Abstract

The rapid and reliable detection of lethal agents such as sarin is of increasing importance. Here, density-functional theory (DFT) is used to compare the interaction of sarin with single-metal-centered phthalocyanine (MPc) and MPc layer structures to a benign model system, i.e., the adsorption of dimethyl methylphosphonate (DMMP). The calculations show that sarin and DMMP behave nearly identical to the various MPcs studied. Among NiPc, CuPc, CoPc, and zinc phthalocyanine (ZnPc), we find the interaction of both sarin and DMMP to be the strongest with ZnPc, both in terms of interaction energy and adsorption-induced work function changes. ZnPc is thus proposed as a promising sensor for sarin detection. Using X-ray photoelectron spectroscopy, the theoretically predicted charge transfer from DMMP to ZnPc is confirmed and identified as a key component in the sensing mechanism.
1. Introduction
Gas sensors have been attracting increasing interest due to their wide range of applications spanning across environmental protection, detection of combustion gases, medical diagnosis, and military and civil safety.1−4 The relevance of these applications has led to intense research in the area of detection techniques and sensing materials. One prominent example in this respect is sarin gas (Figure 1a), a nerve agent that causes death by suffocation within 10 min of exposure to concentrations above 60 ppb.5 Detection of sarin gas has been the subject of numerous investigations in the past decade.6−11 Obviously, sensing devices are required to detect extremely low sarin concentrations and, more importantly, to be able to respond very rapidly. At the same time, the costs associated with sensor production and maintenance are important factors that need to be taken into consideration when proposing new sensors. The thorough, atom-scale understanding of the sensing mechanism is expected to be extremely helpful to tune respective devices and eventually to realize competitive sensor components for industrial production. The gas adsorption and its impact on the sensing material are of crucial importance for the sensor design.12−16
Figure 1.

Schematic representation of (a) sarin, (b) dimethyl methylphosphonate (DMMP), and (c) zinc phthalocyanine (ZnPc). On sarin and DMMP, we label the nonequivalent carbon/oxygen atoms as C1/O1 and C2/O2. On ZnPc, the dashed line is a guide to the eye marking a long molecular axis (LA).
For experimental work, the availability of a benign model system replacing sarin is of crucial importance. Dimethyl methylphosphonate (DMMP) (Figure 1b) is one possible candidate with much lower toxicity in this context.17,18 It features the same characteristic P=O (O1) group and may thus be used to simulate sarin. In the current work, we aim to achieve a thorough understanding of the interaction between sarin and DMMP with zinc phthalocyanine (ZnPc)-based sensing layers. With this, we provide a reference and an effective method for detecting the real nerve agent sarin.
Phthalocyanines (Pc) are structurally related to the well-known porphyrin and corroles in the sense that these molecular species possess tetrapyrrolic macrocycles. Both metal-free (H2Pc) and metal-centered phthalocyanines (MPc) (Figure 1c) are organic semiconductors widely applied in organic electronics,19−22 solar cells,19,20,23,24 and gas sensors.22,25−29 The latter application is based on the strong dependence of the electrical conductivity of Pc films on the chemical species present in the atmosphere.30 It profits from the ease of Pc deposition, their ability to form high-quality films, their remarkable thermal and chemical stabilities, and the possibility to functionalize Pc by changing the central atom or adding substitutes to the phthalocyanine rings.29,31−34
The interaction of MPc with different oxidizing gases, e.g., O2, NOx, and O3, has been found to lead to a p-type conductivity attributed to a redox reaction (see, e.g., refs (35) and (36)). In contrast, the MPc interaction with reducing gases has been reported to decrease the conductivity due to hole trapping by chemisorbed agent-donated electrons.37 Bohrer et al.38 related the adsorption enthalpies with different MPcs (CoPc, CuPc, ZnPc, and NiPc) to the sensitivity expressed by the changes in the film conductivity and found an exponential relation between the sensitivity and the binding enthalpy of various reducing agents. The low sensor response to weak electron donors was attributed to the low availability of the strongest adsorption sites, i.e., metal centers, due to the oxygen adsorption from the ambience. However, there are other conceivable factors that might be responsible such as the orientation of the Pc molecules within the sensing layer: there are, for example, more adsorption sites available for well-ordered flat-laying ZnPc structures compared to vertical orientations. The realization of specific film morphologies, however, requires control of various experimental conditions such as the substrate, deposition technique, deposition temperature, and pressure.39−43
The sensitivity of phthalocyanines and porphyrins (i.e., H2Pc, CuPc, NiPc, PbPc, ZnPc, TiPc, MnTPP, InTPP) to, e.g., DMMP has been explored in refs (38) and (44−48). Despite this potential possibility of using phthalocyanines or porphyrins in, e.g., DMMP sensing, only a few studies regarding this topic have been recently published.28,34,49,50 There is also a number of theoretical studies on molecular adsorption on phthalocyanines and porphyrins.46,51−53 In this work, we combine density-functional theory (DFT) calculations with X-ray photoelectron spectroscopy (XPS) to provide profound knowledge about the sensing mechanism of sarin and DMMP by ZnPc films. Thereby, the present paper is organized as follows: after introducing the theoretical and experimental methods, we confirm almost identical geometric and electronic properties, and by these similar sensing mechanisms of sarin and DMMP by MPcs (M = Co, Ni, Cu, Zn), and rationalize the choice of ZnPc as a sensing layer by comparing various phthalocyanines. Then, the DMMP–MPc adsorption geometries and the related charge transfer are analyzed to provide detailed insight into the sensing mechanism. Finally, the predicted effects induced by detecting DMMP by ZnPc are validated by comparing predicted and measured XPS 1s (N, C) and 2p (Zn) binding energies.
This work provides profound knowledge about the sensing mechanism in the investigated systems (sarin and DMMP), enabling benchmark data for similar systems in the future.
2. Materials and Methods
2.1. DFT Study for the Molecular Substrates
2.1.1. Plane-Wave (PW) Pseudopotential Approach
The PW DFT calculations were performed with the Quantum ESPRESSO package.54 The Perdew–Burke–Ernzerhof (PBE) functional55 and the PBE + U extension (with self-consistently determined U = 4.007 eV)56 complemented with dispersion correction (DFT-D)57 were used to model the electron exchange and correlation. Norm-conserving pseudopotentials were used to describe the electron–ion interaction. Plane waves up to a cutoff energy of 90 Ry were used as basis functions. The atomic structure relaxation for single MPc was done in periodically repeated 30 Å × 30 Å × 24 Å supercells. Molecular monolayers (MLs) were described within lateral periodicities between 6 Å × 6 Å and 15 Å × 15 Å. Convergence criteria of 10–4 eV/Å for forces and 10–8 eV for the total energy were used. The adsorption energies were calculated as
| 1 |
where EMPc–DMMP/sarin is the total energy of the adsystem (DMMP/sarin adsorbed on MPc); EMPc and EDMMP are the total energies of the MPc and DMMP molecule in the gas phase, respectively. The XPS spectra were calculated using a ΔSCF approach, which, for light elements such as nitrogen, has been shown to give highly accurate core-level shifts (CLS).58−61 To model the 1s (2p) core holes of the C and N (Zn) species, multiprojector (gauge including (GI-))projector augmented wave (PAW) pseudopotentials62 with a corresponding occupation of the inner shells were generated. The resulting core-level shifts (CLS) were superimposed and convoluted by assuming a linewidth of 0.7 eV.
2.1.2. All-Electron (AE) Calculations
In the all-electron (AE) calculations, we utilized the hybrid Becke3–Lee–Yang–Parr (B3LYP) exchange–correlation (XC) functional63−65 with the def2-TZVP (split-valence triple-ζ) basis sets.66 The ORCA Package67 has been used to calculate the optimized structures and the adsorption energies of DMMP and sarin on MPc structures. The calculations account for the dispersion corrections through the Grimme approach using atom pairwise additive schemes (the so-called DFT-D3 method).68
2.2. Sample Preparation
2.2.1. ZnPc Deposition
ZnPc (10 nm) was deposited by the thermal evaporation method onto glass substrates with interdigitated gold electrodes (Metrohm DropSens) and a molybdenum oxide (MoO3) thin layer. Substrates were precleaned with isopropanol and purged with pure N and then kept in an ultraviolet (UV) cleaner for 5 min. A 10-nm-thick film of ZnPc was deposited from the sublimed powder (97% dye content; Sigma-Aldrich GmbH) on the substrate at room temperature in high vacuum conditions (base pressure: 10–6 mbar) using the Lesker Spectros II Evaporation System with quartz crystal microbalance (QCM) thickness control. The deposition rate was kept at a level of 0.5 Å/s.
