Summary
The glycome undergoes characteristic changes during histogenesis and organogenesis, but our understanding of the importance of select glycan structures for tissue formation and homeostasis is incomplete. Here, we present a human organotypic platform that allows genetic dissection of cellular glycosylation capacities and systematic interrogation of the roles of distinct glycan types in tissue formation. We used CRISPR-Cas9 gene targeting to generate a library of 3D organotypic skin tissues that selectively differ in their capacity to produce glycan structures on the main types of N- and O-linked glycoproteins and glycolipids. This tissue library revealed distinct changes in skin formation associated with a loss of features for all tested glycoconjugates. The organotypic skin model provides phenotypic cues for the distinct functions of glycoconjugates and serves as a unique resource for further genetic dissection and identification of the specific structural features involved. The strategy is also applicable to other organotypic tissue models.
Keywords: glycobiology, glycans, organotypic model, organoid, CRISPR/Cas9, keratinocytes, skin, epithelia, integrins, Notch
Graphical Abstract

Highlights
-
•
Glycosphingolipids tune cell signaling and impact epithelial barrier formation
-
•
Complex N-glycans govern wound healing and lamellar body functions in human skin
-
•
Core-1 O-glycans are essential for cell-cell adhesion and human tissue differentiation
-
•
Notch O-fucosylation and O-glucosylation have distinct roles in human tissue formation
Dabelsteen et al. present a glycoengineered organotypic epithelial tissue model that allows a systematic interrogation of glycan functions in human tissue formation and homeostasis. The model shows the distinct functions of select glycoconjugates during cellular differentiation and serves as a resource for further identification of glycan molecular functions.
Introduction
Complex carbohydrates found on lipids and proteins are produced through non-template-driven enzymatic processes occurring throughout the secretory pathway of cells. These glycosylation processes are orchestrated by more than 200 glycosyltransferases and a large number of accessory enzymes and transporters (Stanley, 2011) and can be organized into 15 distinct types of glycosylation pathways in mammalian cells, with more than 170 glycosyltransferases reliably assigned to specific pathways (Figure 1D) (Narimatsu et al., 2019). Most of these distinct glycosylation pathways are required for the normal development of mice (Haltiwanger and Lowe, 2004; Varki, 2017), and deficiencies may impact human health and disease (Freeze, 2006; Moremen et al., 2012; Ohtsubo and Marth, 2006; Varki, 2017). Thus, more than 100 rare congenital disorders of glycosylation (CDGs) are caused by genetic defects in genes affecting glycosylation, with 50 CDGs that result in deficiencies in glycosyltransferase genes (Freeze, 2006; Joshi et al., 2018). Genome-wide association studies (GWASs) have predicted that many more glycosyltransferases play subtle roles in more common conditions (Freeze, 2006; Joshi et al., 2018). Numerous studies in model organisms, including mice, fish, Xenopus, Drosophila melanogaster, and Caenorhabditis elegans, have illustrated that many glycosyltransferase genes play important roles in embryogenesis, morphogenesis, and histogenesis (Haltiwanger and Lowe, 2004; Katoh and Tiemeyer, 2013; Moremen et al., 2012; Ohtsubo and Marth, 2006; ten Hagen et al., 2009), and in some cases, these studies have defined more specific cellular functions, with glycosylation in Notch signaling as an important example (Jafar-Nejad et al., 2010). Though these studies clearly document the importance of different types of glycosylation (Varki, 2017), the complexity and heterogeneity of glycosylation pathways in whole animals and whole organisms often preclude dissection of specific structure-function relationships. Furthermore, there are important differences between glycan expression in animals and humans, which may prevent direct translation of the results obtained in model organisms into human-relevant solutions (Varki, 2017).
Figure 1.
Strategy for Dissecting Glycan Function Using CRISPR-Cas9-Engineered Human Skin Organotypic Models
(A) Left: Immortalized human keratinocytes (N/TERT-1) are transduced with lentivirus carrying CRISPR-Cas9 and gRNAs designed to target the gene of interest (Table S1). KO clonal cell lines are used for 3D organotypic human skin equivalents. Right: Schematic depiction of the human epidermis composed of four cell layers: stratum basale, stratum spinosum, stratum granulosum, and stratum corneum. Drawing adapted from Simpson et al. (2011).
(B) Representation of the main human glycosylation pathways: glycosphingolipid biosynthesis initiated by the glucosylceramide synthase (UGCG); biosynthesis of complex-type N-linked glycans from high-mannose structures dependent on MGAT1; mucin-type O-GalNAc-glycosylation, initiated by polypeptide GalNAc-transferases and elongated by the core-1 synthase (C1GALT1) and its obligate chaperone Cosmc (C1GALT1C1); and O-glycosylation of Notch EGF-repeats by Pofut1 (POFUT1) and Poglut1 (POGLUT1). Glycan symbols were drawn according to the symbol nomenclature for glycans (SNFG) format (Varki et al., 2015). Red arrows indicate points of disruption within the glycan structures resulting from knocking out specific glycosyltransferases (GTs).
(C) Genetically engineered human organotypic skin models reveal distinct roles of select glycans in tissue formation. Micrographs depict tissue sections from N/TERT-1 organotypic skin models created with WT or edited cells defective in glycosphingolipid glycosylation (UGCG KO), formation of complex N-linked glycans (MGAT1 KO), GalNAc-type O-glycosylation (C1GALT1 KO), O-fucosylation (POFUT1 KO), and O-glucosylation (POGLUT1 KO). Sections are stained with hematoxylin-eosin (HE, upper panel) or stained for the proliferation marker Ki67 (lower panel). Scale bar represents 20 μm.
(D) CRISPR-Cas9 genetic engineering strategy. Known human GTs are organized into their respective biosynthetic pathways. The concept is visualized by a glycoconjugate sub-library in which KO of the GTs controlling the early steps of glycosphingolipid glycosylation (UGCG), N-glycosylation (MGAT1), O-GalNAc-glycosylation (COSMC), O-fucosylation, (POFUT1), and O-glucosylation (POGLUT1) are selected for CRISPR-Cas9 gene targeting. Glycan symbols were drawn according to the SNFG format (Varki et al., 2015). Genes shown in black are expressed in N/TERT-1 cells.
Recent developments have positioned organotypic cultures as attractive model systems that offer simpler tissue sources and are amenable to high-throughput genetic engineering and screening options (Editorial Comment, 2018). Skin offers a well-defined model for probing cellular maturation, differentiation, and histogenesis (Ridky et al., 2010), and numerous studies have shown that glycosylation influences processes involved in tissue homeostasis, including protein folding, protein trafficking, cell signaling, cell-cell interactions, cell migration, and immune regulation (Ohtsubo and Marth, 2006; Varki, 2017). Therefore, we sought to develop an organotypic skin model that can be used for systematic interrogation of glycosylation pathways by gene targeting. The human N/TERT-1 keratinocyte cell line has the ability to undergo complete histogenesis in three-dimensional (3D) organotypic cultures (Dickson et al., 2000) (Figure 1A) and faithfully models the morphogenesis, cell differentiation, cell-cell interactions, and cell-matrix interactions of normal human epidermis. We used a rational CRISPR-Cas9 gene targeting strategy to develop isogenic N/TERT-1 organotypic 3D skin models with systematic abrogation of individual glycosylation pathways and probed the effects of global elimination of the glycan structures on glycosphingolipids (GSLs), N-glycans, mucin-type O-glycans, O-Fuc O-glycans, and O-Glc O-glycans.
Results
Glycosylation Capacity of Human Immortalized N/TERT-1 Keratinocytes Mimics Primary Human Keratinocytes
We first determined the glycosylation capacity of the immortalized N/TERT-1 keratinocyte cell line (Dickson et al., 2000; Smits et al., 2017; van den Bogaard et al., 2014) by RNA sequencing (RNA-seq) and compared the expression with human primary keratinocytes and previously published data from single-cell transcriptome analysis of human skin (Figure S1) (He et al., 2020). Essentially, all glycosyltransferase genes were similarly expressed in human epidermal keratinocytes, primary keratinocytes, and N/TERT-1 cells (Figure S1). The inherent glycosylation capacity of N/TERT-1 cells was predicted to include monosialodihexosylganglioside (GM3) gangliosides, complex N-glycans, O-GalNAc glycans, and O-fucose, and O-glucose, which are important for neurogenic locus notch homolog protein (Notch) glycosylation (Figures S1 and S2). We also glycoprofiled N- and mucin-type O-glycans from the total lysates of N/TERT-1 cells using mass spectrometry (MS), which confirmed that N-glycans were predominantly high in mannose and complex type with di-, tri-, and tetra-antennary structures partly elongated with poly-LacNAc structures capped by sialic acids (Figure S3). Released GalNAc-type O-glycans had both core-1 and core-2 structures capped by sialic acids (Figure S3).
Engineered Glycosylation Capacity in N/TERT-1 Cells Reflects Predicted Glycan Output
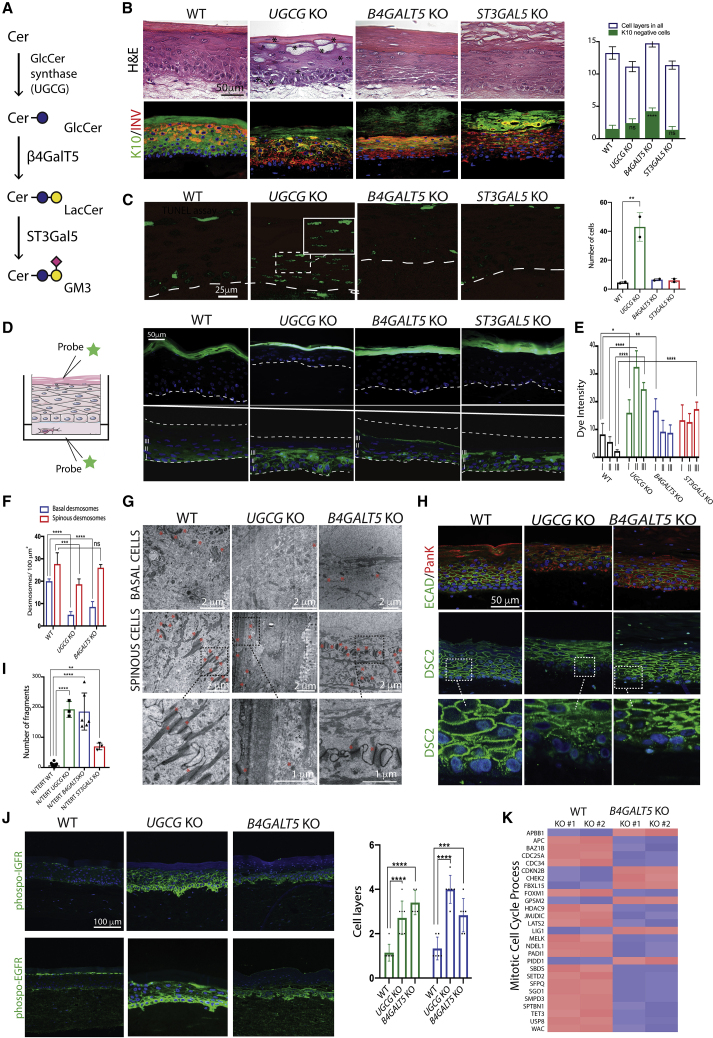
We used CRISPR-Cas9 gene targeting to inactivate non-redundant glycosyltransferase genes directing key steps in the biosynthesis of elaborated glycans on GSLs, N-glycans, and the most common types of O-glycans (Figures 1A–1C; Table S1) (Narimatsu et al., 2019). The effects on the respective glycosylation pathways were confirmed in representative clones by MS-based glycoprofiling (Figure S3). Targeting distinct steps in N-glycan biosynthesis resulted in a loss of complex N-glycans and the presence of high-mannose structures in MGAT1KO cells, whereas targeting the N-glycan branching enzymes (MGAT4AKO or MGAT4BKO; MGAT5KO) only produced the predicted loss of tetra-antennary N-glycans for MGAT5KO (Figure S3). For O-GalNAc glycans, we targeted the core-1 synthase (C1GALT1KO) or its obligate chaperone COSMC (C1GALT1-specific chaperone 1) (C1GALT1C1KO) to truncate O-glycans to the initial GalNAc (Tn) and/or its sialylated variant (STn) (Figure S3), as well as the core-2 synthase (GCNT1KO) to eliminate core-2 structures (Figure S3). For O-Fuc and O-Glc glycans, we targeted the initiating enzymes (POFUT1KO or POGLUT1KO) to eliminate these types of protein glycosylation. Finally, for GSL biosynthesis, we targeted the GlcCer synthase (UGCGKO), which initiates synthesis of all currently elaborated GSLs of the GlcCer type, as well as the subsequent common step in the elongation of GlcCer to LacCer (B4GALT5KO) and the ganglioseries GSL-determining step to produce GM3 (ST3GAL5KO).
We used this library of glyco-engineered cells to build 3D organotypic skin models to probe the effects of the engineered changes in glycosylation capacity. As a baseline, wild-type (WT) and control N/TERT-1 keratinocytes generated normal stratified squamous epithelium with a single basal layer of mitotic cells, multi-layered stratum spinosum, stratum granulosum, and a fully differentiated stratum corneum (Figures 1C and S4). In contrast, N/TERT-1 cells with engineered truncation of GSLs, N-glycans, and GalNAc-type, Fuc-type, and Glc-type O-glycans generated epithelia with distinct aberrations (Figures 1C and S4; Table S1), which demonstrated the suitability of the strategy to convey the functional roles of the major types of glycoconjugates in skin histogenesis. Therefore, we proceeded with a step-by-step dissection of individual glycosylation pathways to further narrow down distinct structure-function relationships.
