Abstract
Background:
Symptom monitoring interventions enhance patient outcomes, including quality of life (QOL), healthcare utilization, and survival, but it remains unclear whether older and younger patients with cancer derive similar benefits. We explored whether age moderates the improved outcomes seen with an outpatient electronic symptom monitoring intervention.
Patients and methods:
We performed a secondary analysis of data from a randomized trial of 766 patients receiving chemotherapy for metastatic solid tumors. Patients received an electronic symptom monitoring intervention integrated with oncology care or usual oncology care alone. The intervention consisted of patients reporting their symptoms, which were provided to their physicians at clinic visits, and nurses received alerts for severe/worsening symptoms. We used regression models to determine if age (older or younger than 70 years) moderated the effects of the intervention on QOL (EuroQol EQ-5D), emergency room (ER) visits, hospitalizations, and survival outcomes.
Results:
Enrollment rates for younger (589/777=75.8%) and older (177/230=77.0%) patients did not differ. Older patients (median age=75, range 70–91) were more likely to have an education level of high school or less (26.6% vs 20.9%, p=0.029) and to be computer-inexperienced (50.3% vs 23.4%, p<0.001) compared with younger patients (median age=58, range 26–69). Younger patients receiving the symptom monitoring intervention experienced lower risk for ER visits (hazard-ratio=0.74, p=0.011) and improved survival (hazard-ratio=0.76, p=0.011) compared with younger patients receiving usual care. However, older patients did not experience significantly lower risk for ER visits (hazard-ratio=0.90, p=0.613) or improved survival (hazard-ratio=1.06, p=0.753) with the intervention. We found no moderation effects based on age for QOL and risk of hospitalizations.
Conclusion:
Among patients with advanced cancer, age moderated the effects of an electronic symptom monitoring intervention on the risk of ER visits and survival, but not QOL. Symptom monitoring interventions may need to be tailored to the unique needs of older adults with cancer.
Keywords: Symptoms, Quality of Life, Advanced Cancer, Geriatric Oncology, Outcomes Research, Hospitalization
Introduction
Studies demonstrate that integrating electronic patient-reported symptom monitoring into oncology care can help to improve patients’ symptom burden, quality of life (QOL), healthcare utilization, and survival outcomes.[1–4] Based on this evidence, many centers have begun integrating electronic symptom monitoring with patient-reported outcomes into routine oncology care.[5] However, we currently lack studies describing the use and benefits of electronic symptom monitoring interventions for the geriatric oncology population.
Older adults with cancer represent the largest group of oncology patients, and these individuals possess unique care needs.[6, 7] Older patients frequently experience a distinct symptom burden and greater risk of chemotherapy toxicity than younger patients.[8–10] When caring for the geriatric oncology population, clinicians often encounter a complex constellation of issues needing to be addressed, such as impaired physical and cognitive function, concurrent comorbid conditions, increased risk of polypharmacy, and limited psychosocial support.[10–13] Thus, oncologists face challenges when trying to address all the multifaceted concerns of older patients with cancer during time-limited clinic visits, thereby leading to under-recognition of these patients’ symptoms.[14] Consequently, electronic symptom monitoring interventions represent a promising solution to ensure clinicians consistently and efficiently assess older patients’ symptoms, yet studies focused on such interventions in the geriatric oncology population are lacking.[15] Moreover, symptom monitoring interventions often require patients to report their symptoms electronically, potentially creating a barrier for less technologically-adept older individuals.[16, 17] Additionally, prior work suggests differential effects of supportive care interventions between older and younger patients, but this has not been studied in symptom monitoring interventions.[18, 19] Therefore, studies are needed to investigate the willingness of older patients to participate in trials of electronic symptom monitoring, while also exploring whether age moderates the improved outcomes seen with these interventions.
In the current study, we sought to investigate differences by age regarding study enrollment and outcomes in a randomized trial of electronic patient-reported symptom monitoring. By comparing rates of study enrollment between older and younger patients, we hope to better understand the willingness of older adults with cancer to participate in a trial of electronic patient-reported symptom monitoring. We also sought to explore whether age moderates the effects of an electronic symptom monitoring intervention on patients’ QOL, healthcare utilization, and survival outcomes. By investigating the differential effects of electronic symptom monitoring based on patients’ age, this study will inform future efforts seeking to integrate electronic patient-reported outcomes into routine cancer care for the rapidly growing geriatric oncology population.
