Abstract
Drug delivery to the central nervous system (CNS) is generally hindered by the selectivity of the blood-brain barrier (BBB). However, there is strong evidence that the integrity of the BBB is compromised under certain pathological conditions, potentially providing a window to deliver drugs to injured brain regions. Recent studies suggest that caveolae-mediated transcytosis, a transport pathway suppressed in the healthy BBB, becomes elevated as an immediate response to ischemic stroke and at early stages of aging, where it may precede irreversible neurological damage. This article reviews early-stage caveolar transcytosis as a novel and promising drug delivery opportunity. We propose that albumin-binding and nanoparticle approaches have the potential to leverage this window of transcellular BBB disruption for trafficking therapeutic agents into the CNS.
Introduction
The blood-brain-barrier (BBB), which is effectively impermeable to all large molecule therapeutics and 98% of small molecules, presents a substantial challenge for the treatment of central nervous system (CNS) disorders [1]. Many engineering strategies have been explored to overcome this obstacle, particularly for the delivery of biologics. Some approaches to increase drug penetration include co-opting endogenous receptor-mediated processes that normally shuttle proteins and amino acids across the BBB, as well as temporarily opening the intact BBB using ultrasound or pharmacological agents [2]. However, using antibodies or peptides to exploit receptor-mediated transcytosis (RMT) is inefficient with only a small fraction of injected dose reaching the brain. Furthermore, drug delivery through RMT is usually not targeted to a focal area of the CNS, as the receptors are ubiquitously expressed in the cerebrovasculature. The alternate strategy of opening the BBB introduces the potential for toxicity from entry of blood-borne proteins, and its relative invasiveness is not well-suited for delivering drugs on a frequent schedule.
Recent work suggests that a promising but underappreciated approach for CNS drug delivery may be selective targeting of receptor-independent pathways that involve trafficking of caveolae, which are small lipid rafts that non-specifically shuttle proteins such as albumin and immunoglobulins across the brain endothelium. Although suppressed at the healthy BBB, robust upregulation of caveolar-dependent transcytosis occurs in early stages of a number of CNS diseases [3–5]. Enhanced caveolae-mediated trafficking of albumin has also been identified as an early hallmark of aging [6]. While sustained upregulation of caveolae-mediated transcytosis may lead to long-term detrimental neurological consequences, the onset of caveolae upregulation in aging and disease likely precedes irreversible neurological damage and may provide a gateway for therapeutic agents to reach brain targets [7]. Here, we review and provide a prospectus on the potential exploitation of early-stage caveolar transcytosis as a novel and promising approach for delivering therapeutic compounds to the CNS.
Neurovascular Unit Structure and Regulation of Transport
The restrictive properties of the BBB arise from the unique molecular signature of brain microvascular endothelial cells (BMECs) and their bidirectional communication with neighboring cells. These polarized BMECs, together with pericytes, glia (astrocytes and microglia), neurons, and the basement membrane, collectively form the neurovascular unit (NVU), which maintains cerebral homeostasis by regulating the transport of compounds between the blood circulation and brain parenchyma [8]. Unlike most peripheral endothelial cells, BMECs are connected by a continuous network of epithelial-like tight junctions that prevent paracellular diffusion of water, ions, and organic molecules. As a result, the NVU effectively controls material exchange between the blood and CNS through selective transporters expressed on the endothelium [9].
The transport pathways at the BBB include passive diffusion, carrier-mediated transport, and vesicular transcytosis (Figure 1). Free diffusion across the endothelium is dictated by the physicochemical properties of the compounds, favoring small (<450 Da) lipophilic molecules with fewer than 6 hydrogen bonds, though these factors are not an absolute prediction of BBB permeability [10]. Most nutrients including glucose, amino acids, and vitamins are too polar to diffuse across the lipid membrane and are therefore transported through specific solute carriers present at both the apical and basolateral membranes of BMECs.
Figure 1. Transport pathways across the BBB.

Passive diffusion through the brain endothelium is restricted to small (<450 Daltons), lipophilic compounds including but not limited to caffeine, alcohol, and a limited subset of small molecule drugs that are not efflux transporter substrates. Transport of small and large molecules between cells (paracellular route) is effectively non-present due to a dense network of specialized tight junctions. Carrier-mediated transport of solutes involves binding of compounds to specific proteins expressed on the brain endothelium. Importantly, this pathway does not involve vesicles, and notable molecules that are transported through this route include glucose, amino acids, and vitamins. In terms of vesicular transport mechanisms, receptor-mediated transport involves specific receptor-ligand interactions that trigger clathrin-dependent endocytosis. After the vesicle is pinched off by dynamin, the clathrin coat is shed and the vesicle fuses with a sorting endosome. Depending on a number of factors, including the affinity of the ligand of the receptor, the vesicle will either be directed to a late endosome and lysosomal degradation pathway or be passed to a transcytotic vesicle. In adsorptive mediated transcytosis, a macromolecule needs to be positively charged, in which binding of the cation to the plasma membrane leads to endocytosis through caveolae. These uncoated vesicles are lipid rafts stabilized by caveolin-1. While many studies suggest that caveolae are directly passed across brain endothelial cells, it is not well understood if there are alternative routes involving degradation pathways.
