Abstract
Introduction
The aim of this study was to determine changes of reactive oxygen species (ROS), serum antioxidant capacity (SAC), oxidative stress index (OSi), and α-tocopherol (α-T) during the periparturient period in healthy and mastitic cows and to further investigate whether these parameters can be used as a tool for identifying cows at higher risk of developing mastitis.
Material and Methods
Blood samples from 110 dairy cows from two commercial farms were obtained at dry-off, calving, and 30 days post-partum. Healthy cows formed group A (n = 90) and mastitic cows B (n = 20). Blood serum was obtained by centrifugation, and the aforementioned parameters were determined. A general linear model was used for analysing the associations among the determined blood parameters, the health of the animals’ udder, and the sampling time.
Results
ROS and OSi values were higher (P < 0.001) by a respective 14% and 26%, and SAC values lower (P < 0.001) by 10% in group B than in group A at calving. ROC curve analysis revealed that all determined parameters at calving and α-T at dry-off and 30 days post-partum had excellent or acceptable predicting ability for mastitis incidence.
Conclusion
This information provides a tool for early identification of cows at high risk of developing mastitis, allowing the implementation of intervention strategies.
Keywords: dairy cows, mastitis, oxidative stress biomarkers, α-tocopherol, early mastitis risk identification
Introduction
Oxidative stress resulting from an imbalance between oxidant and reductant (antioxidant) substances in a living organism plays a pivotal role in several pathological events associated with animal production. The majority of the evidence attests to the fact that high-yielding dairy cows experience such stress during the periparturient period (4, 6, 7). Dairy cows with high body condition scores and high body condition losses are more prone to oxidative stress during this period (4), and increased lipid peroxidation around parturition has been reported (6). Sordilo and Aitken (15) observed that high concentrations of reactive oxygen species (ROS) during this period could expose cows to increased oxidation status. Elevated levels of reactive oxygen metabolites (ROM) at calving have been reported (12). An increase in ROS, also, occurs immediately after parturition (1). The oxidative stress index (OSi), determined as the ratio of the reactive oxygen species (ROS) to serum antioxidant capacity (SAC), has been proposed as a more accurate index for estimating the antioxidant status in a living organism (1). According to the same authors (1), OSi levels were higher in cows post-partum compared to cows in late lactation. Another blood parameter that has been widely associated with oxidative stress in dairy cows is α-tocopherol (α-T), which is considered one of the main antioxidant substances in dairy cows (12, 13). The majority of the studies suggest that lower blood concentrations of α-T during the periparturient period are associated with more oxidation and higher mastitis incidence (12, 13).
Mastitis is a severe infection in both heifers and multiparous cows caused by environmental pathogens, especially coliforms, and is considered as one of the most prevalent and costly production diseases in the dairy sector. The disease lowers milk production and milk quality, resulting in higher production costs. One factor making mastitis a major challenge during the transition from the dry period to lactation, when its incidence is higher (3, 12, 14), is the fact that dairy cows undergo many metabolic and physiological changes including immunosuppression (13, 14, 15). Oxidative stress is considered a major underlying cause of these changes (2, 11). However, as no specific clinical symptoms are directly connected with oxidative stress (in a cause-effect relationship), oxidation biomarkers are used to determine the levels of oxidation and inform the choice of measures to alleviate its impact on cow health (2, 8). Our working hypothesis is that cows with higher oxidation load might be more prone to developing mastitis. Thus, the objectives of the present study were (a) to determine the effect of udder condition (healthy or mastitic) on the levels of the oxidative stress indicators ROS, SAC, and OSi at dry-off, calving, and 30 days (30 d) post-partum in dairy cows; (b) to determine whether differences occur in α-T levels between healthy cows and those with mastitis at these time points; and (c) to examine whether the oxidation biomarkers and α-T levels can be used as a management tool for early identification of cows at higher risk of developing mastitis.
Material and Methods
A total of 110 dairy cows from two commercial farms located in the northern part of Greece participated in this observational field study. Healthy animals were designated as group A (n = 90), and mastitic cows were group B (n = 20). Diets (based on dry matter basis) during the dry period were 42% corn silage, 40% straw hay, 9.6% soybean meal, and 8.4% molasses. After calving, the diet was 48.5% corn silage, 16.5% soybean meal, 14% alfalfa hay, 12% corn, 3.7% molasses, 1.7% rumen-protected fat, and 3.5% vitamin and mineral premix.