Methods
Study Design
We conducted a secondary, exploratory analysis of data collected from a randomized trial of electronic patient-reported symptom monitoring versus usual oncology care.[1, 2] The study procedures have been previously described, but briefly, we randomly assigned patients initiating chemotherapy for metastatic cancer to receive the electronic symptom monitoring intervention or usual oncology care alone.
Patients assigned to the symptom monitoring intervention self-reported 12 symptoms, selected because they are commonly-experienced during treatment and frequently impact the patient experience, via a web-based platform using questions adapted from the National Cancer Institute’s Common Terminology Criteria for Adverse Events (graded from 0 [not present] to 4 [disabling]).[1] If an intervention patient reported a worsening (≥2 points) or severe (absolute grade ≥3) symptom, an e-mail alert was triggered to a clinical nurse responsible for the patient. After hours, participants were encouraged to call the office for concerning symptoms. The treating oncologist also received a report detailing patients’ symptoms at each clinic visit. For the study, clinicians did not receive specific guidance about symptom management.
Patients assigned to the usual care group received the standard-of-care for symptom monitoring in oncology practice, in which patients discuss their symptoms with their clinician(s) during clinical encounters and contact the office between visits for concerning symptoms. Participation was continuous until discontinuing cancer treatment, voluntary withdrawal, or death. The Memorial Sloan Kettering Cancer Center (MSK) and Dana-Farber/Harvard Cancer Center institutional review boards determined that the current secondary study was exempt and did not meet the definition of human-subjects research.
Patient Selection
For the parent trial, 766 consecutive patients initiating chemotherapy for metastatic breast, genitourinary, gynecologic, or lung cancers enrolled at MSK in New York from September 2007 to January 2011. Patients were required to receive their chemotherapy at MSK and be able to read English. We excluded patients if they were participating in an investigational treatment study, as these studies often require symptom reporting for all patients.
Outcome Measures
In the parent trial, change in QOL from baseline to 6-months was the primary outcome. We evaluated patients’ QOL using the EuroQol EQ-5D Index.[20] The EQ-5D Index assesses patients’ QOL across five domains (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) and produces a composite score between 0–1, with lower scores representing worse QOL. To investigate the impact of the intervention on healthcare utilization, we determined time to first emergency room (ER) visit and time to first hospitalization at MSK using data in the medical record.[1] To examine the effects of the intervention on overall survival, we obtained mortality data from the National Death Index.[2] We investigated time from study enrollment to death, censoring patients who had not died at the date of last follow-up.
Statistical Analysis
We used descriptive statistics to describe patient demographics and study variables, comparing younger and older patients (<70 versus ≥70 years at enrollment). We used an age cutoff of ≥70 years for subgroup analyses, as many studies use this cutoff when examining an older population.[13, 16] We ran an exploratory Subpopulation Treatment Effect Pattern Plot (STEPP) analysis, which confirmed age 70 years as an appropriate cut-point for our analyses(Supplemental-Figure-1). The STEPP approach allows investigators to explore the heterogeneity of treatment effects on outcomes across values of a continuous variable (e.g. patient age).[21] We chose the outcomes of QOL, healthcare utilization, and survival, as prior work demonstrated that this intervention had beneficial effects on these outcomes.[1, 2] To assess the degree to which patients’ age moderated the effects of electronic symptom monitoring on QOL (EQ-5D Index mean change scores from baseline to 6-months), healthcare utilization (time to first ER visit and time to first hospitalization), and overall survival (time to death), we computed separate regression models for each outcome that included the following independent variables: group assignment, the moderating variable (age), and an interaction term between group assignment and the moderating variable. We considered interaction terms with p<0.15 to indicate potential moderation worth exploring in subsequent subgroup analyses.[22, 23] We then used competing risk regression (with death treated as a competing event) and Cox proportional hazards regression to determine the effects of electronic symptom monitoring on time to first ER visit and overall survival, respectively, within the age subgroups. Consistent with prior publications of this trial, we adjusted regression models for patient sex, cancer type, race, education level, and computer experience.[1, 2] To help illustrate these findings, we compared the effects of electronic symptom monitoring on time to first ER visit and overall survival between study groups by age using the Kaplan-Meier method.