GSLs: Critical for Epidermal Differentiation and Barrier Function
In humans, mutations in the UGCG gene are lethal and cause, among other manifestations, ichthyosis (Monies et al., 2018). Ugcg knockout (KO) in mice is embryonically lethal (Jennemann et al., 2005), but conditional KO of ugcg in the epidermis resulted in an impaired epidermal barrier with extreme desquamation and excessive water loss, culminating in early death (Amen et al., 2013; Jennemann et al., 2007). We targeted UGCG in N/TERT-1 (UGCGKO) and the two consecutive non-redundant biosynthetic steps to generate LacCer (B4GALT5KO) and the sialylated GM3 product (ST3GAL5KO) (Figure 2A). The tissue generated with UGCGKO exhibited delayed differentiation with multiple keratin 10 (K10)-negative basal cell layers, loss of the normal basal-cell palisade structure, increased intercellular spaces, and parakeratinization with a consistent lack of terminal differentiation (Figures 2B and S4). Furthermore, we detected pyknotic nuclei throughout the tissue, indicative of increased apoptosis, which was verified by the TUNEL assay (Figure 2C). The tissues generated with B4GALT5KO and ST3GAL5KO both exhibited less severe phenotypes with less apparent cellular stress. However, both KO tissues still exhibited delayed differentiation, increased basal proliferation, and a change in basal cell appearance, including loss of the normal palisade structure, and increased thickness of the stratum corneum (Figure 2B). To elucidate how glycolipids impact the integrity of basal cell layers and barrier function, we assayed trans-epidermal permeability by applying biotin as a molecular tracer to the basal compartment of the epidermis. In UGCGKO, B4GALT5KO, and ST3GAL5KO tissues, we found permeability defects in the basal and suprabasal cell layers, with the most pronounced defects observed in UGCGKO (Figures 2D and 2E). No permeability defect was observed when the probe was applied to the surface of the epithelium (Figure 2D). Consequently, we used transmission electron microscopy (TEM) to visualize the integrity of cell-cell contacts in UGCGKO and B4GALT5KO tissues. TEM confirmed compromised cell-cell contact formation in the basal and suprabasal layers of UGCGKO tissue, with a significant reduction in the number of adhesion complexes and increased extracellular space compared with the WT tissue (Figures 2F and 2G). These changes were also observed in B4GALT5KO tissue, though they were less prominent and primarily observed in the basal cell layers compared with the UGCGKO tissue (Figures 2F and 2G). A diminished number of adhesion complexes was confirmed by immunofluorescence of desmocollin-2 and E-cadherin (Figure 2H), and the functional consequences were confirmed by a cellular dissociation assay showing compromised cell-cell adhesion in UGCGKO and B4GALT5KO cells (Figure 2I). Changes in intracellular signaling influence cell-cell adhesion, affecting tissue integrity in human skin (Simpson et al., 2011). Therefore, we speculated that a possible explanation for the reduction in cell-cell adhesion in basal cell layers may be aberrant signaling caused by a loss of gangliosides known to inhibit receptor tyrosine kinases, including the highly expressed insulin growth factor (IGF) and epidermal growth factor (EGF) receptors (IGF-R and EGF-R, respectively) (Bremer et al., 1986; Dam et al., 2017). Immunofluorescence confirmed a significant increase in the activity of IGF-R and EGF-R in both UGCGKO and B4GALT5KO tissues, which was particularly pronounced in basal and several suprabasal cell layers (Figure 2J). This is in contrast to the confined expression of phosphorylated IGF receptor (pIGF-R) and phosphorylated EGF receptor (pEGF-R) in the basolateral membrane of the basal cell layer in WT tissue (Figure 2J). Next, we compared the RNA-seq results in B4GALT5KO cells with WT cells to evaluate the effect on cell-cycle regulators (Figures 2K, S5A, and S5D; Table S2). Consistent with an increased activity of tyrosine kinases in B4GALT5KO cells, we found an increased expression of genes encoding proteins promoting cell-cycle progression and a diminished expression of proteins blocking the cell cycle in B4GALT5KO cells, including negative regulators of the ERK1 and ERK2 pathways (Figure 2K; Table S2). Moreover, consistent with the decrease in cell-cell adhesion, we found a decreased expression of genes encoding proteins with functions in tight junction formation and differentiation (Figures S5A and S4C; Table S2).
Figure 2.
GSLs Impact Permeability, Cell Adhesion, and Differentiation in Human Skin
(A) GSL biosynthesis. The ganglioside GM3 is formed by the consecutive action of the glucosylceramide synthase (UGCG), β4GalT5 (B4GALT5), and ST3GalT5 (ST3GAL5).
(B) Sections from N/TERT-1 WT, UGCGKO, B4GALT5KO, and ST3GAL5KO organotypic culture tissues. The overall tissue organization and the expression of differentiation markers K10 and involucrin (INV) are illustrated. Scale bar represents 50 μm. Asterisks mark pyknotic nuclei in UGCGKO. The graph shows the quantification of K10-negative cell layers as mean ± SEM. Technical replicates = 4, biological replicates = 3.
(C) TUNEL assay detecting fragmented DNA of apoptotic cells. The insert shows a magnified view of the marked rectangle. The epithelial-mesenchyme border is depicted with dashed lines. Scale bar represents 25 μm. The diagram depicts the quantification of apoptotic cells. ∗∗p = 0.0036 using one-way ANOVA, multiple comparison. Technical replicates = 3.
(D) Trans-epidermal permeability assay. Probes were administered to either the apical (upper panel) or basal compartment (lower panel), and fixed organotypic cultures were sectioned and evaluated. Scale bar represents 50 μm. The epithelial-mesenchyme border is depicted with dashed white lines. Roman numerals indicate tissue layers used for quantification in (E). Nuclei are labeled with DAPI. Technical replicates = 5.
(E) The amount of permeabilized label was measured in whole sections from each specimen and genotype (n = 5) using the Photoshop analysis tool in three defined areas (I, II, III) per section. Data are presented as mean ± SEM from three different experiments. ∗∗∗∗p < 0.0001, ∗∗∗p < 0.0005 versus WT using one-way ANOVA followed by multiple comparison analysis.
(F and G) Quantification of the number of desmosomes in basal (blue) and spinous (red) layers in WT, UGCGKO, and B4GALT5KO tissues analyzed by TEM; see (G). Three micrographs were analyzed for each genotype. Data are presented as mean ± SEM. ∗∗∗∗p < 0.0001, ∗∗∗p < 0.0005 versus WT using one-way ANOVA followed by multiple comparison analysis. Technical replicates = 4, biological replicates = 2.
(G) Transmission electron micrographs of organotypic cultures from WT, UGCGKO, and B4GALT5KO tissues. Sections from basal and spinous layers are shown (scale bar = 2 μm). Desmosomes are marked with red asterisks. Magnification of marked areas in the spinous layers are shown in the bottom panel (scale bar = 1 μm).
(H) Immunofluorescent staining of WT, UGCGKO, and B4GALT5KO tissues with E-cadherin (E-CAD), pan-keratin (PanK, co-staining with E-CAD), and desmocollin-2 (DSC2)-specific mAbs. Scale bar represents 50 μm. Technical replicates = 4, biological replicates = 3.
(I) Dissociation assay of WT, UGCGKO, and B4GALT5KO cells grown to confluency, reflective of cell-cell adhesion. Data are presented as mean ± SEM. ∗∗∗∗p < 0.0001, ∗∗p < 0.01 versus WT using one-way ANOVA followed by multiple comparison analysis. Technical replicates = 6, biological replicates = 2.
(J) Immunofluorescent staining of UGCGKO and B4GALT5KO tissues with mAbs recognizing activated pIGF1-R and pEGF-R. Scale bar represents 100 μm. Micrographs were analyzed for each genotype; green bars represent pEGF-R, and blue bars represent pIGFR. Data are presented as mean ± SEM. ∗∗∗∗p < 0.0001, ∗∗∗p = 0.0004 versus WT using one-way ANOVA followed by multiple comparison analysis. Technical replicates = 3, biological replicates = 2.
(K) Differential expression of genes involved in the cell division of WT and B4GALT5KO cells as measured by RNA-seq. Fragments per kilobase of transcript per million mapped reads (FPKM) values for a select list of genes involved in mitotic cell-cycle process (GO:1903047) were loaded into Qlucore Omics Explorer. Genes that significantly contribute to sample variance (q ≤ 0.05) are shown. Red indicates higher expression, and blue indicates lower expression. Biological replicates = 2.
Sialylated Complex-type N-Glycans Play a Role in the Secretion of Lamellar Bodies
MGAT1 KO abrogates the biosynthesis of all complex N-glycans (Figure 1) (Stanley, 2011), and Mgat1 KO in mice leads to early embryonic lethality (Ioffe and Stanley, 1994; Metzler et al., 1994). Tissues generated with MGAT1KO formed skin but with large intracellular vacuoles throughout the epithelium (Figure 3C), a phenotype not observed in MGAT5KO or MGAT4KO tissues. Apart from the large vacuoles, the MGAT1KO skin exhibited reduced initial and terminal differentiation (Figures 3C, 3D, and S5B). To further examine the importance of sialylation in development of the phenotype, we treated organotypic cultures of WT N/TERT-1 cells with a cell-permeable sialylation inhibitor (Ac5SiaFEtoc). Ac5SiaFEtoc is metabolized by cells into a global inhibitor of all sialyltransferase isoenzymes, outcompeting endogenous sialic acids for cytidine monophosphate (CMP) activation by N-acylneuraminate cytidylyltransferase (CMAS) (Heise et al., 2019), thus completely abolishing sialylation (Figures 3F and 3G). Intriguingly, treatment of WT N/TERT-1 cells with Ac5SiaFEtoc phenocopied the large intracellular vacuoles seen in MGAT1KO cells (Figure 3H), demonstrating the involvement of N-glycan sialylation in the observed phenotype. To investigate the nature of the enlarged vacuolar structures, we performed TEM and immunofluorescence with MGAT1KO tissue using markers of secretory pathway components (Figures 3I and 3J). In skin, the main permeability barrier is provided by lipids and proteins delivered to the extracellular spaces of the stratum corneum by the secretion of lamellar bodies with characteristic structures that can be visualized by TEM (Feingold, 2012) (Figure 3A). TEM showed the abnormal presence of multiple lamellar body-like structures in the vacuoles of MGAT1KO cells (Figure 3I), suggesting that N-glycans are involved in mechanisms regulating the specialized secretion of terminal differentiation products in keratinocytes. Consistent with this hypothesis, we found diminished expression of the trans-Golgi marker TGN46 and early endosome marker EEA1 (Figure 3J), whereas no change was observed in the endoplasmic reticulum (ER) and lysosomal markers, though the appearance and location were affected by the altered morphology of MGAT1KO cells (Figures 3I and 3J). RNA-seq also demonstrated that genes involved in lamellar body formation were specifically downregulated in MGAT1KO cells compared with WT cells (Figure 3E; Table S2). The elements secreted by lamellar bodies have multi-factorial roles in the terminal differentiation of keratinocytes in human skin (Elias and Wakefield, 2014), including the proteolytic processing of pro-filaggrin to yield individual filaggrin monomers at the transition between the stratum granulosum and the stratum corneum. Therefore, we investigated the processing of pro-filaggrin to filaggrin monomers in MGAT1KO skin and found both a reduction in pro-filaggrin processing in MGAT1KO skin (Figure 3K) and delayed differentiation (Figures 3C–3E), which are consistent with a problem in lamellar body secretion.
Figure 3.
Complex N-Glycans Have Roles in the Formation of Lamellar Bodies
(A) Schematic model of human skin with an illustration of lamellar body formation. Lamellar bodies are double-membrane structures derived from the trans-Golgi network and important for the delivery and packaging of lipids and proteins required to form the cornified layer in human keratinized epidermis (Elias and Wakefield, 2014).
(B) Biosynthesis of complex N-linked glycans from high-mannose structures, with MGAT1 catalyzing the addition of the first GlcNAc residue required for further synthesis of complex N-linked glycans by MGAT2, MGAT4A or MGAT4B, and MGAT5. Each terminal GlcNAc residue can potentially be elongated with type-2 or type-1 chains as indicated by the dashed arrows.
(C) Micrographs of N/TERT-1 organotypic cultures with essential genes involved in N-glycan synthesis knocked out. HE (top panel) and Ki67 (middle panel) staining. Bottom panel, immunofluorescent labeling of the differentiation markers K10 and INV in tissue sections. Scale bar represents 50 μm. Technical replicates = 3, biological replicates = 2.
(D) Numbers of K10-negative and total cell layers. Three micrographs were analyzed for each genotype. Data are presented as mean ± SEM (n = 4).
(E) The expression of genes involved in lamellar body formation is downregulated in MGAT1KO cells compared with WT cells. FPKM values for a select list of genes involved in lamellar body formation were loaded into Qlucore Omics Explorer. Genes that significantly contribute to sample variance (q ≤ 0.05) are shown.