Blood samples from all cows were collected at dry-off, calving, and 30 d post-partum. Blood serum was obtained after centrifugation at 820 × g for 10 min at 4°C, and it was stored at −80°C for further analysis. The determination of the α-T (μg/mL) in serum was conducted according to a procedure described previously (12). The reactive oxygen species (ROS) capacity, expressed in “Carratelli Units” (CarrU), were determined spectrophotometrically and that of SAC (μmol HClO/mL) was assayed using the OXY-Adsorbent Test (Diacron, Grosseto, Italy), following methodology previously described (16). The oxidative stress index (OSi) was determined as the ratio of ROS to SAC.
Cases of clinical mastitis during the dry period and the subsequent lactation period were diagnosed and recorded by the veterinarian personnel of the farms. Mastitis was initially identified based on the occurrence of gross swelling, heat, redness, pain, disturbed function characterised by a decrease in milk production, or changes in milk composition. The animals were also examined for systemic symptoms (fever, depression, shivering, loss of appetite, and loss of weight) or lesser symptoms (fever or milder depression). Lastly, the milk was checked for watery appearance and the presence of clots. Milder cases of mastitis that occurred during middle and late lactation were left untreated but were recorded. A cow was classified as having mastitis only once, regardless of how many quarters were mastitic or whether the infection recrudesced. Of the 110 cows, 20 developed clinical mastitis. Fifteen cows developed mastitis in early lactation (days in milk less than 45), four cows developed mastitis during middle to late lactation (days in milk 124–245), and one cow did during the dry period. Cows in early lactation and the dry period were treated, while all cows in middle and late lactation were left untreated because they developed milder symptoms. Milk samples from all cows (except that at dry period), which developed symptoms were collected and bacteriological analyses were performed following the methods previously described (9). Staphylococcus aureus was detected in the milk samples of 12 cows, Streptococcus agalactiae in another 3, and 4 cows did not exhibit positive bacteriology results, and thus, their mastitis remained of unknown aetiology.
Animals were divided into two groups based upon udder health. Group A comprised healthy cows (n = 90) and group B (n = 20) cows that developed mastitis. Α general linear model (GLM) was used for analysing the associations between the determined blood parameters (ROS, SAC, OSi and α-T), the health of the udder as defined by group (A or B), and the sampling time (dry-off, calving, or 30 d post-partum). The
statistical model was:
Yij: the mean value of the examined parameter, μ: the general mean,
Gi: the fixed effect of animal group based on udder health (i = 1 – group A, i = 2 – group B),
Lj: the fixed effect of the sampling period (j = 1 – dry-off, 2 – calving, 3 – 30 d post-partum),
Gi*Lj: the effect of interaction between udder health and sampling period, and
eij: the random error.
Receiver operating characteristic curve (ROC) analysis was further implemented to test whether the determined oxidative biomarker concentrations (ROS, SAC, OSi, and α-T) can be used as a management tool for predicting mastitis incidents in dairy cows. The area under the ROC curve (AUC) was used as an index of mastitis prognostic performance. Pairs of sensitivity and specificity were used via the Youden index for estimating an optimal cut-off point widely used in clinical practice. The statistical procedures were performed using the SPSS v.16.0 (SPSS, Chicago, IL, USA) and MedCalc for Windows v.19.1 (MedCalc Software, Ostend, Belgium) packages.
Results
Comparisons of ROS, SAC, and OSi values between group A and group B are presented in Table 1. There were no differences in all three parameters between the two groups at both dry-off and 30 d postpartum. In contrast, at calving time, ROS and OSi levels were higher (P < 0.001) by 13.9% and 25.7%, while SAC levels were lower (P < 0.001) by 10% in group B than in group A. In addition, Table 1 allows for a comparison of ROS, SAC and OSi values between the three sampling points within each group (A and B). The results indicated that fluctuations in the values of all parameters over time were similar in both groups. The highest value for ROS and OSi and the lowest value for SAC occurred at calving.
Table 1.
