Abstract

Films made of colloidal CsPbBr3 nanocrystals packed in isolated or densely-packed superlattices display a remarkably high degree of structural coherence. The structural coherence is revealed by the presence of satellite peaks accompanying Bragg reflections in wide-angle X-ray diffraction experiments in parallel-beam reflection geometry. The satellite peaks, also called “superlattice reflections”, arise from the interference of X-rays diffracted by the atomic planes of the orthorhombic perovskite lattice. The interference is due to the precise spatial periodicity of the nanocrystals separated by organic ligands in the superlattice. The presence of satellite peaks is a fingerprint of the high crystallinity and long-range order of nanocrystals, comparable to those of multilayer superlattices prepared by physical methods. The angular separation between satellite peaks is highly sensitive to changes in the superlattice periodicity. These characteristics of the satellite peaks are exploited to track the superlattice compression under vacuum, as well as to observe the superlattice growth in situ from colloidal solutions by slow solvent evaporation.
Since 2015,1 colloidal nanocrystals (NCs) of lead halide perovskites (LHPs) have emerged as a class of promising nanomaterials for solution-processed light emitters and photovoltaics.2−4 Thin films of randomly-oriented NCs and ordered NC arrays or superlattices (SLs) are forms of solid-state LHP materials alternative to polycrystalline films or single crystals of solution-processed bulk LHPs. Arranging NCs into SLs may reveal novel phenomena arising from the collective behavior of LHP NCs, as has been demonstrated by several studies of light-emission properties of CsPbBr3 NC SLs.5−8
In this work, we investigate another collective phenomenon, namely, the structural coherence in three-dimensional SLs of CsPbBr3 NCs grown on a flat silicon substrate. By structural coherence, we mean the combination of high crystallinity and long-range order of NCs in an SL to an extent comparable with the structural quality of multilayer SLs of metals or semiconductors prepared using molecular beam epitaxy,9 sputtering,10 or electrodeposition.11 CsPbBr3 NC SLs prepared by slow solvent evaporation show not only preferential alignment (commonly observed in arrays of orientationally ordered NCs)12 but also “superlattice reflections”9,13 in wide-angle X-ray diffraction (XRD) experiments (Figures 1a and 2)—the signature of structural coherence. The SL reflections appear as periodically spaced satellite peaks in the region of the Bragg reflections from (110), (11̅0), and (002) planes at 2θ ∼ 15° (Figure 2) and (220), (22̅0), and (004) planes at 2θ ∼ 30.5° (Figure S1) of the orthorhombic unit cell of CsPbBr3 NCs. These satellite peaks are high-order Bragg reflections of the SL10,13 generated by the interference of the reflections from the atomic planes of orthorhombic CsPbBr3 NCs. The structural coherence arises from the combination of two features: (i) the high crystallinity, well-defined, and nearly identical shapes of the individual NCs and (ii) the regular NC packing, with little variation in the inter-NC spacing over large areas in the direction normal to the plane of the sample. Interestingly, this effect appears to be recurrent, as SL reflections of varying intensities can be identified by examination of the asymmetric or split shape of the 2θ ∼ 15° peak in the previously published XRD patterns,14−19 but their origin remained unexplained.
Figure 1.
XRD patterns of (a) a film of densely packed CsPbBr3 NC SLs measured in the out-of-plane reflection geometry. The splitting of the 2θ ∼ 15° peak (highlighted by the magenta box) is due to the SL effect. (b) The same sample measured in the in-plane geometry (a relatively sharp reflection at ∼47.2° is assigned to the silicon substrate20). (c) Randomly oriented NCs mixed with amorphous silica (the reference pattern is based on the lattice parameters from Rietveld refinement: a = 8.2248 Å, b = 8.2741 Å, c = 11.7748 Å; Miller indices are provided for the six most intense reflections). (d) Optical microscopy images of the isolated SLs and a film of densely packed SLs of CsPbBr3 NCs. Note that the peak splitting is observed in both types of samples (Figure S3). (e) Sketch of the film of densely-packed CsPbBr3 NC SLs featuring domains with a coherent structure.
Figure 2.