2.2.2. DMMP Exposure
The sample was placed
in an environmental cell (320 mL) possessing a gas inlet and outlet
and electrical feedthrough. DMMP vapor was prepared from an Owlstone
vapor generator (OVG-4), with a certified permeation tube calibrated
at 70 °C. Nitrogen 5.0 (Air Liquide) was applied as a carrier
gas. The cell was saturated with a nitrogen/DMMP mixture. During DMMP
deposition, the chamber outlet was closed. The permeation oven was
heated to 100 °C. The nitrogen/DMMP mixture flow rate was set
to 50 mL/min. The concentration of DMMP in the mixture for the given
flow can be calculated via 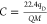 , where C is the concentration
(ppm), qD is the permeation rate (ng/min), Q is the flow rate (50 mL/min), and M is
the DMMP molecular weight (124.08 g/mol). The latter depends strongly
on the temperature. According to the manufacturer, based on the value
at a calibration temperature T1 = 70 °C
(qd1 = 152 ng/min), the permeation
rate qd(T) at a given
temperature T is given by the empirical relation
, where C is the concentration
(ppm), qD is the permeation rate (ng/min), Q is the flow rate (50 mL/min), and M is
the DMMP molecular weight (124.08 g/mol). The latter depends strongly
on the temperature. According to the manufacturer, based on the value
at a calibration temperature T1 = 70 °C
(qd1 = 152 ng/min), the permeation
rate qd(T) at a given
temperature T is given by the empirical relation
For a temperature of 100 °C (oven), we calculate a permeation rate of 747.4 ng/min and, from this, the DMMP concentration in the carrier gas is 6 ppm.
After 2 h, the nitrogen/DMMP flow was stopped and the sample was kept for 3 days in the cell with the closed outlet. To provide a stable temperature during DMMP deposition, the samples were placed on a ceramic heater controlled by a DC power supply. The temperature on the sample’s surface was measured by a Pt100 temperature controller connected to an Agilent 34970A Multimeter.
2.3. Photoelectron Spectroscopy
X-ray photoelectron spectroscopy (XPS) was performed using a PREVAC EA15 hemispherical electron energy analyzer with a two-dimensional (2D) multichannel plate detector. The system base pressure was 9 × 10–11 mbar. The samples were irradiated with an energy of 1486.60 eV provided by an Al Kα X-ray source (PREVAC dual-anode XR-40B source). A pass energy of 200 eV energy was set for survey spectra (scanning step: 0.9 eV), while a pass energy of 100 eV was set (scanning step: 0.05 eV) for particular energy regions. Curved analyzer transfer slits were utilized for enhancing energy resolution. All of the spectra were taken with a normal (i.e., 90° between the sample plane and the analyzer axis) takeoff angle. The energy scale of the analyzer was calibrated to Au 4f7/2 (84.0 eV), while the particular spectra were calibrated to C–C binding energy at 284.6 eV.69,70 For data analysis, spectra were fitted utilizing CasaXPS-embedded algorithms and relative sensitivity factors. For background subtraction, the Shirley function was used. If not explicitly specified in the text, the components were represented by a product of Gaussian (70%) and Lorentzian (30%) lines.
3. Results and Discussion
3.1. Single-Molecule Adsorption
The calculations start by confirming that DMMP is a reliable model for sarin detection and by establishing the most suitable sensor layer material for efficient detection. Thereby, we explore the adsorption of both DMMP and sarin on a variety of single MPc (NiPc, CuPc, CoPc, and ZnPc) molecules. To calculate the stable adsorption structures, a large variety of possible starting configurations for DMMP (sarin) with respect to the MPc has been probed where the considered structures included different spatial, rotational, and relative registries of the adsorbed gas molecules with respect to the MPc. To simplify the discussion, we use ZnPc as a primary example for MPc: it turns out to provide the strongest interaction with DMMP and sarin.
The stable structures of DMMP (sarin) on ZnPc can be categorized into three groups (four groups). In category A (Figure 2a), a covalent bond is formed between the zinc atom from ZnPc and the reactive O1 atom from DMMP (sarin). In category B (Figure 2b), the O2 atom is attached to the Zn atom in ZnPc. In category C (Figure 2c), adsorption of the gas molecules is dominated by the van der Waals (vdW) forces, with no indication of a covalent bond or charge transfer between DMMP and ZnPc (see Figure 2c). Finally, in category D, which appears only in sarin, the fluorine atom from sarin is attached to the Zn atom in ZnPc (Figure 2d). The calculations show that categories B, C, and D in DMMP (and sarin) are clearly less stable than category A by about +0.2 (+0.26), +0.56 (+0.53), and (+0.28) eV, respectively. These categories are therefore of minor relevance compared to category A at room temperature or above. However, a word of caution is in order here. The appearance of a structure from category A strongly depends on the orientation of the ZnPc substrate molecule: monolayers of vertically stacked MPc do not provide adsorption sites for such covalent bonds. We will discuss this point in more detail in Section 3.2.
Figure 2.
Side views of different calculated stable structures of DMMP (sarin) on ZnPc, denoted as categories depending on the type of the formed bond between the adsorbed gas molecule and ZnPc. In category A, a covalent bond is formed between the O1 atom and the Zn atom from ZnPc. In category B, the gas molecule anchors by its O2 atom to the Zn atom. In category C, the adsorption is caused by the vdW interaction exclusively. In category D, of relevance only for sarin, the sarin molecule anchors by its fluorine atom to the Zn atom in ZnPc. Heavier and lighter shades are used to distinguish the atoms of the gas molecules from those of ZnPc, respectively.
In the following, we compare the most stable structures of DMMP with those of sarin, both adsorbed on ZnPc (category A). To describe the adsorption geometries, we denote the O1–Zn distance as r, the upward movement of the central Zn atom as d, the angle Zn–O1–P as α, and the angle between C1–P from DMMP (sarin) and the long axis (LA) of ZnPc as θ, where it describes the azimuthal orientation of the adsorbed gas molecule with respect to ZnPc (see Figure 3). Because of the structural flexibility of the gas molecules, there is no unique stable configuration in category A but a set of one most stable and a few less stable structures, which differ exclusively in the values of α and θ, and can be found in the Supporting Information (SI) (shown exemplarily in S1 for DMMP). In the most stable structure (S type), a DMMP adsorbs on ZnPc by forming a covalent Zn–O1 bond. The adsorption geometry parameters are r = 2.14 Å, d = 0.35 Å, α = 140°, and θ = 12°. The geometric parameters for sarin are almost similar to those for DMMP (within 0.1 Å): r = 2.18 Å, d = 0.35 Å, α = 141°, and θ = 30° (see Figure 3c,d for comparison). Note that changing the value of θ to that of DMMP increases the energy by as little as 2 meV.
Figure 3.
(a/c) Side and (b/d) top views of the most stable adsorption configuration of DMMP/sarin on, e.g., ZnPc (S type). For the definition of the geometry parameters (r, d, α, and θ), see text.
Notably, the adsorption of sarin on other MPcs shows the same tendency as DMMP. For both gas molecules, the covalent interaction is dominating in case of the adsorption on ZnPc, in contrast to other MPcs investigated here: covalent interactions account for 35% (42%) of the adsorption energy of DMMP (sarin) on CoPc, while the adsorptions on CuPc and NiPc are essentially determined by van der Waals interactions (see Table 1). This is also reflected in the binding energies and the values of the adsorption parameters: r and d for each species (see Figure 4 and Table 1). The almost identical behaviors of DMMP and sarin suggest the latter as a suitable model system with respect to the interaction with MPc, in particular, in the case of ZnPc, with almost identical binding energies for DMMP and sarin.
Table 1. Calculated Adsorption Energies of DMMP and Sarin on Different MPcs (Eads) Depicted in Figure 4a.
|
Eads (eV) |
dispersion
energy (eV) (contribution to Eads, %) |
d (Å) |
r (Å) |
|||||
|---|---|---|---|---|---|---|---|---|
| structure of the MPc | DMMP | sarin | DMMP | sarin | DMMP | sarin | DMMP | sarin |
| ZnPc | –0.92 | –0.93 | –0.45 (49%) | –0.43 (46%) | 0.35 | 0.35 | 2.14 | 2.18 |
| CoPc | –0.80 | –0.83 | –0.52 (65%) | –0.49 (58%) | 0.15 | 0.10 | 2.23 | 2.28 |
| CuPc | –0.51 | –0.58 | –0.45 (88%) | –0.44 (77%) | 0.09 | 0.06 | 2.46 | 2.57 |
| NiPc | –0.42 | –0.52 | –0.40 (95%) | –0.46 (90%) | 0.01 | 0.002 | 2.80 | 2.94 |
Figure 4.