Effect of the health of the udder (healthy or mastitic) on ROS (CarrU), SAC (μmol HClO/ml) and OSi (ROS/SAC) in the blood of dairy cows at dry-off, calving and 30d post-partum. Group A – healthy animals; Group B – mastitic animals. Values are presented as LSM ± SEM
| Parameter | Group A | Group B |
|---|---|---|
| Dry-off | ||
| ROS | 51.87a, 1 ± 0.85 | 54.85a, 1 ± 1.87 |
| SAC | 447.31a, 1 ± 2.52 | 431.64a, 1 ± 5.52 |
| OSi | 0.117a, 1 ± 0.002 | 0.127a, 1 ± 0.005 |
| Calving | ||
| ROS | 65.89a, 2 ± 0.86 | 75.04b, 2 ± 1.87 |
| SAC | 347.38a, 2 ± 2.54 | 312.38b, 2 ± 5.52 |
| OSi | 0.191a, 2 ± 0.002 | 0.240b, 2 ± 0.005 |
| 30 d post-partum | ||
| ROS | 53.46a, 1 ± 0.86 | 57.07a, 1 ± 1.87 |
| SAC | 451.42a, 1 ± 2.54 | 440.36a, 1 ± 5.52 |
| OSi | 0.118a, 1 ± 0.002 | 0.130a, 1 ± 0.005 |
Different superscripts within the same row indicate significant difference (P < 0.001) between the two groups. 1,2 Different superscripts within the same column for the same parameter indicate significant difference (P < 0.001) between sampling points
The values of α-T (μg/mL) in the cows of group A were 5.11 ± 0.10, 2.19 ± 0.10, and 4.95 ± 0.10 at dry-off, calving and 30 d post-partum, respectively. The corresponding values for animals of group B were 4.31 ± 0.21, 1.58 ± 0.21, and 4.27 ± 0.21. Proportionally, the α-T values were lower (P < 0.05) in group B by 15.7%, 27.9%, and 13.7% at dry-off, calving, and 30 d post-partum, respectively.
The graphical representation of the ROC analysis curve using each of ROS, SAC, OSi, and α-Τ as a predictor of mastitis incidence in all sampling points is shown in Fig. 1. The diagnostic performance of the implemented ROC curve analysis is also shown in Table 2. The highest AUC for ROS was found at calving (0.869 ± 0.046) resulting in a cut-off point value of >70.2 CarrU. Regarding SAC, the highest AUC value corresponded to calving (0.949 ± 0.027) resulting in a cut-off point value of ≤320 μmol HClO/mL. With respect to OSi, the highest AUC value was determined again at calving (0.952 ± 0.026), giving a cut-off point value of > 0.224. Lastly, the highest AUC value for α-T was determined at calving (0.730 ± 0.048), setting a cutoff point value of ≤1.9 μg/mL. With respect to dry-off and 30 d post-partum time points, among the four parameters determined α-T had the highest AUC values at both time points with cut-off values of ≤5.2 μg/mL and ≤4.5 μg/mL, respectively (Table 2).
Fig. 1.
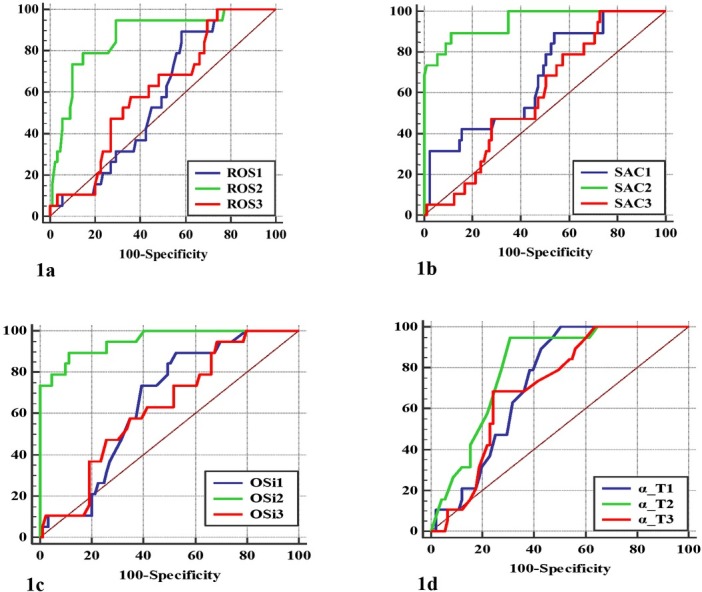
ROC curve analysis in detecting mastitis incidents based on the determined concentrations of the ROS (1a); SAC (1b); OSi (1c); and α-T levels (1d). Numbers (1, 2, 3) after the name of each biomarker correspond to the examined time points (1 – dry-off; 2 – calving; 3 – 30 d post-partum)
Table 2.
