hemorrhoids | Arterial hypertension | – | 8.6 |
| 3 | Group A | Nintedanib | Mild | Hemoptysis for 3 weeks | None | – | 2.7 |
| 4 | Group A | Nintedanib | Mild | Bleeding from hemorrhoids | None | – | 10.0 |
| 5 | Group A | Nintedanib | Mild | Epistaxis and hematochezia | Arterial hypertension | – | 0.3 |
| 6 | Group D | Nintedanib | Moderate | Adrenal hematoma |
Coronary heart disease Arrhythmia—especially AFb Heart failure |
80.5 | 0.3 |
| 7 | Group A | Nintedanib | Mild | Hematuria |
Arterial hypertension Arrhythmia—especially AFc |
– | 3.6 |
| 8 | Group C | Nintedanib | Severe | Gastrointestinal hemorrhage | Arterial hypertension | 11.0 | 9.4 |
AF atrial fibrillation, CVD cardiovascular disease
aFive patients had terminated the anticoagulant/antiplatelet treatment before the bleeding event occurred
bType of arrhythmia not specified; patient was treated with propafenone, rivaroxaban and later dabigatran
cSpecified as ‘supraventricular tachyarrhythmia (probably supraventricular)’ by the attending physician
Table 5.
Incidence of bleeding according to the idiopathic pulmonary fibrosis treatment group
| IPF treatment | |||
|---|---|---|---|
| Pirfenidone | Nintedanib | Other | |
| Patients, n (PY at risk) | |||
| Group A | 589 (549.3) | 465 (312.5) | 774 (579.9) |
| Group B | 86 (111.5) | 31 (25.3) | 110 (97.4) |
| Group C | 220 (225.9) | 161 (166.6) | 278 (271.9) |
| Group D | 38 (63.2) | 16 (16.1) | 26 (26.2) |
| Total | 933 (1123.6) | 673 (514.7) | 1188 (975.5) |
| Number of bleeding events (% of all bleeding events) | |||
| Group A | 0 (0.0) | 5 (62.5) | 0 (0.0) |
| Group B | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Group C | 0 (0.0) | 1 (12.5) | 0 (0.0) |
| Group D | 1 (12.5) | 1 (12.5) | 0 (0.0) |
| Total | 1 (12.5) | 7 (87.5) | 0 (0.0) |
| Incidence of bleeding | |||
| Rate/10,000 PY, total (95% CI) | 8.9 (1.3–63.0) | 136.9 (65.2–287.1) | – |
| Relative incidence ratio nintedanib: pirfenidone (95% CI) |
15.4 (2.0–695.3) P < 0.001 |
||
CI confidence interval, IPF idiopathic pulmonary fibrosis, PY patient-years
Table 6.
Severity of bleeding according to anticoagulation and antiplatelet treatment groups
| Group A (N = 1828) | Group B (N = 227) | Group C (N = 659) | Group D (N = 80) | P value | |
|---|---|---|---|---|---|
| Bleeding | 5 (0.3) | 0 (0.0) | 1 (0.2) | 2 (2.5) | 0.072 |
| Incidence of bleeding/10,000 patient-years | 3.0 | 0 | 1.3 | 18.1 | – |
| Severity of bleeding | |||||
| Mild | 5 (100.0) | – | – | 1 (50.0) | 0.061 |
| Moderate | – | – | – | 1 (50.0) | |
| Severe | – | – | 1 (100.0) | – | |
Data are n (%) or incidence per 10,000 patient-years
Table 7.
