Abstract

The mechanical properties of scaffolds used for mechanically challenging applications such as cardiovascular implants are unequivocally important. Here, the effect of supramolecular additive functionalization on mechanical behavior of electrospun scaffolds was investigated for one bisurea-based model additive and two previously developed antifouling additives. The model additive has no effect on the mechanical properties of the bulk material, whereas the stiffness of electrospun scaffolds was slightly decreased compared to pristine PCL-BU following the addition of the three different additives. These results show the robustness of supramolecular additives used in biomedical applications, in which mechanical properties are important, such as vascular grafts and heart valve constructs.
Keywords: supramolecular chemistry, additives, electrospinning, multiscale, mechanics, biomaterials
Hydrogen-bonded thermoplastic elastomers (TPEs), such as polyureas, poly(urethane urea)s, or polyurethanes, are regularly used in cardiovascular applications such as cardiac patches,1 heart valves,2 and vascular grafts.3,4 The unique mechanical properties of these materials arise from their molecular composition, where macromolecular soft blocks with rubber-like properties are alternated with hard blocks capable of crystallization, for example, through hydrogen bonding.5 These two blocks have the tendency to phase-separate on the nanoscale, and in the case where the hard block is composed of well-defined hydrogen bonding units intriguingly forming fibrous hard segments.6,7 Using molecular recognition of the specific hydrogen-bonding motifs in thermoplastic elastomers, supramolecular biomaterials can be functionalized by modular incorporation of additives,8−10 to formulate materials with antifouling,11 and specific cell-adhesive properties,12,13 and with groups that allow for postfunctionalization.14 The additive-containing material mixtures can be processed into functional scaffolds using electrospinning.11,12,14,15 In general, the mechanical properties of load-bearing scaffolds fabricated with electrospinning, such as vascular grafts or other cardiovascular implants, are particularly important to ensure proper functioning. Therefore, it is crucial to know the influence of functional additives on the mechanical properties of supramolecular materials, and especially of electrospun fibrous scaffolds for load-bearing applications.
Past investigations elucidated the molecular reorganization of hydrogen-bonded fibrous TPEs during elongation.7,16 The orientation of the fibrous hard phase is generally isotropic following solution-casting, as evidenced from atomic force microscopy phase images (example in Figure 1B). Upon stretching, the hard segments orient in the direction of the applied strain. When the yield-point is exceeded, the hard segments fracture, and their orientation transgresses to the direction perpendicular to the strain.7,16 However, when these materials are processed into electrospun scaffolds, another level of complexity is introduced in the mechanical behavior. The mechanical properties of electrospun scaffolds are not only determined by the bulk material properties but also by microscale scaffold properties such as fiber diameter, fiber structure, fiber orientation, and fiber interconnectivity (Figure 1C).17,18 Biaxial deformation of isotropically distributed electrospun fibers results in stretching of the fibers until the fiber–fiber cross-links are strained at higher strains.19 With uniaxial elongation, additional alignment of the fibers occurs. This shows that several principles play important roles in the mechanical behavior in physiologically relevant strain-regimes.20
Figure 1.
Multiscale overview of study. (A) Structural representation of bisurea additives and the PCL-BU base polymer used in this study. (B) Atomic force microscopy phase image with fibrous hard-segments attributed to the bisurea assembly. Scale bar indicates 100 nm. (C) Example of scanning electron micrograph of electrospun PCL-BU scaffold (top, scale bar indicates 10 μm) and the fiber orientation analysis of an electrospun scaffold (bottom). (D) Dogbone-shaped scaffold sample for tensile testing coated with graphite for strain analysis, before (top) and during (bottom) elongation.