Schematic representation of the most stable geometries of exemplary DMMP on (a) ZnPc, compared with those on (b) CoPc, (c) CuPc, and (d) NiPc (only the central parts of MPcs are shown). The black dashed line indicates the plane of an MPc. The upward shifts of the central atoms upon adsorption (d; the scale is enlarged by a factor of 3, i.e., d* = 3 × d) and the distances O1–M (r) are given in Table 1. The latter adsorption geometry features are indicated by dashed red and blue lines, respectively.
It is important to note that the order of the adsorption strength of both gas molecules on MPc (ZnPc > CoPc > CuPc > NiPc) does not depend on the employed XC functional. Very similar results are obtained by the semilocal PBE functional, with and without extension by a Hubbard U,56 which accounts for the strongly correlated 3d electrons, and employing an all-electron (AE) approach using the B3LYP hybrid functional63 (see also Figure S2). Even the adsorption energies are only slightly shifted; all XC functionals provide the same tendency in the order of the adsorption strength, confirming, thus, the validity of the results obtained employing the PBE functional. Therefore, if not otherwise stated, the results achieved with the PBE functional will be discussed. In comparison to hybrid functionals, this reduces the numerical costs by more than one order, allowing for a systematic comparison of many different structures containing a large number of atoms.
It is obvious that the order in the adsorption strength for DMMP/sarin is different from the calculated order in ref (38), where CoPc provides the most sensitive reaction, but reflects the results of a previous DFT study for another reducing gas, namely, NH3.53 An explanation for this order has been reported in ref (71): according to Liao et al., it is related to the occupation of the d-orbitals, in particular, the partially occupied a1g(dz2) orbital. Nevertheless, the higher sensitivity of CoPc in the experiment38 demonstrates that, so far, unknown details of the specific sample, e.g., the arrangement of the MPc molecules in the substrate, are important parameters too.
Irrespective of the employed functional, sarin has larger adsorption energies compared to DMMP for all considered MPcs. Given the small energy difference in the case of ZnPc, however, this does not directly imply that MPcs are more sensitive to sarin compared to DMMP. As will be shown below, other factors, e.g., charge transfer and, in particular, the formation of dipole layers are highly relevant for the sensing mechanism, too. Previous works have tried to explain the differences between DMMP and sarin detected by different sensing materials such as TiO272 or Al2O3,73 but they were not able to identify exactly the factors responsible for this behavior. In the case of the present study, we assume that the obtained differences should be somehow related to the exclusive presence of the F atom in sarin (not present in the DMMP) and its complex interaction/charge redistribution upon covalent bonding with the central metal in MPc. The exact explanation of this point is beyond the scope of this work and requires further, in particular, experimental investigations. Since we are going to use DMMP to relate our theoretical results with experiments, we restrict the discussions in the upcoming sections to DMMP, while keeping in mind that the results can be extended to sarin.
3.2. DMMP Adsorption on ZnPc Monolayers
To evaluate the mechanism of DMMP adsorption on the ZnPc surface and to conclude on the role of the arrangement of ZnPc molecules in the sensing mechanism, we consider a monolayer of phthalocyanine as a representative structure of the sensing part of the MPc substrate.
The phthalocyanine monolayer used to model the substrate is characterized by the lateral lattice constants a, b and the enclosed angle (γ) of the oblique unit cell (see Figure 5a). In the present calculations, the angle γ = 85° measured for ZnPc monolayers on gold (γ = 85°)74 is used, since gold–organic interfaces are typical examples of weakly interacting interfaces.75 Given the phthalocyanine symmetric molecular structure, it is furthermore assumed that a = b (Figure 5a). The monolayer structures were relaxed in unit cells varying between a = 6.5 and 15 Å, and the energy of each structure is calculated. The resulting total energy curve for each of the three types of monolayers yields a minimum energy structure; see Figure 5b (Table 2):
-
1.
planar-oriented flat molecules—monolayer type I with a minimum at a = 14 Å.
-
2.
planar-oriented bent molecules—monolayer type II with a minimum at a = 12 Å.
-
3.
out-of-plane-oriented molecules—monolayer type III with a minimum at a = 7.5 Å.
Figure 5.
(a) Schematic representation of a monolayer of ZnPc with dimensions a and γ (shown here for a = 14 Å and γ = 85°). (b) Calculated total energy/area (E/S) curves for the three types of ZnPc monolayers. The energy of the most stable structure of each monolayer type is considered as an energy reference (0 eV); for the relative total energies, see Table 2. A perspective view of the geometries corresponding to each energy-minimum structure is shown in the side panel.
Table 2. Calculated Adsorption Energies (Eads) for DMMP Molecules Adsorbed on ZnPc in the Isolated Molecule Approach and in Various Monolayer Configurationsa.
| DMMP adsorbed on | Eads (eV) | d (Å) | r (Å) | α (deg) |
|---|---|---|---|---|
| isolated ZnPc molecule | –0.92 | 0.35 | 2.14 | 140 |
| ZnPc monolayer type I | –0.94 | 0.35 | 2.14 | 140 |
| ZnPc monolayer type II | –0.93 | 0.33 | 2.15 | 139 |
| ZnPc monolayer type III | –0.38 |
The closer the Pc molecules come to each other, the stronger the intermolecular interactions leading to reorientation and molecular deformation. As a consequence, interacting molecules tend to stack out of the molecular plane when their distance decreases.
The most stable ZnPc monolayer structures of types I, II, and III were used to study the adsorption of DMMP molecules. The resulting structures are shown in Figure 6. Due to the higher availability of covalent adsorption sites, monolayers of types I and II (see Figure 6a–d) are, of course, more representative for an efficient DMMP-sensing mechanism. In the case of a Pc arrangement of type III monolayers, these adsorption sites are absent or at least reduced to the edges of ZnPc islands, where the interaction is still mainly dominated by the van der Waals (vdW) interactions (see Figure 6e).
Figure 6.
(a/b, c/d) Top/perspective views of DMMP adsorbed on ZnPc monolayers of types I and II. For the dimensions of the unit cells, see text. (e) Perspective view of DMMP adsorbed on ZnPc monolayer type III. The dimensions of the unit cell are twice those shown in Figure 5 (a* = 2a), so that they are comparable to those of monolayer types I and II.
Compared to the adsorption on single ZnPc molecules (S type), the formation of ZnPc monolayers of types I and II yields slightly more stable structures of DMMP (by about 20 and 10 meV, respectively). According to an analysis of the charge redistribution for these arrangements, the formation of ZnPc monolayers (in particular, type I) yields an increase in the charge transfer between DMMP and ZnPc mediated by the intermolecular interactions. Corresponding to these small energy changes, only tiny variations of the DMMP geometries are observed: θ is now about 0°, while r and d as well as α have barely changed. This indicates that the DMMP–substrate interaction does not depend too strongly on the total number of gas particles.
As expected, the adsorption of DMMP on a type III monolayer with strongly tilted ZnPc molecules does not allow for covalent bonding. Purely vdW-bonded DMMP molecules provide about 40% of the adsorption energies found in the other two cases.
It is important to note that the above discussion of adsorption on type I and II monolayers refers to a 1:1 coverage of DMMP on ZnPc. However, upon decreasing the coverage into the sub-ML regime, e.g., assuming 1 of 4 ZnPc decorated with a DMMP molecule, the geometry of the DMMP molecule and its adsorption energy have barely changed. This again confirms the stable sensing potential of the investigated system.
3.3. Charge Transfer and DMMP Adsorption on ZnPc Double Stacks
Besides binding energies, other parameters like charge transfer and/or dipole layers may offer a more direct and, thus, characteristic relation to the sensing capability and the mechanism behind it.76 Since DMMP interacts rather strongly with ZnPc, forming a covalent bond, charge relocation/transfer at the interface is believed to be one of the key components to detect DMMP by ZnPc, while its amount directly influences the sensor performance.
To analyze this charge transfer, it is important to consider models that account for the multilayer aspects of the Pc substrate as realistically as possible. For this purpose, we extend our theoretical DFT modeling to DMMP adsorption on multistacks (up to six layers) of ZnPc. Similar to the monolayer calculations, we first optimized the ZnPc structures and addressed the changes of the adsorption geometry of DMMP on this structure afterward. For the sake of simplicity, we restrict the detailed discussion on ZnPc double stacks but briefly note that the qualitative results hold also in the case of multistacks.