Closer look at the peak profile at 2θ ∼ 15°. A section of the out-of-plane XRD data shown in Figure 1a was replotted on a logarithmic intensity scale vs scattering vector [q (nm–1)]. The circles are experimental data, and the continuous red line is a fit. The vertical drop lines indicate the position of the five satellite peaks originating from the SL. The inset shows the best linear fit of the centers of satellite peaks in q vs n, starting from n = 19 (corresponding to the SL wavelength, Λ ∼ 12.2 nm).
The application of the Bragg law to the analysis of the satellite peaks10 allowed us to extract the SL wavelength, Λ, from a single X-ray measurement in parallel-beam reflection θ/2θ geometry. The utility of this parameter is demonstrated for two case studies: the contraction of NC SLs, hence the contraction of Λ, when the sample is put under vacuum (Figure 3), and the actual formation of the SLs upon slow solvent evaporation (Figure 4). Having a precise estimate of Λ that is independent of electron microscopy measurements is of key relevance for the study of LHP NC arrays, as NC orientation and spacing may strongly affect inter-NC effects, for example, exciton coupling.21,22
Figure 3.
(a) Time evolution of the satellite peaks in the 2θ ∼ 15° region (q ∼ 10.6 nm–1) in the XRD pattern of the film of densely packed CsPbBr3 NC SLs under static vacuum. (b) Corresponding contraction of the SL wavelength, Λ, as a function of time (red circles).
Figure 4.

Emergence of the satellite peaks in the 2θ ∼ 15° region during the growth of a film of densely-packed CsPbBr3 NC SLs from tetrachloroethylene solution. The figure reports a series of XRD patterns collected over 10 h at 30 min intervals. The inset is a sketch of the actually 3D-printed solvent evaporation chamber sealed with X-ray transparent Kapton and equipped with a sliding window on the side (blue-grey rectangle) to allow the placement of the sample.
The samples of CsPbBr3 NCs used in this work were synthesized following the hot-injection method1 with optimized amounts of oleic acid and oleylamine,17 as detailed in the prior work.8 The NC SLs were grown on top of 1 × 1 cm Si substrates from tetrachloroethylene solutions by letting the solvent evaporate over several hours (see Materials and Methods section in Supporting Information (SI)). The XRD pattern of the resulting film measured in the out-of-plane θ/2θ geometry is shown in Figure 1a. Two effects are immediately noticeable: a strong preferential alignment of NCs in the sample, manifested by the presence of two strong peaks at 2θ ∼ 15° and 2θ ∼ 30.5°, and the splitting of the 2θ ∼ 15° peak into narrow peaks. A similar but much weaker splitting is observed for the peak at 2θ ∼ 30.5° (Figure S1). The XRD of the same sample measured in-plane (Figure 1b) or of NCs mixed with amorphous silica (Figure 1c) contained the Bragg reflections expected from orthorhombic CsPbBr3.14,23 The Rietveld refinement of the XRD pattern from randomly-oriented NCs yielded lattice parameters similar to one of the reference structures of orthorhombic CsPbBr3 (ICSD No. 98-009-7851,24Figure S2 and Tables S1 and S3). The effect of peak splitting is reproducible and has been observed on the SL samples prepared from at least five different batches of CsPbBr3 NCs.
The preferential alignment originates from the close-packing of the cubes in the plane parallel to the substrate and leads to the enhanced Bragg reflections from (110), (11̅0), and (002) (2θ ∼ 15°) and (220), (22̅0), and (004) (2θ ∼ 30.5°) planes of the orthorhombic unit cell of CsPbBr3. The splitting of the peaks, most intense at the 2θ ∼ 15° and much weaker at 2θ ∼ 30.5°, arises from the SL effect—an interference of X-rays diffracted by the atomic planes of the orthorhombic perovskite lattice due to the precise spatial periodicity of the NCs in the SL (see discussion of the physical origin below). The SL effect at wide angles in X-ray diffraction is observed in NC films of isolated or densely packed SLs (Figure 1d; see Figure S3 for the XRD pattern of the sample of isolated SLs). The comparison of optical and scanning electron microscopy images of isolated SLs and the films suggests that films consist of densely packed SLs and continuously cover the substrate except in areas next to the sample edges (Figures 1d and e and S4–S7).
