Abstract
We report two cases of HIV positive patients with SARS-CoV-2 infection and a recent diagnosis of opportunistic infections of central nervous system (CNS). We investigated the potential impact of coinfection with SARS-CoV-2 on HIV replication in CNS.
Keywords: COVID-19, HIV infection, Central nervous system, HIV viral escape
Introduction
We report the cases of two HIV-positive patients with coronavirus disease 2019 (COVID-19) and a recent diagnosis of opportunistic infections of the central nervous system (CNS). The potential impact of co-infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on HIV replication in the CNS was investigated.
SARS-CoV-2 is the causative agent of the clinical syndrome COVID-19 and is associated with mild respiratory tract infections and severe pneumonia (Wu and McGoogan, 2020). However, SARS-CoV-2 has been shown to affect other organs both directly, by binding to specific cellular receptors, and indirectly due to the triggered abnormal inflammatory response (Bourgonje et al., 2020, Ye et al., 2020). CNS involvement has been reported and neurological signs and symptoms have been described (Arbour et al., 2000, Helms et al., 2020).
It remains unclear whether persons living with HIV (PLWH) and on antiretroviral treatment, with low CD4 T-cell counts (<200/mm3), may have a higher risk of contracting COVID-19 and developing more serious clinical pictures of the disease (Guo et al., 2020). HIV can itself affect the neuronal compartment, and the interaction between the two viruses in the CNS remains unexplored.
Case reports
Patient 1
In January 2020, a 55-year-old Ethiopian male, suffering from hypertension and aortic steno-valvular insufficiency, was diagnosed with HIV (CDC stage C) during a hospitalization for meningitis caused by Mycobacterium chimaera. Specific treatment with moxifloxacin, rifabutin, azithromycin, and ethambutol was started, and combined antiretroviral therapy (cART) with tenofovir disoproxil fumarate, emtricitabine, and dolutegravir was subsequently prescribed. After 2 months, while the patient was in a rehabilitation facility, a cerebrospinal fluid (CSF) examination revealed no pathological findings; all microbiological tests on the CSF were reported as negative.
On April 20, 2020, reverse transcriptase PCR (RT-PCR) for SARS-CoV-2 on two nasopharyngeal swabs (NPSs) was also performed because of contact with a COVID-19 case, in the absence of symptoms. The result was positive. For this reason the patient was admitted to the COVID hospital associated with the National Institute for Infectious Diseases “L. Spallanzani”. The patient was in a fair clinical condition, afebrile and eupneic. The neurological examination showed hyposthenia of the lower limbs. HIV RNA was <30 copies/mL in plasma and his CD4 T-cell count was 127/mm3 (18%). High-resolution computed tomography (HRCT) of the chest revealed interstitial pneumonia, and hydroxychloroquine therapy was administered for 10 days, according to local guidelines, together with his previous therapy and antithrombotic prophylaxis.
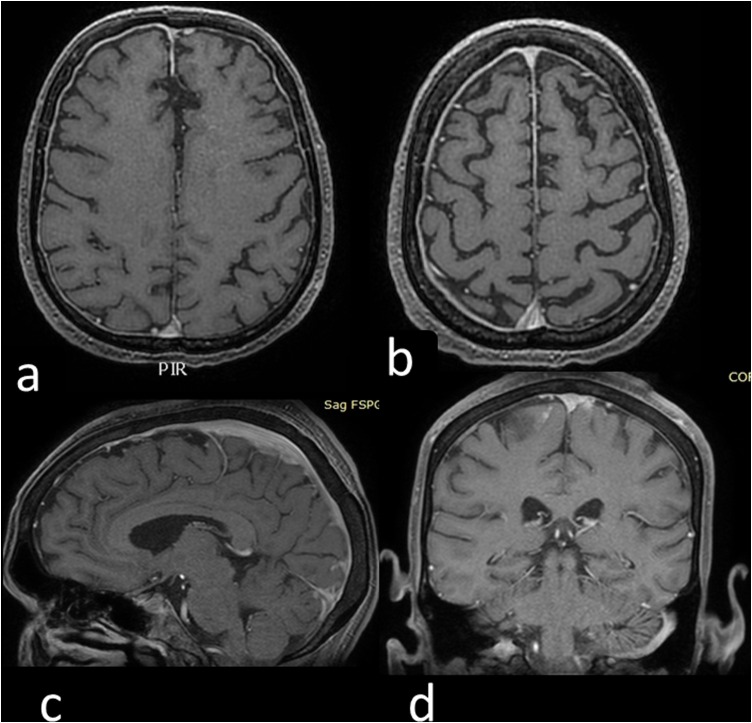
Magnetic resonance imaging (MRI) of the brain was performed for re-evaluation of his disease status (Figure 1 a and b). A lumbar puncture (LP) was performed and CSF examination showed an overlapping picture with the previous one. CSF and plasma albumin were 259 mg/l and 3390 mg/dl, respectively, with a Link index of 0.47. The inflammatory profile of the CSF showed a low level of interleukin (IL)-1β (0.17 pg/mL), IL-6 (8.65 pg/mL), IL-8 (36.17 pg/mL), and tumour necrosis factor alpha (TNF-α) (1.30 pg/mL).