Results
Participant Sample
With 766 patients enrolled in the parent trial, we found no significant differences in the enrollment rates (enrolled/approached) for younger (589/777, 75.8%) and older (177/230, 77.0%) patients(SupplementalFigure-2). Younger patients had a median age of 58 years (range 26–69) and older patients had a median age of 75 years (range 70–91) (Table 1). When comparing differences in baseline characteristics by age, older patients were more likely to be male (73.5% vs 32.6%, p<0.001), have a genitourinary cancer type (68.9% vs 20.9%, p<0.001), education level of high school or less (26.6% vs 20.9%, p=0.029), and to be computer-inexperienced (50.3% vs 23.4%, p<0.001). We did not find a significant difference in the proportion of participants assigned to the usual care or intervention group in the older or younger subgroups. Similarly, baseline QOL scores did not differ between older and younger patients.
Table 1.
Baseline Characteristics of Participants by Age
| Characteristic | Age < 70 Years (N=589) | Age ≥ 70 Years (N=177) | p | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Study arm | 0.730 | ||||
| Usual Care | 252 | 42.8 | 73 | 41.2 | |
| Intervention | 337 | 57.2 | 104 | 58.8 | |
| Age | |||||
| Median (range) | 58 (26 – 69) | 75 (70 – 91) | |||
| Age categories | |||||
| <50 | 138 | 23.4 | - | - | |
| 54–55 | 93 | 15.8 | - | - | |
| 55–59 | 99 | 16.8 | - | - | |
| 60–64 | 143 | 24.3 | - | - | |
| 65–69 | 116 | 19.7 | - | - | |
| 70–74 | - | - | 85 | 48.0 | |
| 75–79 | - | - | 56 | 31.6 | |
| 80+ | - | - | 36 | 20.3 | |
| Sex | <0.001 | ||||
| Female | 397 | 67.4 | 47 | 26.6 | |
| Male | 192 | 32.6 | 130 | 73.5 | |
| Race | 0.072 | ||||
| White | 499 | 84.7 | 161 | 91.0 | |
| Black | 55 | 9.3 | 12 | 6.8 | |
| Asian | 35 | 5.9 | 4 | 2.3 | |
| Cancer type | <0.001 | ||||
| Genitourinary | 123 | 20.9 | 122 | 68.9 | |
| Gynecologic | 160 | 27.2 | 17 | 9.6 | |
| Breast | 140 | 23.8 | 3 | 1.7 | |
| Lung | 166 | 28.2 | 35 | 19.8 | |
| Days since initiation of chemotherapy | 0.087 | ||||
| Median (Range) | 21 (0–840) | 20 (0–1025) | |||
| Education | 0.029 | ||||
| High school or less | 123 | 20.9 | 47 | 26.6 | |
| College | 292 | 49.6 | 68 | 38.4 | |
| Graduate degree | 174 | 29.5 | 62 | 35.0 | |
| Level of prior computer experience | <0.001 | ||||
| Computer-Experienced | 451 | 76.6 | 88 | 49.7 | |
| Computer-Inexperienced | 138 | 23.4 | 89 | 50.3 | |
| Baseline Quality of Life | 0.706 | ||||
| Median (range) | 0.83 (020 to 1.00) | 0.83 (0.26 to 1.00) | |||
Outcomes by Age
Using linear regression, we found that patients’ age did not moderate the effects of electronic symptom monitoring on QOL (age×group assignment, B=−0.02, SE=3.42, p=0.994) or time to first hospitalization (age×group assignment, HR=0.23, SE=0.22, p=0.304). However, patients’ age did appear to moderate the effects of electronic symptom monitoring on time to first ER visit (age×group assignment, HR=0.35, SE=0.24, p=0.148) and overall survival (age×group assignment, HR=0.42, SE=0.20, p=0.034).
Subsequent subgroup analyses by age (Table 2) showed that, among younger patients (age <70 years), the electronic symptom monitoring intervention significantly reduced the hazard for time to ER visit (HR=0.74, SE=0.12, p=0.011) but had no significant effect on this outcome for older patients (HR=0.90, SE=0.22, p=0.613). As illustrated in Figure 1, the incidence of patients visiting the ER was significantly lower in the intervention arm compared with the usual care arm for the younger patients (median time to ER visit: 50.73 months vs 21.72 months, Gray’s test p-value=0.016), yet the difference seen between the intervention and usual care groups was not significant for the older patients (median time to ER visit: 17.61 months vs 21.98 months, Gray’s test p-value=0.738).
Table 2.
