Abstract
Under ambient conditions resorcinol (Res), C6H4(OH)2, favorably crystallizes from methanol and aqueous solutions as the anhydrate, in the form of polymorph α at room temperature. Anhydrous polymorph β can be obtained above 360 K. However, above 0.80 GPa the monohydrate Res·H2O is formed from the aqueous solution. The monohydrate is less stable than the duotritohydrate 3Res·2H2O, which nucleates later. The latter forms a tight passivation layer on the surface of monohydrate crystals and protects them from dissolution. Between 0.20 and 1.0 GPa the duotritohydrate is more favored than the previously reported Res polymorphs α and β. From a methanol solution above 0.40 GPa the methanol monosolvate Res·CH3OH precipitates. In Res·H2O resorcinol molecules assume the syn-syn conformation, and in 3Res·2H2O independent syn-syn and anti-anti conformers are present. The anti-anti molecule is orientationally disordered, despite the fact that usually the disorder requires extra space, while the high pressure suppresses the volume. In all three new solvates, the solvent molecules mediate the H bonding between the hydroxyl groups. The formation of solvates can be rationalized by the low potential energy of syn-syn conformers as well as the volume gain of the solvates in comparison to the summed volumes of the pure resorcinol crystal and stoichiometric amounts of the solvent. The strong preference of the analogous orcinol (5-methylresorcinol) for the monohydrate formation under normal conditions is unchanged under high pressure.
Short abstract
High pressure promotes new MeOH and H2O solvates of resorcinol.
Introduction
Pure compounds and their solvates and cocrystals can significantly differ in their physical and chemical properties. Therefore, such different forms of chemical compounds are often investigated to improve the performance of products.1−3 The investigation of hydrates is of particular importance, because humid air and solvents are usually involved in the manufacturing and formulation processes of (drug) compounds. Hydrate formation concerns at least one-third of organic compounds.4,5 However, presently there are still no general methods of predicting the preference for the formation of the pure or solvated forms. It was shown that high pressure can promote the formation of solvates for compounds that crystallize exclusively in the pure form under ambient conditions: for example, thiourea, C(NH2)S, at 0.5 GPa forms the monohydrate, C(NH2)2S·H2O, and above 0.7 GPa the duotritohydrate, 3C(NH2)S·2H2O,6 and the monohydrate of 1,4-diazabicyclo[2.2.2]octane dihydrobromide (hereafter dabco2HBr; 1,4-diazabicyclo[2.2.2]octane = dabco) above 0.48 GPa forms monohydrate dabco2HBr·H2O polymorphs.7 High-pressure crystallization proved to be an efficient method for obtaining hydrates and other solvates of organic compounds: for example, dabco salts,8−10 4,4′-bipyridinium perchlorate,11 5,6-dimethylbenzimidazole.12 xylazine hydrochloride,13 and diphenylanthracene14 as well as pharmaceutical compounds of β-chlorpropamide15 and deoxycholic acid.16 It was shown that the preferred formation of these hydrates is connected with an increase in their volume, in comparison to the volumes of the exact amount of the pure host compound crystallized separately and with the solvent present in the form of either a liquid or solid depending on the thermodynamic conditions;6−10 also the formation of new types of intermolecular bonds plays an important role.17,18
The reverse effect of pressure favoring the crystallization of separate compounds has also been observed, but less frequently. For example, the isochoric crystallization of thiourea from an aqueous solution above 1.0 GPa yields the pure thiourea and ice VI, the methane hydrate, strongly preferred by pressure, decomposes above 8.0 GPa,19 and Y2(C2O4)3·10H2O undergoes a partial dehydration at 1 GPa, forming monoclinic Y2(C2O4)3·6H2O as single-crystalline inclusions in the original phase.20
Presently, we have investigated the preferences of resorcinol and orcinol (Figure 1) for crystallization as either the pure or solvated compounds under high pressure. Resorcinol crystallizes exclusively as the anhydrate from aqueous solutions under the standard conditions, which contrasts with the analogous orcinol, where hydrate formation is strongly favored. Resorcinol and its derivatives as well as isomeric quinol compounds21 are applied as medicines, and they can also be used as monomers (or as reactive additives) which increase the molecular weight of (pre)polymers. They can also be used in adhesives for wood.22 The active ingredients in the extracts of various plants contain resorcinol derivatives, which are primarily used in the treatment of cancer, osteoporosis, gastric ulcers, and other diseases.23
Figure 1.
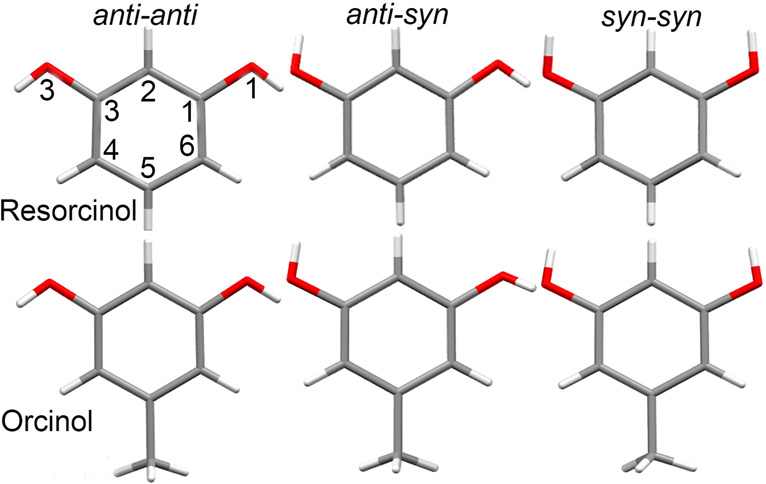
Resorcinol and orcinol conformers. Atomic labels usually applied in the literature and in this report are shown in the first drawing.