The transport of large molecules such as proteins and peptides is restricted predominantly to endocytotic transcellular pathways. Vesicular transport can occur via RMT or non-specific adsorptive-mediated transcytosis (AMT) [10]. The former requires specific protein-receptor interactions to trigger endocytosis, and examples of proteins transported by RMT include transferrin, insulin, and leptin. The less studied AMT pathway involves positively charged proteins accumulating on the negatively charged glycocalyx, triggering caveolar uptake and transport across the BBB. Although the hallmark of the BBB is low baseline rates of AMT from the luminal side of the endothelium into the brain [3], current advances in understanding AMT function and regulation suggest the potential to ‘backpack’ on the AMT pathway to deliver molecules across the injured BBB.
Structure, Transport, and Regulation of Caveolar Transcytosis at the Healthy BBB
Caveolae are flask-shaped plasma membrane invaginations that are vital for the endocytosis and transcytosis of plasma macromolecules [11]. First discovered in the 1950s, caveolae are highly abundant in adipocytes, smooth-muscle cells, fibroblasts, epithelial cells, and most importantly for this review, endothelial cells [11,12]. Brain endothelial caveolae are 50-80 nm in diameter and are highly enriched with cholesterol and glycosphingolipids, forming a lipid raft that is stabilized by caveolin-1 (cav-1) at the plasma membrane [11]. Relative to other endothelia, brain endothelial cells have the lowest frequency of caveolae (<100/μm3) with only a small fraction being internalized [13]. When transcytosis is activated, the vesicle is pinched off from the membrane and subsequently migrates across the brain endothelium to the basolateral membrane (Figure 1). Both activation and fission of caveolae are mediated by Src kinase tyrosine phosphorylation; phosphorylation of cav-1 induces internalization of bound and fluid-phase macromolecules within caveolae [14] and Src phosphorylation of dynamin mediates release of caveolae from the plasma membrane as intracellular vesicles [15]. Upon reaching the basolateral membrane, caveolae fuse and release their contents into the interstitial space [12].
While albumin is one of the most prominent proteins trafficked across the endothelium through caveolae, albumin transport mechanisms are tissue-specific and may be mediated by receptor-dependent or - independent pathways. Albumin binding to albondin (gp60), an endothelial sialoglycoprotein residing in caveolae, initiates non-degradative transport by triggering Src kinase-mediated phosphorylation of gp60 and cav-1[16]. However, despite facilitating over 50% of albumin transport across peripheral endothelia, gp60 is not expressed in healthy brain endothelial cells, further contributing to the inability of albumin to cross the intact BBB [17,18].
While caveolae-mediated transcytosis constitutes a primary albumin transport mechanism in peripheral organs, caveolae vesicle formation is heavily suppressed at the BBB. In 2014, Ben-Zvi et al. discovered that a BBB-specific transporter, Major facilitator super family domain containing 2a (Mfsd2a), is responsible for regulating vesicular transcytosis [19]. Using transgenic Mfsd2a−/− mice, they observed elevated vesicular transport despite the maintenance of tight junctions. Recently, Andreone et al. demonstrated that Mfsd2a−/− mice displayed enhanced caveolar transcytosis without changes to paracellular permeability [3]. Cav1 knockout in the Mfsd2a−/− mice prevented the increase in transcytosis, suggesting that caveolae-mediated transcytosis is the pathway suppressed by Mfsd2a [3]. These researchers proposed that Mfsd2a functions as a lipid flippase that transports docosahexaenoic acid (DHA) onto the inner plasma membrane leaflet. DHA is an omega-3 fatty acid important for neural and cognitive function, but its presence on the plasma membrane displaces cholesterol and cav-1, thereby preventing the formation of caveolae [3,20]. This recent insight into transcytosis mechanisms at the BBB highlights a critical role for the lipid composition of the plasma membrane in regulating caveolae formation in BMECs.
Previous Strategies for CNS Drug Delivery through Transcytotic Routes
In contrast to carrier-mediated transport which is restricted by size limitations, RMT and AMT pathways are well-suited for delivery of proteins, drugs, and nanoparticles across the BBB [2]. BMECs are highly enriched in clathrin-coated vesicles, which mediate RMT. Thus, strategies to deliver biologics across the BBB have primarily exploited the mechanisms of RMT pathways [2]. The general principle of delivering drugs through RMT is to conjugate the therapeutic agent to a vector that selectively binds the target receptor and triggers transcytosis. The most prominent proteins that undergo RMT and have been used to guide drug delivery are transferrin, which shuttles iron into brain, and insulin, which is not produced in the CNS [1]. For example, targeting the transferrin receptor with OX26 (a mouse monoclonal antibody against the rat receptor) successfully enables transcytosis of pharmaceuticals without impeding natural transferrin transport [2]. Fusion of OX26 with proteins such as brain-derived neurotrophic factor [21] and basic fibroblast growth factor [22] has been explored as a possible neuroprotective approach following ischemic stroke. However, while these RMT-leveraging strategies do achieve brain targeting, they are limited in the requirement for substantial and frequent dosing to achieve high extent of uptake into the brain parenchyma. The absolute amount of OX26 transported into the CNS is typically far below 1% of the injected dose per gram, with an upper limit of ~3-4% for bi-specific antibody designs [23,24].