Diagnostic performance (receiver operating characteristic curve coordinates) of ROS, SAC, OSi and α-T as predictors of mastitis incidence at dry-off, calving and 30 d post-partum
| Biomarker /Sampling point | Area (AUC) Under Curve | Cut-off (Threshold) | Sensitivity | Specificity | P-value |
|---|---|---|---|---|---|
| Dry-off | |||||
| ROS | 0.586a ± 0.060 | >50.0 | 89.5 | 42.9 | ns (0.16) |
| SAC | 0.674ab ± 0.067 | ≤450.4 | 89.5 | 45.1 | ** |
| OSi | 0.663b ± 0.057 | >0.113 | 89.5 | 48.4 | ** |
| α-T | 0.730b ± 0.048 | ≤ 5.2 | 100 | 49.5 | *** |
| Calving | |||||
| ROS | 0.869a ± 0.046 | >70.2 | 94.7 | 71.1 | *** |
| SAC | 0.949b ± 0.027 | ≤320.4 | 89.5 | 88.9 | *** |
| OSi | 0.952b ± 0.026 | >0.224 | 89.5 | 88.9 | *** |
| α -T | 0.805a ± 0.045 | ≤1.9 | 94.7 | 69.2 | *** |
| 30 d post-partum | |||||
| ROS | 0.614ab ± 0.064 | >46.7 | 100 | 26.7 | ns (0.075) |
| SAC | 0.592a ± 0.063 | ≤467.3 | 100 | 26.7 | ns (0.14) |
| OSi | 0.636ab ± 0.064 | >0.106 | 94.7 | 32.2 | * |
| α -T | 0.710b ± 0.053 | ≤4.5 | 68.4 | 75.8 | *** |
Different superscripts within the same column for each sampling point (dry-off, calving, and 30 d post-partum) indicate significant difference (ns -not significant; *P < 0.05; **P < 0.01; ***P < 0.001)
Discussion
The first finding emerging from the present study is that there is a strong association between the health of the udder and all three oxidative stress biomarkers determined (ROS, SAC, OSi) at calving but not at dry-off or 30 d post-partum. The association at calving does not imply a cause-effect relationship, but it is tempting to speculate that the increased oxidation load at calving might make cows more prone to develop mastitis during the subsequent lactation. Higher oxidation loads during the perinatal and early lactation period in dairy cows result in dysfunctional immune response activity (2, 14).
This suppression of immune response impairs how the organism counteracts inflammation, and thus, cows are more prone to mastitis (5, 11, 14). Our results are not in agreement with a previous study by our group, which reported that there were no differences between ROM and thiol group (SH) levels, and ferric-reducing ability in the blood serum of healthy cows and these levels and ability in the serum of cows with mastitis at dry-off or at calving (12). There is no explanation as to why these differences between the two studies occurred, except the possibility of the set of parameters determined in this study being more sensitive.
The second finding from the study published here is that the determined levels of the oxidative stress biomarkers and blood α-T exhibited high predictive ability regarding which cows would develop mastitis during the dry period and the subsequent lactation. The accuracy of prediction using ROC curve analysis is classified into one of four categories (10): a) poor (0.5 < AUC < 0.7); b) acceptable (0.7 ≤ AUC ≤ 0.8); c) excellent (0.8 ≤ AUC ≤ 0.9), and d) perfect (AUC ≥ 0.9). Based on these criteria, SAC and OSi at calving exhibited perfect predictive ability and ROS and α-T showed excellent ability. Therefore, these four parameters can be used as extremely valuable tools in the early identification of cows at high risk of developing mastitis because they offer valuable information even before the lactation begins. From a time-point of view, an earlier time point than calving indicating which cows could develop mastitis is the dry-off time point. The dry period (abrupt cessation of lactation) begins approximately 45–60 days prior to calving (3). Unfortunately, the three parameters related to oxidation stress evinced poor predictive ability, but α-T exhibited acceptable ability at dry-off. These data taken together suggest that all four parameters are perfect or excellent indicators at calving, but the α-T value is also valuable at dry-off even though it falls into the acceptable category because it provides information 45–60 days earlier.
The third finding brought to light by this study is the lower α-T values observed at dry-off, calving, and 30 d post-partum in group B compared to group A. These results are in agreement with previously reported findings (12), and this is the reason why they will not be further discussed.
In conclusion, a higher oxidation load was detected in group B (cows that developed mastitis) compared to group A (healthy cows) during the periparturient period. All three oxidative stress biomarkers exhibited perfect or excellent ability at calving for predicting which cows would develop mastitis in the subsequent lactation. The values of α-T at dry-off and calving showed acceptable and excellent predicting ability regarding mastitis incidence at dry-off and calving, respectively. The results of the present study provide valuable, novel information for early identification of cows at high risk of developing mastitis enforcing the verification of such an approach in larger data sets.
Footnotes
Conflict of Interest
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Animal Rights Statement: All rearing conditions and handling procedures were carried out in accordance with the European Commission Recommendation 2007/526/EC and Directive 2010/63/UE on revised guidelines for the accommodation and care of animals used for experimentation and other scientific purposes.