Figure 1.
(a, b) Axial contrast-enhanced three-dimensional T1 SPGR MRI scan showing subtle pachymeningeal enhancement in the bilateral frontotemporal region (patient 1). (c, d) Axial contrast-enhanced three-dimensional T1 SPGR MRI scan showing enhancement of the meningeal plane on the posterior frontal left (patient 2).
The results of all microbiological tests were negative. RT-PCR for SARS-CoV-2 in CSF was negative, and HIV RNA in CSF/plasma pairs was <30 copies/mL. Serology for SARS-CoV-2, performed by indirect immunofluorescence assay (IFA), was negative in CSF, while an IgG titre of 1:320, IgA titre of 1:80, and weak reactivity for IgM were found in plasma. The patient was discharged with three consecutive NPSs negative for SARS-CoV-2 and with the indication to continue anti-mycobacterial therapy and cART.
Patient 2
The second patient was a 32-year-old African American who was diagnosed with HIV infection in 2017; he was CDC stage C3. The patient was on cART with tenofovir disoproxil fumarate, emtricitabine, and efavirenz reaching a poor immunological recovery. He was hospitalized in February 2020 for cryptococcal meningitis and was treated with intravenous liposomal amphotericin B and flucytosine. CSF examination at the switch to consolidation therapy showed a Cryptococcus neoformans antigen titre of 1:4, with a negative PCR and culture; the titre of cryptococcal antigen in serum was 1:400.
On April 1, he was tested for SARS-CoV-2 on two consecutive NPSs as he was a contact of a COVID-19 case; the results were negative. Chest HRCT revealed the presence of an interstitial pneumonia. For this reason, he was transferred to the COVID hospital for investigations. On admission, the patient was in a fair clinical condition, afebrile and eupneic. Serology for SARS-CoV-2 performed at 20 days after the first test, by means of IFA, was positive: IgA titre 1:80, IgM titre 1:80, and IgG titre 1:40. HIV RNA was <30 copies/mL and his CD4 T-cell count was 265/mm3 (8.2%).
MRI of the brain was performed (Figure 1c and d). A LP was also performed and CSF examination showed only protein at 69.8 mg/dl. CSF and plasma albumin were 425 mg/l and 3640 mg/dl, respectively, with a Link index of 0.5. The inflammatory profile in CSF showed IL-1β of 0.21 pg/mL, IL-6 of 44.27 pg/mL, IL-8 of 295.75 pg/mL, and TNF-α of 8.70 pg/mL. All microbiological test results were negative; the cryptococcal antigen titre was 1:8 in CSF and 1:400 in plasma. RT-PCR for SARS-CoV-2 in CSF was negative, and HIV RNA in CSF/plasma pairs was undetectable at <30 copies/mL. Serology for SARS-CoV-2 in CSF showed negative titres for IgA and IgM, while the IgG titre was 1:8. During the hospital stay, consolidation therapy with fluconazole and cART was administered. No therapies for SARS-CoV-2 were prescribed, with the exception of antithrombotic prophylaxis. The patient was discharged after 10 days.
Discussion
HIV viral escape is reported in a variable percentage of cases among cohorts of PLWH; specifically, secondary HIV viral escape due to opportunistic CNS diseases has been observed in clinical reports (Joseph et al., 2016).
The mechanisms of HIV neuro-invasion are still debated. This neuro-invasion has been associated with damage to the blood–brain barrier (BBB) resulting from immune activation or infected cell trafficking in the CNS (Winston et al., 2019).
SARS-CoV-2 might be neurotropic. Indeed, a related necrotizing haemorrhagic encephalopathy has been described and an association with a marked inflammatory response has been hypothesized (Poyiadji et al., 2020).
Neurological involvement in COVID-19 may mimic a heterogeneous spectrum of signs and symptoms, in some cases overlapping with other neurological diseases, including ischemic stroke, alterations of the consciousness state (confusion, sleepy state, seizures), muscle damage, acroparesthesia, and symptoms of encephalitis (Mao et al., 2020). Moreover, limited data are available on the prevalence and timing of COVID-19 neurological symptoms, which often occur in a delayed manner and in severe disease (Pleasure et al., 2020).
It has been hypothesized that SARS-CoV-2 reaches the CNS through several mechanisms: trans-synaptic transfer across infected neurons, entry via the olfactory nerve, infection of the vascular endothelium, or leukocyte migration across the BBB (Zubair et al., 2020).
There are data showing that SARS-CoV-2 infects lymphocytes, granulocytes, and monocytes by binding to angiotensin-converting enzyme 2 (ACE2) receptors (Wang et al., 2020). In addition, the cytokine storm triggered by SARS-CoV-2 might be associated with an increased permeability of the BBB (Zubair et al., 2020).