Previously, the supramolecular functionalization strategy based on molecular recognition by bisurea additives has been used to tune the mechanical properties of bisurea-based thermoplastic elastomers.21 Through incorporation of a small molecular filler, which was shown to coassemble with the bisurea phase in the poly(caprolactone)-bisurea (PCL-BU) base material up to approximately 25 mol %, the ratio of hard block to soft block was altered in a supramolecular fashion. This resulted in an approximately two-fold higher Young’s modulus, without a clear loss of ultimate tensile strength.21 Higher concentrations of the filler additive resulted in two distinct hard-segment domains: below 25 mol %, similar strain-induced orientation of the fibrous hard segments is observed compared to the unmodified base material,22 and above 25 mol %, hard segments form that solely consist of filler, and which do not orient with the hard phase of the base material, as little interaction forces them to do so.22
Here, we further study electrospun functionalized supramolecular materials to get an understanding of the effect supramolecular additives on the mechanical properties of electrospun constructs. To this end, the properties of supramolecular electrospun scaffolds functionalized with antifouling oligo(ethylene glycol) (OEG)-based bisurea additives (BO and OBO)11 and the bisurea-filler (BF)21 as a model compound were studied (Figure 1) and compared to pristine controls. Considering that fiber diameter and orientation directly influence the mechanical properties of electrospun scaffolds,23,24 we verified the similarity in fiber diameter and isotropy of the fiber distribution of the electrospun scaffolds that were fabricated for this study. To determine if the bisurea groups from the additives are incorporated in the same hard segments as those from the TPE base material, the thermal properties of the functionalized materials were assessed with differential scanning calorimetry (DSC). Finally, the mechanical properties of the electrospun scaffolds were quantified using uniaxial tensile testing.
First, the effect on mechanical properties of the BF additive was investigated in both thick films and electrospun scaffolds (thickness of approximately 150 and 200 μm, respectively) at concentrations of 5, 10, and 25 mol % additive. For this additive, the mechanical properties of the composite material are affected if one bisurea hard phase is formed, which was the case for <25 mol % in previously reported literature.21 In that case, a single melting peak associated with the crystalline bisurea domain was observed with DSC. In the current study, the thermogram of both the films and electrospun scaffolds also revealed a single peak (Figure 2A,B), for which the melting temperature decreased slightly as the BF concentration increased (Table S1). However, the melting transition of the pure BF additive is in the same range as the melting transition of the bisurea in the PCL-BU (Figure 2C), which hampers the determination of segregated hard phases through the observation of multiple melting transitions as was shown in the study by Wisse et al.21
Figure 2.
Thermograms of the first heating run of (A) films of PCL-BU with 5, 10, and 25% BF additive, (B) electrospun scaffolds of PCL-BU with 5, 10, and 25 mol % BF additive, and (C) the second heating run of the pure BF additive. Endotherm processes appear as peaks. (D) Scanning electron micrographs of electrospun scaffolds of PCL-BU with 5, 10, and 25% of the BF additive. Scale bars indicate 10 μm. (E) Fiber distributions for the electrospun scaffolds. Open circles represent the data, and the red line represents the fit. (F) Young’s moduli of films (determined between 0 and 5% stretch) of PCL-BU with 0, 5, 10, and 25% BF additive. (G) Young’s moduli (determined between 0 and 20% stretch) of electrospun scaffolds of PCL-BU with 0, 5, 10, and 25% BF additive. Statistically significant differences compared to 0% additive are indicated with an asterisk.
The enthalpy only varied slightly upon incorporation of the BF additive in the electrospun scaffolds, which would indicate minor differences in the crystallinity of the hard phases composed of bisurea from the base material and the BF additive.
Prior to mechanical testing, the morphology of the scaffolds was characterized using scanning electron microscopy and subsequent image analysis. The fiber diameters for the electrospun scaffolds were comparable at 0.6–0.7 μm (Figure 2D, Table S2). Using a previously reported method, the degree of (an)isotropy of the electrospun fibers was quantified.25 Using this method, the fiber directionality histograms obtained after image analysis were fit with a Gaussian distribution on top of a baseline, where the portion below the baseline was considered the isotropic fraction. For these electrospun scaffolds, the approximated anisotropic fraction was low with broad peaks (Figure 2E, Table S2), and therefore, these scaffolds could be considered sufficiently isotropic. The combined similarity of fiber diameter and isotropy substantiated the assumption that these scaffolds also had similar porosity.
Interestingly, the Young’s modulus did not increase for the films with increasing BF additive concentration (Figure 2F), in contrast to the results reported by Wisse et al.21 Compared to these earlier reported results, the Young’s modulus of the pristine PCL-BU films measured in the current study was significantly higher, whereas the amount of hard segments was lower due to an increased soft-block length (PCL, Mn = 2 kg mol–1 in this study26 compared to Mn = 1.4 kg mol–1 in Wisse et al.21). The moduli determined for the electrospun scaffolds were roughly one order of magnitude lower than for the films, which could be explained from the porosity of the scaffolds and different deformation mechanisms involved in changing the scaffold microstructure, compared to deformation of solid materials (Figure 2G). Upon incorporation of BF additive, the stiffness of the scaffolds slightly decreased, in the order of 10–20%, although no BF concentration-dependence was observed (Figure 2).