(F) Illustration of the mechanism of action of the metabolic sialylation inhibitor Ac5SiaFEtoc. The inhibitor passively diffuses into the cell, where it is deacetylated by cytosolic esterases and subsequently outcompetes endogenous Neu5Ac for CMP activation by CMAS. CMP-SiaFEtoc is transported to the Golgi and directly inhibits the sialyltransferase isoenzymes, completely blocking de novo sialylation
(G) Flow cytometry of N/TERT-1 cells grown in the presence of 1-μM Ac5SiaFEtoc or vehicle control for 48 h. Cells were fixed and stained for sialic acids using SiaFind Pan-Specific Lectenz.
(H) Organotypic skin cultures were treated with 1-μM Ac5SiaFEtoc or vehicle control. HE staining and immunofluorescent labeling were performed with differentiation markers K10 and INV (n = 3).
(I) TEM of organotypic cultures with N/TERT-1 WT and MGAT1KO, showing lamellar-body-like structures accumulated in the vesicles of KO cells compared with WT cells (scale bars: left panels 40 μm; top middle panel 1 μm; bottom middle panel 5 μm; right panels 500 nm). The insert shows a lamellar body in WT cells and lamellar-body-like structure in MGAT1KO (asterisk) (n = 3).
(J) N/TERT-1 WT, MGAT1KO, and MGAT5KO cells grown in monolayers were stained for ER (calnexin), Golgi and trans-Golgi network (GalNAc-T2 and TGN46), early endosomes (EEA1), and lysosomes (LAMP2). Scale bar represents 10 μm. Technical replicates = 5, biological replicates = 2.
(K) Western blot of filaggrin in WT, MGAT1KO, and MGAT5KO cells. Pro-filaggrin is proteolytically processed in lamellar bodies to yield individual filaggrin monomers. Technical replicates = 2.
Complex-type and Branching N-Glycans Play Roles in Migration and Wound Healing
Given the large body of evidence that most cell-cell and cell-matrix adhesion proteins are modified with N-linked glycans (Ohtsubo and Marth, 2006), we tested the effects of a global loss of complex N-glycans on cell-cell and cell-matrix interactions. Under homeostasis, MGAT1KO tissues exhibited limited changes in early differentiation, with apparent normal cell-cell interactions (Figure 3C). To further analyze the effects of complex N-glycans on cell-cell interactions, we performed cell-cell dissociation studies (Figure 4A). In both MGAT1KO and MGAT5KO, we did not find any effect on cell-cell dissociation, confirming that the mere presence of an N-glycan is sufficient to preserve cadherin and desmoglein function. In addition, TEM revealed normal desmosome formation in MGAT1KO tissues (Figure 4C, right panels) and normal expression of E-cadherin and desmoglein-1 (Figures 4B and 4C, left panels). Though cadherins and desmogleins are essential for cell-cell interactions under homeostasis, dynamic changes in cell-matrix interactions are particularly important for regenerative processes observed during wound healing. Therefore, we investigated the capacity of MGAT1KO and MGAT5KO keratinocytes to heal tissues after wounding (Figure 4D). MGAT1KO keratinocytes exhibited a decreased capacity to heal, including diminished migratory capacity and loss of proper tissue polarity (Figures 4D and 4E). In contrast, MGAT5KO exhibited an increased migratory capacity and appropriate tissue orientation (Figures 4D and 4E). A potential explanation for dysregulated keratinocyte behavior during wound healing could be the influence of complex N-linked glycans on the functions of integrins, which are known to be heavily N-glycosylated and important for cell-matrix interactions (Cai et al., 2017; Gu and Taniguchi, 2004; Ohtsubo and Marth, 2006). Therefore, we examined the adhesion to extracellular matrix components for WT, MGAT1KO, and MGAT5KO cells. A significant decrease in adhesion to fibronectin and laminin was observed with MGAT1KO cells, whereas no significant differences were observed for MGAT5KO (Figure 4F). In accordance with the diminished binding of MGAT1KO cells to fibronectin, we found an absence of α5 integrin surface expression and a slight decrease in β1 integrin expression in MGAT1KO cells (Figure 4G). No major change in the surface expression of the remaining integrins was observed in MGAT1KO or MGAT5KO cells (Figure 4G). α5 integrin is upregulated during wound healing (Longmate and Dipersio, 2014), and the loss of surface expression of α5 integrin in MGAT1KO cells was further verified in the tissue-wound model (Figure 4H). Here, α5 integrin accumulated inside cells localized in the front of the MGAT1KO wound (Figure 4H). In contrast, α5 integrin was expressed normally in the basal cells of both WT and MGAT5KO tissues, though MGAT5KO tissues exhibited diminished α5 integrin deposition on the basal membrane (Figure 4H). To further test the effect of N-linked glycans on integrin activation during migration, we evaluated the amount of activated β1 integrin at the leading-edge lamellipodia in keratinocytes (Choma et al., 2004) (Figure 4I). We took advantage of the 9EG7 monoclonal antibody, which binds the open conformation of β1 integrin (Byron et al., 2009). As expected, based on the compromised surface expression of α5 integrin, MGAT1 KO cells exhibited a decrease in active β1 integrin complexes at the leading edge compared with WT keratinocytes (Figure 4I). In contrast, MGAT5KO and B4GALT5 KO cells exhibited a slight tendency for hyperactivation of integrin β1, as demonstrated by a marginal increase in the amount of active integrin β1 clusters on the leading edge (Figure 4I). However, the lack of complex N-glycans did not seem to interfere with establishing a polarized migratory phenotype in epithelial cells. A possible explanation for the N-glycan influence on β1-integrin activation could be indirectly through EGF-R, as suggested previously (Chernyavsky et al., 2004; Egles et al., 2010; Sung et al., 1998). Therefore, we assayed the expression of activated EGF-R in the different N-glycan KO cells. We did not find decreased pEGF-R activation in MGAT1KO cells (Figure 4J), but we observed an increase in EGF-R activation in MGAT5KO cells (Figure 4J), possibly explaining their increased migratory capacity (Figures 4D and 4E).
Figure 4.
Complex N-Glycans Have Roles in Migration and Wound Healing
(A) Cell dissociation studies investigating cell-cell adhesion in WT versus MGAT1KO, MGAT5KO, and MGAT4AKO cells, in which the number of dissociated fragments upon mechanical stress is shown (n = 3). Columns depict mean ± SEM. Significance was calculated using one-way ANOVA followed by multiple comparison analysis. NS, non-significant.
(B) Immunofluorescent labeling of adhesion markers E-cadherin (E-CAD), CD44, and desmoglein-1 in 2D grown cells. Scale bar represents 10 μm (n = 5).
(C) Left: Immunofluorescent labeling of adhesion markers E-CAD (co-labeled with pan-keratin [PanK]) and desmoglein-1 in 3D organotypic tissues. Scale bar represents 50 μm (n = 4). Right: TEM with desmosomes marked with asterisks (scale bars: top left panel 2 μm; top right panel 1 μm; bottom left panel 1 μm). Diagram shows the mean number of desmosomes ± SEM (3 micrographs evaluated for each clone). NS, non-significant; analyzed using one-way ANOVA followed by multiple comparison analysis.
(D) Wound healing in 3D organotypic tissues. Organotypic cultures were wounded by incision (vertical row) and growth continued for 24 h (n = 2). Scale bar represents 50 μm.
(E) Scratch assay of mono-cell layers of N/TERT-1 and quantification of the percentage of closure at respective timepoints relative to WT. Data are presented as mean ± SEM. ∗p < 0.05 using one-way ANOVA followed by multiple comparison analysis (n = 3).
(F) Cell adhesion to different matrix proteins (n = 4 for each genotype). Data are presented as mean ± SEM. ∗∗p < 0.01, ∗p = 0.05 using one-way ANOVA followed by multiple comparison analysis.
(G) N/TERT-1 WT, MGAT1KO, and MGAT5KO cells grown in monolayers were stained for α3 integrin, α5 integrin, α6 integrin, β1 integrin, and β4 integrin. Right, double labeling performed with α5 integrin and surface marker CD44. Scale bar represents 10 μm (Technical replicates = 3, biological replicates = 2).
(H) Immunofluorescence of wounded WT, MGAT1KO, and MGAT5KO tissues for the expression of α5 integrin. Scale bar represents 50 μm. Inserts show magnification of selected areas (Technical replicates = 4).
(I) Immunofluorescence of the active β1 integrin conformation in WT, MGAT1KO, MGAT5KO, and B4GALT5KO cells (red asterisks). The mAb (9EG7) is specific to the active conformation of β1 integrin (n = 3).
(J) Immunofluorescence of WT, MGAT1KO, and MGAT5KO cells stained for active phosphorylated EGF receptor (pEGF-R). Increased pEGF-R staining is seen in MGAT5KO cells compared with WT and MGAT1KO. Scale bar represents 10 μm. Quantification of pEGF-R on the cell membrane using the Photoshop quantification tool on >20 membranes per clone. Data are presented as mean ± SEM. ∗∗∗∗p < 0.0001 using one-way ANOVA followed by multiple comparison analysis.
Elongated Mucin-type O-GalNAc Glycans Are Required for Cell-Cell and Cell-Matrix Adhesion
The most abundant type of O-glycans is the mucin-type (GalNAc-type) O-glycans. To explore the biological significance of O-GalNAc glycans in epithelial homeostasis, we developed N/TERT-1 cells with abrogated elongation of the O-glycosylation capacity by targeting the core-1 synthase gene (C1GALT1) or its obligate chaperone COSMC (C1GALT1C1) (Ju and Cummings, 2010). In addition, we targeted the core-2 branching synthase (GCNT1), which is the only core-2 isoenzyme expressed in N/TERT-1 cells (Figure 5A). Mucin-type O-glycans undergo characteristic changes during cell differentiation and maturation in stratified squamous epithelia (Mandel et al., 1991). Immunolabeling with mAbs and lectins specific for O-glycans representing the initial biosynthetic steps confirmed that basal cells expressed sialyl-T O-glycans, whereas the branched core-2-based O-glycans were found in the suprabasal layers (Figure 5B). The expression of core-2-based structures was deduced from the observation of increased expression of non-sialylated core-1 structures in GCNT1KO tissue, which is consistent with the existence of core-2-based structures in the profile of O-glycans in the cell lines (Figure S3). Phenotypically, COSMCKO and G1GALT1KO tissues exhibited variable cell sizes in the basal cell layers, as well as delayed differentiation as visualized by K10 immunolabeling (Figures 5C and 5D). In agreement with our previous observations (Radhakrishnan et al., 2014), cell-cell fragmentation assays demonstrated compromised cell-cell adhesion in both COSMCKO and C1GALT1KO N/TERT-1 keratinocytes (Figure 5E), whereas no difference was found in GCNT1KO cells (Figure 5E). The compromised cell-cell association in C1GALT1KO N/TERT-1 keratinocytes was restored by inhibition of PKC (Figure 5E). To identify potential protein candidates involved in mediating cell-cell interactions, we performed an in-depth analysis of the O-glycoproteome of N/TERT-1 keratinocyte (Figures 5G and 5H; Table S3), which resulted in the identification of approximately 400 O-GalNAc glycoproteins, with a total of 1,500 O-glycosites (Table S3), including several proteins directly involved in cell-cell and cell-matrix adhesions (Figure 5H; Table S3) (Ponta et al., 2003) (King et al., 2017). The RNA-seq results for COSMCKO and C1GALT1KO keratinocytes showed downregulation of differentiation markers and upregulation of several markers of cellular stress (Figures S5E and S5G; Table S2), consistent with the observed phenotypes.
Figure 5.
O-glycans Are Required for Normal Epidermal Differentiation
(A) O-GalNAc glycan biosynthesis. Glycan symbols were drawn according to the SNFG format (Varki et al., 2015).
(B) Immunofluorescence of the main O-glycans in sections from normal human skin and N/TERT-1 organotypic cultures with KO of essential genes involved in O-glycan synthesis: C1GALT1, C1GALT1C1, and GCNT1. Technical replicates = 4, biological replicates = 2. Scale bar represents 50 μm.
(C) Histological sections illustrate the overall tissue organization, as well as the expression of proliferation marker Ki67 and differentiation markers K10, and INV Scale bar represents 50 μm (n = 3).
(D) Number of K10-negative layers and the total number of cell layers. Three micrographs were analyzed for each genotype. Data are presented as mean ± SEM. ∗p < 0.05 using one-way ANOVA followed by multiple comparison analysis (n = 4).
(E) Cell dissociation studies investigating cell-cell adhesion in COSMCKO and C1GALT1KO cells. Treatment of C1GALT1KO cells with a PKC inhibitor restored the cell-cell adhesion capacity (n = 3). ∗∗∗∗p < 0.001 using one-way ANOVA followed by multiple comparison analysis.
(F) Common downregulated transcripts identified in grouped analysis of N/TERT-1 WT, C1GALT1KO, and COSMCKO (q ≤ 0.05). Transcripts downregulated at least 1.5-fold in both C1GALT1KO and COSMCKO and contributing to protein interaction networks (Figure S5E) are shown. Red indicates higher expression, and blue indicates lower expression.
(G) Glycoproteomics workflow.
(H) Glycoproteomic analysis shows O-glycosite positions on a selection of adhesion proteins.