Severity of bleeding according to antifibrotic treatment groups
| Total (N = 1606) | Pirfenidone (n = 933) | Nintedanib (n = 673) | P value | |
|---|---|---|---|---|
| Bleeding | 8 (0.5) | 1 (0.1) | 7 (1.0) | 0.007 |
| Severity of bleeding | ||||
| Mild | 6 (75.0) | 1 (100.0) | 5 (71.4) | 0.733 |
| Moderate | 1 (12.5) | – | 1 (14.3) | |
| Severe | 1 (12.5) | – | 1 (14.3) | |
Data are presented as n (%). Bold formatting indicates significance
Discussion
In this study, we present data on anticoagulant use and bleeding risk in patients with IPF from Central and Eastern Europe enrolled in EMPIRE. Overall, pirfenidone was widely used irrespective of anticoagulation treatment, whereas nintedanib was less frequently received by patients taking regular anticoagulation medication. While the incidence of bleeding overall was low (8/2794 patients [0.29%]), the majority of patients who experienced a bleeding event had received nintedanib treatment (seven of eight patients) without anticoagulant and antiplatelet therapy (five of eight patients). The incidence of bleeding was 3.0, 0, 1.3, and 18.1 per 10,000 patient-years in groups A, B, C, and D, respectively, and the relative rate of bleeding in patients treated with nintedanib was 136.9 per 10,000 patient-years. To date, no real-world data on patient characteristics and antifibrotic treatment have been available as patients treated with full-dose anticoagulants and high-dose antiplatelet therapy have previously been excluded from clinical trials. Ours is the first study to describe patients with IPF, treated with antifibrotics, stratified into groups depending on the type of anticoagulant or antiplatelet medication received.
Previous reports on bleeding events in patients with IPF and vascular disease have been published, although the overall risk remains unclear [21, 22]. Studies have linked a prothrombotic state with promotion of fibrosis [23, 24], and there is strong evidence for avoiding warfarin anticoagulation in patients with IPF [25]. A recent review of antiplatelet therapy found that while bleeding risk was often considered a minor problem, it is associated with an increased risk of mortality and morbidity [26]. Despite this, registry data suggest that anticoagulants/antiplatelet drugs are still prescribed to patients with IPF [27]. In our study, approximately one-third of the patients received anticoagulant and/or antiplatelet treatment (966/2974 [32.5%]); however, administration of antiplatelet medication was not associated with an increase in bleeding events, even when used in combination with anticoagulant treatment, and the majority of patients with bleeding events had not received anticoagulant or antiplatelet therapy. Patients with cardiovascular disorders in the present study typically had comorbidities that required anticoagulation and/or antiplatelet therapy as described for groups B, C, and D. This suggests a need for real-world data that include patients receiving co-medications that alter homeostasis that may have excluded them from previous clinical trials of IPF treatments such as nintedanib. In addition, most recorded bleeding events were mild or moderate in severity (seven of eight).
In studies of the vascular endothelial growth factor tyrosine kinase inhibitors (e.g., sunitinib or sorafenib or dasatinib), gastrointestinal bleeding and epistaxis were observed in a substantial proportion of patients [28, 29]. Because of its inhibition of the VEGFR, nintedanib may be associated with an increased risk of bleeding and is recommended for use in patients with known risk of bleeding only if the anticipated benefit outweighs the potential risk [4, 5, 16, 17]. In a pooled analysis of the INPULSIS trials of nintedanib in patients with IPF, bleeding events were observed in 10.3% of patients in the nintedanib group versus 7.8% in the placebo group [30]. The majority of bleeding events in these trials were non-serious, with epistaxis (nintedanib group 4.1%; placebo group 3.1%) and contusion (nintedanib group 1.6%; placebo group 0.9%) the most frequently reported [30]. A US postmarketing surveillance analysis of 3838 patients with IPF treated with nintedanib identified 120 bleeding AEs, two of which had a fatal outcome [31]. Overall, the relative incidence of bleeding events in patients treated with nintedanib was low in our study (7/673 patients [1.0%]) and did not appear to be related to anticoagulant or antiplatelet treatment (five of eight patients with bleeding events had not received either anticoagulant or antiplatelet treatment). Because of the potential risk of bleeding associated with nintedanib, more attention may have been paid to this AE in patients treated with nintedanib in the randomized controlled trials, compared with trials of pirfenidone, and bleeding may have been under-reported in real-world studies. However, based on real-world data from EMPIRE, a direct causal link between bleeding events and antifibrotic therapies could not be confirmed.
Treatment of IPF with nintedanib in patients with a known risk of bleeding or cardiovascular disease should be approached with caution [32]. Direct oral anticoagulants (DOACs) have a better efficacy and safety profile than vitamin K antagonists such as warfarin, with lower bleeding rates [33]. Patient selection, anticoagulant dose choice (based on patient age, renal function, weight, and presence of drug interactions), and bleeding risk scoring systems may help limit the risk factors associated with DOAC use [33]. However, in our study, we did not have data on the specific oral medications used for anticoagulation, and this may affect outcome. It should be noted that the use of these drugs is not contraindicated for patients receiving nintedanib, and the benefits and risks should be discussed with individual patients when deciding appropriate treatment.