Effects of Electronic Patient-Reported Symptom Monitoring on ER Visits and Survival by Age
| Outcomes* | Age < 70 | Age ≥ 70 | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | SE | P | HR | 95% CI | SE | P | |
| Hazard for ER Visit for Intervention vs Usual Care | 0.740 | 0.588 to 0.933 | 0.118 | 0.011 | 0.895 | 0.581 to 1.378 | 0.220 | 0.613 |
| Hazard for Death for Intervention vs Usual Care | 0.760 | 0.616 to 0.938 | 0.107 | 0.011 | 1.056 | 0.751 to 1.486 | 0.174 | 0.753 |
Abbreviations: ER, emergency room; HR, hazard ratio; 95% CI, 95% confidence interval; SE, standard error.
Adjusted for sex, cancer type, race, education level, and computer experience.
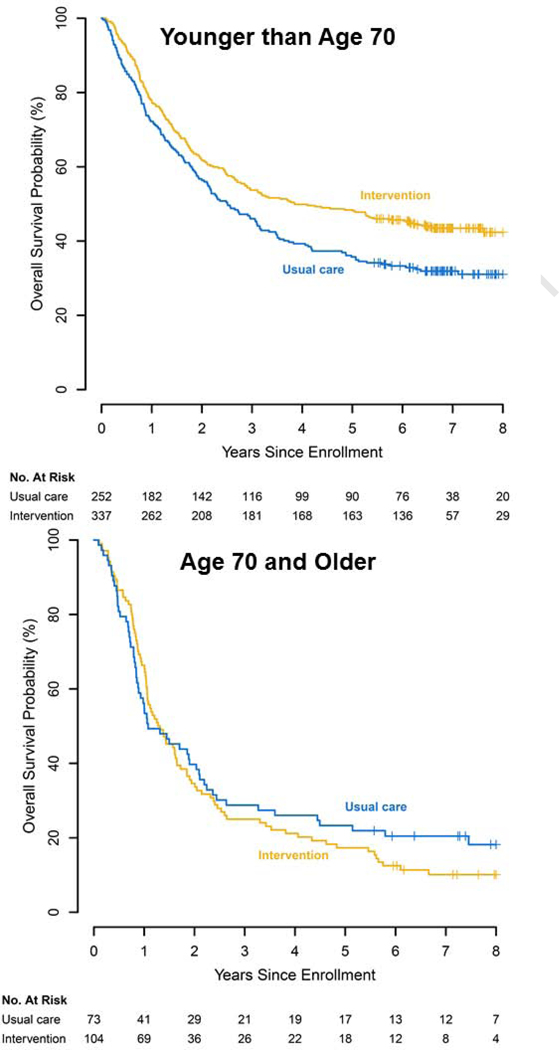
Figure 1. Effects of Electronic Patient-Reported Symptom Monitoring on ER Visits by Age.
Abbreviations: ER, emergency room; No., number.
Additionally, we observed a significant moderation effect by age on overall survival, and subsequent subgroup analyses revealed that the electronic symptom monitoring intervention led to decreased hazard for death (HR=0.76, SE=0.11, p=0.011) among younger patients. However, we did not find significant survival benefits for patients assigned to the intervention among the older patients (HR=1.06, SE=0.17, p=0.753). Figure 2 illustrates that younger patients assigned to the electronic symptom monitoring intervention experienced significantly longer median overall survival compared with younger patients assigned to usual care (46.23 vs 30.14 months, log-rank p-value=0.004), whereas we found no significant differences between intervention and usual care for the older patients (15.57 vs 12.94, log-rank p-value=0.512).
Figure 2. Effects of Electronic Patient-Reported Symptom Monitoring on Survival by Age.
Abbreviations: No., number.
Discussion
In this exploratory analysis of data from a randomized trial assessing the impact of an electronic symptom monitoring intervention among patients with advanced cancer, we demonstrated that age moderated some of the benefits derived from the intervention. We found that patients’ age moderated the effects of electronic symptom monitoring on time to first ER visit and overall survival. Specifically, younger patients assigned to the electronic symptom monitoring intervention had lower risk for ER visits and better overall survival than younger patients assigned to usual care, yet older patients did not experience these intervention effects. Collectively, these findings suggest the potential for differential effects of electronic symptom monitoring based on patients’ age.