Notch Glycosylation Impacts Terminal Differentiation of Human Keratinocytes
The glycosyltransferase POFUT1, which initiates O-fucosylation, and POGLUT1, which initiates O-glucosylation (Figure 6A) (Takeuchi and Haltiwanger, 2014), possess rather specific substrate specificities and primarily glycosylate Notch receptors that are important for signal transduction in human skin formation (Demehri et al., 2009; Dotto, 2009). To investigate the impact of O-fucosylation and O-glucosylation on tissue formation, we knocked out the POFUT1 and POGLUT1 genes, as well as the NOTCH1 receptor and one of its primary ligands, JAG1 (Figure 6B). Upon NOTCH activation, the cleaved intracellular domain of Notch1 (NICD) is translocated to the nucleus. As predicted, we found no nuclear localization of NICD in NOTCH1KO tissue (Figure 6C). Moreover, in POFUT1KO and POGLUT1KO tissues, we found an absence or a significant decrease in the number of NICD-positive cells (Figure 6C), which was confirmed by western blot of lysates from keratinocytes grown in monolayers (Figure 6G). Phenotypically, POFUT1KO and POGLUT1KO tissues exhibited delayed differentiation, with an increased number of K10-negative cells at the basal layer, and a reduced number of differentiated K10-positive cell layers (Figure 6D). In POFUT1KO tissue, the induction of terminal differentiation was abrupt, with almost complete elimination of the spinous layer (Figures 6B and 6C). The morphological result was a marked decrease in epidermal thickness compared with WT, as well as NOTCH1KO and POGLUT1KO, tissues (Figure 6C). In contrast, a similarly abrupt shift to terminal differentiation was not observed in POGLUT1KO tissue (Figure 6D), and cell proliferation was observed throughout the skin (Figure 6C). Furthermore, evaluation of ΔNp63 expression demonstrated differences between POFUT1KO and POGLUT1KO tissues. ΔNp63 is present in proliferative keratinocytes and is involved in skin development and stem cell and progenitor cell regulation (Crum and McKeon, 2010). In POFUT1KO tissue, ΔNp63 was primarily present in the basal 3–4 cell layers, whereas the distribution was broader and expanded to 6–8 cell layers in POGLUT1KO tissue (Figure 6E). We also evaluated the phenotype of JAG1KO tissue based on the known modulation of NOTCH1-JAG1 interactions by O-fucosylation and O-glucosylation (Jafar-Nejad et al., 2010). Interestingly, the JAG1KO tissue had an almost complete loss of the stratum spinosum and a distribution of ΔNp63 similar to POFUT1KO tissue (Figures 6B–6D). Therefore, we show that a lack of POFUT1 and POGLUT1 differentially impacts differentiation in the human epidermis. This differential effect on the cellular senescence programs in human skin cells was confirmed by RNA-seq in POFUT1KO and POGLUT1KO cells (Figures 6F and S5F; Table S2). Consistent with the broad importance of Pofut1 in Notch signaling, we found a downregulation of genes involved in cellular senescence in POFUT1KO cells and corresponding differentiation markers (Figure 6F). In contrast, lack of POGLUT1 had a more selective effect on cell-cycle regulators and intracellular signaling components involved in cellular senescence, possibly through ΔNp63 signaling (Figure 6F; Table S2).
Figure 6.
Notch Glycosylation Impacts Epidermal Differentiation
(A) Schematic model of the Notch pathway. POGLUT1KO lacks O-Glc on EGF-like repeats, POFUT1KO lacks O-Fuc on EGF-like repeats, JAG1KO lacks the main ligand (Jagged-1) for Notch receptors, and NOTCH1KO lacks the Notch1 receptor. Drawing adapted from Haltiwanger et al. (2015).
(B) Micrographs illustrating HE staining and immunolabeled tissue sections of organotypic cultures. The expression of differentiation markers K10 and INV is shown. Scale bar represents 50 μm (n = 3).
(C) Immunohistochemical staining of tissue sections from N/TERT-1 WT and POFUT1KO, POGLUT1KO, NOTCH1KO, and JAG1KO tissues stained with antibodies recognizing cleaved (activated) Notch intracellular domains, p63, and the proliferation marker Ki67. Scale bar represents 50 μm (n = 3).
(D) Diagrams showing the number of cell layers negative for K10 (left panel) or involucrin (IVL, right panel) and the total number of cell layers. The columns depict mean ± SEM. ∗∗p < 0.01, ∗∗∗p = 0.0001 using one-way ANOVA followed by multiple comparison analysis for WT versus KO cells (n = 4).
(E) Number of p63-positive cells for each cell layer in POFUT1KO and POGLUT1KO tissues (n = 4).
(F) Differential expression of cellular senescence and differentiation genes, as evaluated by RNA-seq, of conventionally grown WT, POFUTKO, and POGLUT1KO cells. FPKM values for a select list of genes involved in cellular senescence (left panel) or cellular differentiation (right panel) were loaded into Qlucore Omics Explorer. Genes that significantly contribute to sample variance (q ≤ 0.05) are shown. Red indicates higher expression, and blue indicates lower expression.
(G) Western blot of N/TERT-1 WT, POFUT1KO, and POGLUT1KO cells with an antibody against NICD (cleaved Notch 1). EGTA induces ligand-independent Notch1 activation and is used as a positive control, whereas γ-secretase inhibitor DAPT blocks Notch signaling. DMSO is used as a control. The downstream target of Notch1, HES1, is included (n = 2).
(H) O-fucose and O-glucose glycosylation of Notch differentially affects keratinocyte differentiation. In the interfollicular epidermis, the reciprocal signaling between Notch and p63 contribute to the balance between self-renewing and proliferating keratinocytes at various stages of differentiation. Notch 1–4 activity suppresses the expression of p63 and vice versa, controlling the expression of HES1, CDKN1A (p21), and WNT4 differentiation, counteracting the effects of Notch on their expression. Our data suggest that both Pofut1 and Poglut1 differentially regulate the dynamic equilibrium between putative stem cells and cells committed to terminal differentiation. Figure adapted from Dotto (2009).
Discussion
Here, we used the N/TERT-1 3D organotypic skin model of fully differentiated human skin to perform strategic engineering of early non-redundant biosynthetic steps in distinct glycosylation pathways. We demonstrate that the formation of a normal stratified squamous epithelium requires the capacity for biosynthesis of all tested types of lipid and protein glycosylation.
We found that GSLs are important for the integrity of the basal skin compartment and barrier formation, and that these effects are solely due to the direct influence on the epithelium. The main effect of GSLs on skin permeability was attributed previously to unique epidermal ceramides with long-chain acyl moieties that function as key components of the extracellular lipid lamellae binding cornified envelope proteins in the suprabasal compartments of the skin (Amen et al., 2013). In skin biology, most previous murine studies have used conditional targeting of UGCG. Our observation of multiple apoptotic cells in UGCGKO tissues suggests that targeting UGCG could be problematic. In contrast, the phenotypes seen in B4GALT5KO and ST3GAL5KO skin were less severe and, importantly, almost identical. In this context, it is essential to note that UGCG KO abrogates all GlcCer-based GSLs (Stolfa et al., 2016), resulting in the accumulation and imbalance of ceramide metabolites known to induce apoptosis (Uchida et al., 2002). When targeting UGCG, it is difficult to translate the findings from murine studies into molecular functions of the more elaborate GSL species. Our strategy circumvents this problem and demonstrates how specific elongated GSL species are essential for the confinement of IGF-1 and EGF signaling to the basal skin compartment. These findings are in line with previous studies demonstrating that GM3 plays an inhibitory role in IGF-1R and EGF-R signaling (Bremer et al., 1986; Dam et al., 2017) and suggest that a lack of elongated GSLs indirectly affects cell-cell adhesion and proliferation (Dahlgaard et al., 2012; Getsios et al., 2009; Simpson et al., 2011).
Furthermore, we demonstrated that keratinocytes devoid of complex-type N-glycans (MGAT1KO) exhibit an abnormal, vacuolar phenotype with impaired terminal differentiation (Figure 3C) and intracellular accumulation of structures resembling lamellar bodies. Lamellar bodies are essential for the transport of components required for the terminal differentiation of keratinocytes and in the formation of the cornified envelope (Elias and Wakefield, 2014). The accumulation of lamellar bodies suggests that complex N-glycans are required for specialized transport in keratinocytes, with a deficiency leading to a secretory blockade. Similar vesicular bodies were identified previously in surfactant-producing type II pneumocytes from mice deficient in the two mannosidases functioning prior to MGAT2 (Akama et al., 2006), indicating that complex N-glycans are required for specialized transport in several cell types sharing the requirement of packaging large amounts of lipidified material. To further define the glycan structures involved in the observed phenotype, we combined our genetic dissection with the use of Ac5SiaFEtoc, a small molecule inhibitor of sialylation (Heise et al., 2019). We demonstrated that the phenotype is mediated specifically by sialylated complex N-glycans, providing a powerful example of how the genetic KO strategy can be combined with chemical inhibitors to further dissect the importance of defined glycan structures.
Cell-cell and cell-matrix adhesion are paramount to skin formation (Burgeson and Christiano, 1997; Simpson et al., 2011; Watt and Jones, 1993), and several studies have suggested that N-linked glycosylation is essential for the function of E-cadherin, P-cadherin, desmogleins, and integrins (Hang et al., 2016; Jin and Chung, 2018; Pinho and Reis, 2015). The preservation of cell-cell adhesion between MGAT1KO keratinocytes was therefore a surprise, but in accordance with the limited effects on general skin formation observed in MGAT1KO tissues, and suggests that cell-cell adhesion is largely unaffected by complete elimination of complex N-glycans. An explanation for this difference between our results and previous reports, could be the use of site-directed mutagenesis to eliminate N-glycans entirely in most of the previous studies. This complete loss of an N-glycan may affect the folding and stability of proteins (Carvalho et al., 2016; Zhao et al., 2008). In contrast, by maintaining the initial steps in N-glycosylation and only targeting later maturation steps in N-glycan biosynthesis, major folding and stability effects are not expected.
We next investigated the influence of N-glycans on wound healing. We found that wound healing was severely delayed and the migratory behavior of MGAT1KO cells impaired with disrupted surface presentation of α5 integrin, an adhesin upregulated in the migratory front of wound closure. α5 integrin is largely absent from resting epidermis and expressed de novo in wounded epidermis, where it is thought to assist in epidermal migration over fibronectin in the provisional matrix of the wound (Longmate and Dipersio, 2014). Thus, the failure of MGAT1KO cells to present and incorporate α5 integrin into the provisional basement membrane could explain the impaired cell migration of MGAT1KO cells. However, we cannot exclude an effect on other surface receptors and matrix proteins involved in migration and wound closure.
Dissecting GalNAc-type O-glycosylation in the N/TERT-1 model confirmed that complete loss of the elaborated O-glycans (C1GALT1KO and COSMCKO) causes delayed differentiation and cell-cell interactions in a PKC-dependent manner, whereas loss of only branched core-2 O-glycans (GCNT1KO) affected suprabasal differentiation to a minor degree and did not affect cell-cell interactions. Our findings confirm that core-1 O-glycans are primarily expressed in basal keratinocytes and essential for endogenous epithelial functions, including cell-cell adhesion, and that core-2 structures not are essential for cell-cell adhesion of basal keratinocytes. Instead, the core-2 structures were predominantly expressed in suprabasal cell layers, where they could be speculated to serve non-endogenous functions, such as in interactions with immune cells.
Another type of O-glycosylation pathway mediates the glycosylation of Notch proteins, a family of cell-surface receptors that regulate differentiation, especially the transition from basal (continual division) to suprabasal (terminal differentiation) cell fates, in human skin. In agreement with Notch receptors influencing skin differentiation (Blanpain et al., 2006; Nicolas et al., 2003; Williams et al., 2011), we found that NOTCH1 KO in human skin causes a relatively mild phenotype with delayed differentiation at the basal layer and impaired terminal keratinization. Notch receptors are regulated by multiple types of O-glycosylation (Figure 6A) (Harvey and Haltiwanger, 2018). O-Fucosylation directed by Pofut1 is important for folding Notch and increases its ligand affinity (Luca et al., 2017), whereas O-glucosylation directed by Poglut1 increases Notch activation through upregulation of membrane-adjacent intracellular Notch cleavage (Fernandez-Valdivia et al., 2011; Okajima and Irvine, 2002; Takeuchi et al., 2017). In contrast to the mild phenotype observed in NOTCH1KO tissue, elimination of POFUT1 causes a severe and complex defect in regulating the differentiation program, with delayed differentiation of the basal cells, complete ablation of the spinous layer, and premature differentiation of the apical cell layers, resulting in a markedly reduced epidermal thickness (Basmanav et al., 2014; Li et al., 2013). Skin lacking Jagged-1 corresponds well with the POFUT1KO phenotype, which is consistent with previous findings that Notch signaling is required for spinous transition (Blanpain et al., 2006). The results suggest the primary function of Pofut1 in human skin could be to facilitate binding of Jagged-1, which serves as the main ligand across all classes of Notch receptors, and are in accordance with previous studies showing that Pofut1 regulates Notch-2 function in human keratinocytes (Li et al., 2013). In comparison with the phenotype observed in POFUT KO tissue, we found a different differentiation pattern in POGLUT1KO tissue underscoring the importance of both Pofut1 and Poglut1 in the differentiation of human epidermis.