In contrast, AMT has a far greater binding capacity than RMT because AMT is not limited by the expression of specific receptors and is therefore saturated at much higher concentrations [25]. Intriguingly, some approaches to brain drug delivery have targeted AMT pathways by cationizing serum proteins such as albumin and immunoglobulin. The negative charge arising from sialoglycoproteins and heparin sulfate proteoglycans in the endothelial glycocalyx preclude the transport of native albumin, which is negatively charged at neutral pH due to an abundance of acidic amino acid residues [26]. For delivering chimeric peptides through the healthy brain endothelium, Kang and Partridge originally used cationized human serum albumin (cHSA) bound to neutral avidin and demonstrated increased brain delivery of H3-biotin conjugated to the cHSA-avidin vector [27]. Overall, cationized albumin improved pharmacokinetic properties by greatly enhancing half-life and brain targeting compared to other organs [26]. However, these studies were published in 1994 when the transport of albumin and regulation of AMT pathways at the BBB were poorly understand, and few efforts have built upon this nascent work. Below, we delve into the mechanisms by which caveolar transport pathways become elevated during disease and aging as a potential motivation for revisiting caveolar-based drug delivery strategies.
Changes to BBB Permeability Following Ischemic Stroke
Caused by thrombosis or embolism, ischemic stroke accounts for over 80% of strokes and is characterized by reduced cerebral blood supply resulting in poor delivery of oxygen and glucose [28]. Nutrient inaccessibility causes rapid neuronal death in the ischemic core. However, salvageable neurons in the surrounding penumbra slowly die due to a complex secondary injury cascade involving glutamate-mediated excitotoxicity, neuroinflammation, and oxidative stress [28]. Clinical and experimental findings indicate that sustained BBB breakdown is a hallmark of ischemic stroke, and that greater BBB disruption exacerbates secondary injuries, thereby contributing to adverse long-term prognoses [29,30]. BBB integrity is compromised within several hours of ischemic injury permitting the uncontrolled leakage of blood-borne molecules into the brain parenchyma, which increases the risk of hemorrhage and induces vasogenic edema through disruption of osmotic gradients, leading to elevated intracranial pressure [31,32]. While BBB breakdown as a cause or consequence of secondary injury is debated, recent studies demonstrated that an increased number of endothelial caveolae after medial cerebral artery occlusion (MCAO) correlated with the extent of BBB damage and widespread extravasation of large plasma proteins such as albumin and IgG [33,34].
Further, while the prevailing belief over the last few decades was that BBB breakdown in ischemic injury is caused by destabilization of endothelial tight junction proteins, recent studies instead support a biphasic BBB breakdown profile with an initial early period of increased caveolar transcytosis and therefore elevated transcellular permeability, followed by a later period of tight junction remodeling and elevated paracellular permeability (Figure 2) [33]. Early evidence for this novel mechanism came from ultrastructural studies that revealed minimal damage to tight junctions in areas of massive albumin accumulation following embolic stroke in rats [35]. A further report from Knowland et al. demonstrated that caveolae-mediated transcytosis precedes tight junction structural defects in a transient-MCAO (t-MCAO) stroke model [4]. By expressing green fluorescent protein fused with claudin-5 in a transgenic mouse and visualizing junctions with in vivo time lapse two-photon microscopy, the authors delineated the contribution of caveolar transport of fluorescent albumin from tight junction breakdown-induced transport [4]. They further found that caveolar vesicle number and transcytosis rate increase within 6 hours of t-MCAO without tight junction disruption, which becomes evident after 48-58 hours, concurrent with a second phase of albumin hyperpermeability, where both the paracellular and transcellular routes contribute to albumin transport. Mechanistically, others have shown that an ischemic stroke induces charge neutralization of the endothelial glycocalyx, potentially enabling caveolar transport of native (anionic) albumin [36,37]. Overall, microvascular pathology following ischemic stroke appears to be characterized by an early window of caveolae-mediated transport that precedes extensive neuronal damage.
Figure 2. Overview of temporal changes in BBB integrity following an ischemic stroke.

The healthy BBB is characterized by low rates of transcytosis, an intact negatively charged glycocalyx, and a stringent paracellular barrier that collectively prevent non-specific transport of material into the brain parenchyma. At early stages of injury post-stroke (<6 hours), the dissolution of the glycocalyx coupled with activation of caveolae contribute to a robust transcytosis of plasma proteins across the brain endothelium without evidence of disruption to tight junctions. At late stages after injury (48-72 hours), breakdown of tight junctions allows paracellular transport of proteins in addition to caveolar transport. At this stage, the secondary injury cascade has developed to include neuroinflammation, excitotoxicity, and oxidative stress that all contribute to neuronal cell death.
Age-associated Changes in BBB Integrity
Structural and biochemical changes to the cerebrovasculature have long been observed in aging, which is the greatest risk factor for many neurodegenerative diseases [38]. A meta-analysis of 1,953 individuals demonstrated that BBB permeability increases both in normal aging and in patients with vascular or Alzheimer’s disease, whereas the age-dependent BBB disruption was greater in the diseased groups [39]. It is clear that aging causes pathological changes to vascular structure and function and that in many cases BBB dysfunction precedes neurodegeneration and cognitive impairment [40–42]. Furthermore, while the mechanisms and timing of aging-induced BBB dysfunction are not fully understood, recent studies suggest a prominent role for elevated endothelial caveolae-mediated transcytosis as an early BBB disruption event that contributes to neurodegeneration [6].
