1. Introduction
Cancer antigen 125 (CA 125) is a transmembrane glycoprotein expressed on the surface of germinal epithelium and other tissues derived from embryonic coelomic epithelium (Bischof, 1993). CA 125 is a useful biomarker to detect treatment response and recurrence of ovarian malignancy (Montagnana et al., 2017), however, its use as a diagnostic marker is precluded by its limited specificity. CA 125 can be elevated in several non-ovarian malignancies including cervical, lung, and colorectal cancers (Johnson et al., 2008), and have also been found to be elevated in benign diseases involving coelomic epithelium, such as endometriosis, fibroids, ovarian cysts, and pelvic inflammatory disease (Sevinc et al., 2007).
Recently, one retrospective study found that among all admitted patients with coronavirus disease 2019 (COVID-19) infection, increases in CA 125 and other cancer biomarkers were associated with severe COVID-19 infection (Wei et al., 2020). Thus, it is important for us to understand the potential impact COVID-19 has on CA 125 which may confound the treatment team of patients with gynecologic malignancy. Here, we report the case of a women with FIGO stage IVA ovarian high grade serous carcinoma (HGSC) during the COVID-19 pandemic who had a transient increase in CA 125 without evidence of progression of disease on imaging, and who was later found to have a positive COVID-19 antibody test.
2. Case
A 54-year-old BRCA1 mutation carrier presented to her gynecologic oncologist for follow-up for advanced platinum resistant ovarian HGSC in New York City during the COVID-19 pandemic. Her treatment history is detailed as follows. The patient was initially diagnosed with advanced stage HGSC by omental biopsy. She was deemed unresectable and completed four cycles of neoadjuvant taxol and carboplatin chemotherapy prior to an interval debulking surgery in November 2017 and completed chemotherapy in February 2018. She was enrolled in a maintenance Niraparib vs placebo clinical trial (S16-00663) but was taken off the trial after she was found to have progression of disease. She was subsequently enrolled in a pegylated liposomal doxirubicin (PLD)/atezolizumab/bevacizumab clinical trial (S18-00430), with PLD held since cycle 6 due to toxicities and she continued on the atezolizumab/bevacizumab.
During the COVID-19 pandemic, the patient was able to follow social distancing guidelines at her home in Brooklyn except for in person medical visits and had no known sick contacts. Since March 2020, the patient has had two telemedicine visits and three in person visits with a gynecologic oncologist. As a participant in an immunotherapy clinical trial, the patient underwent COVID-19 screening prior to further treatment. She was found to be negative by real time PCR on May 5, 2020.
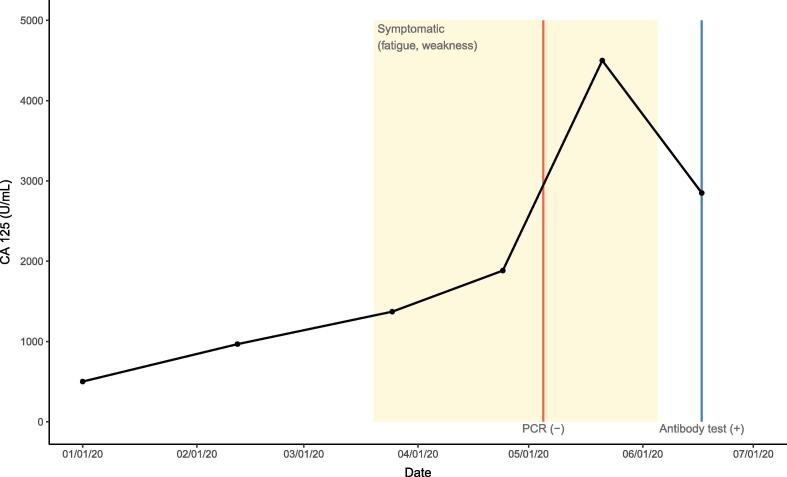
The patient’s CA 125 levels prior to and during the COVID-19 pandemic are illustrated in Fig. 1. During the months of the pandemic in NYC, the patient missed 3 dates of atezolizumab/bevacizumab treatment due to anxiety, extreme fatigue, weakness, and declining performance status on April 8, May 8, and June 4, 2020. During this time, she continued to receive treatment as scheduled on March 25, April 24, May 21, and June 17, 2020. She denies any history of fever or respiratory symptoms including cough or shortness of breath. During the course of her extreme fatigue, CA 125 levels were monitored at routine visits (Fig. 1). On June 17, 2020 she underwent COVID-19 IgG antibody testing and was found to have a level of 1.4 S/C, consistent with a positive result. Retrospectively, her symptoms of fatigue and weakness were thought to have reflected active COVID-19 infection. CT imaging of the chest, abdomen, and pelvis on May 22, 2020 demonstrated stable disease compared to prior imaging.