Five polymorphs of pure resorcinol, labeled α and β (the same space group Pna21),24,25 γ (Pnna),26,27 δ (space group unknown),26,27 and ε (space group P212121)28 have been reported. The density of the α polymorph, which is stable under normal conditions, is lower than that of the high-temperature polymorph β.29 Their structures were determined by X-ray diffraction in the 1930s,24,25 the structure of the α polymorph was one of the first for an organic compound to be determined by neutron diffraction in 1956,30 and the ε polymorph was discovered in 2016. Despite such a long and varied record of experimental studies, no hydrates of resorcinol have been reported, which contrasts with a strong preference of orcinol (a close analogue of resorcinol) to form the hydrate. Our present study is aimed at determining the thermodynamic preferences of resorcinol solvates and to form an understanding of the mechanisms favoring the formation of solvates at high pressure. To study these preferences, we have performed high-pressure recrystallizations of resorcinol in aqueous, methanol, and ethanol solutions. We have also performed high-pressure crystallizations of orcinol, to allow a comparison of the results obtained from the study of these two similar compounds.
Experimental Section
The high-pressure crystallizations of the aqueous and methanol solutions were performed in a modified diamond anvil cell (DAC).31 Resorcinol solutions of various concentrations were used in order to start the nucleation at pressure under a range of isochoric and isothermal conditions.32 The in situ isothermal crystallization of the aqueous solutions, at 296 K, yielded the monohydrate C6H4(OH)2·H2O at 0.8 GPa (Figure 2). The monohydrate precipitates as a powdered mass, and when the pressure was released to 0.35 GPa, all grains but one were dissolved and a bigger single crystal was grown by slowly increasing the pressure to 0.93 GPa (Figure 2). The crystallization process took about 2 h, and we noticed that after about 20 min at 0.93 GPa the crystal surface was covered by a layer of tightly packed tiny crystals of another new form (Figure 2). This monohydrate sample could be kept for days without visible changes. However, we have established that a release of pressure causes the passivation layer, most likely formed of hydrate 3Res·2H2O, to dissolve followed by the monohydrate dissolving gradually, while a single crystal of the duotritohydrate grew until the monohydrate disappeared (Figure 3). The conclusion that the passivation layer is formed of the duotritohydrate is based on the observation that this is the stable form of resorcinol in the aqueous solution between 0.2 and 1.0 GPa and that no other form of resorcinol could be obtained under these conditions. The Res·H2O and 3Res·2H2O crystals can be easily distinguished by their morphology, unit cell parameters, and symmetry (Figures 2 and 3 and Table 1); however, the crystal grains constituting the passivation layer are too small for such considerations. It can be noted that the 3:2 host molecules to crystallization water molecules ratio is relatively rare among hydrates. For this reason a common name for this hydrate stoichiometry is used, and in a few research papers33,34 a “2/3 hydrate” term was used. We have suggested the term “duotrito”, used in the literature.35,36
Figure 2.
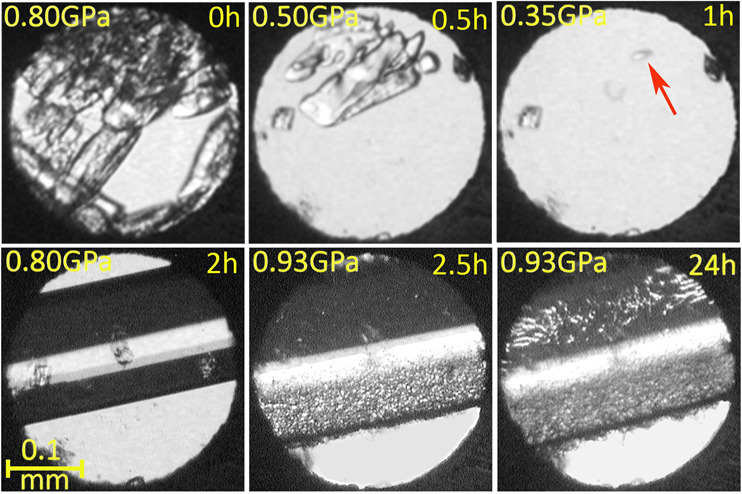
Isothermal crystallization of Res·H2O at 296 K up to 0.93 GPa and of 3Res·2H2O at 0.93 GPa. Two small ruby chips for the pressure calibration lie at the edge of the DAC chamber. The seed is indicated by the red arrow.
Figure 3.
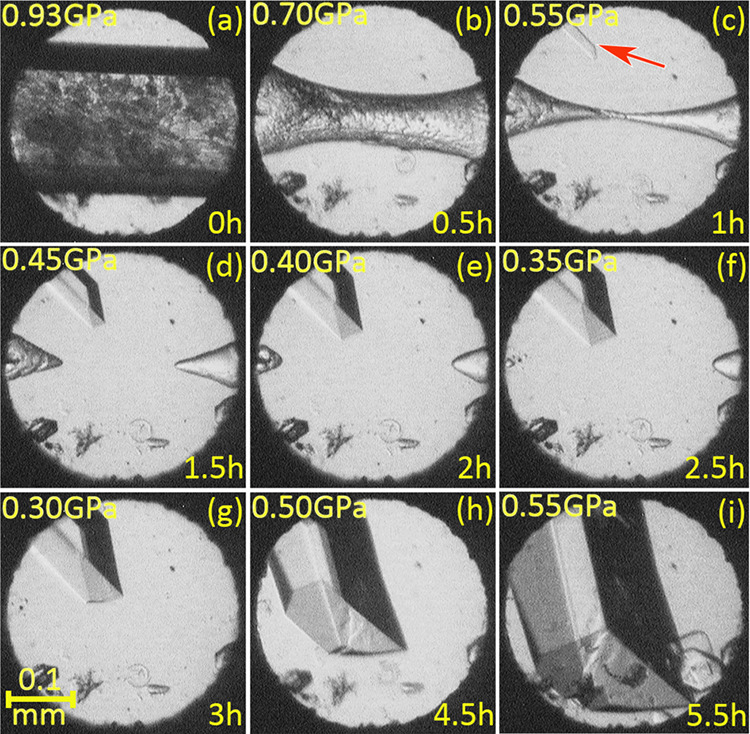
A Res·H2O crystal (cf. Figure 2) covered by tiny 3Res·2H2O crystals at 296 K/0.93 GPa (a). After the pressure is released (b–d), dissolution concomitant with the single-crystal nucleation of 3Res·2H2O ((c), the seed indicated by the red arrow) and its further growth causes a further gradual reduction of pressure (e–g). Then (h, i), the pressure was increased to continue the growth. Several small ruby chips for the pressure calibration lie along the bottom edge of the DAC chamber.