As the most prominent protein in the blood that is trafficked intracellularly by caveolae, albumin extravasation may be one of the earliest hallmarks of the aging brain [7]. A recent study by Senatorov et al. demonstrated that in naturally aged mice (12-15 months), there is marked increase of albumin extravasation into the CNS where it binds to the transforming growth factor beta receptor (TGFβR) on astrocytes and triggers hyperexcitability in the hippocampus to yield a phenotype that is indicative of early-stage mild cognitive impairment [7]. Infusion of albumin into the brains of young mice was sufficient to mimic these age-related neurological deficits, while genetic knockdown of TGFβR or injection of a TGFβR small molecule inhibitor reversed these pathological effects. Thus, albumin infiltration through the BBB leading to TGFβ hyperactivation, as mediated by astrocytes, represents an early event in age-related cognitive decline.
The study by Senatorov et al. did not examine why albumin infiltration into the CNS occurred with aging, and indeed, the possibility of upregulated caveolae transport as an age-related BBB defect represents a relatively unexplored connection to neurodegeneration. Along these lines, a seminal paper by Park et al. causally linked acid sphingomyelinase (ASM), a sphingolipid metabolizing enzyme secreted by brain endothelial cells, with age-related neurological phenotypes [6]. The authors suggest that ASM becomes elevated with age and upregulates caveolae-mediated transcytosis via protein phosphatase-1 mediated ezrin/radixin/moesin dephosphorylation. Genetic inhibition of ASM reduced cav-1 phosphorylation, leading to improved BBB integrity and cognitive abilities compared to age-matched controls. Furthermore, conditional transgenic mice engineered to overexpress ASM at a young age demonstrated significant memory impairment and neurodegeneration [6]. These findings suggest that ASM triggers BBB disruption through upregulation of caveolae-mediated transcytosis, which enables albumin transport and accelerates aging-associated cognitive changes.
More broadly, these studies reveal that the loss of BBB integrity, particularly the ability to suppress caveolae-mediated transcytosis, is a hallmark of natural aging. These studies, along with recent revelations in mechanisms of BBB disruption during ischemia, highlight upregulation of caveolae transport as an early pathological event and reveal a possible opportunity to co-opt transport of albumin for brain drug delivery.
Drug Delivery Opportunities
Delivering efficacious concentrations of drug to diseased brain regions is a challenge for developing effective treatments to combat neurodegeneration. As detailed above, caveolae-mediated transcytosis is a transport pathway that is suppressed in healthy BMECs but becomes elevated as an immediate response to ischemic stroke and at early stages of aging [5,6], where it may precede irreversible neurological damage [7,43]. While blood-borne protein transport into the brain parenchyma may contribute to neurodegeneration, these extravasation events also may enable the selective delivery of normally excluded drugs to regions of CNS injury (Figure 3). Nanoparticles and albumin conjugates are promising drug delivery strategies that may be well suited to leverage this window of enhanced caveolae trafficking into the CNS.
Figure 3. Approaches for leveraging caveolae-mediated transcytosis as a drug delivery route to the CNS.
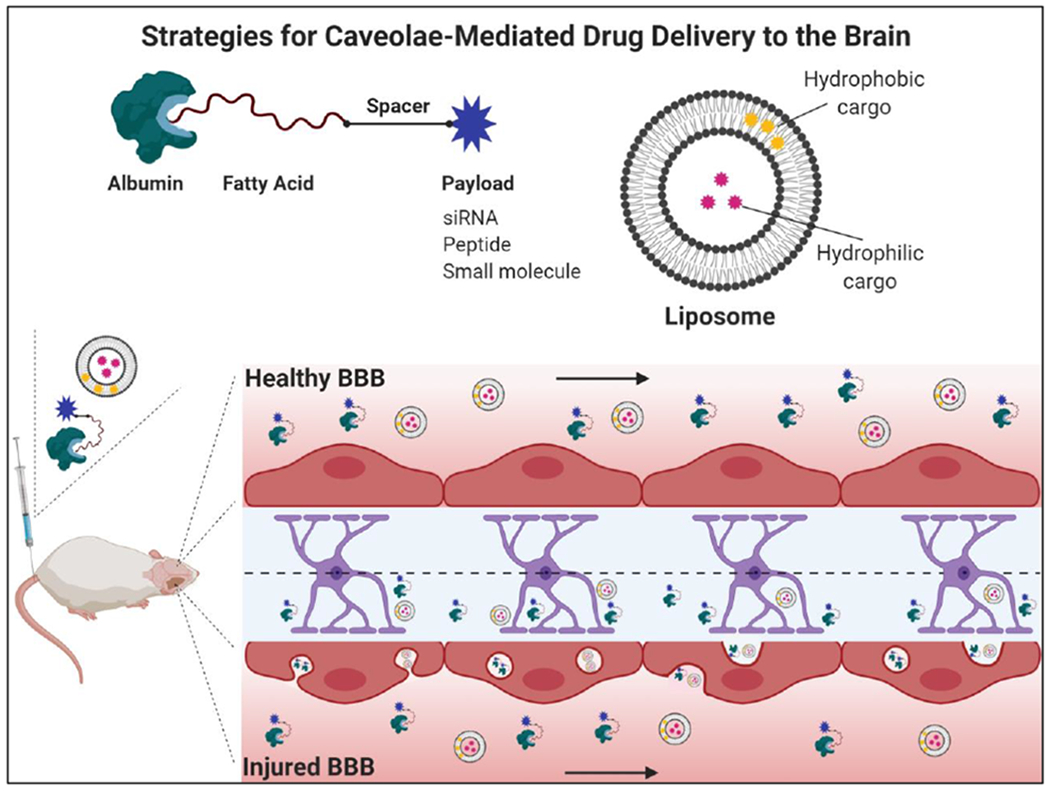
One possibility for targeting caveolae is to conjugate therapeutic compounds to endogenous albumin, which is highly abundant in blood and accumulates in brain regions where BBB integrity is compromised. As a natural lipid carrier, albumin contains binding pockets that interact with fatty acid moieties, which can be chemically engineered to carry a variety of payloads. Liposomal nanocarriers have also been shown to traffic through caveolae following stroke and can deliver both hydrophobic and hydrophilic compounds. In each case, delivery would be expected only at sites of injury where caveolae-mediated transcytosis is selectively upregulated.