According to a systematic literature review of comorbidities associated with IPF, respiratory comorbidities such as chronic obstructive pulmonary disease, pulmonary hypertension, and obstructive sleep apnea were common in many of the studies examined, although estimates varied depending on study population [34]. Anticoagulation treatment is often required for patients with atrial fibrillation, different forms of thrombosis, and embolism (affecting ~ 10% of patients; Table 2), as well as for some forms of valvular heart defect. In addition, almost all cases of myocardial infarction, coronary heart disease, cerebrovascular accident, transient ischemic attack, and lower limb ischemia require antiplatelet therapy (~ 28% received this treatment; Table 2). Non-respiratory comorbidities such as systemic arterial hypertension, ischemic heart disease, gastroesophageal reflux disease, and diabetes were also highly prevalent among patients with IPF [34]. In our study, a large proportion of patients had at least one comorbidity, and the vast majority had more than one additional disease; this was more pronounced for patients in group D who received anticoagulant and antiplatelet treatment.
Patients with IPF usually exhibit age-related comorbidities, including pulmonary hypertension, cardiovascular diseases, osteoporosis, gastroesophageal reflux disease, obstructive sleep apnea, emphysema, lung cancer, and diabetes mellitus, that alter the natural course of the disease [35]. Women are typically better protected than men against cardiovascular disease, which is attributed, at least in part, to the hormone estrogen [36]. Group A included patients who had not received anticoagulant or antiplatelet treatment and had fewer cardiovascular problems, and therefore included more women, younger patients with better GAP scores, and milder/less advanced IPF (lower New York Heart Association score and fewer lung crackles). Among patients in EMPIRE, cardiovascular comorbidities were the most common type and were significantly higher in patients treated with anticoagulants and/or antiplatelet treatment versus patients who received no anticoagulant or antiplatelet therapy (P < 0.001); the next most common comorbidities were gastrointestinal disorders and pulmonary comorbidities. With the exception of gastrointestinal and pulmonary issues, patients in group D (on both antiplatelet and anticoagulant medication) were the most frequently affected by comorbidities.
Limitations included missing data for some of the lung function parameters, i.e., information was not available for 886 patients for FVC%, 1187 patients for FEV1%, and 1069 patients for DLCO%; however, lung function data were used for the description of patients at baseline only, and the impact of missing data on the results of the study has not been monitored or analyzed. The proportion of patients from different countries was similar in groups B–D; differences were noted in group A, where there was a lower proportion of patients from the Czech Republic and a higher proportion of patients from Turkey; this may reflect differences in treatment practices between countries. Since EMPIRE primarily focused on IPF, rather than cardiovascular diseases, it is possible that patients began anticoagulant and/or antiplatelet therapy earlier than was recorded on the follow-up form. Detailed information on anticoagulation and/or antiplatelet therapy (i.e., dose or type, or distribution of cardiovascular disorder within each group) was not available.
Conclusions
The study shows that the incidence of bleeding was low in patients treated with antifibrotics in a real-world setting. Despite caution for the use of nintedanib in patients receiving anticoagulant and/or antiplatelet treatment, the overall incidence of bleeding AEs was acceptable. Pirfenidone is widely used irrespective of concomitant anticoagulant and/or antiplatelet treatment, whereas patients receiving regular anticoagulation treatment were less likely to receive nintedanib. Bleeding was reported in only seven patients with IPF who received nintedanib (0.25% of all patients in the analysis), with five of these bleeding events recorded in patients who received nintedanib without anticoagulant and antiplatelet treatment. Overall, however, the very low number of non-severe bleeding events provides reassurance on the safety concerns of nintedanib in anticoagulant- and/or antiplatelet-treated patients with IPF in a real-world setting.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Open access funding provided by Semmelweis University. The authors thank the patients, their families, the nurses, and the investigators who participated in this study. Medical writing assistance was supported financially by Boehringer Ingelheim and provided by Islay Steele, PhD, of Nucleus Global under the authors’ conceptual direction and based on feedback from the authors. Boehringer Ingelheim supported the analysis of EMPIRE registry data reported here.
Compliance with Ethical Standards
Author Contributions
All authors were involved in acquisition, analysis, or interpretation of data for the work. MV is the principal investigator of EMPIRE. All authors were involved in drafting the work or critically revising it for important intellectual content, and provided final approval of the version to be published.