A deeper inspection of the out-of-plane XRD pattern unraveled a fine structure for the 2θ ∼ 15° peak, better represented on a logarithmic intensity versus scattering vector scale (Figure 2). The conversion from the scattering angle, θ (in radians), to the scattering vector, q (in nm–1), was done through the formula q = (4π/λX-ray) sinθ, where λX-ray = 0.15406 nm. The peak profile is composed of several contributions, which were fit to the total of six peaks described by Gaussian profiles on top of the background approximated by a 2nd order polynomial (Figure S8 and Table S2). Five out of six fitted peaks were assigned to the SL reflections, and one, with q =10.72 nm–1 (2θ ∼ 15.1°), was ascribed to the atomic Bragg reflection of the CsPbBr3 NCs on the basis that its position coincides with a corresponding peak from the XRD pattern of randomly oriented NCs.
The SL wavelength, Λ, order of satellite (n), and the corresponding peak center, qn, are related by the formula qn = 2πn/Λ, derived
by rearranging the original expression for the orders of SL reflections: 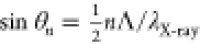 (10) (the expression is reproduced here with different
notation). According to the best fit, qn = 0.51483n (R2 = 0.998, Figure 2, inset) and Λ ∼ 12.2 nm, and the first resolved
satellite peak (at q = 9.76 nm–1) corresponds to n = 19. However, n values 18 and 20 (Λ ∼ 11.6 and 12.8 nm, respectively)
also yielded a reasonable linear fit. Unequivocal discrimination between
the different values of n is hindered by the broadening
of the peaks. The best fit of SL wavelength Λ ∼ 12.2
nm is comparable to ∼11.6–12.5 nm for the period extracted
from the fast Fourier transforms of the transmission electron microscopy
images of close-packed CsPbBr3 NCs (Figures S9–S11). The value of Λ is interpreted
as an average center-to-center distance between the inorganic CsPbBr3 cores inside the SL. The fitting of the XRD pattern in the
region of 2θ ∼ 15° is meant to provide an accurate
identification of the SL satellites for determination of the most
likely value of Λ and is not a quantitative model of the observed
effect.
(10) (the expression is reproduced here with different
notation). According to the best fit, qn = 0.51483n (R2 = 0.998, Figure 2, inset) and Λ ∼ 12.2 nm, and the first resolved
satellite peak (at q = 9.76 nm–1) corresponds to n = 19. However, n values 18 and 20 (Λ ∼ 11.6 and 12.8 nm, respectively)
also yielded a reasonable linear fit. Unequivocal discrimination between
the different values of n is hindered by the broadening
of the peaks. The best fit of SL wavelength Λ ∼ 12.2
nm is comparable to ∼11.6–12.5 nm for the period extracted
from the fast Fourier transforms of the transmission electron microscopy
images of close-packed CsPbBr3 NCs (Figures S9–S11). The value of Λ is interpreted
as an average center-to-center distance between the inorganic CsPbBr3 cores inside the SL. The fitting of the XRD pattern in the
region of 2θ ∼ 15° is meant to provide an accurate
identification of the SL satellites for determination of the most
likely value of Λ and is not a quantitative model of the observed
effect.
The physical origins of the satellite peaks in XRD of thin film SLs and epitaxial quantum dots are well-documented in the literature.13,25−27 For a qualitative explanation of the SL satellites observed in this work, let us consider the SL of CsPbBr3 NCs as a periodic 3D array of X-ray scatterers separated by the period Λ. CsPbBr3 NCs in the SL are crystalline particles which diffract X-rays in specific directions as determined by their crystal structure. The X-rays first diffracted by the atomic planes of the NCs undergo interference because of the phase shift caused by the periodicity of the SL. The diffraction from such a periodic array follows Bragg’s law, producing reflections at qn = 2πn/Λ: the consequent constructive interference of diffracted X-rays leads to a series of closely and equally spaced reflections (qn+1 – qn = 2π/Λ). Consequently, the satellite peaks appear only in the q range where there is diffraction by the atomic planes, and the intensities of the satellite peaks in the resulting diffraction pattern are modulated by the intensity profile of the NC diffraction peak.