One approach to improving the pharmacokinetic properties of rapidly metabolized or excreted compounds is encapsulation in nanoparticles. Nanoparticles are versatile drug carriers that can be functionalized with peptides, proteins, or antibodies to target regions of injury [44]. Liposomes are well-studied carriers for treating ischemic stroke as they can be engineered to enhance CNS bioavailability by appropriately tuning physical and chemical properties (size, charge, and surface properties) [45]. For example, to elucidate the window of maximum liposomal delivery following stroke, Al-Ahmedy et al. monitored fluorescently labeled liposome accumulation in the ischemic hemisphere of mice following t-MCAO; they identified an early window of accumulation (0.5 hours after reperfusion) due to transcellular transport and a delayed window (48 hours after t-MCAO) where both the paracellular and transcellular pathways contribute to liposome delivery [43]. Interestingly, in contrast to treatment at 48 hours, delivery of liposomes in the acute phase preceded histological evidence of neurological damage, suggesting that targeting the neurovascular unit itself to improve BBB integrity may improve outcomes. To investigate if liposome transport was mediated by caveolae, fluorescently labeled liposomes were intravenously administered and subsequently colocalized with brain regions of enhanced cav-1 expression and immunoglobulin penetration [43].
An alternate approach to CNS drug delivery is to design nanoparticles that actively target caveolae as a transcytosis pathway. For example, to leverage the overexpression of gp60 on the glioma endothelium, Lin et al. intravenously administered chemotherapeutics encapsulated in albumin nanoparticles and observed enhanced BBB penetration and glioma targeting via gp60-mediated caveolar transport [46]. However, this approach has only been explored in glioblastoma and the large size (~150 nm) of these biomimetic albumin nanoparticles likely renders them ill-suited for uptake in caveolae. Voigt et al. developed polyanionic lipid nanoparticles that selectively undergo caveolae-mediated transcytosis in endothelial cells [47]. The principle of this technology is that high affinity hydrophobic interactions promotes association of the lipid nanoparticle with caveolar lipid rafts and the negatively charged sulfonate polymer on the nanoparticle surface prevents non-specific electrostatic interaction with the cell membrane [47]. While these nanoparticles were developed for cancer applications and not CNS delivery, this formulation may have potential for targeting the injured cerebrovasculature following stroke.
Despite substantial pre-clinical research on nanoparticle efficacy, a major obstacle to their widespread clinical translation is the possibility of cytotoxicity and preferential accumulation in the liver and spleen [48]. In contrast, albumin-mediated drug delivery can increase circulation half-life of therapeutic cargo and enhance target site accumulation without overt dose-limiting toxicity [49]. As a native carrier of fatty acids, albumin is well-suited for a variety of conjugation platforms, the majority of which have been developed for diabetes, vaccine, and cancer applications [50]. Commercially available Semaglutide leverages a fatty acid modification to promote in vivo association of glucagon-like peptide 1 (GLP-1) with native albumin to extend the peptide bioavailability in the treatment of type 2 diabetes [51]. In the context of vaccines, ‘albumin backpacking’ of antigen and adjuvants with lipophilic albumin binding domains improves lymph node targeting and T-cell priming [52]. Albumin is also an attractive drug carrier for cancer applications due to preferential accumulation in tissues with leaky vasculature [50]. For example, Sarett et al. developed a PEGylated diacyl lipid moiety to link endogenous albumin to siRNAs and achieved uptake in 99% of breast cancer cells with significant target protein knockdown in mice [53]. This approach could possibly be translated to treating CNS diseases, as the albumin-binding conjugate greatly enhances the pharmacokinetic properties of siRNA therapeutics, and the hyperpermeability of tumor vasculature is analogous in many ways to increased caveolin-mediated transport in BMECs under disease conditions. While the transport processes contain a number of differences as well, a notable similarity is that the transport of both albumin and nanoparticles across the leaky tumor endothelium is predicted to be predominantly mediated by active trans-endothelial processes that result in accumulation in the tumor interstitium [54,55].
Overall, while leveraging albumin as a drug carrier has been primarily investigated for cancer and diabetes therapeutics, we suggest this approach is a promising strategy for CNS delivery, as albumin is one of the most prominent proteins in the blood trafficked across the damaged BBB by caveolae. Furthermore, it is likely that the albumin-associated cargos would preferentially target cells in regions of BBB damage, as the healthy BMECs should not transport albumin. The proposed strategy for albumin backpacking involves conjugating drugs to fatty acid molecules that non-covalently interact with endogenous albumin after intravenous injection (Figure 3). An advantage of this approach is that albumin is readily passed through the brain endothelium and is not associated with early endosomes or lysosomal pathways (Figure 1) [4]. After leaking through the BBB, evidence suggests albumin is endocytosed to some degree by astrocytes, neurons, and microglia [56], where it could theoretically deliver a drug payload. To our knowledge, albumin-drug conjugates have not yet been translated to the treatment of CNS disorders in this manner, but they may represent a versatile avenue for localized drug delivery in the early stages of neurodegeneration.