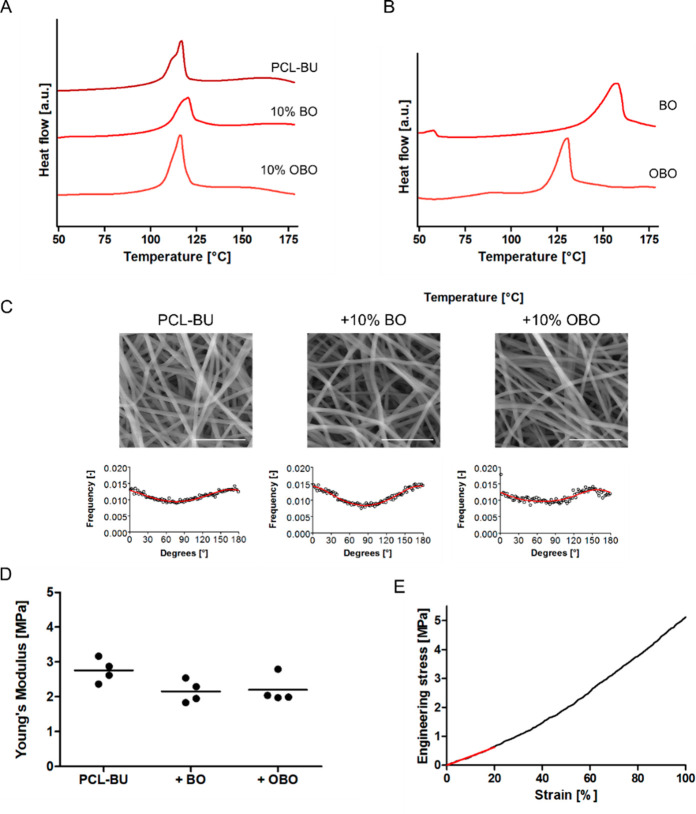
The electrospun scaffolds functionalized with the antifouling additives were characterized in a similar fashion with DSC. The melting temperature of the PCL-BU only varied a few degrees upon functionalization with the two OEG-based additives (Figure 3A, Tables S3 and S4). Interestingly, the melting temperatures for the pure additives were substantially higher (Figure 3B). The single melting transition in the thermograms of the functionalized scaffolds indicated a single hard phase and thereby that the bisurea blocks of the additives were incorporated in the hard phase of the PCL-BU base material. The increased melting temperature of the bisurea additives, along with a clear melting transition that was not necessarily wider compared to PCL-BU, indicated proper assembly of the larger hard segments with additional hydrogen bonding urethane groups in these additives.10 The different melting temperatures for the two additives can be attributed to the slightly different composition of the hydrophobic parts in the additives (urethane-C12-urea-C4-urea-C12-urethane and C6-urea-C4-urea-C12-urethane for OBO and BO, respectively). The fiber diameter in the scaffolds ranged from 0.7 ± 0.1 μm to 0.8 ± 0.1 μm and can therefore be considered similar for the purpose of these experiments (Table S5). Moreover, the overall morphology and degree of isotropy of the scaffolds were comparable (Figure 3C, Table S5). The stiffness of the electrospun scaffolds decreased slightly upon functionalization with either one of the two OEG-based additives, approximately to the same extent as was observed for the addition of 10% of the model BF compound (Figure 3D). Notably, a slight strain-stiffening trend can be observed in the tensile test up to 100% strain, which is typically observed for biopolymer materials (Figure 3E).27
Figure 3.
(A) Thermogram of first heating run of electrospun scaffolds of PCL-BU with 10% BO, and OBO additive. Endotherm processes appear as peaks. (B) Second heating run of pure BO and OBO additive. Endotherm processes appear as peaks. (C) Top row: scanning electron micrographs of electrospun scaffolds of PCL-BU with 10% BO and OBO additive. Scale bars indicate 10 μm. Bottom row: fiber distributions for the electrospun scaffolds. Open circles represent the data, and the red line represents the fitted distribution. (D) Young’s moduli of electrospun scaffolds of pristine PCL-BU and with 10% BO and OBO additive. (E) Representative engineering stress–strain curve for electrospun scaffold, up to 100% strain. The Young’s modulus is determined from the slope of the red fitted line (between physiologically relevant strains of 0–20%).