An immediate utility of the satellite peaks for the characterization of CsPbBr3 NC SLs comes from the recognition that the spacing between the peaks is inversely proportional to the SL wavelength, Δqn ∝ 1/Λ, and that spacing can be accessed through a straightforward X-ray diffraction measurement in a parallel-beam out-of-plane geometry. For example, a series of XRD patterns recorded on the CsPbBr3 NC SLs placed under medium vacuum (∼10–2 mbar) for ∼70 min revealed a continuous change in the intensity, angular position, and separation of the satellite peaks in the 2θ ∼ 15° region, as shown in Figure 3a. The fitting of the satellite peaks following the procedure described above results in a progressively shrinking Λ (Figure 3b) from ∼12.21 to ∼11.95 nm (the number of figures after the decimal point reflects the average value of Λ). The SL contraction is irreversible, and the XRD pattern remains unchanged after the sample has been returned to atmospheric pressure (Figure S12). The contraction of Λ under vacuum has been reproduced on two different samples (Figures S13 and S14). Notably, the magnitude of a Λ contraction of ∼0.25 nm (Figure 3b) is comparable to the length of the tetrachloroethylene molecule (∼0.32 nm, calculated from the equilibrium distances and angles of C2Cl4),28 suggesting the solvent removal as the main reason for a Λ contraction and explaining its irreversibility upon return to ambient pressure.
The presence of the satellite peaks is characteristic of the structural coherence in the material. Tracking the emergence of the satellite peaks with X-ray diffraction in situ during the formation of SLs is a non-invasive way of monitoring the growth process.29Figure 4 shows the appearance of the satellite peaks during tetrachloroethylene evaporation from the CsPbBr3 NC solution deposited on top of the Si wafers (a replica of the process used for the preparation of the films described in this study, see Materials and Methods in SI). The process of tetrachloroethylene evaporation to dryness under ambient conditions takes ∼10 h; however, the satellite peaks begin to emerge after ∼6 h from the start of the experiment (cyan curve in Figure 4) and practically stop evolving after the total of ∼8 h have passed, possibly indicating the completion of the SL formation process. Interestingly, the angular positions of the satellite peaks do not shift throughout the process of growth (the apparent shift of the peak at 2θ ∼ 15.2° during the ∼4–6.5 h period is due to the transition from NCs suspended in the liquid to the SL structure), suggesting the retaining of the nearly constant Λ (∼12.1 nm from the fitting) during the film growth.
In conclusion, the observation and analysis of the satellite peaks at wide angles in the X-ray diffraction of CsPbBr3 NC SLs is presented. The structural coherence in the CsPbBr3 NC SLs arises from the combination of high crystallinity, well-defined and nearly identical shapes of individual NCs, and regular packing with little variation in the inter-NC spacing over a large area. The fitting of the satellite peaks enables the determination of the SL wavelength, Λ, and its modulation under external conditions (e.g., contraction under vacuum). It is also shown how the emergence of the satellite peaks can be used to monitor the formation of the NC SLs in situ during the solvent evaporation. Remarkably, the presence of satellite peaks makes all these insights into the structure of CsPbBr3 NC SLs accessible with a common X-ray diffraction setup.
Acknowledgments
The work of S.T. and L.M. was supported by the European Union under grant agreement no. 614897 (ERC Grant TRANS-NANO). The work of D.B. was supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement no. 794560 (RETAIN).
Glossary
Abbreviations
- XRD
X-ray diffraction
- NC
nanocrystal
- SL
superlattice
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmaterialslett.9b00217.