To optimize the structure of ZnPc multilayers, we relaxed their structures by testing a variety of stacking possibilities. Thereby, the angle between the long axis (LA) (see Figure 1) of both stacking molecules is considered as a reference for the molecular orientation (φ), while the relative lateral distance (m) between the Zn atoms is taken as a reference for the molecular registries. Both parameters have been widely varied and the energy of each structure has been calculated. The calculations show two energy minima. A local energy minimum is obtained if the planes of both ZnPcs are parallel but with a tilting angle φ = 45° (see Figure 7a). In the global minimum (energetically more stable by 0.26 eV), the LAs of both molecules are parallel to each other (φ = 0°). However, their centers are laterally shifted by m = 1.32 Å, while their centers are vertically separated by 2.92 Å (see Figure 7b). Denoted as slipped–stack packing, this mode of crystallization is commonly known for phthalocyanines.77,78
Figure 7.
Top views of ZnPc double layers in the (a) energetically local minimum and (b) global minimum structures. The lines indicate the long axes of the molecules, the parameter m indicates the relative lateral shift between the Zn atoms in the stacked molecules, while φ indicates the relative azimuthal orientation of stacked molecules. (c) Top and (d) side views of DMMP adsorbed on double-stacked ZnPc in the most stable structure. The uppermost ZnPc molecule (most related to the sensing aspects) is shown in colors similar to those of the previous figures (as a guide to the eye, the second molecule is shown in light brown).
Again, upon the adsorption of DMMP on the double-stack structure of ZnPcs (see Figure 7c,d), its geometries exhibit no significant changes compared to these related to the S type. The adsorption energy for this structure equals −0.89 eV. This value is around 30 meV lower than that for the single ZnPc molecule, an effect that can be attributed to a slightly modified charge transfer (see below).
To report on the amount and the direction of the charge transfer between the attached DMMP and the modeled ZnPc part of the substrate, we perform a Löwdin analysis and compare the charge distribution between the constituent atoms of both molecules (ZnPc and DMMP) before and after the adsorption. Our calculations indicate a net charge transfer by about 0.35 e from DMMP to ZnPc. The charge density (electron density) has been almost uniformly depleted from all DMMP atoms but mainly from the region between P and O1 atoms (e.g., the depleted charge on the O1 atom is calculated to be 0.14 e). It is accumulated at the center of the ZnPc molecule, at the Zn atom (by 0.19 e), and in the region between the Zn and the attached O1 atom (see Figure 8a,b). The other part of the ZnPc molecule undergoes a polarization effect. Thereby, a slight charge accumulation on the inner nitrogen atoms by 0.03 e and a depletion of 0.04 e on the outer ones have been calculated. On carbon atoms, we calculate a total charge accumulation of about 0.2 e (∼0.005 e per C atom).
Figure 8.
(a/c) Charge density difference calculated for DMMP/sarin adsorbed on a single ZnPc in its most stable structure, expressing the charge redistribution upon DMMP/sarin adsorption on ZnPc (an isosurface value of ±0.004 e/Å3 was chosen). (b/d) Side view of the same structure of DMMP/sarin. The charge distribution indicates the formation of a surface dipole moment, denoted by p⃗.
It is illustrative that a possible charge transfer toward neighboring ZnPc deeper in the substrate does not take place, at least not to a critical extent: our calculations showed, in the case of a neutral DMMP-double ZnPc system, that almost 90% of transferred charge density accumulates on the uppermost ZnPc molecule, while only 10% is further transferred to the second one. As a consequence, our DFT analysis indicates the formation of a surface dipole layer as a main ingredient of the sensing mechanism; see also Figure 8. It is also important to note that the aforementioned findings, in particular, the formation of the surface dipole and its direction, hold as well for the case of sarin (see Figure 8c,d). Below, we confirm these claims by XPS spectroscopy applied on DMMP.
It has to be pointed out that the ZnPc sensing layer is deposited on another material, e.g., MoO3. The thickness of the ZnPc layer (10 nm) does not allow for a complete atomistic modeling of the complete system. Therefore, we approximate a possible electron transfer across the second interface (ZnPc/MoO3) by considering charged models of DMMP–ZnPc with additional net charges up to ±2 e. The calculations show that irrespective of the sign and value of the net charge, the extra charge is completely distributed on the DMMP-decorated ZnPc molecules without changing the geometrical properties of a covalently bound DMMP considerably. The secondary interface, thus, might modify the Fermi level and some aspects of the induced surface dipole but it will not cancel it.
For further characterization of the induced surface dipole, we
calculate the work function changes (Δϕ) upon DMMP adsorption
(Δϕ = ϕZnPc–DMMP – ϕZnPc). The work function ϕ (or, in the case of a molecular
system, ionization energy) is defined as the difference between the
respective Fermi level (the highest occupied molecular orbital (HOMO))
and the vacuum level. Thus, 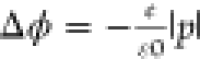 reflects the nonuniform electrostatic potential
at the surface and provides a direct measure for the induced surface
dipole p⃗. For pristine ZnPc, we calculate
a value of 1.76 eV, which decreases to 1.22 eV if a DMMP molecule
adsorbs at ZnPc in the S-type structure. Due to spurious electron
self-energy effects, DFT is expected to underestimate the work function
compared to the measured values. Indeed, experimentally, a value of
4.4 eV is reported for ZnPc.79 Work function
changes (Δϕ) due to molecular adsorption can be expected
to be trustworthy, however.80 The nonvanishing
change, more precisely the considerable reduction of the work function
by 0.54 eV, thus confirms the formation of the surface dipole upon
DMMP adsorption, pointing from the ZnPc substrate toward the DMMP
molecule (see also Figure 8). Note that Δϕ is largely reduced to a few meV
if DMMP is vdW adsorbed on ZnPc (category C), where no covalent bond
is formed, no direct charge transfer takes place, and, thus, no relevant
dipole is induced. For other MPc, the adsorption of DMMP results in
a reduction of the work function by 0.21, 0.18, and 0.20 eV for CoPc,
CuPc, and NiPc, respectively. This again confirms the already reported
trend in the adsorption energies of DMMP on different MPcs, and renders
ZnPc as the potentially most sensitive MPc for DMMP adsorption.
reflects the nonuniform electrostatic potential
at the surface and provides a direct measure for the induced surface
dipole p⃗. For pristine ZnPc, we calculate
a value of 1.76 eV, which decreases to 1.22 eV if a DMMP molecule
adsorbs at ZnPc in the S-type structure. Due to spurious electron
self-energy effects, DFT is expected to underestimate the work function
compared to the measured values. Indeed, experimentally, a value of
4.4 eV is reported for ZnPc.79 Work function
changes (Δϕ) due to molecular adsorption can be expected
to be trustworthy, however.80 The nonvanishing
change, more precisely the considerable reduction of the work function
by 0.54 eV, thus confirms the formation of the surface dipole upon
DMMP adsorption, pointing from the ZnPc substrate toward the DMMP
molecule (see also Figure 8). Note that Δϕ is largely reduced to a few meV
if DMMP is vdW adsorbed on ZnPc (category C), where no covalent bond
is formed, no direct charge transfer takes place, and, thus, no relevant
dipole is induced. For other MPc, the adsorption of DMMP results in
a reduction of the work function by 0.21, 0.18, and 0.20 eV for CoPc,
CuPc, and NiPc, respectively. This again confirms the already reported
trend in the adsorption energies of DMMP on different MPcs, and renders
ZnPc as the potentially most sensitive MPc for DMMP adsorption.
3.4. Experimental Verification by Photoelectron Spectroscopy (XPS)
Motivated by the results of our DFT calculations, we employ XPS spectroscopy to experimentally verify the theoretical findings. In principle, both effects, (i) the induced charge transfer from DMMP to the sensing ZnPc material (surface dipole) and (ii) the existence of the underlying substrate (MoO3), provide the potential to yield energy shifts in the spectroscopic fingerprints. Based on this, we report on the related changes in X-ray spectra upon adsorption of DMMP molecules, namely, XPS at the C 1s, N 1s, and Zn 2p core levels.