Financial Disclosure Statement: The present study was funded by the participating institutions: Agricultural University of Athens and University of Milan.
References
- 1.Abuelo A., Hernández J., Benedito J.L., Castillo C.. Oxidative stress index (OSi) as a new tool to assess redox status in dairy cattle during the transition period. Animal. 2013;7:1374–1378. doi: 10.1017/S1751731113000396. [DOI] [PubMed] [Google Scholar]
- 2.Abuelo A., Hernández J., Benedito J.L., Castillo C.. The importance of the oxidative status of dairy cattle in the periparturient period: revisiting antioxidant supplementation. J Anim Physiol Anim Nutr. 2015;99:1003–1016. doi: 10.1111/jpn.12273. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson O.. Management of transition cows in dairy practice. In Practice. 2016;38:229–240. [Google Scholar]
- 4.Bernabucci U., Ronchi B., Lacetera N., Nardone A.. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J Dairy Sci. 2005;88:2017–2026. doi: 10.3168/jds.S0022-0302(05)72878-2. [DOI] [PubMed] [Google Scholar]
- 5.Cai T.Q., Weston P.G., Lund L.A., Brodie B., McKenna D.J., Wagner W.C.. Association between neutrophil functions and periparturient disorders in cows. Am J Vet Res. 1994;55:934–943. [PubMed] [Google Scholar]
- 6.Castillo C., Hernández J., Bravo A., Lopez-Alonso M., Pereira M., Benedito J.L.. Oxidative status during late pregnancy and early lactation in dairy cows. Vet J. 2005;169:286–292. doi: 10.1016/j.tvjl.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Castillo C., Hernández J., Valverde I., Pereira V., Sotillo J., Alonso M.L., Benedito J.L.. Plasma malonaldehyde (MDA) and total antioxidant status (TAS) during lactation in dairy cows. Res Vet Sci. 2006;80:133–139. doi: 10.1016/j.rvsc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Celi P.. Biomarkers of oxidative stress in ruminant medicine. Immunopharmacol Immunotoxicol. 2011;33:233–240. doi: 10.3109/08923973.2010.514917. [DOI] [PubMed] [Google Scholar]
- 9.Hogan J.S., Gonzalez R.N., Harmon R.J., Nickerson S.C., Oliver S.P., Pankey J.W., Smith K.L. Laboratory and field handbook on bovine mastitis. National Mastitis Council; Madison: 1999. pp. 1–33. [Google Scholar]
- 10.Hosmer D.W., Lemeshow S., Sturdivant R.X. Applied Logistic Regression. John Wiley & Sons; Hoboken, NJ: 2013. pp. 173–182. [Google Scholar]
- 11.Jóźwik A., Krzyżewski J., Strzałkowska N., Poławska E., Bagnicka E., Wierzbicka A., Niemczuk A.K., Lipińska P., Horbańczuk O.. Relations between the oxidative status, mastitis, milk quality, and disorders of reproductive functions in dairy cows – A review. Anim Sci Pap Rep. 2012;30:297–307. [Google Scholar]
- 12.Politis I.. Reevaluation of vitamin E supplementation of dairy cows: bioavailability, animal health, and milk quality. Animal. 2012;6:1427–1434. doi: 10.1017/S1751731112000225. [DOI] [PubMed] [Google Scholar]
- 13.Politis I., Theodorou G., Lampidonis A.D., Kominakis A., Baldi A.. Oxidative status and incidence of mastitis relative to blood α-tocopherol concentrations in the postpartum period in dairy cows. Short communication. J Dairy Sci. 2012;95:7331–7335. doi: 10.3168/jds.2012-5866. [DOI] [PubMed] [Google Scholar]
- 14.Sordillo L.M.. Factors affecting mammary gland immunity and mastitis susceptibility. Livestock Prod Sci. 2005;98:89–99. [Google Scholar]
- 15.Sordillo L.M., Aitken S.L.. Impact of oxidative stress on the health and immune function of dairy cattle. Vet Immunol Immunopathol. 2009;128:104–109. doi: 10.1016/j.vetimm.2008.10.305. [DOI] [PubMed] [Google Scholar]
- 16.Trotti R., Carratelli M., Barbieri M., Micieli G., Bosone D., Rondanelli M., Bo P.. Oxidative stress and a thrombophilic condition in alcoholics without severe liver disease. Haematologica. 2001;86:85–91. [PubMed] [Google Scholar]