Conclusion
In summary, the restrictive properties of the BBB hinder drug transport into the CNS, spurring research into approaches that exploit endogenous transcytosis pathways to enhance drug delivery. While extensive research has focused on RMT, we posit that emerging data suggests that leveraging caveolae-mediated transport pathways for CNS delivery of biologics is a promising strategy for treating neurodegenerative diseases or combating age-associated cognitive decline. Recent advances highlight robust albumin transport through caveolae as an early pathological response to stroke and natural aging. As such, backpacking of drugs on albumin or other serum proteins could be an unrecognized approach for localized drug delivery during early stages of BBB dysfunction prior to widespread CNS disease.
Acknowledgments
Funding in our laboratories related to the topic of this review is graciously provided by a Chan Zuckerberg Initiative Ben Barres Early Career Acceleration Award (grant 2018-191850 to ESL) and NIH grant R01 HL122347 (to CLD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Pardridge WM, Drug transport across the blood–brain barrier, J. Cereb. Blood Flow Metab. 32 (2012) 1959–1972. 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jones AR, Shusta EV, Blood-Brain Barrier Transport of Therapeutics via Receptor-Mediation, Pharm. Res 24 (2007) 1759–1771. 10.1007/s11095-007-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, Deik AA, Ginty DD, Clish CB, Gu C, Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis, Neuron. 94 (2017) 581–594.e5. 10.1016/j.neuron.2017.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Knowland D, Arac A, Sekiguchi KJ, Hsu M, Lutz SE, Perrino J, Steinberg GK, Barres BA, Nimmerjahn A, Agalliu D, Stepwise Recruitment of Transcellular and Paracellular Pathways Underlies Blood-Brain Barrier Breakdown in Stroke, Neuron. 82 (2014) 603–617. 10.1016/j.neuron.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nag S, Venugopalan R, Stewart DJ, Increased caveolin-1 expression precedes decreased expression of occludin and claudin-5 during blood-brain barrier breakdown, Acta Neuropathol. (Berl.). 114 (2007) 459–469. 10.1007/s00401-007-0274-x. [DOI] [PubMed] [Google Scholar]
- [••6].Park MH, Lee JY, Park KH, Jung IK, Kim K-T, Lee Y-S, Ryu H-H, Jeong Y, Kang M, Schwaninger M, Gulbins E, Reichel M, Kornhuber J, Yamaguchi T, Kim H-J, Kim SH, Schuchman EH, Jin HK, Bae J, Vascular and Neurogenic Rejuvenation in Aging Mice by Modulation of ASM, Neuron. 100 (2018) 167–182.e9. 10.1016/j.neuron.2018.09.010. [DOI] [PubMed] [Google Scholar]; This study identified brain endothelial cell-derived ASM as a cause of BBB disruption in aging, as mediated by increased caveolae internalization. They demonstrate that elevated ASM accelerates neural dysfunction and cognitive impairment.
- [••7].Senatorov VV, Friedman AR, Milikovsky DZ, Ofer J, Saar-Ashkenazy R, Charbash A, Jahan N, Chin G, Mihaly E, Lin JM, Ramsay HJ, Moghbel A, Preininger MK, Eddings CR, Harrison HV, Patel R, Shen Y, Ghanim H, Sheng H, Veksler R, Sudmant PH, Becker A, Hart B, Rogawski MA, Dillin A, Friedman A, Kaufer D, Blood-brain barrier dysfunction in aging induces hyperactivation of TGFβ signaling and chronic yet reversible neural dysfunction, Sci. Transl. Med 11 (2019). 10.1126/scitranslmed.aaw8283. [DOI] [PubMed] [Google Scholar]; The authors found that BBB disruption and subsequent albumin accumulation in the brain are early hallmarks of aging. Furthermore, serum albumin activates the TGFβ pathway in astrocytes, accelerating neurodegeneration. Infusion of albumin into young rodents was sufficient to induce age-related cognitive impairment, while inhibiting TGFβ signaling in old mice reversed pathological outcomes.