Fig. 1.
Patient’s CA 125 values over 2020. The patient had a negative COVID-19 PCR on May 5, 2020 and had a positive COVID-19 IgG antibody test on June 17, 2020.
3. Discussion
As cases of COVID-19 surpass 6 million in the US, it is critical to understand how infection with COVID-19 manifests in gynecologic oncology patients and how infection impacts oncologic management. This report of a women with advanced ovarian cancer and COVID-19 infection with a transient rise and fall of CA 125 biomarkers without radiographic evidence of disease progression demonstrates the importance of considering COVID-19 infection as an etiology after ruling the patient out for active or progression of cancer. Although the patient was not known to have COVID-19 infection at the time of CA 125 increase, the marked fatigue and weakness reported by the patient at this time coupled with the subsequent positive COVID-19 antibody test and resolution of CA 125 spike without change in treatment plan make COVID-19 infection the most likely cause of the increase in CA 125.
This case supplements the results reported by Wei et al. (2020), who demonstrated significantly higher CA 125 levels in hospitalized non-oncology patients with severe COVID-19 infection compared to those with non-severe infection, and suggests that this pattern may be true in cancer patients as well. Moreover, the increase of COVID-19 reported by Wei et al. seen in severe COVID-19 infections compared to non-severe infections was modest (5.4 ± 4.1 vs 3.2 ± 1.4 U/mL respectively; p < .001), while our patient had a peak CA 125 of 4499 U/mL, an increase of 2617 U/mL from the presumed pre-COVID-19 level and only had mild infection not requiring hospitalization. This case illustrates that in the presence of underlying malignancy and elevated CA 125, COVID-19 infection may produce a dramatic increase in CA 125 that resembles cancer progression.
CA 125 is a large mucinous glycoprotein expressed on apical surfaces of tissue derived from coelomic epithelium including the female reproductive tract, respiratory tract, and ocular surfaces (Bischof, 1993). The mechanism by which CA 125 is increased in inflammatory states is not well described, but secretion of CA 125 has been shown to increase in response to inflammatory cytokines such as IL-1beta, TNF-alpha, and lipopolysaccharide (Zeillemaker et al., 1994). Elevated CA 125 has been documented in infections including pulmonary tuberculosis (Sahin and Serum, 2012) and pelvic inflammatory disease (Moley et al., 1996), and in addition to certain malignancies can be elevated in non-infectious states such as chronic obstructive pulmonary disease, endometriosis, ovarian cysts, and fibroids (Sevinc et al., 2007, Barouchos et al., 2015). Increased CA 125 seen in COVID-19 infection may be secondary to inflammatory damage to lungs as increased CA 125 has also been documented in other pulmonary processes, however interestingly our patient reported no pulmonary symptoms. While COVID-19 has been primarily thought of as a pulmonary disease, there has been increasing evidence that it can also infect other tissues throughout the body. Literature suggests that the SARS-Cov2 receptor ACE2 is expressed in ovaries of reproductive age and postmenopausal women as well as other gynecologic tissue, raising the possibility that COVID-19 can infect these organs as well (Jing et al., 2020). Given the elevation of CA 125 in other infectious etiologies, future studies would be informative to evaluate the role of CA 125 in COVID-19 infection, particularly among patients with a history of gynecologic cancer.