Funding
This study was supported by Boehringer Ingelheim International GmbH (BI). Medical writing assistance was provided by Islay Steele, PhD, of Nucleus Global, which was contracted and funded by Boehringer Ingelheim International GmbH. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Conflicts of interest
JK has received financial support, including consultant fees, support for travel to international conferences, lectures, honoraria, and clinical trials investigator payments from Boehringer Ingelheim and Roche. MH has received lecture fees and consulting fees and served as an advisory board member for Boehringer Ingelheim. VM has received consulting fees from Roche and Boehringer Ingelheim. DJ has received consulting fees or honorarium and payment for lectures from Roche and Boehringer Ingelheim. JT-T has received payment for lectures from Roche and Boehringer Ingelheim and consulting fees from Boehringer Ingelheim. MV has received an independent grant from Roche and consultancy, lecture, and advisory board fees from Boehringer Ingelheim and Roche. MS has received consultation fees from Boehringer Ingelheim. AK-F, NM, SL, KH have no personal conflicts of interest that are directly relevant to the content of this article.
Authorship
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors.
Ethical approval and informed consent
The EMPIRE registry protocol was approved in each country by the respective ethical committee. Written informed consent was obtained from all individual patients, who were enrolled in accordance with the Helsinki Declaration.
Data sharing
Data sharing is not applicable to this article as no datasets were analyzed during the current study.
References
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molina-Molina M, Aburto M, Acosta O, Ancochea J, Rodríguez-Portal JA, Sauleda J, et al. Importance of early diagnosis and treatment in idiopathic pulmonary fibrosis. Expert Rev Respir Med. 2018;12:537–539. doi: 10.1080/17476348.2018.1472580. [DOI] [PubMed] [Google Scholar]
- 3.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e44–e68. [DOI] [PubMed]
- 4.European Medicines Agency. OFEV® (nintedanib): Summary of Product Characteristics. 2019. https://www.ema.europa.eu/documents/product-information/ofev-epar-product-information_en.pdf. Accessed 10 Jan 2020.
- 5.U.S. Food & Drug Administration. OFEV® (nintedanib) prescribing information. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205832s000lbl.pdf. Accessed 28 Nov 2019.
- 6.Genentech. ESBRIET® (pirfenidone) capsules and film-coated tablets, for oral use. 2017. https://www.gene.com/download/pdf/esbriet_prescribing.pdf. Accessed 11 Nov 2019.
- 7.European Medicines Agency. Esbriet (pirfenidone): Summary of Product Characteristics. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002154/WC500103049.pdf. Accessed 15 Nov 2019.
- 8.Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–829. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 9.Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev. 2011;20:85–97. doi: 10.1183/09059180.00001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 11.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 12.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 13.Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68:4774–4782. doi: 10.1158/0008-5472.CAN-07-6307. [DOI] [PubMed] [Google Scholar]
- 14.Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther. 2014;349:209–220. doi: 10.1124/jpet.113.208223. [DOI] [PubMed] [Google Scholar]
- 15.Wollin L, Distler JHW, Redente EF, Riches DWH, Stowasser S, Schlenker-Herceg R, et al. Potential of nintedanib in treatment of progressive fibrosing interstitial lung diseases. Eur Respir J. 2019;54:1900161. doi: 10.1183/13993003.00161-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roodhart JM, Langenberg MH, Witteveen E, Voest EE. The molecular basis of class side effects due to treatment with inhibitors of the VEGF/VEGFR pathway. Curr Clin Pharmacol. 2008;3:132–143. doi: 10.2174/157488408784293705. [DOI] [PubMed] [Google Scholar]
- 17.Touyz RM, Herrmann S, Herrmann J. Vascular toxicities with VEGF inhibitor therapies-focus on hypertension and arterial thrombotic events. J Am Soc Hypertens. 2018;12:409–425. doi: 10.1016/j.jash.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richeldi L, Cottin V, Flaherty KR, Kolb M, Inoue Y, Raghu G, et al. Design of the INPULSIS™ trials: two phase 3 trials of nintedanib in patients with idiopathic pulmonary fibrosis. Respir Med. 2014;108:1023–1030. doi: 10.1016/j.rmed.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 19.EMPIRE. EMPIRE Registry home page. 2019. http://empire.registry.cz/index-en.php. Accessed 7 Feb 2019.