In conclusion, the developed strategy offers a powerful genetic approach, potentially combined with small compound inhibitors, to unravel specific functions of complex metabolic networks, such as cellular glycosylation. The strategy eliminates the problems that often occur with interference from immune components, the microbiome, or other elements that can make it difficult to determine the molecular nature of phenotypes observed in whole animals despite tissue-specific ablation of select genes. We believe that the model may be useful for screening a broad range of glycan functions in tissue formation and regeneration, for stem cell biology, and for screening environmental factors, including microbial adhesion and infection.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| mouse anti-Ki-67 (IF 1:100) | Dako (Agilent) | Cat# M7240; RRID:AB_2142367 |
| mouse anti-Cytokeratin 10 (IF 1:100; WB 1:250) | Dako (Agilent) | Cat# M7002 |
| rabbit anti-Cytokeratin | Dako (Agilent) | Cat# Z0622; RRID:AB_2650434 |
| mouse anti-Involucrin, clone SY5 (WB 1:100) | Thermo Fisher | Cat# MA5-11803; RRID:AB_10982738 |
| rabbit anti-Involucrin (IF 1:200) | Thermo Fisher | Cat# PA1-37934; RRID:AB_2265014 |
| goat anti-Desmoglein-1 | R&D Systems | Cat# AF944; RRID:AB_2277393 |
| rabbit anti-Desmocollin-2 | Proteintech | Cat# 13876-1-AP |
| goat anti-E-cadherin (IF 1:200) | R&D Systems | Cat# AF648; RRID:AB_355504 |
| mouse anti-human CD44s, clone 2C5 | R&D Systems | Cat# BBA10; RRID:AB_356933 |
| mouse anti-Integrin alpha 3/CD49c, clone IA3 (IF 1:20) | R&D Systems | Cat# MAB1345; RRID:AB_2129765 |
| rabbit anti-Integrin alpha 5 (IF 1:100) | Abcam | Cat# ab150361; RRID:AB_2631309 |
| mouse anti-Integrin alpha 6, clone mp4f10 (IF 1:50) | R&D Systems | Cat# MAB1350; RRID:AB_2249284 |
| mouse mAb to integrin Beta 1 (P5D2) (IF 1:100) | Abcam | Cat# ab24693; RRID:AB_448230 |
| rat anti-CD29, clone 9EG7 (IF 1:200) | BD Biosciences | Cat# 553715; RRID:AB_395001 |
| rat anti-Integrin Beta 4 | LSBio | Cat# LS-B3778-100; RRID:AB_10659338 |
| rabbit anti-Calnexin (IF 1:100) | Abcam | Cat# ab75801; RRID:AB_1310022 |
| rabbit anti-TGN46 (IF 1:100) | Abcam | Cat# ab50595; RRID:AB_2203289 |
| rat anti-LAMP2 [GL2A7] | Abcam | Cat# ab13524; RRID:AB_2134736 |
| rabbit anti-EEA1, clone C45B10 (IF 1:100) | CST | Cat# 3288; RRID:AB_2096811 |
| rabbit anti-Collagen IV | LSBio | Cat# LS-B2717-50; RRID:AB_1812160 |
| mouse anti-Cyclin A (IF 1:100), | Novocastra (Leica Biosystems) | Cat# NCL-CYCLIN A; RRID:AB_563675 |
| rabbit anti-cleaved Notch1 (Val1744) (D3B8) (IF 1:100, WB 1:1000) | CST | Cat# 4147; RRID:AB_2153348 |
| rabbit anti-Hes1 (WB 1:1000) | Abcam | Cat# ab71559; RRID:AB_1209570 |
| mouse anti-Filaggrin, clone 15c10 (WB 1:1000) | Novocastra (Leica Biosystems_ | Cat# NCL-FILAGGRIN; RRID:AB_563731 |
| rabbit anti-alpha tubulin (WB 1:2000) | Abcam | Cat# ab4074; RRID:AB_2288001 |
| rabbit anti-phospho-IGF-I receptor beta (Tyr1135/1136)/Insulin receptor beta (Tyr1150/1151) (19H7) (1:100) | CST | Cat# 3024; RRID:AB_331253 |
| mouse anti-phospho-EGF receptor (Tyr1068) (1H12) (1:100) | CST | Cat# 2236; RRID:AB_331792 |
| mouse anti-human p63 (1:100) | Dako (Agilent) | Cat# M731729-2 |
| mouse anti-Tn mAb, clone 5F4 | our laboratory | N/A |
| mouse anti-STn mAb, clone 3F1 | our laboratory | N/A |
| mouse anti-GalNAc-T2 mAb, clone 4C4 | our laboratory | N/A |
| Biotinylated Maackia amurensis lectin II (MAL II) antibody | Vector Laboratories | Cat# B-1265; RRID:AB_2336569 |
| Biotinylated Peanut agglutinin (PNA) antibody | Vector Laboratories | Cat# B-1075; RRID:AB_2313597 |
| goat anti-mouse IgG (H+L) Antibody, Alexa Fluor™ 488 Conjugated (IF1:500) | Molecular Probes | Cat# A-11029; RRID:AB_138404 |
| goat anti-rat IgG (H+L) Antibody, Alexa Fluor™ 488 Conjugated (IF1:500) | Molecular Probes | Cat# A-11006; RRID:AB_141373 |
| goat anti-rat IgG, Alexa FluorR 488 conjugated (IF 1:1500) | Abcam | Cat# ab150157; RRID:AB_2722511 |
| rabbit anti-mouse IgG (H+L), Alexa Fluor™ 488 Conjugated (IF 1:500) | Molecular Probes | Cat# A-11059; RRID:AB_142495 |
| goat anti-rabbit IgG (H+L) Antibody, Alexa Fluor™ 488 Conjugated (IF1:500) | Molecular Probes | Cat# A-11008; RRID:AB_143165 |
| donkey anti-goat IgG (H+L) Antibody, Alexa Fluor™ 488 Conjugated (IF1:500) | Molecular Probes | Cat# A-11055; RRID:AB_2534102 |
| streptavidin-conjugated Alexa Fluor 488 (IF 1:1000) | Thermo Fisher | Cat# S11223 |
| goat anti-mouse IgG (H+L) Antibody, Alexa Fluor™ 594 Conjugated (IF1:500) | Molecular Probes | Cat# A-11005; RRID:AB_141372 |
| goat anti-rabbit IgG (H+L) Antibody, Alexa Fluor™ 594 Conjugated (IF1:500) | Molecular Probes | Cat# A-11012; RRID:AB_141359 |
| rabbit anti-mouse immunoglobulins/HRP (WB 1:4000) | Dako (Agilent) | Cat# P0260; RRID:AB_2636929 |
| goat anti-rabbit immunoglobulins/HRP (WB 1:4000) | Dako (Agilent) | Cat# P0448; RRID:AB_2617138 |
| Bacterial and Virus Strains | ||
| One Shot™ Stbl3™ Chemically Competent E. coli | Thermo Fisher | Cat# C7373-03 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| DAPT | CST | Cat# 15020 |
| EGTA | Sigma | Cat# CAS 67-42-5 |
| Methyl (3-(R)-Fluoro-5-N-ethyloxycarbonyl-2,4,7,8,9-penta-O-acetyl-3,5-dideoxy-D-α/β-glycero-D-galacto-2-nonulopyranose)onate(Ac5SiaFEtoc) | Thomas J. Boltje, Cluster for Molecular Chemistry, Institute for Molecules and Materials, Radboud University Nijmegen, The Netherlands | N/A |
| Lucifer Yellow CH dilithium salt (1:100) | Sigma | Cat# L0259 |
| EZ-Link™ Sulfo-NHS-LC-Biotin | Thermo FIsher | Cat# 21335 |
| Collagen I | Made in lab | N/A |
| cOmplete™, Mini, EDTA-free Protease Inhibitor Cocktail | Roche | Cat# 11836170001 |
| Critical Commercial Assays | ||
| UltraVision™ Quanto Detection System HRP | Thermo Fisher Scientific | Cat# TL_125_QHL |
| CytoSelect™ 48-well Cell Adhesion Assay | Cell Biolabs | Cat# CBA-070, |
| RNeasy®kit | Qiagen | Cat# 75144 |
| High pH Reverse-Phase Fractionation Kit | Thermo Fisher | Cat# 84868 |
| Macherey-Nagel™ NucleoBond™ Xtra Midi EF | Thermo Fisher | Cat# 12798412 |
| SiaFind™ Pan-Specific Lectenz®, Biotinylated Kit | Lectenz Bio | Cat# SK0501B |
| LIVE/DEAD™ Fixable Violet Dead Cell Stain Kit | Thermo Fisher | Cat# L34955 |
| Pierce BCA Protein Assay Kit | Thermo Fisher | Cat# 23225 |
| Pierce™ ECL Western Blotting Substrate | Thermo Fisher | Cat# 32106 |
| SuperSignal™ West Pico PLUS Chemiluminescent Substrate | Thermo Fisher | Cat# 34580 |
| Deposited Data | ||
| RNA-seq data | NCBI, Genome Expression Omnibus | GSE148284 |
| MS data | Proteome Central/Proteome Exchange | PXD018418 |
| Experimental Models: Cell Lines | ||
| HEK293T | ATCC | Cat# CRL-3216; RRID:CVCL_0063 |
| MRC-5 | ATCC | Cat# CCL-171; RRID:CVCL_0440 |
| N/TERT-1 | James G. Rheinwald, Harvard Institute of Medicine | N/A |
| N/TERT-1 COSMC KO | This study | N/A |
| N/TERT-1 C1GALT1 KO | This study | N/A |
| N/TERT-1 GCNT1 OK | This study | N/A |
| N/TERT-1 MGAT1 KO | This study | N/A |
| N/TERT-1 MGAT4A KO | This study | N/A |
| N/TERT-1 MGAT4B KO | This study | N/A |
| N/TERT-1 MGAT5 KO | This study | N/A |
| N/TERT-1 POFUT1 KO | This study | N/A |
| N/TERT-1 POGLUT1 KO | This study | N/A |
| N/TERT-1 UGCG KO | This study | N/A |
| N/TERT-1 B4GALT5 KO | This study | N/A |
| N/TERT-1 ST3GAL5 KO | This study | N/A |
| N/TERT-1 JAG1 KO | This study | N/A |
| N/TERT-1 NOTCH1 KO | This study | N/A |
| Oligonucleotides | ||
| Sequences for the gRNAs are available in Table S1. | ||
| Recombinant DNA | ||
| psPAX2 | Addgene | RRID:Addgene_12260 |
| pCMV-VSV-G | Addgene | RRID:Addgene_8454 |
| lentiCRISPR v2 | Addgene | RRID:Addgene_52961 |
| Software and Algorithms | ||
| Adobe Illustrator (CS6) | Adobe | RRID: SCR_010279 |
| Adobe Photoshop (CS6) | Adobe | RRID: SCR_014199 |
| ZEN | Zeiss | RRID: SCR_013672 |
| GraphPad Prism version 8.4.0 | GraphPad | RRID:SCR_002798 |
| Peak Scanner Software V1.0 | Thermo Fischer | Cat#: 4381867 |
| STRING | STRING | RRID:SCR_005223 |
| Leica Application Suite v. 2.6.0 software | Leica Microsystems | N/A |
| Proteome Discoverer 1.4 software | Thermo Fisher | N/A |
| iTEM software | Olympus | https://www.emsis.eu/home/ |
| Qlucore Omics Explorer | Qlucore | https://www.qlucore.com/ |
| FlowLogic software | Inivai Technologies | N/A |
| R | RCore Team | www.R-project.org/ |
| Other | ||
| Zeiss LSM 710 Confocal Microscope | Zeiss | N/A |
| Olympus LH50A | Olympus | N/A |
| Bruker Autoflex MALDI-TOF | Bruker Daltonik GmbH | N/A |
| Philips CM100 Transmission Electron Microscope | Philips | N/A |
| SA3800 Spectral Analyzer | Sony | N/A |
| Orbitrap Fusion™ Tribrid™ Mass Spectrometer | Thermo Fisher | N/A |
| ABI PRISM™ 3010 Genetic Analyzer | Thermo Fisher | N/A |
| ImageQuant LAS 4000 | GE Healthcare | N/A |
Resource Availability
All cell lines are available on request under a standard MTA with University of Copenhagen for academic research purposes.
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Hans H. Wandall (hhw@sund.ku.dk).
Materials Availability
All cell lines are available on request under a standard MTA with the University of Copenhagen for academic research purposes.
Data and Code Availability
The accession number for the RNA-Seq data reported in this paper is NCBI, Genome Expression Omnibus: GSE148284. The accession number for the MS data reported in this paper is Proteome Central/Proteome Exchange: PXD018418.
Experimental Model and Subject Details
Cell Lines and Culture
N/TERT-1 immortalized human keratinocytes (male) were kindly provided by James G. Rheinwalds lab, Harvard Institute of Medicine, Brigham & Women’s Hospital. Cells were maintained in K-SFM (Gibco) supplemented with 25 μg/ml BPE (Gibco), 0.2 ng/ml EGF (Thermo Scientific), and 0.3 mmol/l CaCl2 (Sigma) at 37°C with 5% CO2 as previously described (Dickson et al., 2000). Cells were cultured until they reached ~30% confluence, at which time they were passaged by trypsinizing with TrypLE (Gibco). For experiments where cell were grown to complete confluence, medium was shifted to 1:1 vol/vol K-SFM/DF-K (DMEM/F12) supplemented with 25 μg/ml BPE, 0.2 ng/ml EGF, and 2 mM L-glutamine (Thermo Fisher). HEK293T cells were cultured in DMEM (Gibco) containing 10% FBS (HyClone) at 37°C with 5% CO2 and passaged at ~90% confluence.