Our case underscores the importance of ruling out COVID-19 infection in patients with increase in CA 125 from their baseline as well as consulting imaging modalities for evidence of disease progression. Further investigation into the effects of COVID-19 infection on CA 125 levels in patients with ovarian malignancy and other gynecologic cancers is warranted.
4. Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
CRediT authorship contribution statement
Maria Smith: Investigation, Visualization, Writing - original draft.
Declaration of Competing Interest
M.S. and O.L. have nothing to disclose. B.P. reports grants, personal fees and non-financial support outside the submitted work; institutional PI for industry sponsored trials from Tesaro/GSK, AstraZeneca, Merck, Genentech/Roche, and Clovis Oncology. Compensated advisory boards include Tesaro/GSK, AstraZeneca, Merck and Eisai.
References
- Barouchos N., Papazafiropoulou A., Iacovidou N., Vrachnis N., Barouchos N., Armeniakou E., Dionyssopoulou V., Mathioudakis A.G., Christopoulou E., Koltsida S., Bassiakou E. Comparison of tumor markers and inflammatory biomarkers in chronic obstructive pulmonary disease (COPD) exacerbations. Scand. J. Clin. Lab. Invest. 2015;75(2):126–132. doi: 10.3109/00365513.2014.992944. [DOI] [PubMed] [Google Scholar]
- Bischof P. What do we know about the origin of CA 125? Eur. J. Obstetrics Gynecol. Reprod. Biol. 1993;49(1-2):93–98. doi: 10.1016/0028-2243(93)90131-u. [DOI] [PubMed] [Google Scholar]
- Jing Y., Run-Qian L.i., Hao-Ran W., Hao-Ran C., Ya-Bin L., Yang G., Fei C. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol. Hum. Reprod. 2020;26(6):367–373. doi: 10.1093/molehr/gaaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.C., Kessel B., Riley T.L., Ragard L.R., Williams C.R., Xu J.-L., Buys S.S. The epidemiology of CA-125 in women without evidence of ovarian cancer in the Prostate, Lung, Colorectal and Ovarian Cancer (PLCO) Screening Trial. Gynecol. Oncol. 2008;110(3):383–389. doi: 10.1016/j.ygyno.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moley K.H., Massad L.S., Mutch D.G. Pelvic inflammatory disease. Correlation of severity with CA-125 levels. J. Reprod. Med. 1996;41(5):341–346. [PubMed] [Google Scholar]
- Montagnana M., Benati M., Danese E. Circulating biomarkers in epithelial ovarian cancer diagnosis: from present to future perspective. Ann. Transl. Med. 2017;5(13):276. doi: 10.21037/atm.2017.05.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin F., Yildiz P. Serum CA-125: biomarker of pulmonary tuberculosis activity and evaluation of response to treatment. Clin. Invest. Med. 2012;35(4):E223–E228. Published 2012 Aug 4. [PubMed] [Google Scholar]
- Sevinc A., Adli M., Kalender M.E., Camci C. Benign causes of increased serum CA-125 concentration. Lancet Oncol. 2007;8(12):1054–1055. doi: 10.1016/S1470-2045(07)70357-1. [DOI] [PubMed] [Google Scholar]
- Wei X., Su J., Yang K. Elevations of serum cancer biomarkers correlate with severity of COVID-19 [published online ahead of print, 2020 Apr 29] J. Med. Virol. 2020 doi: 10.1002/jmv.25957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeillemaker A.M., Verbrugh H.A., Hoynck van Papendrecht A.A., Leguit P. CA 125 secretion by peritoneal mesothelial cells. J. Clin. Pathol. 1994;47(3):263–265. doi: 10.1136/jcp.47.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]