- 20.Tran T, Šterclová M, Mogulkoc N, Lewandowska K, Müller V, Hájková M, et al. The European MultiPartner IPF registry (EMPIRE): validating long-term prognostic factors in idiopathic pulmonary fibrosis. Respir Res. 2020;21:11. doi: 10.1186/s12931-019-1271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubbard RB, Smith C, Le Jeune I, Gribbin J, Fogarty AW. The association between idiopathic pulmonary fibrosis and vascular disease: a population-based study. Am J Respir Crit Care Med. 2008;178:1257–1261. doi: 10.1164/rccm.200805-725OC. [DOI] [PubMed] [Google Scholar]
- 22.Sprunger DB, Olson AL, Huie TJ, Fernandez-Perez ER, Fischer A, Solomon JJ, et al. Pulmonary fibrosis is associated with an elevated risk of thromboembolic disease. Eur Respir J. 2012;39:125–132. doi: 10.1183/09031936.00041411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chambers RC. Procoagulant signalling mechanisms in lung inflammation and fibrosis: novel opportunities for pharmacological intervention? Br J Pharmacol. 2008;153:S367–S378. doi: 10.1038/sj.bjp.0707603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navaratnam V, Fogarty AW, McKeever T, Thompson N, Jenkins G, Johnson SR, et al. Presence of a prothrombotic state in people with idiopathic pulmonary fibrosis: a population-based case-control study. Thorax. 2014;69:207–215. doi: 10.1136/thoraxjnl-2013-203740. [DOI] [PubMed] [Google Scholar]
- 25.Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. An official ATS/ERS/JRS/ALAT Clinical Practice Guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015;192:e3–e19. [DOI] [PubMed]
- 26.Esmonde S, Sharma D, Peace A. Antiplatelet agents in uncertain clinical scenarios—a bleeding nightmare. Cardiovasc Diagn Ther. 2018;8:647–662. doi: 10.21037/cdt.2018.06.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behr J, Kreuter M, Hoeper MM, Wirtz H, Klotsche J, Koschel D, et al. Management of patients with idiopathic pulmonary fibrosis in clinical practice: the INSIGHTS-IPF registry. Eur Respir J. 2015;46:186–196. doi: 10.1183/09031936.00217614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brave M, Goodman V, Kaminskas E, Farrell A, Timmer W, Pope S, et al. Sprycel for chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant to or intolerant of imatinib mesylate. Clin Cancer Res. 2008;14:352–359. doi: 10.1158/1078-0432.CCR-07-4175. [DOI] [PubMed] [Google Scholar]
- 29.Je Y, Schutz FAB, Choueiri TK. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. Lancet Oncol. 2009;10:967–974. doi: 10.1016/S1470-2045(09)70222-0. [DOI] [PubMed] [Google Scholar]
- 30.Corte T, Bonella F, Crestani B, Demedts MG, Richeldi L, Coeck C, et al. Safety, tolerability and appropriate use of nintedanib in idiopathic pulmonary fibrosis. Respir Res. 2015;16:116. doi: 10.1186/s12931-015-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noth I, Allinger A, Kaul M, Conoscenti CS, Oelberg D. Safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis (IPF): one-year data from post-marketing surveillance in the United States. Am J Respir Crit Care Med. 2016;193:A2692. [Google Scholar]
- 32.Case AH, Johnson P. Clinical use of nintedanib in patients with idiopathic pulmonary fibrosis. BMJ Open Respir Res. 2017;4:e000192. doi: 10.1136/bmjresp-2017-000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hellenbart EL, Faulkenberg KD, Finks SW. Evaluation of bleeding in patients receiving direct oral anticoagulants. Vasc Health Risk Manag. 2017;13:325–342. doi: 10.2147/VHRM.S121661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raghu G, Amatto VC, Behr J, Stowasser S. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. Eur Respir J. 2015;46:1113–1130. doi: 10.1183/13993003.02316-2014. [DOI] [PubMed] [Google Scholar]
- 35.Buendía-Roldán I, Mejía M, Navarro C, Selman M. Idiopathic pulmonary fibrosis: clinical behavior and aging associated comorbidities. Respir Med. 2017;129:46–52. doi: 10.1016/j.rmed.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ. 2017;8:33. doi: 10.1186/s13293-017-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.