Combining the data on mechanical testing of electrospun scaffolds of PCL-BU modified with the BF model compound and with the antifouling OEG-based additives indicated that incorporation of a supramolecular additive can result in a slight change in initial modulus of the electrospun scaffolds. For the BF additive, this cannot be related to a change in mechanical properties of the bulk material, which was not affected by filler incorporation, contrary to earlier reports.21 Therefore, the observed decrease in modulus of the electrospun scaffolds, which had apparent similar morphologies, must have had a cause in a different aspect of the scaffold properties. Other studies have reported on the mechanical properties of electrospun scaffolds with changing chemical composition. In a study by Ye et al., electrospun scaffolds were fabricated from a PCL-based poly(urethane urea), which had increasing sulfobetaine content incorporated in the polymer backbone, and corresponding increasing hard phase:soft phase ratios. The initial modulus of the bulk material did not change up to 50% sulfobetaine content when measured in dry conditions, and it decreased slightly when measured in wet conditions. The initial modulus of their electrospun scaffolds also decreased considerably as sulfobetaine content increased, which illustrates the effect chemical adaptations can have on electrospun scaffold properties.28
The degree of fiber–fiber interactions in electrospun scaffolds has been shown to affect mechanical behavior.19 Electrospinning with different material compositions may require different spinning condition and may result in different spinning outcomes and thereby a change in fiber–fiber interactions, which would not be obvious from the optical characterization implemented here. Furthermore, a change in surface chemistry of the fibers, which was present at least for the scaffolds modified with OEG-based additives,11 can also result in changed fiber–fiber interactions. However, decreased interactions between the fibers were not expected for the BF additive because of an expected limited effect on surface chemistry. Besides the fiber–fiber interactions, the overall porosity of the scaffold might be influenced by the incorporation of additives. Here, we assumed similar porosity between the different scaffolds based on the similarities in fiber diameter and isotropy. Yet, changes in porosity would influence the true stress in the material. An increased porosity for the additive modified scaffolds would explain the decrease in initial stiffness. Therefore, porosity should be determined accurately in future efforts.29
The choice for thermoplastic elastomers with defined hard blocks, such as the PCL-BU studied here, follows from their superior mechanical properties compared to TPEs with less defined hard segments.16 This was attributed to an increased nanoscale segregation of the hard and soft phases, in combination with increased crystallinity. The comparable melting transitions observed here for films and scaffolds of PCL-BU (Figure 2) suggest that similar degrees of crystallinity are obtained, which would point toward the same amount of segregation between the soft and hard phase.
Additionally, tensile deformation of the bulk material involves reorientation of the fibrous hard phase from isotropic to anisotropic, parallel to the strain directions. For other polymeric electrospun fibers, orientation of the polymer chains parallel to the fiber direction was observed.17 Furthermore, in supramolecular TPE electrospun fibers, evidence from wide-angle X-ray scattering and atomic force microscopy measurements pointed toward the presence of nanofibers, similar to those seen in solution-cast films.30 These findings, in combination with the comparable degree of bisurea hard-phase crystallinity in the electrospun scaffolds, may indicate a molecular hard-segment conformation with alignment already perpendicular to the fiber direction, which would correspond to a bulk material beyond the yield point. It would be particularly interesting to more closely investigate the molecular conformation of the bisurea-based TPEs in electrospun fibers with X-ray diffraction methods under strain, in a similar fashion as for the bulk materials, to get a more fundamental understanding of the nanoscale processes that are involved in deformation of supramolecular electrospun scaffolds. Furthermore, the opportunity to probe strain at a molecular level using mechanically sensitive additives can be used in a complementary fashion.31−33 Additionally, numerical modeling efforts may result in increased understanding of the principles involved in these complex, multiscale questions.23,34
In this study, we have shown that despite a lack of change in bulk mechanical properties due to supramolecular additive modification, the initial modulus of electrospun scaffolds can be slightly affected thereby. The small decrease in initial scaffold stiffness was comparable for all three additives studied here. These results show that the combination of supramolecular modification and processing into complex 3D shapes via electrospinning is a multiscale problem that requires more in-depth understanding of the interactions between the modification and the mechanical behavior of the 3D construct. Furthermore, in designing functional biomaterial constructs, care should be taken with the (minor) effect of the functionalization on mechanical properties of the electrospun scaffolds.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsapm.0c00658.