- [8].Gomes MJ, Martins S, Sarmento B, siRNA as a tool to improve the treatment of brain diseases: Mechanism, targets and delivery, Ageing Res. Rev. 21 (2015) 43–54. 10.1016/j.arr.2015.03.001. [DOI] [PubMed] [Google Scholar]
- [9].Bock MD, Haver VV, Vandenbroucke RE, Decrock E, Wang N, Leybaert L, Into rather unexplored terrain—transcellular transport across the blood-brain barrier, Glia. 64 (2016) 1097–1123. 10.1002/glia.22960. [DOI] [PubMed] [Google Scholar]
- [10].Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ, Structure and function of the blood–brain barrier, Neurobiol. Dis 37 (2010) 13–25. 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- [11].Parton RG, Simons K, The multiple faces of caveolae, Nat. Rev. Mol. Cell Biol. 8 (2007) 185–194. 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- [12].Zhao Y-L, Song J-N, Zhang M, Role of caveolin-1 in the biology of the blood-brain barrier, Rev. Neurosci 25 (2014) 247–254. 10.1515/revneuro-2013-0039. [DOI] [PubMed] [Google Scholar]
- [13].Tuma PL, Hubbard AL, Transcytosis: crossing cellular barriers, Physiol. Rev 83 (2003) 871–932. 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- [14].Kim MP, Park SI, Kopetz S, Gallick GE, Src family kinases as mediators of endothelial permeability: effects on inflammation and metastasis, Cell Tissue Res. 335 (2009) 249–259. 10.1007/s00441-008-0682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lajoie P, Nabi IR, Chapter 3 - Lipid Rafts, Caveolae, and Their Endocytosis, in: Jeon KW (Ed.), Int. Rev. Cell Mol. Biol, Academic Press, 2010: pp. 135–163. 10.1016/S1937-6448(10)82003-9. [DOI] [PubMed] [Google Scholar]
- [16].Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB, Gp60 Activation Mediates Albumin Transcytosis in Endothelial Cells by Tyrosine Kinase-dependent Pathway, J. Biol. Chem 272 (1997) 25968–25975. 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- [17].Schnitzer JE, Oh P, Albondin-mediated capillary permeability to albumin. Differential role of receptors in endothelial transcytosis and endocytosis of native and modified albumins., J. Biol. Chem 269(1994)6072–6082. [PubMed] [Google Scholar]
- [18].Merlot AM, Kalinowski DS, Richardson DR, Unraveling the mysteries of serum albumin—more than just a serum protein, Front. Physiol 5 (2014). 10.3389/fphys.2014.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C, MSFD2A is critical for the formation and function of the blood brain barrier, Nature. 509 (2014) 507–511. 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh ELK, Silver DL, Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid, Nature. 509 (2014) 503–506. 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- [21].Zhang Y, Pardridge WM, Blood–brain barrier targeting of BDNF improves motor function in rats with middle cerebral artery occlusion, Brain Res. 1111 (2006) 227–229. 10.1016/j.brainres.2006.07.005. [DOI] [PubMed] [Google Scholar]
- [22].Song B-W, Vinters HV, Wu D, Pardridge WM, Enhanced Neuroprotective Effects of Basic Fibroblast Growth Factor in Regional Brain Ischemia after Conjugation to a Blood-Brain Barrier Delivery Vector, J. Pharmacol. Exp. Ther 301 (2002) 605–610. 10.1124/jpet.301.2.605. [DOI] [PubMed] [Google Scholar]
- [23].Johnsen KB, Burkhart A, Thomsen LB, Andresen TL, Moos T, Targeting the transferrin receptor for brain drug delivery, Prog. Neurobiol 181 (2019) 101665 10.1016/j.pneurobio.2019.101665. [DOI] [PubMed] [Google Scholar]
- [24].Boado RJ, Zhou Q-H, Lu JZ, Hui EK-W, Pardridge WM, Pharmacokinetics and Brain Uptake of a Genetically Engineered Bifunctional Fusion Antibody Targeting the Mouse Transferrin Receptor, Mol. Pharm 7 (2010) 237–244. 10.1021/mp900235k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hervé F, Ghinea N, Scherrmann J-M, CNS Delivery Via Adsorptive Transcytosis, AAPS J. 10 (2008) 455–472. 10.1208/s12248-008-9055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bickel U, Yoshikawa T, Pardridge WM, Delivery of peptides and proteins through the blood–brain barrier, Adv. Drug Deliv. Rev. 46 (2001) 247–279. 10.1016/S0169-409X(00)00139-3. [DOI] [PubMed] [Google Scholar]
- [27].Kang Y-S, Pardridge WM, Brain Delivery of Biotin Bound to a Conjugate of Neutral Avidin and Cationized Human Albumin, Pharm. Res 11 (1994) 1257–1264. 10.1023/A:1018982125649. [DOI] [PubMed] [Google Scholar]
- [28].Terasaki Y, Liu Y, Hayakawa K, Pham LD, Lo EH, Ji X, Arai K, Mechanisms of Neurovascular Dysfunction in Acute Ischemic Brain, Curr. Med. Chem 21 (2014) 2035–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Brouns R, Wauters A, Surgeloose DD, Mariën P, Deyn PPD, Biochemical Markers for Blood-Brain Barrier Dysfunction in Acute Ischemic Stroke Correlate with Evolution and Outcome, Eur. Neurol 65 (2011) 23–31. 10.1159/000321965. [DOI] [PubMed] [Google Scholar]
- [30].Prakash R, Carmichael ST, Blood-brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury, Curr. Opin. Neurol 28 (2015) 556–564. 10.1097/WCO.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Stokum JA, Gerzanich V, Simard JM, Molecular pathophysiology of cerebral edema, J. Cereb. Blood Flow Metab. 36 (2016) 513–538. 10.1177/0271678X15617172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Andrea Kassner, Zamir Merali, Assessment of Blood–Brain Barrier Disruption in Stroke, Stroke. 46 (2015) 3310–3315. 10.1161/STROKEAHA.115.008861. [DOI] [PubMed] [Google Scholar]