Figure 9a–d shows a set of high-resolution XPS spectra recorded for pristine ZnPc (bottom rows) and DMMP-exposed (upper rows) samples. From the chemical structure of the sensing part of the sample, ZnPc, and DMMP molecules, it is intuitively clear that an appearance of oxygen or phosphorous is indicative of the adsorption of DMMP molecules. However, since the MoO3 substrate includes oxygen in its structure, measuring the O 1s XPS signal yields a less reliable indicator for DMMP adsorption. Thus, being present in the adsorbed molecular species exclusively, P appears, at first glance, to be the best indicator for DMMP adsorption at the ZnPc surface.
Figure 9.
High-resolution XPS spectra ((a) P 2p, (b) C 1s, (c) N 1s, and (d) Zn 2p3/2) of the DMMP-exposed samples (upper panels) in comparison to the pristine ZnPc samples (bottom panels). (a) The weak P 2p signal is decomposed into 2p1/2 and 2p3/2 signals. The other recorded energy regions (b–d) have been decomposed into components from nonequivalent constituent atoms. The characteristic DMMP-related shifts in the N 1s and Zn 2p3/2 binding energies of the DMMP-exposed samples are also indicated.
3.4.1. P 2p Region
Figure 9a shows a comparison in the P 2p region. As expected, there is no distinct P-related signal in the pristine (P-free) sample at all. For the DMMP-exposed sample, however, we detect a slight rise of a spin–orbit split signal around ∼130 eV, which is a clear fingerprint of a P 2p core level.81 The decomposition into P 2p1/2 and 2p3/2 spin–orbit split components is also supported by our additional relativistic DFT calculations predicting a splitting of 1.226 eV. The intensity of the signal is, however, extremely weak. Note that this does not mean that the coupling to the ZnPc is weak. The weakness of the signal simply reflects the dominant contribution of the ZnPc layers and renders signals from this sensing part of the sample as dominating and much more promising for sensing. It may also indicate that only a part of the available adsorption sites is occupied by DMMP. This appears furthermore likely as the surface is not homogeneous, whereby a part of ZnPcs might possess orientations different from the most favorable flat-lying structure.
3.4.2. C 1s Region
Figure 9b compares the C 1s in pristine ZnPc with that of the DMMP-exposed sample. The decomposition of the C 1s region into components of different carbon types provides signals that can be unambiguously assigned to C–C and C–N signals accompanied by their respective satellites (SC–C and SC–N).82 Such a decomposition for the DMMP-exposed sample, however, reveals no clear extra signals. The explanation of this aspect is again straightforward: as in the case of the P 2p signal, the overwhelming majority of the carbon signal stems from the ZnPc film. According to our calculations (see Figure S3 in SI), the C–P and C=O components of this region should contribute to signals at energies of about 286 and 287.5 eV, respectively. The latter is notably overlapping with the SC–N region in the measured spectrum. Indeed, the measurements exhibit a slight increase in this region in the DMMP-exposed sample. However, while the calculations indicate a DMMP adsorption-induced electron accumulation at the ZnPc carbon atoms resulting in a slight shift of about 0.2 eV toward smaller binding energies, no significant energy shift could be observed experimentally. Besides the already mentioned overlap with other contaminating signals, this may be related to the comparatively high X-ray attenuation length in the C 1s energy regime. In any case, the C 1s energy region appears to be only a very indirect indicator for residual DMMP adsorption at the ZnPc surface. It appears to be not really sensitive to DMMP adsorption and, thus, cannot be used for further evaluation of the sensing mechanism.
3.4.3. N 1s Region
More relevant information on DMMP adsorption can be expected from the core-level shifts in the N 1s region. Figure 9c shows the existence of three components that can be attributed to (starting from the lowest binding energy) residual substrate-related Mo 3p3/2, N–C, and N–H. The latter is a contribution of residual base-free Pc existing in the evaporated source material (see Figure S4 in SI). Among them, the N–C component is of particular interest, since it is one of the main constituents of the examined ZnPc structure. Apparently, this component undergoes a significant shift toward smaller binding energies by 0.8 eV after DMMP adsorption (Figure 9c), which is in qualitative agreement with a DFT-calculated shift of about 0.5 eV. Note that the DFT-predicted asymmetry in the charge redistribution over the N ligands (+0.04 vs −0.03 e, even with different signs) is able to explain variations of up to 0.1 eV (see the calculated XPS N 1s spectra, S5, in SI). While the present measurements do not provide direct information on the specific arrangement of the ZnPc molecules, the relatively large chemical shifts that take place indicate covalent bonding between DMMP and ZnPc. In other words, there is strong evidence that the topmost layer of ZnPc is a monolayer of type I or II.
3.4.4. Zn 2p Region
Given the covalent adsorption of DMMP at the Zn atom and, according to our charge analysis, it can be expected that the Zn 2p region presents the most powerful signature verifying that DMMP adsorption has taken place. Figure 9d compares the Zn 2p3/2 region of pristine ZnPc (bottom) and the DMMP-exposed samples (top). In both cases, the region consists of a single Zn-related component showing a simple symmetric form of Zn 2p with no hint of additional peaks from different states. Most importantly, the peak in the case of the DMMP-exposed sample is indeed (and surprisingly strongly) shifted by 3 eV compared to the sample of pristine ZnPc. Again, the shift is toward smaller binding energies, consistent with our calculated (DFT-predicted) DMMP-induced accumulation of valence charge in the Zn vicinity. By calculating the Zn 2p core-level shift for the DMMP–ZnPc system, we actually obtain a rather moderate decrease of the corresponding binding energy by roughly 1 eV.
Although theory and experiment show qualitative agreement in terms of the direction, they strongly differ in the size of the Zn 2p shift. Whereas the calculated values provide an upper limit for the shifts (for purely vdW-bound DMMP, smaller shifts are expected), the experimental values are unusually high. A measured Zn 2p binding energy of 1017 eV is clearly out of the usual range between 1022 and 1019 eV. According to our DFT calculations, however, the high value could be explained by negative recharging upon DMMP adsorption by, e.g., one electron: then a total shift of the Zn 2p by 2.9 eV is obtained. Simultaneously, also, the shifts of the N 1s and C 1s binding energies are considerably increased, resulting in actually too high values of 2.1 eV and 1.4 eV, respectively. This fact renders rigid negative charging of the DMMP–ZnPc to be less probable. One may speculate that the recharging is related to the high-energy treatment in the case of the Zn 2p XPS measurements. Since other experimental side effects cannot be excluded, a detailed evaluation of the origin behind a possible energy/edge-selective recharging remains for future work.
4. Conclusions
DFT calculations on the adsorption of sarin and its less toxic simulant DMMP on metal-centered phthalocyanines, namely, ZnPc, NiPc, CuPc, and CoPc, are presented. The calculations show a strong similarity of the interaction of both species to various MPcs. The strongest interaction was found for ZnPc, suggesting the latter for real sensor applications. The most stable conformation is found for both DMMP and sarin, covalently bound via the O=P oxygen with the central Zn atom of the ZnPc. We further found that among possible types of ZnPc monolayers, the most preferable for DMMP sensing are those where the molecules are well-ordered in the planar form so that covalent adsorption sites are highly available. Since the orientation of MPc in the real sensing structure depends on the substrate, deposition techniques as well as deposition temperature and pressure, it appears of special importance to take this factor into account during device design. According to our DFT predictions, which were confirmed by experimental XPS studies, the sensing mechanism is related to the DMMP-induced charge transfer resulting in a surface-dipole layer. Our results indicate that further modification of the surface dipole is instrumental for an optimization of the sensing efficiency and, thus, needs to be further evaluated in future sensing device design.
This study represents a cornerstone for understanding the sensing mechanisms of a warfare agent and provides an efficient aid for perspective sensing device development.
Acknowledgments
Numerical calculations were performed using grants of computer time from the Paderborn Center for Parallel Computing (PC2). The Deutsche Forschungsgemeinschaft (DFG) is acknowledged for financial support, project number 231447078 (TRR142). P. Powroźnik additionally acknowledges the support of the Polish National Science Center (NCN), grant no. 2016/21/N/ST5/03335. M. Krzywiecki acknowledges Rector’s pro-quality grant No. 14/990/RGJ19/0115. The authors acknowledge the ORZEL project (European Union’s Horizon 2020 research and innovation programme) under grant agreement no 691684. The authors thank the ESPEFUM laboratory for access to the XPS facility.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpcc.9b11116.