N/TERT-1 glycogenes KO library was generated using CRISPR/Cas9 technology by targeting particular glycogene exons by validated gRNAs (Narimatsu et al., 2018) or gRNAs predicted by GPP (Doench et al., 2016). gRNAs were cloned (Ran et al., 2013) using oligos (TAGC, Denmark) into lentiCRISPR-v2-Puro plasmid backbone (Addgene #52961) or lentiCRISPR-v2-Blast with a blasticidin resistance gene replacing the puromycin resistance gene (Brakebusch laboratory, BRIC, UCPH, DK). Directional cloning and insertion of the gRNA duplex using BsmBI and T4 ligase into the LentiCRISPR-v2 plasmid backbone was done as described earlier (Sanjana et al., 2014). All plasmids were propagated in One Shot™ Stbl3™ Chemically Competent E. coli cells (Thermo Fisher). Endonuclease free plasmid preparations were made using Midi Prep kit (Thermo Fisher). For lentivirus production HEK293T cells were seeded at 1x105 cells/well density in a 6-well plate and grown for 72 h until 80-90% confluence. For transfection, 200 μL OPTI-MEM (Gibco), 8 μL of 1 mg/m PEI (Sigma), 0.8 μg LentiCRISPR-V2-gRNA plasmid, 0.6 μg pCMV-VSV-G plasmid (Addgene #8454), and 0.6 μg psPAX2 plasmid (Addgene #12260) were mixed and incubated for 10 min at RT before added to the adherent HEK293T cells. After 24 h the transfection medium was replaced by K-SFM for N/TERT-1 transduction. Medium containing viral particles was collected 48/72 h post-transfection, i.e. when virus had accumulated for 24 h after medium change, filtered (0.45 μm pore size). Filtered virus-containing medium was mixed 1:1 with fresh complete K-SFM and 1:1000 polybrene (Sigma), and used to transduce N/TERT-1 cells overnight (Ehrhardt et al., 2006). Selection of KO cell lines started 48-96 hours after transduction, with either 5 μg/ml blasticidin S (Gibco) or 1 μg/ml puromycin (Gibco) including biweekly cell passaging. Single clones were obtained by serial dilution in 96 well plates and KO clones were identified by IDAA using ABI PRISM™ 3010 Genetic Analyzer (Thermo Fisher) and Sanger sequencing (GATC, Germany). Two to five clones were selected for each gene with out of frame indel formation. IDAA results were analyzed using Peak Scanner Software V1.0 (Thermo Fisher) (Yang et al., 2015a). N/TERT-1 with silent mutations provided phenotypic control cell lines.
Organotypic Culture
Organotypic cultures were prepared as described previously (Dabelsteen et al., 2009). Briefly, human fibroblasts were suspended in acid-extracted Type I collagen (4 mg/ml), and allowed to polymerize over a 1-ml layer of acellular collagen in six-well culture inserts with 3-μm-pore polycarbonate filters (BD Biosciences NJ, USA). Gels were allowed to contract for 4–5 days before seeding with 3x105 N/TERT-1 keratinocytes in DMEM/F12 raft medium supplemented with 1.5% FCS (HyClone), 5 μg/ml insulin (Humulin, Eli Lilly), 0.1 nM choleratoxin (Sigma), 400 ng/ml hydrocortisone (Sigma), 0.02 nM triiodothyronine, and 0.18 mM adenine (Sigma). Inserts were raised to the air-liquid interface 4 days after cell seeding, and media was changed every second day for additional 10 days. At least 3 independent experiments at different time points with two different clones of each knockout cell line were conducted, resulting in organotypic cultures with similar morphologies. For experiments using the sialyltransferase inhibitor Ac5SiaFEtoc, culture medium was supplemented with 1 μM Ac5SiaFEtoc when the inserts were raised to the air-liquid interface, and the concentration of the inhibitor was replenished with subsequent medium changes. For wound healing studies, the organotypic cultures were divided in half on day 9 of the air-lift interface. One half was placed onto a new collagen gel with fibroblast and cultured for 24 hours before fixation in 4% PFA. Organotypic sections were prepared and stained histochemically as previously described (Dabelsteen et al., 2009).
Method Details
Immunofluorescent Labeling and Imaging
For immunofluorescent staining N/TERT-1 isogenic cells were grown either on sterile glass coverslips (Thermo Fisher) or seeded on diagnostic imaging printed slides (Clearcell; Histolab). Cells were fixed and permeabilized either in cold methanol/acetone (1:1), or in fresh 4% paraformaldehyde and 0.1% Triton X-100, followed by blocking in 2.5% BSA in PBS. Immunostaining was performed using primary antibodies listed in Key Resources Table with matching secondary Alexa Fluor-conjugated antibodies (Molecular Probes). Organotypic cultures were fixed for 2 h at 4°C in 10% neutral buffered formalin and paraffin embedded. 3-5μm sections were used for hematoxylin-eosin staining or immunohistochemistry. Heat-induced antigen retrieval was performed in Tris-EDTA (pH= 9.0) or Citrate buffer (pH=6.0) before applying primary antibodies. Immunohistochemistry was performed using fluorescently labeled secondary antibodies or Ultra Vision Quanto Detection System HRP (Thermo Fisher). For some antibodies (pEGFR, pIGFR) the SuperBoost Tyramide kit (Thermo Fisher) was used according to manufacturer’s protocol. After washing 3 times with PBS, sections or coverslips were incubated in 1 μg/ml DAPI (Sigma) for 5 min at RT, washed again 3 times with PBS, and mounted with ProLong Gold Antifade Reagent without DAPI (Thermo Fisher). For experiments using the sialyltransferase inhibitor, the culture medium was supplemented with 1 μM Ac5SiaFEtoc 24 hours after seeding of the cells on the coverslips and cells were cultured for 48 hours before fixation in 4% paraformaldehyde. Images were collected using LSM710 confocal microscope (Zeiss) with 405, 488 and 561 laser lines and either an oil 60x/1.4 DIC M27 or 20x/0.8 M27 objective.
Permeability Assay
To measure changes in barrier formation important for prevention of trans-epidermal water loss (TEWL), the inside-out epidermal barrier function was studied by turning the organotypic skin upside-down and applying 3.3 mg/ml EZ-link sulfo-NHS-LC-biotin (Thermo Fisher) to the bottom of the filters or Lucifer Yellow CH dilithium salt (1:100 dilution of 5% stock solution) (Sigma) on the top of the organotypic skin cultures for 60 min at RT. Excess probe was removed and organotypic skin was fixed in 4% formalin and prepared for histological processing as described previously (Dabelsteen et al., 2009; Smits et al., 2017; van den Bogaard et al., 2014). Deparaffinized sections were stained with Alexa Fluor 488 streptavidin (1:500) (Thermo Fisher) for detection of biotin. Sections were mounted with ProLong Gold Antifade with DAPI (Thermo Fisher).
Western Blotting
Cells were grown to confluence and lysed in modified RIPA buffer (50 mM Tris pH=7.5, 150 mM NaCl, 1% NP-40, 0.1% Na-deoxycholate, 1 mM EDTA) supplemented with protease inhibitors (Roche) and phosphatase inhibitors at 4°C for 30 min with agitation. For Notch1 activation assay, cells were treated with 50 μM DAPT for 24 h, 4 mM EGTA for 15 min or 1 μl/ml DMSO for 24h for untreated controls. Lysates were transferred to microcentrifuge tubes and sonicated using sonic probe (3 pulses for 5 sec, 40 % amplitude). Lysates were spun for 10 min at 20000 g at 4°C and supernatant was used for quantification of protein concentration using Pierce BCA Protein Assay Kit (Thermo Fisher). Protein concentration was adjusted with lysis buffer and samples were heated at 70°C for 10 min in a 1x dilution of NuPAGE LDS Sample Buffer (Thermo Fisher) and 10 mM DTT. Denatured cell lysates were separated by PAGE in 4-12 % Bis-Tris gradient gels (Thermo Fisher) along with SeeBlue Plus 2 Prestained Standard (Thermo Fisher) using NuPAGE MES SDS running buffer (Thermo Fisher) on ice at 200 V for 45 min. Proteins were transferred onto nitrocellulose membrane (Bio-RAD) using transfer buffer (20% methanol in NuPAGE MES SDS running buffer) on ice at 320 mA for 60 min. Blots were washed 3 x 5 min with 0.05% Tween-TBS at RT with agitation and blocked with 5% skim milk powder (Merck Millipore) in TBS-T for 1 h at RT with agitation. Blots were incubated in primary antibody in 5% skimmed milk in TBS-T overnight at 4°C with slow agitation. Blots were washed 3 x 5 min with TBS-T at RT with agitation and incubated in HRP-conjugated secondary antibody in 5 % skimmed milk in TBS-T for 45 min at RT with slow agitation. Blots were washed as described above and visualized using Pierce ECL Western Blotting Substrate (Thermo Fisher) or SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher), and ImageQuant LAS 4000 (GE Healthcare).
Flow Cytometry
Wild type N/TERT-1 cells were cultured in the presence of 1μM Ac5SiaFEtoc for 48 h. Cells were harvested using TrypLE (Gibco) and were washed in PBS before being stained for viability using LIVE/DEAD™ Fixable Violet Dead Cell Stain Kit (Invitrogen) according to manufacturer’s instructions. Cells were washed twice in 0.5% BSA in PBS and stained using SiaFind™ Pan-Specific Lectenz® (1:400) (Lectenz Bio) pre-complexed with streptavidin-conjugated Alexa Fluor 488 (1:1000) (Thermo Fisher) for 30 min on ice. The data was acquired on a SA3800 Sony Spectral Analyzer (Sony Biotechnology) and was analyzed using the FlowLogic software (Inivai Technologies).
Scratch Assay
N/TERT-1 wild type cells and N/TERT-1 knockout cells were seeded in a 24-well plate in triplicates and grown to confluence. Two linear scratches were performed in each well using a P1000 pipet tip, cells debris were removed by washing with PBS, and fresh media was added. Cells were grown for 72 h at 37°C in tissue culture incubator and images of representative parts of the scratches were recorded immediately after scratching, and after 12 h, 24 h, 48 h and 72 h with an Olympus LH50A microscope with a Leica DFC290 camera using Leica Application Suite v. 2.6.0 software (Leica Microsystems, Germany).
Dissociation Assay
N/TERT-1 wild type cells and N/TERT-1 knockout cells were grown to confluence and left confluent for 48 h. Cells were washed in HBSS (Gibco) and treated with 2.4 mg/ml dispase II (Roche) for 20 min at 37°C to release cells as monolayers. Dispase was removed gently and the detached monolayers were exposed to mechanical stress by shaking on an orbital shaker (500 rpm for 30 sec). The resulted number of fragments were counted (Radhakrishnan et al., 2014).
Cell-Matrix Adhesion Assay
Changes in binding to ECM components were measured using CytoSelect™ 48-well Cell Adhesion Assay (Cell Biolabs) according to manufacturer’s protocol. Briefly, keratinocytes were seeded in wells pre-coated with different ECM components. After 1 h at 37°C, non-adherent cells were removed and remaining adherent cells were stained and lysed for colorimetric quantification. Each knock out was setup in two technical replicates and results represent four independent experiments.
Transmission Electron Microscopy
TEM was performed as described previously (Radhakrishnan et al., 2014). The samples were fixed with 2% (vol/vol) glutaraldehyde in 0.05 M sodium phosphate buffer (pH=7.2), rinsed three times in 0.15 M sodium cacodylate buffer (pH=7.2), and subsequently post-fixed in 1% (wt/vol) OsO4 in 0.12 M sodium cacodylate buffer (pH=7.2) for 2 h. The specimens were dehydrated in a graded series of ethanol, transferred to propylene oxide, and embedded in Epon according to standard procedures. Sections (~80-nm-thick) were cut with a Reichert-Jung Ultracut E microtome and collected on one-hole copper grids with Formvar supporting membranes, stained with uranyl acetate and lead citrate, and subsequently examined with a Philips CM 100 transmission electron microscope (Philips, Eindhoven) operated at an accelerating voltage of 80 kV and equipped with an OSIS Veleta digital slow scan 2,000 × 2,000 CCD camera. Digital images were recorded using the iTEM software package.
RNA Isolation, RNA Sequencing and Network Analysis
Total RNA was isolated using RNeasy®kit (Qiagen). RNA quality and RNA integrity number (RIN) was determined using Bioanalyzer instrumentation (Agilent Technologies). RNA from N/TERT-1 WT and two clones of isogenic N/TERT-1 KO cell lines was sequenced on the BGISEQ-500 NGS platform (BGI, Hong Kong) with subsequent mapping of sequenced reads using the HISAT/Bowtie2 package for R. Gene expression levels were quantified using RNA sequencing library RNA-seq Quantification Library (Normal Library) was used and RNA was sequenced with Illumina HiSeq 4000 System (Illumina, USA) using paired-end sequencing. Clean reads were filtered and mapped to reference using HISAT/Bowtie2 Tool. Global, grouped and pairwise analyses between individual samples were performed using Qlucore Omics Explorer. Only transcripts with expression values above 1 FPKM in at least one of compared samples were considered. Student’s t-test and ANOVA were used to identify transcripts significantly contributing to sample variance for pairwise and grouped analyses, respectively. Benjamini & Hochberg procedure was used to control for false discovery (q ≤ 0.05). STRING resource was used to visualize some of the identified networks. For targeted analyses, defined lists of genes involved in experimentally identified pathways were pulled out and analyzed separately. The single cell RNAseq data were adopted from published scRNAseq dataset on skin biopsy specimens (He et al., 2020). Average expression profiles (transcripts per kilobase million (TPM)-like values) of different cell types (vascular endothelial cells, macrophages, sweat gland cells, melanocytes, keratinocytes and fibroblasts) from one heathy control subject (S9_H) were used. Twelve glycosyltransferase genes (B4GALT5, C1GALT1, C1GLAT1C1, GCNT1, MGAT1, MGAT4A, MGAT4B, MGAT5, POFUT1, POGLUT1, ST3GAL5 and UGCG) in this study were compared in different samples and cell types. The corrplot package was used to generate the correlation matrix among selected samples.