The potential role of the BBB in containing and preventing access to the brain tissue could be crucial in patients with HIV co-infection, even if other concomitant opportunistic CNS pathologies, able to alter BBB permeability, are present.
We have described SARS-CoV-2 infections during follow-up for CNS pathologies and hypothesized that SARS-CoV-2 could have altered the BBB permeability, resulting in HIV escape in the CNS, with potential consequent neuronal damage. However, this hypothesis was not confirmed by virological and immunological tests performed on the CSF. Indeed, neither SARS-CoV-2 RNA nor HIV RNA was detected in CSF. In one of the two cases, IgG was found to be present in the CSF, showing a passage across the BBB of these antibodies into the CSF.
The absence of HIV in the CNS could be explained by the fact that both patients were correctly taking the specific therapy for the opportunistic infections as well as cART. Moreover, the few symptoms associated with COVID-19 could suggest a contained inflammatory response in the CNS/plasma, probably due to a low reactive immune response. Indeed these patients had a poor immunological recovery, which might have contributed to the lack of immune-pathogenetic processes and mitigated the symptoms (Tay et al., 2020).
In conclusion, a CNS study should be proposed for patients with SARS-CoV-2 and HIV co-infection, in order to clarify the interaction between HIV and SARS-CoV-2 and the associated inflammatory response, and to optimize any ongoing treatments.
Author contributions
CP, AV, and A Antinori followed the patients during the diagnostic and therapeutic path, conceived the study, drafted the first manuscript, and revised the final version. AM, RG, SC, and FB followed the patients during the diagnostic and therapeutic path and discussed the results of the study. CC, A Amendola, and MRC provided for virological assay on plasma and CSF samples. CA and SN provided for biomarkers level in CSF. PC provided for brain MRI. All authors gave their final approval of the version to be submitted.
Funding source
This study was supported by Ricerca Corrente Linea 2 funded by the Italian Ministry of Health.
Ethical approval
Informed consent was obtained from the patients for publication of this case report and respective images (Study Project RetroSNC Ethical approval n.78/2016, 14/06/2016).
Conflict of interest
CP has received travel grants and honoraria from Gilead and Janssen-Cilag. AV has received research institutional grants from Gilead Sciences, travel grants from Gilead Sciences and Janssen-Cilag, and speaker’s honoraria from Janssen-Cilag and MSD. RG has received travel grants from Janssen, Gilead Sciences, and Viiv; grants for speakers’ honoraria/educational activities from ViiV Healthcare and MSD; and grants for advisory board from ViiV Healthcare. AM has received honoraria from Gilead and ViiV Healthcare. SC has served as a paid consultant to Gilead Sciences, Janssen-Cilag, Merck, and ViiV Healthcare, and has received research funding through the National Institute for Infectious Diseases “Lazzaro Spallanzani” IRCCS from Gilead Sciences. AA has served as a paid consultant to Gilead Sciences, Janssen-Cilag, Merck, and ViiV Healthcare, and has received research institutional grants from Gilead Sciences, Janssen-Cilag, and ViiV Healthcare. All of the other authors declare no conflicts of interest.
Acknowledgements
We gratefully acknowledge the medical and nursing staff of the HIV/AIDS Clinical Unit and the two patients for their availability. We gratefully acknowledge the collaborating members of the INMI COVID-19 study group.
References
- Arbour N., Day R., Newcombe J., Talbot P.J. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74(19):8913–8921. doi: 10.1128/JVI.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgonje A.R., Abdulle A.E., Timens W., et al. Angiotensin-converting enzyme-2 (ACE2), SARS-CoV-2 and pathophysiology of coronavirus disease 2019 (COVID-19) [published online ahead of print, 2020 May 17] J Pathol. 2020 doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Ming F., Dong Y., et al. A survey for COVID-19 among HIV/AIDS patients in two districts of Wuhan, China. Preprint research paper. Lancet. 2020 [Google Scholar]
- Helms J., Kremer S., Merdji H., et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J., Cinque P., Colosi D., et al. Highlights of the global HIV-1 CSF escape consortium meeting, 9 June 2016, Bethesda, MD, USA. J Virus Erad. 2016;2:243–250. doi: 10.1016/S2055-6640(20)30879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasure S.J., Green A.J., Josephson S.A. The spectrum of neurologic disease in the severe acute respiratory syndrome coronavirus 2 pandemic infection: neurologists move to the frontlines. JAMA Neurol. 2020;77(6):679–680. doi: 10.1001/jamaneurol.2020.1065. [DOI] [PubMed] [Google Scholar]
- Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features [published online ahead of print, 2020 Mar 31] Radiology. 2020:201187. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020:1–12. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Xu W., Hu G., et al. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston A., Antinori A., Cinque P., et al. Defining cerebrospinal fluid HIV RNA escape: editorial review AIDS. AIDS. 2019;33 Suppl 2:S107–S111. doi: 10.1097/QAD.0000000000002252. [DOI] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention [published online ahead of print, 2020 Feb 24] JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2065. Published online May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]