Less stable structures of DMMP on ZnPc in category A; adsorption energies obtained by different XC functionals; calculated C 1s energy region of ZnPc–DMMP compared to ZnPc; calculated N 1s energy region of H2Pc; and calculated N 1s energy region of ZnPc compared to ZnPc–DMMP (PDF)
Author Contributions
∥ H.A. and P.P. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Dey A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng., B 2018, 229, 206–217. 10.1016/j.mseb.2017.12.036. [DOI] [Google Scholar]
- Mirzaei A.; Leonardi S. G.; Neri G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. 10.1016/j.ceramint.2016.06.145. [DOI] [Google Scholar]
- Kaushik A.; Kumar R.; Arya S. K.; Nair M.; Malhotra B. D.; Bhansali S. Organic-Inorganic Hybrid Nanocomposite-Based Sensors for Environmental Monitoring. Chem. Rev. 2015, 115, 4571–4606. 10.1021/cr400659h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righettoni M.; Amann A.; Pratsinis S. E. Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors. Mater. Today 2015, 18, 163–171. 10.1016/j.mattod.2014.08.017. [DOI] [Google Scholar]
- National Research Council . Acute Exposure Guideline Levels for Selected Airbone Chemicals; National Academies Press: Washington, DC, 2003; Vol. 3. [Google Scholar]
- Li X.; Dutta P. K. Interaction of Dimethylmethylphosphonate with Zeolite Y: Impedance-Based Sensor for Detecting Nerve Agent Simulants. J. Phys. Chem. C 2010, 114, 7986–7994. 10.1021/jp100088w. [DOI] [Google Scholar]
- Matatagui D.; Marti J.; Fernandez M. J.; Fontecha J. L.; Gutierrez J.; Garcia I.; Cane C.; Horrillo M. C. Chemical warfare agents simulants detection with an optimized SAW sensor array. Sens. Actuators, B 2011, 154, 199–205. 10.1016/j.snb.2010.01.057. [DOI] [Google Scholar]
- Wild A.; Winter A.; Hager M. D.; Schubert U. S. Fluorometric, water-based sensors for the detection of nerve gas G mimics DMMP, DCP and DCNP. Chem. Commun. 2012, 48, 964–966. 10.1039/C1CC15978J. [DOI] [PubMed] [Google Scholar]
- Yoo R.; Yoo S.; Lee D.; Kim J.; Cho S.; Lee W. Highly selective detection of dimethyl methylphosphonate (DMMP) using CuO nanoparticles/ZnO flowers heterojunction. Sens. Actuators, B 2017, 240, 1099–1105. 10.1016/j.snb.2016.09.028. [DOI] [Google Scholar]
- Jun J.; Lee J. S.; Shin D. H.; Oh J.; Kim W.; Na W.; Jang J. Fabrication of a one-dimensional tube-in-tube polypyrrole/tin oxide structure for highly sensitive DMMP sensor applications. J. Mater. Chem. A 2017, 5, 17335–17340. 10.1039/C7TA02725G. [DOI] [Google Scholar]
- Wang Y.; Yang M.; Liu W.; Dong L.; Chen D.; Peng C. Gas sensors based on assembled porous grapheme multilayer frameworks for DMMP detection. J. Mater. Chem. C 2019, 7, 9248–9256. 10.1039/C9TC02299F. [DOI] [Google Scholar]
- Schedin F.; Geim A. K.; Morozov S. V.; Hill E. W.; Blake P.; Katsnelson M. I.; Novoselov K. S. Detection of individual gas molecules adsorbed on grapheme. Nat. Mater. 2007, 6, 652–655. 10.1038/nmat1967. [DOI] [PubMed] [Google Scholar]
- Korotcenkov G.; Ivanov M.; Blinov I.; Stetter J. R. Kinetics of indium oxide-based thin film gas sensor response: The role of “redox” and adsorption/desorption processes in gas sensing effects. Thin Solid Films 2007, 515, 3987–3996. 10.1016/j.tsf.2006.09.044. [DOI] [Google Scholar]
- Kou L.; Frauenheim T.; Chen C. Phosphorene as a Superior Gas Sensor: Selective Adsorption and Distinct I-V Response. J. Phys. Chem. Lett. 2014, 5, 2675–2681. 10.1021/jz501188k. [DOI] [PubMed] [Google Scholar]
- Aghaei S. M.; Monshi M. M.; Calizo I. A theoretical study of gas adsorption on silicone nanoribbons and its application in highly sensitive molecule sensor. RSC Adv. 2016, 6, 94417–94428. 10.1039/C6RA21293J. [DOI] [Google Scholar]
- Zhao L.; Wang K.; Wei W.; Wang L.; Han W. High-performance flexible sensing devices based on polyaniline/MXene nanocomposites. InfoMat 2019, 1, 407–416. 10.1002/inf2.12032. [DOI] [Google Scholar]
- Wang J.; Du X.; long Y.; Tang X.; Tai X.; Jiang Y. The response comparison of a hydrogen-bond acidic polymer to sarin, soman and dimethyl-methylphosphonate based on a surface acoustic wave sensor. Anal. Methods 2014, 6, 1951. 10.1039/c3ay42214c. [DOI] [Google Scholar]
- Wang L.; Chen S.; Li W.; Wang K.; Lou Z.; Shen G. Grain-Boundary-Induced Drastic Sensing Performance Enhancement of Polycrystalline-Microwire Printed Gas Sensors. Adv. Mater. 2019, 31, 1804583 10.1002/adma.201804583. [DOI] [PubMed] [Google Scholar]
- Kumar C. V.; Sfyri G.; Raptis D.; Stathatos E.; Lianos P. Perovskite solar cell with low cost Cu-phthalocyanine as hole transporting material. RSC Adv. 2015, 5, 3786–3791. 10.1039/C4RA14321C. [DOI] [Google Scholar]
- Cho K. T.; Rakstys K.; Cavazzini M.; Orlandi S.; Pozzi G.; Nazeeruddin M. K. Perovskite Solar Cells Employing Molecularly Engineered Zn(II) Phthalocyanines as Hole-transporting Materials. Nano Energy 2016, 30, 853–857. 10.1016/j.nanoen.2016.09.008. [DOI] [Google Scholar]
- Kumar A.; Brunet J.; Varenne C.; Ndiaye A.; Pauly A.; Penza M.; Alvisi M. Tetra-tert-cutyl-copper phthalocyanine based QCM sensor for toluene detection in air at room temperature. Sens. Actuators, B 2015, 210, 398–407. 10.1016/j.snb.2015.01.010. [DOI] [Google Scholar]
- Rydosz A.; Maciak E.; Wincza K.; Gruszczynski S. Microwave-based sensors with phthalocyanine films for acetone, ethanol and methanol detection. Sens. Actuators, B 2016, 237, 876–886. 10.1016/j.snb.2016.06.168. [DOI] [Google Scholar]
- Cho K. T.; Trukhina O.; Roldan-Carmona C.; Ince M.; Gratia P.; Grancini G.; Gao P.; Marszalek T.; Pisula W.; Reddy P. Y.; Torres T.; Nazeeruddin M. K. Molecularly Engineered Phthalocyanines as Hole-Transporting Materials in Perovskite Solar Cells Reaching Power Conversion Efficiency of 17.5%. Adv. Energy Mater. 2016, 7, 1601733 10.1002/aenm.201601733. [DOI] [Google Scholar]
- Urbani M.; Ragousi M. E.; Nazeeruddin M. K.; Torres T. Phthalocyanines for dye-sensitized solar cells. Coord. Chem. Rev. 2019, 381, 1–64. 10.1016/j.ccr.2018.10.007. [DOI] [Google Scholar]
- Sun Q.; Feng W.; Yang P.; You G.; Chen Y. Highly selective room-temperature NO2 sensor based on a fluoroalkoxy-substituted phthalocyanine. New J. Chem. 2018, 42, 6713–6718. 10.1039/C8NJ00622A. [DOI] [Google Scholar]
- Dong Z.; Kong X.; Wu Y.; Zhang J.; Chen Y. High-sensitive room-temperature NO2 sensor based on a aoluble n-type phthalocyanine semiconductor. Inorg. Chem. Commun. 2017, 77, 18–22. 10.1016/j.inoche.2017.01.023. [DOI] [Google Scholar]
- Demir F.; Yenilmez H. Y.; Koca A.; Bayir Z. A. Metallo-phthalocyanines containing thiazole moieties: Synthesis, characterization, electrochemical and spectroelectrochemical properties and sensor applications. J. Electroanal. Chem. 2019, 832, 254–265. 10.1016/j.jelechem.2018.11.003. [DOI] [Google Scholar]