Experimental details and supporting data (PDF)
This work was funded by the European Research Council (FP7/2007–2013) ERC Grant Agreement No. 308045, the Ministry of Education, Culture and Science (Gravity Program 024.001.035), and ZonMW as part of the LSH 2Treat program (Project No. 436001003).
The authors declare no competing financial interest.
Supplementary Material
References
- Gu X.; Matsumura Y.; Tang Y.; Roy S.; Hoff R.; Wang B.; Wagner W. R. Sustained Viral Gene Delivery from a Micro-Fibrous, Elastomeric Cardiac Patch to the Ischemic Rat Heart. Biomaterials 2017, 133, 132–143. 10.1016/j.biomaterials.2017.04.015. [DOI] [PubMed] [Google Scholar]
- Kluin J.; Talacua H.; Smits A. I. P. M.; Emmert M. Y.; Brugmans M. C. P.; Fioretta E. S.; Dijkman P. E.; Söntjens S. H. M.; Duijvelshoff R.; Dekker S.; Janssen-Van den Broek M. W. J. T.; Lintas V.; Vink A.; Hoerstrup S. P.; Janssen H. M.; Dankers P. Y. W.; Baaijens F. P. T.; Bouten C. V. C. In Situ Heart Valve Tissue Engineering Using a Bioresorbable Elastomeric Implant – From Material Design to 12 Months Follow-up in Sheep. Biomaterials 2017, 125, 101–117. 10.1016/j.biomaterials.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Seifalian A. M.; Salacinski H. J.; Tiwari A.; Edwards A.; Bowald S.; Hamilton G. In Vivo Biostability of a Poly(Carbonate-Urea)Urethane Graft. Biomaterials 2003, 24 (14), 2549–2557. 10.1016/S0142-9612(02)00608-7. [DOI] [PubMed] [Google Scholar]
- Hong Y.; Ye S.; Nieponice A.; Soletti L.; Vorp D. A.; Wagner W. R. A Small Diameter, Fibrous Vascular Conduit Generated from a Poly(Ester Urethane)Urea and Phospholipid Polymer Blend. Biomaterials 2009, 30 (13), 2457–2467. 10.1016/j.biomaterials.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spontak R. J.; Patel N. P. Thermoplastic Elastomers: Fundamentals and Applications. Curr. Opin. Colloid Interface Sci. 2000, 5 (5–6), 333–340. 10.1016/S1359-0294(00)00070-4. [DOI] [Google Scholar]
- Koevoets R. A.; Versteegen R. M.; Kooijman H.; Spek A. L.; Sijbesma R. P.; Meijer E. W. Molecular Recognition in a Thermoplastic Elastomer. J. Am. Chem. Soc. 2005, 127 (9), 2999–3003. 10.1021/ja0451160. [DOI] [PubMed] [Google Scholar]
- Sauer B. B.; McLean R. S.; Gaymans R. J.; Niesten M. C. J. E. Crystalline Morphologies in Segmented Copolymers with Hard Segments of Uniform Length. J. Polym. Sci., Part B: Polym. Phys. 2004, 42 (9), 1783–1792. 10.1002/polb.20060. [DOI] [Google Scholar]
- Botterhuis N. E.; Karthikeyan S.; Veldman D.; Meskers S. C. J.; Sijbesma R. P. Molecular Recognition in Bisurea Thermoplastic Elastomers Studied with Pyrene-Based Fluorescent Probes and Atomic Force Microscopy. Chem. Commun. 2008, (33), 3915–3917. 10.1039/b804457k. [DOI] [PubMed] [Google Scholar]
- Botterhuis N. E.; Karthikeyan S.; Spiering A. J. H.; Sijbesma R. P. Self-Sorting of Guests and Hard Blocks in Bisurea-Based Thermoplastic Elastomers. Macromolecules 2010, 43 (2), 745–751. 10.1021/ma902585w. [DOI] [Google Scholar]
- Wisse E.; Spiering A. J. H.; van Leeuwen E. N. M.; Renken R. A. E.; Dankers P. Y. W.; Brouwer L. A.; van Luyn M. J. A.; Harmsen M. C.; Sommerdijk N. A. J. M.; Meijer E. W. Molecular Recognition in Poly(ε-Caprolactone)-Based Thermoplastic Elastomers. Biomacromolecules 2006, 7 (12), 3385–3395. 10.1021/bm060688t. [DOI] [PubMed] [Google Scholar]