Table 1. Selected Crystallographic Data of Resorcinol Solvatesa.
| Res·CH3OH |
||||
|---|---|---|---|---|
| Res·H2O | 3Res·2H2O | |||
| pressure | 0.80 GPa | 0.93 GPa | 0.49 MPa | 0.70 GPa |
| space group | P212121 | C2/c | P212121 | P212121 |
| unit cell | ||||
| a (Å) | 5.6567(18) | 8.0312(8) | 6.0242(12) | 5.9240(3) |
| b (Å) | 7.6544(5) | 8.1080(6) | 8.1523(12) | 8.1201(3) |
| c (Å) | 13.226(3) | 26.01(4) | 14.32(3) | 14.087(9) |
| β (deg) | 90 | 95.31 | 90 | 90 |
| V (Å3) | 572.7(2) | 1686(2) | 703.4(14) | 677.6(4) |
| Z/Z′ | 4/1 | 12/1.5 | 4/1 | 4/1 |
| Dx (g/cm3) | 1.486 | 1.435 | 1.342 | 1.393 |
| conformation | syn-syn | syn-syn and anti-anti | syn-syn | syn-syn |
Cf. Table S1 in the Supporting Information.
In the second series of experiments, resorcinol was crystallized in the DAC of the methanol solution. The new resorcinol monosolvate C6H4(OH)2·CH3OH nucleated under isochoric conditions at 0.4 GPa, and then it was isothermally compressed up to 0.70 GPa (Figure 4 and Table 1). Finally, orcinol was recrystallized in order to also check its stability under the high-pressure conditions. The orcinol monohydrate C7H6(OH)2·H2O was fully dissolved either in methanol or in water. The isochoric recrystallizations from the methanol solution yielded the monohydrate C7H6(OH)2·H2O at 0.2 GPa; likewise, the recrystallizations of aqueous solution at 0.2 GPa also yielded the monohydrate (Figures S1 and S2).
Figure 4.
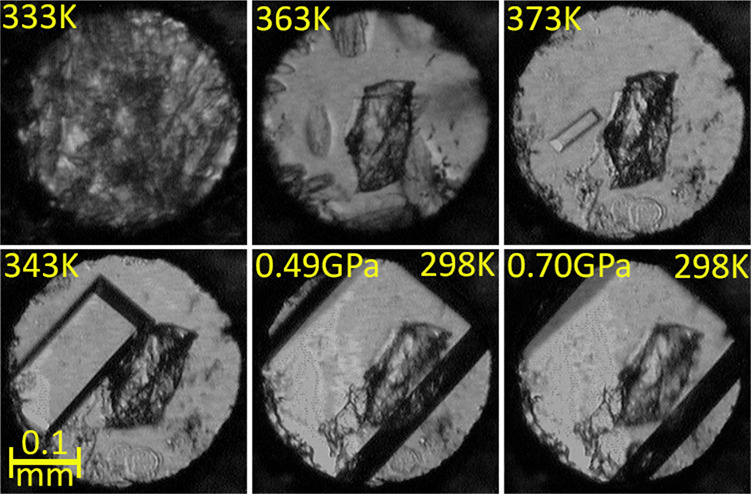
Isochoric crystallization of Res·CH3OH at 0.49 GPa and subsequent (the last panel) isothermal compression of the single crystal to 0.70 GPa. An irregular ruby chip for pressure calibration lies at the center of the chamber.
The pressure in the DAC chamber was calibrated by the ruby-fluorescence method with a Photon Control Inc. spectrometer, with an accuracy of 0.02 GPa.37 The calibration was repeated before and after each diffraction measurement.38 The single-crystal data have been measured with a KUMA KM4-CCD diffractometer. The CrysAlis software39 was used for the data collections40 and preliminary reduction of data after correcting the intensities for the effects of DAC absorption, sample shadowing by the gasket, and sample absorption.41,42 The reflections overlapping with diamond reflections were eliminated. The structures were refined with full-matrix least squares on F2 using SHELX-L.43,44 Phenyl hydrogen atoms were ideally positioned according to the molecular geometry. The hydroxyl and water H atoms were located on the difference Fourier maps and then included in the refinements in the positions consistent with the molecular dimensions (O–H distance, 0.85 Å, C–O–H angle, 109.30°; H–O–H angle, 109.5°). The crystallographic and experimental details are given in Table S1 and deposited in the CIF format in the Cambridge Structure Database with CCDC numbers 1974181–1974186 and 1974294–1974295. The CIFs can be requested free of charge from https://www.ccdc.cam.ac.uk.
Discussion
Chemical compounds often display a strong preference for crystallization in the form of either anhydrates or hydrates, when the crystallization is performed in the laboratory in open vials. In many cases, the presence of moisture in the atmospheric air suffices for the hydrate formation, even when the pure compound is dissolved in (initially) dry solvent but the solution is not then sealed from the atmosphere. There are also compounds that form anhydrates or hydrates depending on the composition of the solution, air humidity, temperature, etc. Many compounds crystallize as anhydrates even from aqueous solutions. Resorcinol belongs to this latter group. Resorcinol has been thoroughly studied for decades, with five polymorphs (α, β, γ, δ, and ε) reported, but no hydrate of resorcinol was obtained. At the same time resorcinol favorably forms cocrystals with various compounds and presently there are 121 such multicomponent deposits in the Cambridge Structure Database (version 1.23). Moreover, orcinol (5-methylresorcinol), despite being a close analogue of resorcinol, displays a strong preference for the formation of hydrates. Namely, under standard conditions orcinol favorably forms a monohydrate and dry solvents are required to obtain the anhydrate (two polymorphs of pure orcinol are known).45 Hence, our present study is aimed at understanding the strong preference of resorcinol to form the anhydrate. We have applied high-pressure crystallization to establish whether this promotes hydrate formation by resorcinol. Recrystallizations from methanol and aqueous solutions in the DAC yielded new forms of resorcinol, as either polymorph α or β, up to 0.5 GPa. However, presently we have established that the pressure efficiently induces the formation of hydrates and a methanol solvate of resorcinol. The resorcinol monohydrate is formed in isothermal crystallization at 0.80 GPa. Then the surface of the monohydrate was tightly covered by many tiny crystals of duotritohydrate, 3C6H4(OH)2·2H2O (Figure 2). We have established that Res·H2O is metastable, but it is protected from dissolution by the passivation layer of 3Res·2H2O crystals. In the crystal structure of Res·H2O, the water molecule mediates the hydrogen bonds between the hydroxyl groups (Figure 5). There are four symmetry-independent OH···O hydrogen bonds. Their dimensions are given in Table S3 and plotted in Figure S3. All of the OH···O bonds bind water and resorcinol molecules, while no water···water or resorcinol···resorcinol bonds are present (Figures 5 and 6).