- Chaabene M.; Gassoumi B.; Mignon P.; Chaabane R. B.; Allouche A.-R. New zinc phthalocyanine derivatives for nitrogen dioxide sensors: A theoretical optoelectronic investigation. J. Mol. Graphics Modell. 2019, 88, 174–182. 10.1016/j.jmgm.2019.01.008. [DOI] [PubMed] [Google Scholar]
- Kaya E. N.; Şenocak A.; Klyamer D. D.; Dembiraş E.; Basova T. V.; Durmuş M. Ammonia sensing performance of thin films of cobalt(II) phthalocyanine bearing fluorinated substituents. J. Mater. Sci.: Mater. Electron. 2019, 30, 7543–7551. 10.1007/s10854-019-01068-8. [DOI] [Google Scholar]
- Mukherjee D.; Manjunatha R.; Sampath S.; Kumar Ray A.. Phthalocyanines as Sensitive Materials for Chemical Sensors. In Materials for Chemical Sensing;Paixão T. C.; Reddy S., Eds.; Springer, 2017. [Google Scholar]
- Tasaltin C.; Gurol I.; Harbeck M.; Musluoglu E.; Ahsen V.; Ozturk Z. Z. Synthesis and DMMP sensing properties of fluoroalkyloxy and fluoroaryloxy substituted phthalocyanines in acoustic sensors. Sens. Actuators, B 2010, 150, 781–787. 10.1016/j.snb.2010.07.056. [DOI] [Google Scholar]
- Bilgiçli A. T.; Günsel A.; Kandaz M.; Altindal A.; Cömert H. Double-decker senor phthalocyanines functionalizes with 1-hydroxyhexane-3-ylthio moieties; synthesis, characterization, electrical properties and H- or J- type aggregation studies. J. Organomet. Chem. 2015, 785, 112–121. 10.1016/j.jorganchem.2015.02.048. [DOI] [Google Scholar]
- Kumar A.; Brunet J.; Varenne C.; Ndiaye A.; Pauly A.; Penza M.; Alvisi M. Tetra-tert-cutyl-copper phthalocyanine based QCM sensor for toluene detection in air at room temperature. Sens. Actuators, B 2015, 210, 398–407. 10.1016/j.snb.2015.01.010. [DOI] [Google Scholar]
- Sharma A. K.; Mahajan A.; Bedi R. K.; Kumar S.; Debnath A. K.; Aswal D. K. Non-covalently anchored multi-walled carbon nanotubes with hexa-decafluorinated zinc phthalocyanine as ppb level chemiresistive chlorine sensor. Appl. Surf. Sci. 2018, 427, 202–209. 10.1016/j.apsusc.2017.08.040. [DOI] [Google Scholar]
- Temofonte T. A.; Schoch K. F. Phthalocyanine semiconductor sensors for room-temperature ppb level detection of toxic gases. J. Appl. Phys. 1989, 65, 1350. 10.1063/1.343031. [DOI] [Google Scholar]
- Tongpool R.; Yoriya S. Kinetics of nitrogen dioxide exposure in lead phthalocyanine sensors. Thin Solid Films 2005, 477, 148–152. 10.1016/j.tsf.2004.08.125. [DOI] [Google Scholar]
- Lee Y. L.; Tsai W. C.; Chang C. H.; Yang Y. M. Effects of heat annealing on the film characteristics and gas sensing properties of substituted and un-substituted copper phthalocyanine films. Appl. Surf. Sci. 2001, 172, 191–199. 10.1016/S0169-4332(00)00846-1. [DOI] [Google Scholar]
- Bohrer F. I.; Colesniuc C. N.; Park J.; Ruidiaz M. E.; Schuller I. K.; Kummel A. C.; Trogler W. C. Comparative gas sensing in cobalt, nickel, copper, zinc, and metal-free phthalocyanine chemiresistors. J. Am. Chem. Soc. 2009, 131, 478–485. 10.1021/ja803531r. [DOI] [PubMed] [Google Scholar]
- Suto K.; Yoshimoto S.; Itaya K. Two-Dimensional Self-Organization of Phthalocyanine and Porphyrin: Dependence on the Crystallographic Orientation of Au. J. Am. Chem. Soc. 2003, 125, 14976–14977. 10.1021/ja038857u. [DOI] [PubMed] [Google Scholar]
- Kera S.; Casu M. B.; Bauchspies K. R.; Batchelor D.; Schmidt T.; Umbach E. Growth mode and molecular orientation of phthalocyanine molecules on metal single crystal substrates: A NEXAFS and XPS study. Surf. Sci. 2006, 600, 1077–1084. 10.1016/j.susc.2005.12.042. [DOI] [Google Scholar]
- Gaffo L.; Cordeiro M. R.; Freitas A. R.; Moreira W. C.; Girotto E. M.; Zucolotto V. The effects of temperature on the molecular orientation of zinc phthalocyanine films. J. Mater. Sci. 2010, 45, 1366–1370. 10.1007/s10853-009-4094-3. [DOI] [Google Scholar]
- Cox J. J.; Bayliss S. M.; Jones T. S. Influence of substrate orientation on the formation of ordered copper phthalocyanine overlayers on InAs. Surf. Sci. 1999, 425, 326–333. 10.1016/S0039-6028(99)00218-6. [DOI] [Google Scholar]
- Louis J. S.; Lehmann D.; Friedrich M.; Zahn D. R. T. Study of dependence of molecular orientation of zinc phthalocyanine grown under two different pressure conditions. J. Appl. Phys. 2007, 101, 013503 10.1063/1.2403845. [DOI] [Google Scholar]
- Yang R. D.; Park J.; Colesniuc C. N.; Schuller I. K.; Royer J. E.; Trogler W. C.; Kummel A. C. Analyte chemisorption and sensing on n- and p- channel copper phthalocyanine thin film transistors. J. Chem. Phys. 2009, 130, 164703 10.1063/1.3078036. [DOI] [PubMed] [Google Scholar]
- Tiwari D. C.; Sharma R.; Vyas K. D.; Boopathi M.; Singh V. V.; Pandey P. Electrochemical incorporation of copper phthalocyanine in conducting polypyrrole for the sensing od DMMP. Sens. Actuators, B 2010, 151, 256–264. 10.1016/j.snb.2010.09.004. [DOI] [Google Scholar]
- Wu M.; Yang H.; Wei H.; Hu X.; Qu B.; Chen M. Self-Assembled Nanoscaled Metalloporphyrin for Optical Detection of Dimethylmethylphosphonate. BioMed Res. Int. 2019, 7689183 10.1155/2019/7689183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şen Z.; Tarakci D. K.; Gürol I.; Ahsen V.; Harbeck M. Governing the sorption and sensing properties of titanium phthalocyanines by means of axial ligands. Sens. Actuators, B 2016, 229, 581–586. 10.1016/j.snb.2016.01.145. [DOI] [Google Scholar]
- Harbeck M.; Sen Z.; Erbhar D.; Gumus G.; Musluoglu E. Gas sensing with hexafluoroisopropanol substituted phthalocyanines and vic-dioximes: a comparative study. Turk. J. Chem. 2019, 43, 890–901. 10.3906/kim-1811-27. [DOI] [Google Scholar]
- Kus F.; Tasaltin C.; Albakour M.; Gurek A. G.; Gurol I. Macromolecular hexa-asymmetric zinc(II) phthalocyanines bearing triazole-modified triphenylene core: Synthesis, spectroscopy and analysis towards volatile organic compounds on Surface Acoustic Wave devices. J. Porphyrins Phthalocyanines 2019, 23, 477–488. 10.1142/S1088424619500342. [DOI] [Google Scholar]
- Soganci T.; Baygu Y.; Kabay N.; Gok Y.; Ak M. Comparative Investigation of Peripheral and Nonperipheral Zinc Phthalocyanine-Based Polycarbazoles in Terms of Optical, Electrical, and Sensing Properties. ACS Appl. Mater. Interfaces 2018, 10, 21654–21665. 10.1021/acsami.8b06206. [DOI] [PubMed] [Google Scholar]
- Powroźnik P.; Grządziel L.; Jakubik W.; Krzywiecki M. Sarin-simulant detection by phthalocyanine/palladium structures: From modeling to real sensor response. Sens. Actuators, B 2018, 273, 771–777. 10.1016/j.snb.2018.06.101. [DOI] [Google Scholar]
- Zou D.; Zhao W.; Cui B.; Li D.; Liu D. Adsorption of gas molecules on a manganese phthalocyanine molecular device and its possibility as a gas sensor. Phys. Chem. Chem. Phys. 2018, 20, 2048–2056. 10.1039/C7CP06760G. [DOI] [PubMed] [Google Scholar]