MS-Based Glycan Profiles
For O-GalNAc profiles, the Cellular O-Glycome Reporter/Amplification (CORA) method was used as described in (Kudelka et al., 2016). In brief, N/TERT-1 cells were seeded in 6-well plates at 5x104 cells/well density. When cells reached 70% confluence medium was supplemented with 100 μM Bn-α-GalNAc or DMSO for untreated controls. Media was harvested after 48 h and passed through a 10-kDa Omega membrane (Pall Corporation) followed by purification with a Sep-Pak 3-cc C18 cartridge (Waters). Samples were then concentrated by SpeedVac to remove organic solvent and lyophilized. For profiling of N-linked glycans, N/TERT-1 cell pellets (approximately 5x106 cells) were dissolved in 0.1% Rapigest (Waters) and incubated on ice and vortexed every 5 minutes. Samples were then centrifuged at 10000 g for 10 min and the supernatant was collected and reduced with 5 mM DTT (Sigma) for 1 h at 60°C followed by alkylation with iodoacetamide (Sigma) for 30 minutes at RT while kept dark. Samples were digested with 5 μg trypsin (Roche Diagnostics) overnight at 37°C and peptides were purified with a Sep-Pak 1-cc C18 cartridge (Waters). Organic solvent was removed by SpeedVac and pH was adjusted to 7-8 with 50 mM ammonium bicarbonate, followed by overnight digestion with 3 U PNGase F (Roche diagnostics) at 37°C. Released N-glycans were cleaned up on a Sep-Pak 1-cc C18 cartridge and lyophilized. For both O-GalNAc and N-glycan profiles, dried samples were permethylated as described previously (Yang et al., 2015b) and analysed by positive reflector mode MALDI-TOF (AutoFlex Speed, Bruker Daltonics) with data acquisition in the 1000-7000 m/z range.
O-Glycoproteome Analysis
10 mg protein of total cell lysate was prepared from N/TERT-1 WT cells in 0.1% Rapigest (Waters) in 50-mM ammonium bicarbonate. After reduction with 5 mM DTT (Sigma) for 1 h at 60°C and alkylation with iodoacetamide (Sigma) for 30 min at RT, the sample was split in three and digested overnight at 37°C with either trypsin, chymotrypsin or endoproteinase Glu-C (Roche). Samples were then acidified with trifluoroacetic acid and peptides were purified on C18 Sep-Pak (Waters). Each sample was then desialylated with 1 U neuraminidase (Clostridium perfringens (C. welchii), Sigma) in 50 mM sodium acetate (pH=5.0) for 5 h at 37°C. T and Tn glycopeptides and non-glycopeptides were separated using a long Jacalin-agarose lectin weak affinity chromatography (LWAC) as described in (Steentoft et al., 2011). LWAC elutions were cleaned up on StageTips containing C8 and C18 membranes (3M Science), pooled and fractionated using Pierce™ High pH Reverse-Phase Fractionation Kit (Thermo Fisher) according to manufacturer’s instructions and submitted for MS analysis. EASY-nLC 1000 UHPLC (Thermo Fisher) interfaced via PicoView nanoSpray ion source to an Orbitrap Fusion mass spectrometer (Thermo Scientific) was used for glycoproteomic studies. All MS1, HCD-MS2, and ETD-MS2 spectra were acquired in the Orbitrap sector. MS data analysis was performed using Proteome Discoverer 1.4 software, assisted by manual validation.
Quantification and Statistical Analysis
Graphs were produced using Prism (GraphPad). Bar charts show mean with error bars showing standard error and N numbers are indicated in the figure legends. Statistical significance was determined using one-way ANOVA test followed by multiple comparison analysis.
Acknowledgments
This work was supported by the European Commission (GlycoSkin H2020-ERC), European Commission (Imgene H2O20), Lundbeck Foundation, The Danish Research Councils (Sapere Aude Research Leader grant to H.H.W.), Danish National Research Foundation (DNRF107), the Friis Foundation, The Michelsen Foundation, A.P. Møller og Hustru Chastine Mc-Kinney Møllers Fond til Almene Formaal, Danish Strategic Research Council, Lundbeck Foundation, and the program of excellence from the University of Copenhagen (CDO2016). We want to thank Vladislav Panin for highly valuable discussions and critical review of the manuscript. We are also grateful for excellent technical assistance by Karin Uch Hansen. We acknowledge the Core Facility for Integrated Microscopy, Faculty of Health and Medical Sciences, University of Copenhagen. Finally, we would like to thank James G. Rheinwald, Harvard Institute of Medicine, for providing the N/TERT-1 cells.
Author Contributions
E.M.H.P., S.D., and H.H.W. conceived and designed the study; E.M.H.P., S.D., M.I.N., I.N.M., D.T.A., A.L., E.P.B., I.B., M.A., T.B.R., H.C., C.B., T.B., S.J.M., Z.Y., S.Y.V., and H.H.W. contributed with experimental data and interpretations; T.B.R. and H.H.W. wrote the manuscript; and all authors edited and approved the final version.
Declaration of Interests
The authors declare no competing interests.
Published: July 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.devcel.2020.06.039.
Supplemental Information
References
- Akama T.O., Nakagawa H., Wong N.K., Sutton-Smith M., Dell A., Morris H.R., Nakayama J., Nishimura S., Pai A., Moremen K.W. Essential and mutually compensatory roles of {alpha}-mannosidase II and {alpha}-mannosidase IIx in N-glycan processing in vivo in mice. Proc. Natl. Acad. Sci. USA. 2006;103:8983–8988. doi: 10.1073/pnas.0603248103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amen N., Mathow D., Rabionet M., Sandhoff R., Langbein L., Gretz N., Jäckel C., Gröne H.J., Jennemann R. Differentiation of epidermal keratinocytes is dependent on glucosylceramide:ceramide processing. Hum. Mol. Genet. 2013;22:4164–4179. doi: 10.1093/hmg/ddt264. [DOI] [PubMed] [Google Scholar]
- Basmanav F.B., Oprisoreanu A.M., Pasternack S.M., Thiele H., Fritz G., Wenzel J., Größer L., Wehner M., Wolf S., Fagerberg C. Mutations in POGLUT1, encoding protein O-glucosyltransferase 1, cause autosomal-dominant Dowling-Degos disease. Am. J. Hum. Genet. 2014;94:135–143. doi: 10.1016/j.ajhg.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C., Lowry W.E., Pasolli H.A., Fuchs E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 2006;20:3022–3035. doi: 10.1101/gad.1477606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer E.G., Schlessinger J., Hakomori S. Ganglioside-mediated modulation of cell growth. Specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J. Biol. Chem. 1986;261:2434–2440. [PubMed] [Google Scholar]
- Burgeson R.E., Christiano A.M. The dermal-epidermal junction. Curr. Opin. Cell Biol. 1997;9:651–658. doi: 10.1016/s0955-0674(97)80118-4. [DOI] [PubMed] [Google Scholar]
- Byron A., Humphries J.D., Askari J.A., Craig S.E., Mould A.P., Humphries M.J. Anti-integrin monoclonal antibodies. J Cell Sci. 2009;122:4009–4011. doi: 10.1242/jcs.056770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Thinn A.M.M., Wang Z., Shan H., Zhu J. The importance of N-glycosylation on β3 integrin ligand binding and conformational regulation. Sci. Rep. 2017;7:4656. doi: 10.1038/s41598-017-04844-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho S., Catarino T.A., Dias A.M., Kato M., Almeida A., Hessling B., Figueiredo J., Gärtner F., Sanches J.M., Ruppert T. Preventing E-cadherin aberrant N-glycosylation at Asn-554 improves its critical function in gastric cancer. Oncogene. 2016;35:1619–1631. doi: 10.1038/onc.2015.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyavsky A.I., Arredondo J., Wess J., Karlsson E., Grando S.A. Novel signaling pathways mediating reciprocal control of keratinocyte migration and wound epithelialization through M3 and M4 muscarinic receptors. J. Cell Biol. 2004;166:261–272. doi: 10.1083/jcb.200401034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choma D.P., Pumiglia K., DiPersio C.M. Integrin α3β1 directs the stabilization of a polarized lamellipodium in epithelial cells through activation of Rac1. J Cell Sci. 2004;117:3947–3959. doi: 10.1242/jcs.01251. [DOI] [PubMed] [Google Scholar]
- Crum C.P., McKeon F.D. p63 in epithelial survival, germ cell surveillance, and neoplasia. Annu. Rev. Pathol. 2010;5:349–371. doi: 10.1146/annurev-pathol-121808-102117. [DOI] [PubMed] [Google Scholar]
- Dabelsteen S., Hercule P., Barron P., Rice M., Dorsainville G., Rheinwald J.G. Epithelial cells derived from human embryonic stem cells display p16INK4A senescence, hypermotility, and differentiation properties shared by many P63+ somatic cell types. Stem Cells. 2009;27:1388–1399. doi: 10.1002/stem.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgaard K., Jung A., Qvortrup K., Clausen H., Kjaerulff O., Wandall H.H. Neurofibromatosis-like phenotype in Drosophila caused by lack of glucosylceramide extension. Proc. Natl. Acad. Sci. USA. 2012;109:6987–6992. doi: 10.1073/pnas.1115453109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam D.H.M., Wang X.Q., Sheu S., Vijay M., Shipp D., Miller L., Paller A.S. Ganglioside GM3 mediates glucose-induced suppression of IGF-1 receptor-Rac1 activation to inhibit keratinocyte motility. J. Invest. Dermatol. 2017;137:440–448. doi: 10.1016/j.jid.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demehri S., Turkoz A., Kopan R. Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer Cell. 2009;16:55–66. doi: 10.1016/j.ccr.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson M.A., Hahn W.C., Ino Y., Ronfard V., Wu J.Y., Weinberg R.A., Louis D.N., Li F.P., Rheinwald J.G. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 2000;20:1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doench J.G., Fusi N., Sullender M., Hegde M., Vaimberg E.W., Donovan K.F., Smith I., Tothova Z., Wilen C., Orchard R., Virgin H.W., Listgarten J., Root D.E. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 2016;34:184–191. doi: 10.1038/nbt.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto G.P. Crosstalk of Notch with p53 and p63 in cancer growth control. Nat. Rev. Cancer. 2009;9:587–595. doi: 10.1038/nrc2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Editorial Comment Method of the year 2017: organoids. Nat Methods. 2018;15:1. [Google Scholar]
- Egles C., Huet H.A., Dogan F., Cho S., Dong S., Smith A., Knight E.B., McLachlan K.R., Garlick J.A. Integrin-blocking antibodies delay keratinocyte re-epithelialization in a human three-dimensional wound healing model. PLoS One. 2010;5:e10528. doi: 10.1371/journal.pone.0010528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt C., Schmolke M., Matzke A., Knoblauch A., Will C., Wixler V., Ludwig S. Polyethylenimine, a cost-effective transfection reagent. Signal Transduct. 2006;6:179–184. [Google Scholar]
- Elias P.M., Wakefield J.S. Mechanisms of abnormal lamellar body secretion and the dysfunctional skin barrier in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2014;134:781–791.e1. doi: 10.1016/j.jaci.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold K.R. Lamellar bodies: the key to cutaneous barrier function. J. Invest. Dermatol. 2012;132:1951–1953. doi: 10.1038/jid.2012.177. [DOI] [PubMed] [Google Scholar]
- Fernandez-Valdivia R., Takeuchi H., Samarghandi A., Lopez M., Leonardi J., Haltiwanger R.S., Jafar-Nejad H. Regulation of mammalian Notch signaling and embryonic development by the protein O-glucosyltransferase Rumi. Development. 2011;138:1925–1934. doi: 10.1242/dev.060020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze H.H. Genetic defects in the human glycome. Nat. Rev. Genet. 2006;7:537–551. doi: 10.1038/nrg1894. [DOI] [PubMed] [Google Scholar]
- Getsios S., Simpson C.L., Kojima S., Harmon R., Sheu L.J., Dusek R.L., Cornwell M., Green K.J. Desmoglein 1-dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. J. Cell Biol. 2009;185:1243–1258. doi: 10.1083/jcb.200809044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Taniguchi N. Regulation of integrin functions by N-glycans. Glycoconj. J. 2004;21:9–15. doi: 10.1023/B:GLYC.0000043741.47559.30. [DOI] [PubMed] [Google Scholar]
- Haltiwanger R.S., Lowe J.B. Role of glycosylation in development. Annu. Rev. Biochem. 2004;73:491–537. doi: 10.1146/annurev.biochem.73.011303.074043. [DOI] [PubMed] [Google Scholar]
- Haltiwanger R.S., Wells L., Freeze H.H., Stanley P. Other classes of eukaryotic glycans. In: Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Darvill A.G., Kinoshita T., Packer N.H., editors. Essentials of Glycobiology. Cold Spring Harbor; 2015. pp. 151–160. [Google Scholar]