Figure 5.
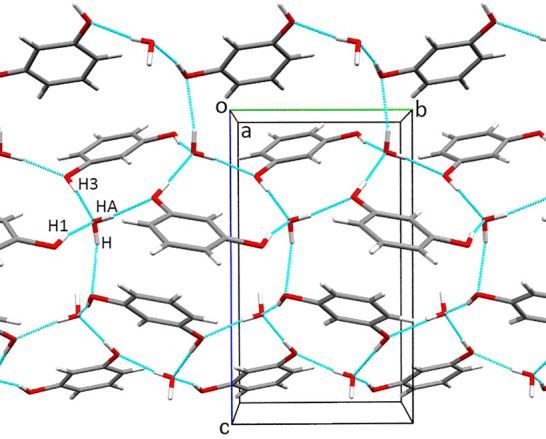
Autostereogram51 of the molecular packing in Res·H2O at 0.80 GPa/296 K. The OH···O hydrogen bonds are indicated as cyan lines, and the labels of H atoms participating in the H bonds are specified.
Figure 6.
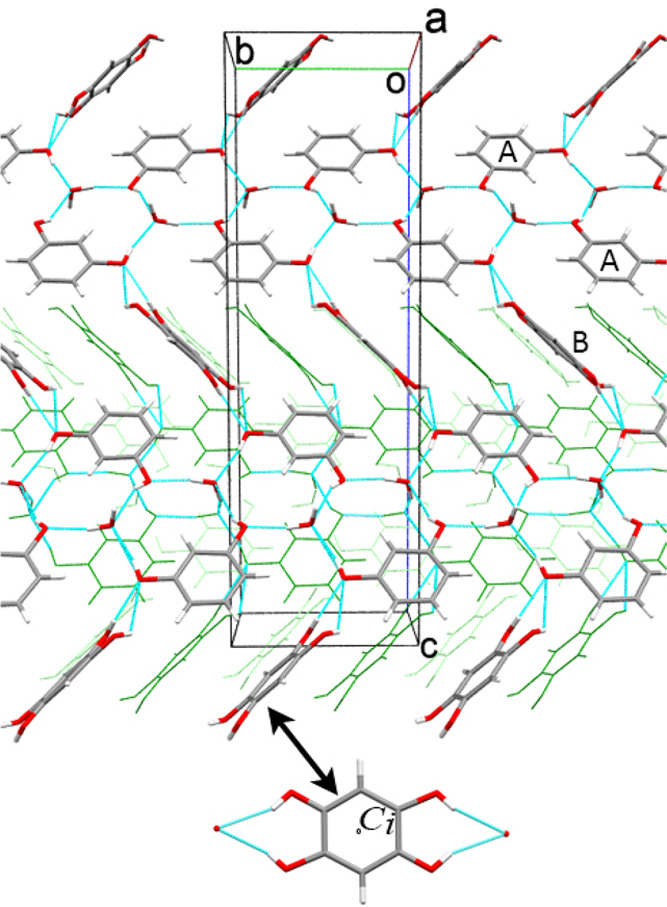
Molecular packing in 3Res·2H2O at 0.93 GPa/296 K. H-B bonds are indicated by cyan lines. Capital letters A and B label the independent resorcinol molecules. Two overlapping positions of disordered half-occupied molecules B are shown, also viewed perpendicular to the ring at the bottom (indicated by the arrow).
In the structure of 3Res·2H2O, there are two independent molecules of resorcinol; one of them (labeled A) is ordered in a general position, whereas the other (molecule B) is disordered and located on an inversion center (Figure 6). The water molecule lies in a general position. The water molecule forms three hydrogen bonds to resorcinol molecule A and one H2O···H2O hydrogen bond. Each resorcinol molecule A is OH···O bonded to three water molecules and to one molecule B. Each molecule B is OH···O bonded to two molecules A. The H-bonding pattern is shown in Figure 6 (cf. Table S3). The disorder of molecule B can be described as a nearly perfect superposition of the benzene ring with its half-occupied sites of hydroxyl group substituents at C1, C3, and their Ci-transformed sites C1′ and C3′. The conformation of the hydroxyl groups is syn-syn in molecule A and anti-anti in molecule B. It is characteristic that a very similar type of disordered molecular orientation was reported for resorcinol cocrystals with hexamine46 and bis(5-ferrocenylpyrimidine).47 In these two cocrystals the disordered molecules are in an anti-anti configuration. In resorcinol cocrystallized with N-phthaloylglycine,48 the syn-syn conformer of resorcinol is disordered. Other types of disorder of resorcinol molecules were observed in cocrystals with terpyridine49 and several complexes of methylviologen.50
The structure of Res-CH3OH determined at 0.49 and 0.70 GPa, of orthorhombic space group P212121, is isostructural with resorcinol monohydrate (cf. Table 1 and Figures 5 and 7). In the structure of Res·CH3OH, the resorcinol and methanol molecules are H-bonded into ribbons along [010].
Figure 7.
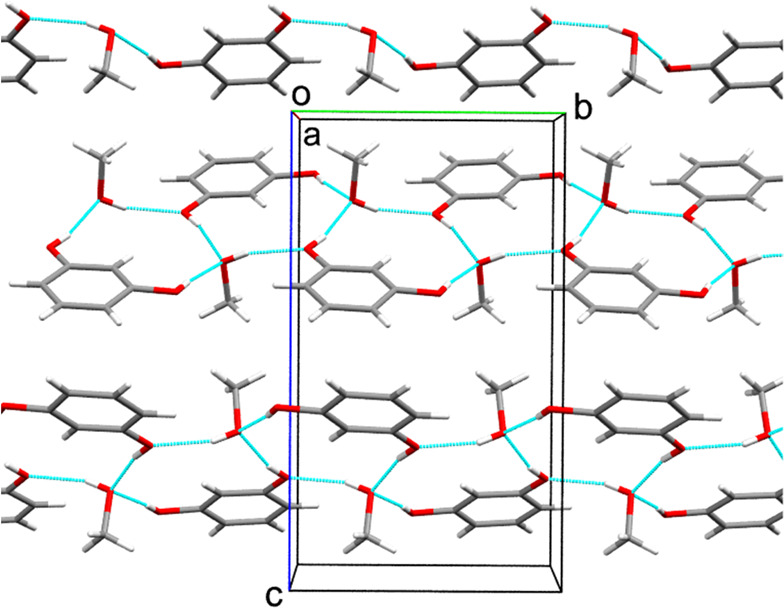
Autosterographic view of the structure of Res·CH3OH at 0.49 GPa/296 K. The H bonds are indicated by cyan lines.