- Baggio A. R.; Machado D. F. S.; Carvalho-Silva V. H.; Patemo L. G.; De Oliveira H. C. B. Rovibrational spectroscopic constants of the interaction between ammonia and metallo-phthalocyanines: a theoretical protocol for ammonia sensor design. Phys. Chem. Chem. Phys. 2017, 19, 10843–10853. 10.1039/C6CP07900H. [DOI] [PubMed] [Google Scholar]
- Giannozzi P.; Baroni S.; Bonini N.; Calandra M.; Car R.; Cavazzoni C.; Ceresoli D.; Chiarotti G. L.; Cococcioni M.; Dabo I.; et al. QUANTUM ESPRESSO: a Modular and Open-Source Software Project for Quantum Simulations of Materials. J. Phys.: Condens. Matter 2009, 21, 395502 10.1088/0953-8984/21/39/395502. [DOI] [PubMed] [Google Scholar]
- Perdew J. P.; Burke K.; Ernzerhof M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- Cococcioni M.; de Gironcoli S. Linear Response Approach to the Calculation of the Effective Interaction Parameters in the LDA+U Method. Phys. Rev. B: Condens. Matter Mater. Phys. 2005, 71, 035105 10.1103/PhysRevB.71.035105. [DOI] [Google Scholar]
- Ortmann F.; Bechstedt F.; Schmidt W. G. Semiempirical van der Waals Correction to the Density Functional Description of Solids and Molecular Structures. Phys. Rev. B: Condens. Matter Mater. Phys. 2006, 73, 205101 10.1103/PhysRevB.73.205101. [DOI] [Google Scholar]
- Aldahhak H.; Paszkiewicz M.; Allegretti F.; Duncan D. A.; Tebi S.; Deimel P. S.; Casado Aguilar P.; Zhang Y.-Q.; Papageorgiou A. C.; Koch R.; et al. X-ray Spectroscopy of Thin Film Free-Base Corroles: A Combined Theoretical and Experimental Characterization. J. Phys. Chem. C 2017, 121, 2192–2200. 10.1021/acs.jpcc.6b09935. [DOI] [Google Scholar]
- Tebi S.; Paszkiewicz M.; Aldahhak H.; Allegretti F.; Gonglach S.; Haas M.; Waser M.; Deimel P. S.; Aguilar P. C.; Zhang Y.-Q.; et al. On-Surface Site-Selective Cyclization of Corrole Radicals. ACS Nano 2017, 11, 3383–3391. 10.1021/acsnano.7b00766. [DOI] [PubMed] [Google Scholar]
- Aldahhak H.; Paszkiewicz M.; Rauls E.; Allegretti F.; Tebi S.; Papageorgiou A. C.; Zhang Y. Q.; Zhang L.; Lin T.; Paintner T.; et al. Identifying On-Surface Site-Selective Chemical Conversions by Theory-Aided NEXAFS Spectroscopy: The Case of Free-Base Corroles on Ag(111). Chem. – Eur. J. 2018, 24, 6787–6797. 10.1002/chem.201705921. [DOI] [PubMed] [Google Scholar]
- Paszkiewicz M.; Biktagirov T.; Aldahhak H.; Allegretti F.; Rauls E.; Schöfberger W.; Schmidt W. G.; Barth J. V.; Gerstmann U.; Klappenberger F. Unraveling the Oxidation and Spin State of Mn–Corrole through X-ray Spectroscopy and Quantum Chemical Analysis. J. Phys. Chem. Lett. 2018, 9, 6412–6420. 10.1021/acs.jpclett.8b02525. [DOI] [PubMed] [Google Scholar]
- Blöchl P. E. Projector Augmented-Wave Method. Phys. Rev. B: Condens. Matter Mater. Phys. 1994, 50, 17953. 10.1103/PhysRevB.50.17953. [DOI] [PubMed] [Google Scholar]
- Becke A. D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic-Behavior. Phys. Rev. A: At., Mol., Opt. Phys. 1988, 38, 3098–3100. 10.1103/PhysRevA.38.3098. [DOI] [PubMed] [Google Scholar]
- Becke A. D. Density-Functional Thermochemistry. 3. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]
- Lee C. T.; Yang W. T.; Parr R. G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron-Density. Phys. Rev. B 1988, 37, 785. 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- Weigend F.; Ahlrichs R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. 10.1039/b508541a. [DOI] [PubMed] [Google Scholar]
- Neese F. Software Update: the ORCA Program System, version 4.0. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2018, 8, e1327 10.1002/wcms.1327. [DOI] [Google Scholar]
- Grimme S.; Antony J.; Ehrlich S.; Krieg H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104 10.1063/1.3382344. [DOI] [PubMed] [Google Scholar]
- https://xpssimplified.com/elements/carbon.php.
- Beamson G.; Briggs D.. High Resolution XPS of Organic Polymers; John Wiley & Sons: Chichester, 1992. [Google Scholar]
- Liao M.-S.; Scheiner S. Electronic Structure and Bonding in Metal Phthalocyanines, Metal = Fe, Co, Ni, Cu, Zn, Mg. J. Chem. Phys. 2001, 114, 9780. 10.1063/1.1367374. [DOI] [Google Scholar]
- Quintero Y.; Nagarajan R. Molecular and dissociative adsorption of DMMP, Sarin and Soman on dry and wet TiO2(110) using density functional theory. Surf. Sci. 2018, 675, 26–35. 10.1016/j.susc.2018.04.002. [DOI] [Google Scholar]
- Bermudez V. M. Quantum-Chemical Study of the Adsorption of DMMP and Sarin on γ-Al2O3. J. Phys. Chem. C 2007, 111, 3719–3728. 10.1021/jp066439g. [DOI] [Google Scholar]
- Guo H.; Wang Y.; Du S.; Gao H. High-resolution scanning tunnelling microscopy imaging of Si (1 1 1)-7 × 7 structure and intrinsic molecular states. J. Phys.: Condens. Matter 2014, 26, 394001 10.1088/0953-8984/26/39/394001. [DOI] [PubMed] [Google Scholar]
- Ahmadi S.; Shariati M. N.; Yu S.; Gothelid M. Molecular layers of ZnPc and FePc on Au(111) surface: Charge transfer and chemical interaction. J. Chem. Phys. 2012, 137, 084705 10.1063/1.4746119. [DOI] [PubMed] [Google Scholar]
- Oprea A.; Barsan N.; Weimar U. Work function changes in gas sensitive materials: Fundamentals and applications. Sens. Actuators, B 2009, 142, 470–493. 10.1016/j.snb.2009.06.043. [DOI] [Google Scholar]
- Hiesgen R.; Rabisch M.; Bottcher H.; Meissner D. STM investigation of the growth structure of Cu-phthalocyanine films with submolecular resolution. Sol. Energy Mater. Sol. Cells 2000, 61, 73–85. 10.1016/S0927-0248(99)00098-7. [DOI] [Google Scholar]
- Sims T. D.; Pemberton J. E.; Lee P.; Armstrong N. R. Comparison of supermolecular aggregate structure and spectroscopic and photoelectrochemical properties of tetravalent and trivalent metal phthalocyanine thin films. Chem. Mater. 1989, 1, 26–34. 10.1021/cm00001a010. [DOI] [Google Scholar]
- Gao W.; Kahn A. Controlled p-doping of zinc phthalocyanine by coevaporation with tetrafluorotetracyanoquinodimethane: A direct and inverse photoemission study. Appl. Phys. Lett. 2001, 79, 4040. 10.1063/1.1424067. [DOI] [Google Scholar]
- Hofmann O. T.; Deinert J. C.; Xu Y.; Rinke P.; Stähler J.; Wolf M.; Scheffler M. Large work function reduction by adsorption of a molecule with negative electron affinity: Pyridine on ZnO (1010). J. Chem. Phys. 2013, 139, 174701 10.1063/1.4827017. [DOI] [PubMed] [Google Scholar]
- https://srdata.nist.gov/xps/EnergyTypeValSrch.aspx.
- Evans D. A.; Steiner H. J.; Evans S.; Middleton R.; Jones T. S.; Park S.; Kampen T. U.; Zahn D. R. T.; Cabailh G.; McGovern I. T. Copper phthalocyanine on InSb(111)A-interface bonding, growth mode and energy band alignment. J. Phys.: Condens. Matter 2003, 15, S2729. 10.1088/0953-8984/15/38/011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.