To our knowledge, this is the first study to report that the effects of electronic symptom monitoring differ based on the age of patients with cancer. We found that an electronic symptom monitoring intervention helped younger patients with regards to their risk of ER visits and overall survival, but these benefits were not significant for older patients. Potentially, older patients did not derive benefits in terms of ER visits and survival due to the constellation of factors that may influence these outcomes in older adults with cancer, such as mobility, cognitive function, and the availability of social supports, which are not comprehensively addressed with a symptom monitoring intervention.[11, 12] We also found that older patients in our study were more likely to be computer-inexperienced, which could theoretically influence their experience with this type of intervention.[15, 16] However, prior work demonstrated that the benefits of this symptom monitoring intervention were greater for participants with limited computer experience.[1] Importantly, we did not find that age moderated the impact of electronic symptom monitoring on patients’ QOL, thereby suggesting that older and younger patients both experienced significant QOL benefits from this intervention. These findings underscore that when investigating supportive care interventions for the geriatric cancer population, researchers should consider outcomes that are important to older adults, such as QOL, functional independence, and treatment tolerability.[24, 25] Thus, our findings are hypothesis-generating and additional work is needed to confirm the results and help us fully understand the mechanisms underlying the differential benefits of symptom monitoring interventions for younger and older patients with cancer.
Notably, older patients in our study were equally as likely to enroll in this randomized trial as their younger counterparts, which highlights their willingness to participate in a trial testing an electronic symptom monitoring intervention. Although cancer disproportionately impacts older adults, little research has sought to test age-specific interventions focused on the supportive care needs of the geriatric oncology population. The population of aging individuals is expected to continue to rise exponentially, and therefore it is imperative to design models of oncology care tailored to the complex needs of older adults with cancer.[6, 7] Additionally, we need more supportive care trials that enroll older individuals, thereby providing more age-diversity, and fostering investigations exploring differential effects between older and younger patients. Ultimately, additional research is needed to allow us to understand how best to develop interventions targeting the geriatric oncology population.
Our work underscores the need to study age as a moderator of intervention effects in supportive care trials. Prior research has demonstrated that older and younger patients have differing supportive care needs,[18, 19] yet studies had not yet shown that the impact of electronic symptom monitoring interventions differentially vary by age among patients with cancer. Understanding differential effects of interventions on younger versus older patients can be informative in: (1) enhancing current models of care by highlighting where existing standards of care may not address all the unique concerns of certain subgroups of patients; (2) developing innovative care models personalized to patients’ distinct care needs; and (3) designing age-specific interventions for the geriatric oncology population to enhance care delivery and outcomes for this largest subgroup of patients with cancer. By demonstrating differential effects of electronic symptom monitoring based on patients’ age, this work supports the need for population-specific interventions tailored to older individuals with cancer and should inform future efforts to support these patients with complex and diverse needs.
Our study has several limitations. First, this was an exploratory analysis, with a relatively modest sample size, and thus our hypothesis-generating findings merit confirmation in follow-up studies. Second, we only investigated moderation based on patient age, and future prospective studies should test the differential effects of electronic symptom monitoring interventions across other patient characteristics. Notably, we observed differences in the sexes and cancer types between older and younger patients in this study, and although we adjusted for this in our regression models, unmeasured confounding could still affect our findings. Third, our study sample included patients initiating treatment for advanced solid tumors at a tertiary cancer center, which limits our ability to generalize findings to patients outside of this population and care setting. Fourth, the current analysis was limited to QOL, healthcare utilization, and survival outcomes, and thus we lack information about potential differential effects on other important outcomes, such as patients’ symptoms, physical function, and treatment tolerance. In addition, we cannot account for potentially important unmeasured confounds, including comorbid conditions, cognitive function, and social support.
In summary, we demonstrated differential effects of an electronic symptom monitoring intervention based on patient age regarding the risk of ER visits and survival, but not QOL. Specifically, we found that younger patients receiving electronic symptom monitoring experienced fewer ER visits and longer survival than those receiving usual care. Conversely, older patients did not experience these same benefits with electronic symptom monitoring. Thus, symptom monitoring interventions may need to be tailored to patients’ age-specific care needs. Expanding on this work, future studies should seek to develop strategies for tailoring and personalizing symptom monitoring interventions to the individual supportive care needs of all patients with advanced cancer.
Supplementary Material
Abbreviations: No., number.
Abbreviations: N, number; QOL, quality of life.
Key Message:
In this analysis of randomized trial data, age moderated the impact of electronic symptom monitoring on patients’ risk of emergency room (ER) visits and survival, but not quality of life. Younger patients receiving the intervention had lower risk of ER visits and improved survival compared to younger patients receiving usual care, yet older patients did not experience these intervention effects.