Resorcinol molecules in pure polymorphs, solvates, and cocrystals assume the anti-anti, anti-syn, and syn-syn configurations (Figure 1). The DFT calculations at the RB3LYP/6-31G level of theory of the potential energy for isolated molecules indicate that the anti-syn conformer is 0.94 kJ mol–1 more stable than anti-anti conformer and 2.15 kJ mol–1 more stable than the syn-syn conformer. These values are smaller than the energy of cohesion forces in H-bonded crystals, but they can be significant enough to help the stability of the observed resorcinol forms.
We established that one of the H bonds in the monohydrate, O–H···O2664, has a dimension of nearly 3 Å (Figure 8) and is much longer than the analogous H bonds in other solvates and in pure resorcinol. It is plausible that some steric hindrances in the structure of Res·H2O prevent the formation of the H-bonding network, efficiently using up all the available H donors and H acceptors. This could be the reason for the metastability of the Res·H2O and its subsequent transformation to 3Res·2H2O, where all hydroxyl groups and H2O molecules can be linked by H bonds between 2.65 and 2.85 Å (Figure 8).
Figure 8.
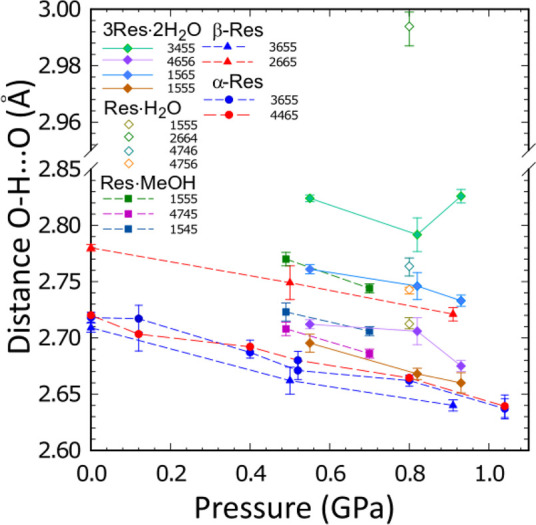
Pressure dependence of O···O H bond lengths in Res·H2O, 3Res·2H2O, and Res·CH3OH, as well as in resorcinol polymorphs α and β. Symmetry operations indicated by ORTEP codes53 are explicitly given in Table S9.
The molecular volumes of Res·H2O, 3Res·2H2O, and Res·CH3OH are somewhat smaller than the sums of volumes of the stoichiometric amounts of pure resorcinol and solvent molecules
where Vm is the molecular volume of the solvates, the pure resorcinol polymorph β (stable in the considered range of pressure), and the solvents (Figure 9). In these comparisons we have applied volumes of the separate components (resorcinol polymorph β and liquid solvents) compressed to the appropriate pressure conditions (Figure 10).52,54
Figure 9.
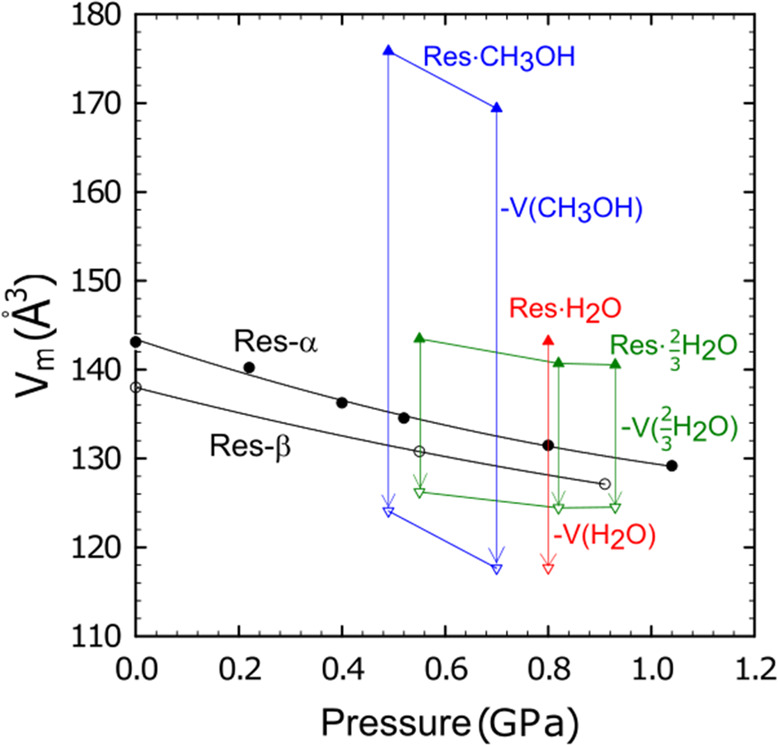
Pressure dependence of molecular volume referred to one resorcinol molecule in polymorphs α (black circles), β (empty black circles),52 Res·H2O (red triangle), 3Res·2H2O (green triangles), and Res·CH3OH (blue triangles). The vertical arrows indicate the difference in the solvate and appropriate amounts of the solvate volume (water or methanol).54
Figure 10.