Materials and methods, XRD patterns of the samples of densely packed and isolated SLs, Rietveld refinement for randomly oriented NCs, fitting parameters for the satellite peaks, optical and scanning electron microscopy images of SLs, transmission electron microscopy images of CsPbBr3 NCs, and additional XRD patterns for SL samples before and after application of vacuum (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Protesescu L.; Yakunin S.; Bodnarchuk M. I.; Krieg F.; Caputo R.; Hendon C. H.; Yang R. X.; Walsh A.; Kovalenko M. V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. 10.1021/nl5048779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalenko M. V.; Protesescu L.; Bodnarchuk M. I. Properties and potential optoelectronic applications of lead halide perovskite nanocrystals. Science 2017, 358, 745–750. 10.1126/science.aam7093. [DOI] [PubMed] [Google Scholar]
- Akkerman Q. A.; Rainò G.; Kovalenko M. V.; Manna L. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat. Mater. 2018, 17, 394–405. 10.1038/s41563-018-0018-4. [DOI] [PubMed] [Google Scholar]
- Shamsi J.; Urban A. S.; Imran M.; De Trizio L.; Manna L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. 10.1021/acs.chemrev.8b00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurow M. J.; Lampe T.; Penzo E.; Kang J.; Koc M. A.; Zechel T.; Nett Z.; Brady M.; Wang L.-W.; Alivisatos A. P.; Cabrini S.; Brütting W.; Liu Y. Tunable Anisotropic Photon Emission from Self-Organized CsPbBr3 Perovskite Nanocrystals. Nano Lett. 2017, 17, 4534–4540. 10.1021/acs.nanolett.7b02147. [DOI] [PubMed] [Google Scholar]
- Rainò G.; Becker M. A.; Bodnarchuk M. I.; Mahrt R. F.; Kovalenko M. V.; Stöferle T. Superfluorescence from Lead Halide Perovskite Quantum Dot Superlattices. Nature 2018, 563, 671–675. 10.1038/s41586-018-0683-0. [DOI] [PubMed] [Google Scholar]
- Tong Y.; Yao E.-P.; Manzi A.; Bladt E.; Wang K.; Döblinger M.; Bals S.; Müller-Buschbaum P.; Urban A. S.; Polavarapu L.; Feldmann J. Spontaneous Self-Assembly of Perovskite Nanocrystals into Electronically Coupled Supercrystals: Toward Filling the Green Gap. Adv. Mater. 2018, 30, 1801117. 10.1002/adma.201801117. [DOI] [PubMed] [Google Scholar]
- Baranov D.; Toso S.; Imran M.; Manna L. Investigation into the Photoluminescence Red Shift in Cesium Lead Bromide Nanocrystal Superlattices. J. Phys. Chem. Lett. 2019, 10, 655–660. 10.1021/acs.jpclett.9b00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segmuller A.; Krishna P.; Esaki L. X-ray diffraction study of a one-dimensional GaAs-AlAs superlattice. J. Appl. Crystallogr. 1977, 10, 1–6. 10.1107/S0021889877012679. [DOI] [Google Scholar]
- Schuller I. K. New Class of Layered Materials. Phys. Rev. Lett. 1980, 44, 1597–1600. 10.1103/PhysRevLett.44.1597. [DOI] [Google Scholar]
- Lashmore D. S.; Dariel M. P. Electrodeposited Cu-Ni Textured Superlattices. J. Electrochem. Soc. 1988, 135, 1218–1221. 10.1149/1.2095930. [DOI] [Google Scholar]
- Murray C. B.; Kagan C. R.; Bawendi M. G. Self-Organization of CdSe Nanocrystallites into Three-Dimensional Quantum Dot Superlattices. Science 1995, 270, 1335–1338. 10.1126/science.270.5240.1335. [DOI] [Google Scholar]
- Holý V. C.; Pietsch U.; Baumbach T.. High-Resolution X-ray Scattering from Thin Films and Multilayers; Springer: Berlin, 1999; p xi, 256 p. [Google Scholar]