- Ippel B. D.; Keizer H. M.; Dankers P. Y. W. Supramolecular Antifouling Additives for Robust and Efficient Functionalization of Elastomeric Materials: Molecular Design Matters. Adv. Funct. Mater. 2019, 29 (1), 1805375. 10.1002/adfm.201805375. [DOI] [Google Scholar]
- Muylaert D. E. P.; van Almen G. C.; Talacua H.; Fledderus J. O.; Kluin J.; Hendrikse S. I. S.; van Dongen J. L. J.; Sijbesma E.; Bosman A. W.; Mes T.; Thakkar S. H.; Smits A. I. P. M.; Bouten C. V. C.; Dankers P. Y. W.; Verhaar M. C. Early In-Situ Cellularization of a Supramolecular Vascular Graft Is Modified by Synthetic Stromal Cell-Derived Factor-1α Derived Peptides. Biomaterials 2016, 76, 187–195. 10.1016/j.biomaterials.2015.10.052. [DOI] [PubMed] [Google Scholar]
- Ippel B. D.; Arts B.; Keizer H. M.; Dankers P. Y. W. Combinatorial Functionalization with Bisurea-peptides and Antifouling Bisurea Additives of a Supramolecular Elastomeric Biomaterial. J. Polym. Sci., Part B: Polym. Phys. 2019, 57 (24), 1725–1735. 10.1002/polb.24907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goor O. J. G. M.; Keizer H. M.; Bruinen A. L.; Schmitz M. G. J.; Versteegen R. M.; Janssen H. M.; Heeren R. M. A.; Dankers P. Y. W. Efficient Functionalization of Additives at Supramolecular Material Surfaces. Adv. Mater. 2017, 29 (5), 1604652. 10.1002/adma.201604652. [DOI] [PubMed] [Google Scholar]
- van Almen G. C.; Talacua H.; Ippel B. D.; Mollet B. B.; Ramaekers M.; Simonet M.; Smits A. I. P. M.; Bouten C. V. C.; Kluin J.; Dankers P. Y. W. Development of Non-Cell Adhesive Vascular Grafts Using Supramolecular Building Blocks. Macromol. Biosci. 2016, 16 (3), 350–362. 10.1002/mabi.201500278. [DOI] [PubMed] [Google Scholar]
- Versteegen R. M.; Kleppinger R.; Sijbesma R. P.; Meijer E. W. Properties and Morphology of Segmented Copoly(Ether Urea)s with Uniform Hard Segments. Macromolecules 2006, 39 (2), 772–783. 10.1021/ma051874e. [DOI] [Google Scholar]
- Baji A.; Mai Y.; Wong S.; Abtahi M.; Chen P. Electrospinning of Polymer Nanofibers: Effects on Oriented Morphology, Structures and Tensile Properties. Compos. Sci. Technol. 2010, 70 (5), 703–718. 10.1016/j.compscitech.2010.01.010. [DOI] [Google Scholar]
- Amoroso N. J.; D’Amore A.; Hong Y.; Wagner W. R.; Sacks M. S. Elastomeric Electrospun Polyurethane Scaffolds: The Interrelationship Between Fabrication Conditions, Fiber Topology, and Mechanical Properties. Adv. Mater. 2011, 23 (1), 106–111. 10.1002/adma.201003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella J. A.; Wagner W. R.; Sacks M. S. Scale-Dependent Fiber Kinematics of Elastomeric Electrospun Scaffolds for Soft Tissue Engineering. J. Biomed. Mater. Res., Part A 2009, 93 (3), 1032–1042. 10.1002/jbm.a.32593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haaften E.; Bouten C.; Kurniawan N. Vascular Mechanobiology: Towards Control of In Situ Regeneration. Cells 2017, 6 (3), 19. 10.3390/cells6030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse E.; Govaert L. E.; Meijer H. E. H.; Meijer E. W. Unusual Tuning of Mechanical Properties of Thermoplastic Elastomers Using Supramolecular Fillers. Macromolecules 2006, 39 (21), 7425–7432. 10.1021/ma060986i. [DOI] [Google Scholar]
- Wisse E.; Spiering A. J. H.; Pfeifer F.; Portale G.; Siesler H. W.; Meijer E. W. Segmental Orientation in Well-Defined Thermoplastic Elastomers Containing Supramolecular Fillers. Macromolecules 2009, 42 (2), 524–530. 10.1021/ma801668k. [DOI] [Google Scholar]