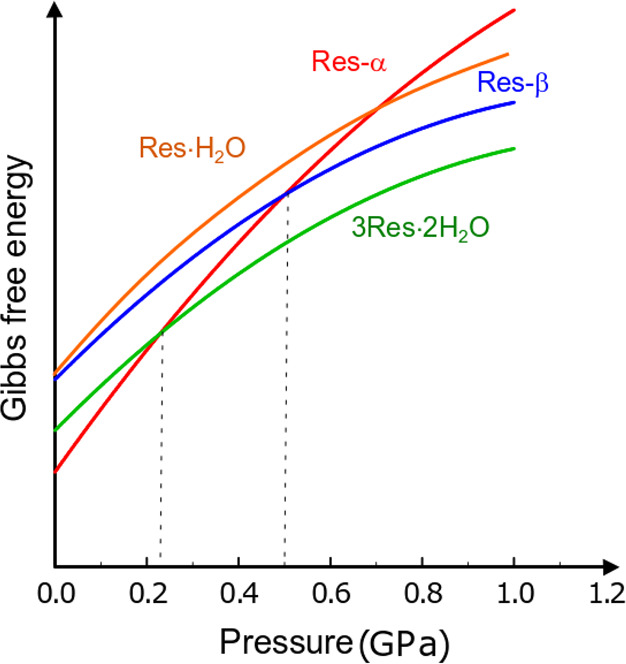
Schematic diagram of Gibbs free energy versus pressure at 296 K for resorcinol polymorphs α (red line),52 β (blue),52 Res·H2O (orange), and 3Res·2H2O (green).
This result indicates that the volume gain is a significant factor for the formation of these solvates. The energy associated with this volume difference in comparison to β-resorcinol is 4.8 kJ mol–1 for Res·H2O at 0.8 GPa, 1.2 kJ mol–1 for 3Res·2H2O at 0.45 GPa and 1.7 kJ mol–1 at 0.82 GPa, and 3.6 kJ mol–1 for Res·CH3OH at 0.6 GPa. It appears that the effect of the largest work component gain for Res·H2O is diminished by the inadequate H-bonding pattern in the structure (one of the possible H bonds cannot be formed)—hence the internal energy of the Res·H2O structure increases and it is metastable in comparison to 3Res·2H2O, as schematically illustrated in Figure 10. The energy of one hydrogen bond between two hydroxyl groups can be roughly estimated as several kJ mol–1; therefore, this value can be significant for changing the balance between the resorcinol solvates.
Conclusions
We have found that high pressure favors the formation of new solvates, which are unstable under ambient conditions. These new forms are resorcinol monohydrate, duotritohydrate, and monomethanol solvates. The stability of these new solvates at high pressure can be associated with the formation of new H bonds and with a volume gain of the solvates in comparison to the summed volume of their components. The H bonding plays a direct role in the molecular aggregation, as the solvent molecules mediate most of the H bonds between the resorcinol hydroxyl groups. The metastability of the monohydrate has been associated with a disadvantageous molecular arrangement hampering the formation of one of the possible OH···O bonds. Surprisingly, the more stable duotritohydrate is disordered, as one of its independent molecules assumes two orientations in the structure. This contrasts with the general assumption that high pressure eliminates orientational disorder. Although none of the new solvates could be recovered to ambient conditions, the revealed mechanisms of the pressure effects can prove useful for obtaining new stable solvates of this and other compounds. The comparison of resorcinol and orcinol illustrates that a relatively small structural difference (the methanol group at C5 in orcinol) can cause a drastic effect in the solvation of similar compounds. Such differences can be reduced, to some extent, by pressure, as has been presently observed for resorcinol hydrates promoted by high pressure. However, none of the resorcinol hydrates is isostructural with the orcinol monohydrate (similarly, as none of their anhydrate polymorphs are isostructural either).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.cgd.9b01732.
Additional photos of resorcinol crystals, crystal data and structure refinement details, and a plot showing the pressure dependence of geometric dimensions of hydrogen bonds OH···O as a function of pressure (PDF)
Accession Codes
CCDC 1974181–1974186 and 1974294–1974295 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This study was supported by the Polish Ministry of Higher Education; F.S. is grateful to the EU European Social Fund, Operational Program Knowledge Education Development, grant POWR.03.02.00–00-I026/16 and project OPUS 10 UMO-2015/19/B/ST5/00262 from the Polish National Science Centre.
The authors declare no competing financial interest.
Supplementary Material
References
- Bernstein J. Polymorphism – A Perspective. Cryst. Growth Des. 2011, 11, 632–650. 10.1021/cg1013335. [DOI] [Google Scholar]
- Singhal D.; Curatolo W. Drug Polymorphism and Dosage form Design: a Practical Perspective. Adv. Drug Delivery Rev. 2004, 56, 335–347. 10.1016/j.addr.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Blagden N.; Matas M.; Gavan P. T.; York P. Crystal Engineering of Active Pharmaceutical Ingredients to Improve Solubility and Dissolution rates. Adv. Drug Delivery Rev. 2007, 59, 617–630. 10.1016/j.addr.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Stahly G. P. Diversity in Single- and Multiple-Component Crystals. The Search for and Prevalence of Polymorphs and Co-Crystals. Cryst. Growth Des. 2007, 7, 1007–1026. 10.1021/cg060838j. [DOI] [Google Scholar]
- Cruz-Cabeza A. J.; Reutzel-Edens S. M.; Bernstein J. Facts and Fictions about Polymorphism. Chem. Soc. Rev. 2015, 44, 8619–8635. 10.1039/C5CS00227C. [DOI] [PubMed] [Google Scholar]
- Tomkowiak H.; Olejniczak A.; Katrusiak A. Pressure-Dependent Formation and Decomposition of Thiourea Hydrates. Cryst. Growth Des. 2013, 13 (1), 121–125. 10.1021/cg301254a. [DOI] [Google Scholar]
- Andrzejewski M.; Olejniczak A.; Katrusiak A. Humidity Control of Isostructural Dehydration and Pressure-induced Polymorphism in 1,4-Diazabicyclo [2.2.2] octane Dihydrobromide Monohydrate. Cryst. Growth Des. 2011, 11, 4892–4899. 10.1021/cg200743n. [DOI] [Google Scholar]
- Olejniczak A.; Podsiadło M.; Katrusiak A. High Pressure Used for Producing a New Solvate of 1,4-Diazabicyclo [2.2.2] octane Hydroiodide. New J. Chem. 2016, 40, 2014–2020. 10.1039/C5NJ01654A. [DOI] [Google Scholar]
- Olejniczak A.; Katrusiak A. Pressure-Induced Hydration of 1,4-Diazabicyclo [2.2.2] octane Hydroiodide (dabcoHI). Cryst. Growth Des. 2011, 11, 2250–2256. 10.1021/cg101653x. [DOI] [Google Scholar]