Acknowledgments
Funding sources: NCI K24 CA181253 (Temel)
Footnotes
Conflict of Interest Disclosures: No authors have conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Basch E, Deal AM, Kris MG et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J Clin Oncol 2016; 34: 557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basch E, Deal AM, Dueck AC et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA 2017; 318: 197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strasser F, Blum D, von Moos R et al. The effect of real-time electronic monitoring of patient-reported symptoms and clinical syndromes in outpatient workflow of medical oncologists: E-MOSAIC, a multicenter cluster-randomized phase III study (SAKK 95/06). Ann Oncol 2016; 27: 324–332. [DOI] [PubMed] [Google Scholar]
- 4.Nipp RD, El-Jawahri A, Ruddy M et al. Pilot Randomized Trial of an Electronic Symptom Monitoring Intervention for Hospitalized Patients with Cancer. Ann Oncol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holch P, Warrington L, Bamforth LCA et al. Development of an integrated electronic platform for patient self-report and management of adverse events during cancer treatment. Ann Oncol 2017; 28: 2305–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yancik R. Population aging and cancer: a cross-national concern. Cancer J 2005; 11: 437–441. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro CL. Cancer Survivorship. N Engl J Med 2018; 379: 2438–2450. [DOI] [PubMed] [Google Scholar]
- 8.Cheung WY, Le LW, Gagliese L, Zimmermann C. Age and gender differences in symptom intensity and symptom clusters among patients with metastatic cancer. Support Care Cancer 2011; 19: 417–423. [DOI] [PubMed] [Google Scholar]
- 9.Repetto L. Greater risks of chemotherapy toxicity in elderly patients with cancer. J Support Oncol 2003; 1: 18–24. [PubMed] [Google Scholar]
- 10.Mohile SG, Dale W, Somerfield MR et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol 2018; JCO2018788687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroenke CH, Kubzansky LD, Schernhammer ES et al. Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol 2006; 24: 1105–1111. [DOI] [PubMed] [Google Scholar]
- 12.Pamoukdjian F, Aparicio T, Zelek L et al. Impaired mobility, depressed mood, cognitive impairment and polypharmacy are independently associated with disability in older cancer outpatients: The prospective Physical Frailty in Elderly Cancer patients (PF-EC) cohort study. J Geriatr Oncol 2017; 8: 190–195. [DOI] [PubMed] [Google Scholar]
- 13.Derks MG, de Glas NA, Bastiaannet E et al. Physical Functioning in Older Patients With Breast Cancer: A Prospective Cohort Study in the TEAM Trial. Oncologist 2016; 21: 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon DH, Chera BS, Deal AM et al. Clinician-observed and patient-reported toxicities and their association with poor tolerance to therapy in older patients with head and neck or lung cancer treated with curative radiotherapy. J Geriatr Oncol 2019; 10: 42–47. [DOI] [PubMed] [Google Scholar]
- 15.Loh KP, McHugh C, Mohile SG et al. Using Information Technology in the Assessment and Monitoring of Geriatric Oncology Patients. Curr Oncol Rep 2018; 20: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCleary NJ, Wigler D, Berry D et al. Feasibility of computer-based self-administered cancer-specific geriatric assessment in older patients with gastrointestinal malignancy. Oncologist 2013; 18: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine DM, Lipsitz SR, Linder JA. Trends in Seniors’ Use of Digital Health Technology in the United States, 2011–2014. JAMA 2016; 316: 538–540. [DOI] [PubMed] [Google Scholar]
- 18.Nipp RD, Greer JA, El-Jawahri A et al. Age and Gender Moderate the Impact of Early Palliative Care in Metastatic Non-Small Cell Lung Cancer. Oncologist 2016; 21: 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nipp RD, El-Jawahri A, Traeger L et al. Differential effects of early palliative care based on the age and sex of patients with advanced cancer from a randomized controlled trial. Palliat Med 2018; 32: 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.EuroQol G. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 21.Yip WK, Bonetti M, Cole BF et al. Subpopulation Treatment Effect Pattern Plot (STEPP) analysis for continuous, binary, and count outcomes. Clin Trials 2016; 13: 382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall SW. Power for tests of interaction: effect of raising the Type I error rate. Epidemiol Perspect Innov 2007; 4: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang R, Ware JH. Detecting moderator effects using subgroup analyses. Prev Sci 2013; 14: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med 2002; 346: 1061–1066. [DOI] [PubMed] [Google Scholar]
- 25.Hurria A, Dale W, Mooney M et al. Designing Therapeutic Clinical Trials for Older and Frail Adults With Cancer: U13 Conference Recommendations. J Clin Oncol 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Abbreviations: No., number.
Abbreviations: N, number; QOL, quality of life.