- Hang Q., Isaji T., Hou S., Zhou Y., Fukuda T., Gu J. N-Glycosylation of integrin α5 acts as a switch for EGFR-mediated complex formation of integrin α5β1 to α6β4. Sci. Rep. 2016;6:33507. doi: 10.1038/srep33507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey B.M., Haltiwanger R.S. Regulation of notch function by O-glycosylation. Adv. Exp. Med. Biol. 2018;1066:59–78. doi: 10.1007/978-3-319-89512-3_4. [DOI] [PubMed] [Google Scholar]
- He H., Suryawanshi H., Morozov P., Gay-Mimbrera J., Del Duca E., Kim H.J., Kameyama N., Estrada Y., Der E., Krueger J.G. Single-cell transcriptome analysis of human skin identifies novel fibroblast subpopulation and enrichment of immune subsets in atopic dermatitis. J. Allergy Clin. Immunol. 2020;145:1615–1628. [Google Scholar]
- Heise T., Pijnenborg J.F.A., Büll C., van Hilten N., Kers-Rebel E.D., Balneger N., Elferink H., Adema G.J., Boltje T.J. Potent metabolic sialylation inhibitors based on C-5-modified fluorinated sialic acids. J. Med. Chem. 2019;62:1014–1021. doi: 10.1021/acs.jmedchem.8b01757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioffe E., Stanley P. Mice lacking N-acetylglucosaminyltransferase I activity die at mid-gestation, revealing an essential role for complex or hybrid N-linked carbohydrates. Proc. Natl. Acad. Sci. USA. 1994;91:728–732. doi: 10.1073/pnas.91.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafar-Nejad H., Leonardi J., Fernandez-Valdivia R. Role of glycans and glycosyltransferases in the regulation of Notch signaling. Glycobiology. 2010;20:931–949. doi: 10.1093/glycob/cwq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennemann R., Sandhoff R., Langbein L., Kaden S., Rothermel U., Gallala H., Sandhoff K., Wiegandt H., Gröne H.J. Integrity and barrier function of the epidermis critically depend on glucosylceramide synthesis. J. Biol. Chem. 2007;282:3083–3094. doi: 10.1074/jbc.M610304200. [DOI] [PubMed] [Google Scholar]
- Jennemann R., Sandhoff R., Wang S., Kiss E., Gretz N., Zuliani C., Martin-Villalba A., Jäger R., Schorle H., Kenzelmann M. Cell-specific deletion of glucosylceramide synthase in brain leads to severe neural defects after birth. Proc. Natl. Acad. Sci. USA. 2005;102:12459–12464. doi: 10.1073/pnas.0500893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S.P., Chung J.H. Inhibition of N-glycosylation by tunicamycin attenuates cell-cell adhesion via impaired desmosome formation in normal human epidermal keratinocytes. Biosci. Rep. 2018;38 doi: 10.1042/BSR20171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi H.J., Hansen L., Narimatsu Y., Freeze H.H., Henrissat B., Bennett E., Wandall H.H., Clausen H., Schjoldager K.T. Glycosyltransferase genes that cause monogenic congenital disorders of glycosylation are distinct from glycosyltransferase genes associated with complex diseases. Glycobiology. 2018;28:284–294. doi: 10.1093/glycob/cwy015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju T., Cummings R.D. Functional assays for the molecular chaperone cosmc. Methods Enzymol. 2010;479:107–122. doi: 10.1016/S0076-6879(10)79006-6. [DOI] [PubMed] [Google Scholar]
- Katoh T., Tiemeyer M. The N's and O's of Drosophila glycoprotein glycobiology. Glycoconj. J. 2013;30:57–66. doi: 10.1007/s10719-012-9442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S.L., Joshi H.J., Schjoldager K.T., Halim A., Madsen T.D., Dziegiel M.H., Woetmann A., Vakhrushev S.Y., Wandall H.H. Characterizing the O-glycosylation landscape of human plasma, platelets, and endothelial cells. Blood Adv. 2017;1:429–442. doi: 10.1182/bloodadvances.2016002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudelka M.R., Antonopoulos A., Wang Y., Duong D.M., Song X., Seyfried N.T., Dell A., Haslam S.M., Cummings R.D., Ju T. Cellular O-Glycome Reporter/amplification to explore O-glycans of living cells. Nat. Methods. 2016;13:81–86. doi: 10.1038/nmeth.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Cheng R., Liang J., Yan H., Zhang H., Yang L., Li C., Jiao Q., Lu Z., He J. Mutations in POFUT1, encoding protein O-fucosyltransferase 1, cause generalized Dowling-Degos disease. Am. J. Hum. Genet. 2013;92:895–903. doi: 10.1016/j.ajhg.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmate W.M., Dipersio C.M. Integrin regulation of epidermal functions in wounds. Adv. Wound Care (New Rochelle) 2014;3:229–246. doi: 10.1089/wound.2013.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca V.C., Kim B.C., Ge C., Kakuda S., Wu D., Roein-Peikar M., Haltiwanger R.S., Zhu C., Ha T., Garcia K.C. Notch-Jagged complex structure implicates a catch bond in tuning ligand sensitivity. Science. 2017;355:1320–1324. doi: 10.1126/science.aaf9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel U., Petersen O.W., Sørensen H., Vedtofte P., Hakomori S., Clausen H., Dabelsteen E. Simple mucin-type carbohydrates in oral stratified squamous and salivary gland epithelia. J. Invest. Dermatol. 1991;97:713–721. doi: 10.1111/1523-1747.ep12484064. [DOI] [PubMed] [Google Scholar]
- Metzler M., Gertz A., Sarkar M., Schachter H., Schrader J.W., Marth J.D. Complex asparagine-linked oligosaccharides are required for morphogenic events during post-implantation development. EMBO J. 1994;13:2056–2065. doi: 10.1002/j.1460-2075.1994.tb06480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monies D., Anabrees J., Ibrahim N., Elbardisy H., Abouelhoda M., Meyer B.F., Alkuraya F.S. Identification of a novel lethal form of autosomal recessive ichthyosis caused by UDP-glucose ceramide glucosyltransferase deficiency. Clin. Genet. 2018;93:1252–1253. doi: 10.1111/cge.13180. [DOI] [PubMed] [Google Scholar]
- Moremen K.W., Tiemeyer M., Nairn A.V. Vertebrate protein glycosylation: diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012;13:448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narimatsu Y., Joshi H.J., Nason R., Van Coillie J., Karlsson R., Sun L., Ye Z., Chen Y.H., Schjoldager K.T., Steentoft C. An atlas of human glycosylation pathways enables display of the human glycome by gene engineered cells. Mol. Cell. 2019;75:394–407.e5. doi: 10.1016/j.molcel.2019.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narimatsu Y., Joshi H.J., Yang Z., Gomes C., Chen Y.H., Lorenzetti F.C., Furukawa S., Schjoldager K.T., Hansen L., Clausen H., Bennett E.P., Wandall H.H. A validated gRNA library for CRISPR/Cas9 targeting of the human glycosyltransferase genome. Glycobiology. 2018;28:295–305. doi: 10.1093/glycob/cwx101. [DOI] [PubMed] [Google Scholar]
- Nicolas M., Wolfer A., Raj K., Kummer J.A., Mill P., van Noort M., Hui C.C., Clevers H., Dotto G.P., Radtke F. Notch1 functions as a tumor suppressor in mouse skin. Nat. Genet. 2003;33:416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- Ohtsubo K., Marth J.D. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Okajima T., Irvine K.D. Regulation of notch signaling by o-linked fucose. Cell. 2002;111:893–904. doi: 10.1016/s0092-8674(02)01114-5. [DOI] [PubMed] [Google Scholar]
- Pinho S.S., Reis C.A. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- Ponta H., Sherman L., Herrlich P.A. CD44: from adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan P., Dabelsteen S., Madsen F.B., Francavilla C., Kopp K.L., Steentoft C., Vakhrushev S.Y., Olsen J.V., Hansen L., Bennett E.P. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc. Natl. Acad. Sci. USA. 2014;111:E4066–E4075. doi: 10.1073/pnas.1406619111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridky T.W., Chow J.M., Wong D.J., Khavari P.A. Invasive three-dimensional organotypic neoplasia from multiple normal human epithelia. Nat. Med. 2010;16:1450–1455. doi: 10.1038/nm.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjana N.E., Shalem O., Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson C.L., Patel D.M., Green K.J. Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nat. Rev. Mol. Cell Biol. 2011;12:565–580. doi: 10.1038/nrm3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits J.P.H., Niehues H., Rikken G., van Vlijmen-Willems I.M.J.J., van de Zande G.W.H.J.F., Zeeuwen P.L.J.M., Schalkwijk J., van den Bogaard E.H. Immortalized N/TERT keratinocytes as an alternative cell source in 3D human epidermal models. Sci. Rep. 2017;7:11838. doi: 10.1038/s41598-017-12041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. Golgi glycosylation. Cold Spring Harbor Perspect. Biol. 2011;3:a005199. doi: 10.1101/cshperspect.a005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steentoft C., Vakhrushev S.Y., Vester-Christensen M.B., Schjoldager K.T., Kong Y., Bennett E.P., Mandel U., Wandall H., Levery S.B., Clausen H. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat. Methods. 2011;8:977–982. doi: 10.1038/nmeth.1731. [DOI] [PubMed] [Google Scholar]
- Stolfa G., Mondal N., Zhu Y., Yu X., Buffone A., Jr., Neelamegham S. Using CRISPR-Cas9 to quantify the contributions of O-glycans, N-glycans and glycosphingolipids to human leukocyte-endothelium adhesion. Sci. Rep. 2016;6:30392. doi: 10.1038/srep30392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung C.C., O'Toole E.A., Lannutti B.J., Hunt J., O'Gorman M., Woodley D.T., Paller A.S. Integrin alpha 5 beta 1 expression is required for inhibition of keratinocyte migration by ganglioside GT1b. Exp. Cell Res. 1998;239:311–319. doi: 10.1006/excr.1997.3897. [DOI] [PubMed] [Google Scholar]
- Takeuchi H., Haltiwanger R.S. Significance of glycosylation in Notch signaling. Biochem. Biophys. Res. Commun. 2014;453:235–242. doi: 10.1016/j.bbrc.2014.05.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H., Yu H., Hao H., Takeuchi M., Ito A., Li H., Haltiwanger R.S. O-Glycosylation modulates the stability of epidermal growth factor-like repeats and thereby regulates Notch trafficking. J. Biol. Chem. 2017;292:15964–15973. doi: 10.1074/jbc.M117.800102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Hagen K.G., Zhang L., Tian E., Zhang Y. Glycobiology on the fly: developmental and mechanistic insights from Drosophila. Glycobiology. 2009;19:102–111. doi: 10.1093/glycob/cwn096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y., Murata S., Schmuth M., Behne M.J., Lee J.D., Ichikawa S., Elias P.M., Hirabayashi Y., Holleran W.M. Glucosylceramide synthesis and synthase expression protect against ceramide-induced stress. J. Lipid Res. 2002;43:1293–1302. [PubMed] [Google Scholar]
- van den Bogaard E.H., Tjabringa G.S., Joosten I., Vonk-Bergers M., van Rijssen E., Tijssen H.J., Erkens M., Schalkwijk J., Koenen H.J.P.M. Crosstalk between keratinocytes and T cells in a 3D microenvironment: a model to study inflammatory skin diseases. J. Invest. Dermatol. 2014;134:719–727. doi: 10.1038/jid.2013.417. [DOI] [PubMed] [Google Scholar]
- Varki A. Biological roles of glycans. Glycobiology. 2017;27:3–49. doi: 10.1093/glycob/cww086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A., Cummings R.D., Aebi M., Packer N.H., Seeberger P.H., Esko J.D., Stanley P., Hart G., Darvill A., Kinoshita T. Symbol nomenclature for graphical representations of glycans. Glycobiology. 2015;25:1323–1324. doi: 10.1093/glycob/cwv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt F.M., Jones P.H. Expression and function of the keratinocyte integrins. Dev. Suppl. 1993:185–192. [PubMed] [Google Scholar]
- Williams S.E., Beronja S., Pasolli H.A., Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–358. doi: 10.1038/nature09793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Steentoft C., Hauge C., Hansen L., Thomsen A.L., Niola F., Vester-Christensen M.B., Frödin M., Clausen H., Wandall H.H., Bennett E.P. Fast and sensitive detection of indels induced by precise gene targeting. Nucleic Acids Res. 2015;43:e59. doi: 10.1093/nar/gkv126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Wang S., Halim A., Schulz M.A., Frodin M., Rahman S.H., Vester-Christensen M.B., Behrens C., Kristensen C., Vakhrushev S.Y. Engineered CHO cells for production of diverse, homogeneous glycoproteins. Nat. Biotechnol. 2015;33:842–844. doi: 10.1038/nbt.3280. [DOI] [PubMed] [Google Scholar]
- Zhao H., Liang Y., Xu Z., Wang L., Zhou F., Li Z., Jin J., Yang Y., Fang Z., Hu Y. N-glycosylation affects the adhesive function of E-cadherin through modifying the composition of adherens junctions (AJs) in human breast carcinoma cell line MDA-MB-435. J. Cell. Biochem. 2008;104:162–175. doi: 10.1002/jcb.21608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession number for the RNA-Seq data reported in this paper is NCBI, Genome Expression Omnibus: GSE148284. The accession number for the MS data reported in this paper is Proteome Central/Proteome Exchange: PXD018418.