- Bertolotti F.; Protesescu L.; Kovalenko M. V.; Yakunin S.; Cervellino A.; Billinge S. J. L.; Terban M. W.; Pedersen J. S.; Masciocchi N.; Guagliardi A. Coherent Nanotwins and Dynamic Disorder in Cesium Lead Halide Perovskite Nanocrystals. ACS Nano 2017, 11, 3819–3831. 10.1021/acsnano.7b00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzon M.; Sortino L.; Akkerman Q.; Accornero S.; Pedrini J.; Prato M.; Pinchetti V.; Meinardi F.; Manna L.; Brovelli S. Role of Nonradiative Defects and Environmental Oxygen on Exciton Recombination Processes in CsPbBr3 Perovskite Nanocrystals. Nano Lett. 2017, 17, 3844–3853. 10.1021/acs.nanolett.7b01253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazon F.; Almeida G.; Akkerman Q. A.; De Trizio L.; Dang Z.; Prato M.; Manna L. Changing the Dimensionality of Cesium Lead Bromide Nanocrystals by Reversible Postsynthesis Transformations with Amines. Chem. Mater. 2017, 29, 4167–4171. 10.1021/acs.chemmater.7b00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida G.; Goldoni L.; Akkerman Q.; Dang Z.; Khan A. H.; Marras S.; Moreels I.; Manna L. Role of Acid–Base Equilibria in the Size, Shape, and Phase Control of Cesium Lead Bromide Nanocrystals. ACS Nano 2018, 12, 1704–1711. 10.1021/acsnano.7b08357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A.; Dutta S. K.; Das Adhikari S.; Pradhan N. Tuning the Size of CsPbBr3 Nanocrystals: All at One Constant Temperature. ACS Energy Letters 2018, 3, 329–334. 10.1021/acsenergylett.7b01226. [DOI] [Google Scholar]
- Imran M.; Ijaz P.; Baranov D.; Goldoni L.; Petralanda U.; Akkerman Q.; Abdelhady A. L.; Prato M.; Bianchini P.; Infante I.; Manna L. Shape-Pure, Nearly Monodispersed CsPbBr3 Nanocubes Prepared Using Secondary Aliphatic Amines. Nano Lett. 2018, 18, 7822–7831. 10.1021/acs.nanolett.8b03598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard C. R.; Swanson H. E.; Mauer F. A. A silicon powder diffraction standard reference material. J. Appl. Crystallogr. 1975, 8, 45–48. 10.1107/S0021889875009508. [DOI] [Google Scholar]
- Vovk I. A.; Tepliakov N. V.; Baimuratov A. S.; Leonov M. Y.; Baranov A. V.; Fedorov A. V.; Rukhlenko I. D. Excitonic phenomena in perovskite quantum-dot supercrystals. Phys. Chem. Chem. Phys. 2018, 20, 25023–25030. 10.1039/C8CP04724C. [DOI] [PubMed] [Google Scholar]
- Nestoklon M. O.; Goupalov S. V.; Dzhioev R. I.; Ken O. S.; Korenev V. L.; Kusrayev Y. G.; Sapega V. F.; de Weerd C.; Gomez L.; Gregorkiewicz T.; Lin J.; Suenaga K.; Fujiwara Y.; Matyushkin L. B.; Yassievich I. N. Optical orientation and alignment of excitons in ensembles of inorganic perovskite nanocrystals. Phys. Rev. B: Condens. Matter Mater. Phys. 2018, 97, 235304. 10.1103/PhysRevB.97.235304. [DOI] [Google Scholar]
- Cottingham P.; Brutchey R. L. On the crystal structure of colloidally prepared CsPbBr3 quantum dots. Chem. Commun. 2016, 52, 5246–5249. 10.1039/C6CC01088A. [DOI] [PubMed] [Google Scholar]
- Rodová M.; Brožek J.; Knížek K.; Nitsch K. Phase transitions in ternary caesium lead bromide. J. Therm. Anal. Calorim. 2003, 71, 667–673. 10.1023/A:1022836800820. [DOI] [Google Scholar]
- Bowen D. K.; Tanner B. K.. High Resolution X-ray Diffractometry and Topography; Taylor & Francis: London, 1998; p x, 252 p. [Google Scholar]
- Dhez P.; Weisbuch C.; North Atlantic Treaty Organization, Scientific Affairs Division. Physics, Fabrication, and Applications of Multilayered Structures; Plenum Press: New York, 1988; p xiii, 416 p. [Google Scholar]
- Schmidt O.Lateral Alignment of Epitaxial Quantum Dots; Springer: Berlin, 2007; p xv, 707 p. [Google Scholar]
- Karle I. L.; Karle J. The Structure of the Tetrachloroethylene Molecule. J. Chem. Phys. 1952, 20, 63–65. 10.1063/1.1700196. [DOI] [Google Scholar]
- Bein B.; Hsing H.-C.; Callori S. J.; Sinsheimer J.; Chinta P. V.; Headrick R. L.; Dawber M. In situ X-ray diffraction and the evolution of polarization during the growth of ferroelectric superlattices. Nat. Commun. 2015, 6, 10136. 10.1038/ncomms10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