- Anioła M.; Olejniczak A.; Katrusiak A. Pressure-Induced Solvate Crystallization of 1,4-Diazabicyclo [2.2.2] octane Perchlorate with Methanol. Cryst. Growth Des. 2014, 14, 2187–2191. 10.1021/cg4016959. [DOI] [Google Scholar]
- Anioła M.; Katrusiak A. Pressure Effects on Crystallization, Polymorphism, and Solvation of 4,4′-Bipyridinium Perchlorate. Cryst. Growth Des. 2017, 17, 3134–3141. 10.1021/acs.cgd.7b00085. [DOI] [Google Scholar]
- Zielinski W.; Katrusiak A. Pressure-Induced Preference for Solvation of 5,6-Dimethylbenzimidazole. CrystEngComm 2016, 18, 3211–3215. 10.1039/C6CE00419A. [DOI] [Google Scholar]
- Olejniczak A.; Kru̅kle-Be̅rziņa K.; Katrusiak A. Pressure-Stabilized Solvates of Xylazine Hydrochloride. Cryst. Growth Des. 2016, 16, 3756–3762. 10.1021/acs.cgd.6b00264. [DOI] [Google Scholar]
- Fabbiani F. P. A.; Bergantin S.; Gavezzotti A.; Rizzato S.; Moret M. X-ray Diffraction and Computational Studies of the Pressure-Dependent Tetrachloroethane Solvation of Diphenylanthracene. CrystEngComm 2016, 18, 2173–2181. 10.1039/C6CE00055J. [DOI] [Google Scholar]
- Zakharov B. A.; Seryotkin Y. V.; Tumanov N. A.; Paliwoda D.; Hanfland M.; Kurnosov A. V.; Boldyreva E. V. The Role of Fluids in High-Pressure Polymorphism of Drugs: Different Behaviour of β-Chlorpropamide in Different Inert Gas and Liquid Media. RSC Adv. 2016, 6, 92629–92637. 10.1039/C6RA17750F. [DOI] [Google Scholar]
- Tozuka Y.; Kawada D.; Oguchi T.; Yamamoto T. Supercritical Carbon Dioxide Treatment as a Method for Polymorph Preparation of Deoxycholic Acid. Int. J. Pharm. 2003, 263, 45–50. 10.1016/S0378-5173(03)00344-2. [DOI] [PubMed] [Google Scholar]
- Drebushchak V. A.; Ogienko A. G.; Boldyreva E. V. Polymorphic Effects at the Eutectic Melting in the H2O–Glycine System. J. Therm. Anal. Calorim. 2013, 111, 2187–2191. 10.1007/s10973-012-2761-0. [DOI] [Google Scholar]
- Zieliński W.; Katrusiak A. Hydrate Smaller Than the Anhydrate. CrystEngComm 2015, 17, 5468–5473. 10.1039/C5CE00694E. [DOI] [Google Scholar]
- Hirai H.; Uchihara Y. High-Pressure Structures of Methane Hydrate Observed up to 8 GPa at Room Temperature. J. Chem. Phys. 2001, 115, 7066–7070. 10.1063/1.1403690. [DOI] [Google Scholar]
- Zakharov B.; Gribov P.; Matvienko A.; Boldyreva E. Isostructural Crystal Hydrates of Rare-Earth Metal Oxalates at High Pressure: from Strain Anisotropy to Dehydration. Z. Kristallogr. - Cryst. Mater. 2017, 232, 751–757. 10.1515/zkri-2016-2038. [DOI] [Google Scholar]
- Caspari W. A. The Crystal Structure of Quinol. Part II. J. Chem. Soc. 1927, 0, 1093–1095. 10.1039/JR9270001093. [DOI] [Google Scholar]
- Alan R.; Sabongi J.. Resorcinol, Its Uses and Derivatives; Springer: 1994; pp 5–86. 10.1007/978-1-4899-0999-2. [DOI] [Google Scholar]
- Durairaj R. B.Resorcinol Chemistry, Technology and Applications; Springer: 2005; pp 633–634. 10.1007/3-540-28090-1. [DOI] [Google Scholar]
- Robertson J. M. The Space Group of Resorcinol C6H6O2. Z. Kristallogr. - Cryst. Mater. 1934, 89, 518–518. 10.1524/zkri.1934.89.1.518. [DOI] [Google Scholar]
- Ubbelohde A. R.; Robertson J. M. A New Form of Resorcinol. Proc. R. Soc. A 1937, 140, 239–239. 10.1038/140239a0. [DOI] [Google Scholar]
- Rao R.; Sakuntala T.; Godwal B. K. Evidence for High-Pressure Polymorphism in Resorcinol. Phys. Rev. B: Condens. Matter Mater. Phys. 2002, 65, 054108. 10.1103/PhysRevB.65.054108. [DOI] [Google Scholar]
- Kichanov S. E.; Kozlenko D. P.; Bilski P.; Wąsicki J.; Nawrocik W.; Medek A.; Hancock B. C.; Lukin E. V.; Lathe C.; Dubrovinsky L. S.; Savenko B. N. The Polymorphic Phase Transformations in Resorcinol at High Pressure. J. Mol. Struct. 2011, 1006, 337–343. 10.1016/j.molstruc.2011.09.029. [DOI] [Google Scholar]
- Zhu Q.; Shtukenberg A. G.; Carter D. J.; Yang T.; Yu J.; Chen M.; Raiteri P.; Oganov A. R.; Pokroy B.; Polishchuk I.; Bygrave P. J.; Day G. M.; Rohl A. L.; Tuckerman M. E.; Kahr B. Resorcinol Crystallization From the Melt: a New Ambient Phase and New “Riddles. J. Am. Chem. Soc. 2016, 138, 4881–4889. 10.1021/jacs.6b01120. [DOI] [PubMed] [Google Scholar]
- Lautz H. Über die Beziehungen Instabiler Formen zu Stabilen. Z. Phys. Chem. 1913, 84U, 611–641. 10.1515/zpch-1913-0140. [DOI] [Google Scholar]
- Bacon G. E.; Curry N. A. A Study of α-Resorcinol by Neutron Diffraction. Proc. R. Soc. Lon. A 1956, 235, 552–559. 10.1098/rspa.1956.0105. [DOI] [Google Scholar]
- Merrill L.; Bassett W. A. Miniature Diamond Anvil Pressure Cell for Single Crystal X-ray Diffraction Studies. Rev. Sci. Instrum. 1974, 45, 290–294. 10.1107/S2052252516000725. [DOI] [Google Scholar]
- Katrusiak A. High-pressure crystallography. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, 64, 135–148. 10.1107/S0108767307061181. [DOI] [PubMed] [Google Scholar]
- Jovanovski G.; Kamenar B. Two Ionic Saccharinates: (1a) Sodium Saccharinate 2/3 Hydrate, C7H4NO3SNa.2/3H2O (1b) Magnesium Disaccharinate Heptahydrate, (C7H4NO3S)2Mg.7H2O. Cryst. Struct. Comm. 1982, 11, 247–255. [Google Scholar]
- Bishop R.Aspects of Crystallization and Chirality. In Chirality in Supramolecular Assemblies: Causes and Consequences; Keene F. R., Ed.; Wiley: London, UK, 2016; p 81. 10.1002/9781118867334.ch3. [DOI] [Google Scholar]
- Watts N.The Oxford New Greek Dictionary; Berkley Books: 2008; pp 201, 445. [Google Scholar]
- https://www.foundalis.com/lan/grknum.htm.