- Argento G.; Simonet M.; Oomens C. W. J.; Baaijens F. P. T. Multi-Scale Mechanical Characterization of Scaffolds for Heart Valve Tissue Engineering. J. Biomech. 2012, 45 (16), 2893–2898. 10.1016/j.jbiomech.2012.07.037. [DOI] [PubMed] [Google Scholar]
- Keun Kwon I.; Kidoaki S.; Matsuda T. Electrospun Nano- to Microfiber Fabrics Made of Biodegradable Copolyesters: Structural Characteristics, Mechanical Properties and Cell Adhesion Potential. Biomaterials 2005, 26 (18), 3929–3939. 10.1016/j.biomaterials.2004.10.007. [DOI] [PubMed] [Google Scholar]
- van Haaften E. E.; Wissing T. B.; Rutten M. C. M.; Bulsink J. A.; Gashi K.; van Kelle M. A. J.; Smits A. I. P. M.; Bouten C. V. C.; Kurniawan N. A. Decoupling the Effect of Shear Stress and Stretch on Tissue Growth and Remodeling in a Vascular Graft. Tissue Eng., Part C 2018, 24 (7), 418–429. 10.1089/ten.tec.2018.0104. [DOI] [PubMed] [Google Scholar]
- Duijvelshoff R.; van Engeland N.; Gabriels K.; Söntjens S.; Smits A.; Dankers P.; Bouten C. Host Response and Neo-Tissue Development during Resorption of a Fast Degrading Supramolecular Electrospun Arterial Scaffold. Bioengineering 2018, 5 (3), 61. 10.3390/bioengineering5030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm C.; Pastore J. J.; MacKintosh F. C.; Lubensky T. C.; Janmey P. A. Nonlinear Elasticity in Biological Gels. Nature 2005, 435 (7039), 191–194. 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- Ye S.; Hong Y.; Sakaguchi H.; Shankarraman V.; Luketich S. K.; D’Amore A.; Wagner W. R. Nonthrombogenic, Biodegradable Elastomeric Polyurethanes with Variable Sulfobetaine Content. ACS Appl. Mater. Interfaces 2014, 6 (24), 22796–22806. 10.1021/am506998s. [DOI] [PubMed] [Google Scholar]
- Guo A.; Roso M.; Modesti M.; Maire E.; Adrien J.; Colombo P. Characterization of Porosity, Structure, and Mechanical Properties of Electrospun SiOC Fiber Mats. J. Mater. Sci. 2015, 50 (12), 4221–4231. 10.1007/s10853-015-8973-5. [DOI] [Google Scholar]
- Mollet B. B.; Bogaerts I. L. J.; van Almen G. C.; Dankers P. Y. W. A Bioartificial Environment for Kidney Epithelial Cells Based on a Supramolecular Polymer Basement Membrane Mimic and an Organotypical Culture System. J. Tissue Eng. Regener. Med. 2017, 11 (6), 1820–1834. 10.1002/term.2080. [DOI] [PubMed] [Google Scholar]
- Karthikeyan S.; Sijbesma R. P. Probing Strain in Thermoplastic Elastomers Using Fluorescence Resonance Energy Transfer. Macromolecules 2009, 42 (14), 5175–5178. 10.1021/ma900739d. [DOI] [Google Scholar]
- Filonenko G. A.; Lugger J. A. M.; Liu C.; van Heeswijk E. P. A.; Hendrix M. M. R. M.; Weber M.; Müller C.; Hensen E. J. M.; Sijbesma R. P.; Pidko E. A. Tracking Local Mechanical Impact in Heterogeneous Polymers with Direct Optical Imaging. Angew. Chem. 2018, 130 (50), 16623–16628. 10.1002/ange.201809108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filonenko G. A.; Khusnutdinova J. R. Dynamic Phosphorescent Probe for Facile and Reversible Stress Sensing. Adv. Mater. 2017, 29 (22), 1700563. 10.1002/adma.201700563. [DOI] [PubMed] [Google Scholar]
- D’Amore A.; Amoroso N.; Gottardi R.; Hobson C.; Carruthers C.; Watkins S.; Wagner W. R.; Sacks M. S. From Single Fiber to Macro-Level Mechanics: A Structural Finite-Element Model for Elastomeric Fibrous Biomaterials. J. Mech. Behav. Biomed. Mater. 2014, 39, 146–161. 10.1016/j.jmbbm.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.