- Piermarini G. J.; Block S.; Barnett J. D.; Forman R. A. Calibration of the Pressure Dependence of the R1 Ruby Fluorescence Line to 195 kbar. J. Appl. Phys. 1975, 46, 2774–2780. 10.1063/1.321957. [DOI] [Google Scholar]
- Mao H. K.; Xu J.; Bell P. M. Calibration of the Ruby Pressure Gauge to 800 kbar Under Quasi-Hydrostatic Conditions. J. Geophys. Res. 1986, 91, 4673–4676. 10.1029/JB091iB05p04673. [DOI] [Google Scholar]
- Budzianowski A.; Katrusiak A.. High-Pressure Crystallographic Experiments with a CCD Detector. In High-Pressure Crystallography; Katrusiak A., McMillan P. F., Eds.; Kluwer: Dordrecht, The Netherlands, 2004; pp 101–112. 10.1007/978-1-4020-2102-2_7. [DOI] [Google Scholar]
- Xcalibur CCD system, CrysAlisPro Software System, Ver. 1.171.33; Oxford Diffraction Ltd.: Wrocław, Poland, 2009.
- Katrusiak A.REDSHABS, Program for the correcting reflections intensities for DAC absorption, gasket shadowing and sample crystal absorption; Adam Mickiewicz University: Poznań, Poland, 2003.
- Katrusiak A. Shadowing and Absorption Corrections of Single-Crystal High-Pressure Data. Z. Kristallogr. - Cryst. Mater. 2004, 219, 461–467. 10.1524/zkri.219.8.461.38328. [DOI] [Google Scholar]
- Dolomanov O. V.; Bourhis L. J.; Gildea R. J.; Howard J. A. K.; Puschmann H. OLEX2: a Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. 10.1107/S0021889808042726. [DOI] [Google Scholar]
- Sheldrick G. M. A Short History of SHELX. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, 64, 112–122. 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- Mukherjee A.; Grobelny P.; ThakurGautam S. T.; Desiraju R. Polymorphs, Pseudopolymorphs and Co-Crystals of Orcinol: Exploring the Structural Landscape with High Throughput Crystallography. Cryst. Growth Des. 2011, 11, 2637–2653. 10.1021/cg200361x. [DOI] [Google Scholar]
- Ng S. W.; Naumov P.; Ibrahim A. R.; Fun H. K.; Chantrapromma S.; Wojciechowski G.; Brzezinski B. X-Ray and Spectroscopic Re-investigation of the 1:1 Complex Formed Between Urotropine and Resorcinol. J. Mol. Struct. 2002, 609, 89–95. 10.1016/S0022-2860(01)00945-0. [DOI] [Google Scholar]
- Horikoshi R.; Nambua C.; Mochida M. Supramolecular Assembly of Ferrocenesvia Hydrogen Bonds: Dimensional Variation in Ferrocenylpyrimidine Complexes with Carboxylic Acids and Aromatic Alcohols. New J. Chem. 2004, 28, 26–33. 10.1039/b306699a. [DOI] [Google Scholar]
- Barooah N.; Sarmaa R. J.; Batsanov A. S.; Baruaha J. B. Structural Aspects of Adducts of N-Phthaloylglycine and Its Derivatives. J. Mol. Struct. 2006, 791, 122–130. 10.1016/j.molstruc.2006.01.025. [DOI] [Google Scholar]
- Trokowski R.; Akinea S.; Nabeshima T. Selective Binding of Benzenediol Derivatives by Simultaneous Non-Covalent Interactions in Bis-Pt (II) Aza-Aromatic Host–Guest System. Dalton Trans. 2009, 46, 10359–10366. 10.1039/B911602H. [DOI] [PubMed] [Google Scholar]
- Imai Y.; Kamona K.; Kinuta K.; Nobuob T.; Sato S.; Kuroda R.; Matsubara Y. An Isoselective and Visual Inclusion Host System Using Charge-Transfer Complexes of 3,3′-Disubstituted-1,1′-bi-2-Naphthol and Methylviologen. Tetrahedron Lett. 2007, 48, 6321–6325. 10.1016/j.tetlet.2007.07.023. [DOI] [Google Scholar]
- Katrusiak A. Crystallographic Autostereograms. J. Mol. Graphics Modell. 2001, 19, 363–367. 10.1016/S1093-3263(00)00085-1. [DOI] [PubMed] [Google Scholar]
- Safari F.; Olejniczak A.; Katrusiak K. Pressure-Dependent Crystallization Preference of Resorcinol Polymorphs. Cryst. Growth Des. 2019, 19, 5629–5635. 10.1021/acs.cgd.9b00610. [DOI] [Google Scholar]
- Johnson C. K.ORTEPII; Oak Ridge National Laboratory: Oak Ridge, TN, 1976; Report ORNL-5138.
- Bridgman P. W. Thermodynamic Properties of Twelve Liquids Between 20° and 80° and up to 12000 kgm. per sq. cm. Proc. Am. Acad. Arts Sci. 1913, 49, 3–113. 10.2307/20025445. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