- [33].Nahirney PC, Reeson P, Brown CE, Ultrastructural analysis of blood–brain barrier breakdown in the peri-infarct zone in young adult and aged mice, J. Cereb. Blood Flow Metab. 36 (2016) 413–425. 10.1177/0271678X15608396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Haley MJ, Lawrence CB, The blood–brain barrier after stroke: Structural studies and the role of transcytotic vesicles, J. Cereb. Blood Flow Metab. 37 (2017) 456–470. 10.1177/0271678X16629976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Krueger M, Härtig W, Reichenbach A, Bechmann I, Michalski D, Blood-Brain Barrier Breakdown after Embolic Stroke in Rats Occurs without Ultrastructural Evidence for Disrupting Tight Junctions, PLoS ONE. 8 (2013) e56419 10.1371/journal.pone.0056419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].DellaValle Brian, Hasseldam Henrik, Johansen Flemming F., Iversen Helle K., Rungby Jørgen, Hempel Casper, Multiple Soluble Components of the Glycocalyx Are Increased in Patient Plasma After Ischemic Stroke, Stroke. 50 (2019) 2948–2951. 10.1161/STROKEAHA.119.025953. [DOI] [PubMed] [Google Scholar]
- [37].Ikonomidis I, Frogoudaki A, Vrettou A-R, Andreou I, Palaiodimou L, Katogiannis K, Liantinioti C, Vlastos D, Zervas P, Varoudi M, Lambadiari V, Triantafyllidi H, Pavlidis G, Efentakis P, Tsoumani M, Tsantes AE, Parissis J, Revela I, Andreadou I, Tsivgoulis G, Impaired Arterial Elastic Properties and Endothelial Glycocalyx in Patients with Embolic Stroke of Undetermined Source, Thromb. Haemost 119 (2019) 1860–1868. 10.1055/s-0039-1694752. [DOI] [PubMed] [Google Scholar]
- [38].Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, Schneider JA, Wellington C, Wilcock DM, Zipfel GJ, Zlokovic B, Bain LJ, Bosetti F, Galis ZS, Koroshetz W, Carrillo MC, Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease, Alzheimers Dement. 11 (2015) 710–717. 10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Farrall AJ, Wardlaw JM, Blood–brain barrier: Ageing and microvascular disease – systematic review and meta-analysis, Neurobiol. Aging 30 (2009) 337–352. 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- [•40].Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Chui HC, Law M, Toga AW, Zlokovic BV, Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction, Nat. Med 25 (2019) 270–276. 10.1038/s41591-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using cerebrospinal fluid markers and magnetic resonance imaging, the authors demonstrate that BBB breakdown is an early marker of cognitive impairment, independent of tau or amyloid-β. This study highlights the importance of the neurovasculature as a biomarker for age-associated cognitive decline.
- [41].Zlokovic BV, Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders, Nat. Rev. Neurosci 12 (2011) 723–738. 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zoltan Ungvari, Stefano Tarantini, Donato Anthony J., Veronica Galvan, Anna Csiszar, Mechanisms of Vascular Aging, Circ. Res 123 (2018) 849–867. 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [•43].Al-Ahmady ZS, Jasim D, Ahmad SS, Wong R, Haley M, Coutts G, Schiessl I, Allan SM, Kostarelos K, Selective Liposomal Transport through Blood Brain Barrier Disruption in Ischemic Stroke Reveals Two Distinct Therapeutic Opportunities, ACS Nano. 13 (2019) 12470–12486. 10.1021/acsnano.9b01808. [DOI] [PubMed] [Google Scholar]; This study identifies two windows of enhanced liposomal delivery, consistent with the biphasic profile of BBB permeability following an ischemic stroke. The authors demonstrate that the liposomes are selectively delivered to regions of brain injury via caveolae-mediated transcytosis across the brain endothelium.
- [44].Saraiva C, Praga C, Ferreira R, Santos T, Ferreira L, Bernardino L, Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases, J. Controlled Release. 235 (2016) 34–47. 10.1016/j.jconrel.2016.05.044. [DOI] [PubMed] [Google Scholar]
- [45].Lai F, Fadda AM, Sinico C, Liposomes for brain delivery, Expert Opin. Drug Deliv. 10 (2013) 1003–1022. 10.1517/17425247.2013.766714. [DOI] [PubMed] [Google Scholar]
- [46].Lin T, Zhao P, Jiang Y, Tang Y, Jin H, Pan Z, He H, Yang VC, Huang Y, Blood–Brain-Barrier-Penetrating Albumin Nanoparticles for Biomimetic Drug Delivery via Albumin-Binding Protein Pathways for Antiglioma Therapy, ACS Nano. 10 (2016) 9999–10012. 10.1021/acsnano.6b04268. [DOI] [PubMed] [Google Scholar]
- [47].Voigt J, Christensen J, Shastri VP, Differential uptake of nanoparticles by endothelial cells through polyelectrolytes with affinity for caveolae, Proc. Natl. Acad. Sci 111 (2014) 2942–2947. 10.1073/pnas.1322356111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bahadar H, Maqbool F, Niaz K, Abdollahi M, Toxicity of Nanoparticles and an Overview of Current Experimental Models, Iran. Biomed. J 20 (2016) 1–11. 10.7508/ibj.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kratz F, Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles, J. Controlled Release. 132 (2008) 171–183. 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- [•50].Hoogenboezem EN, Duvall CL, Harnessing albumin as a carrier for cancer therapies, Adv. Drug Deliv. Rev. 130 (2018) 73–89. 10.1016/j.addr.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper comprehensively reviews design strategies for leveraging albumin as a drug carrier in cancer applications. In addition to highlighting the motivation and advantages of albumin-based drug delivery approaches, this review describes an array of clinical and pre-clinical studies.
- [51].Lau J, Bloch P, Schaffer L, Pettersson I, Spetzler J, Kofoed J, Madsen K, Knudsen LB, McGuire J, Steensgaard DB, Strauss HM, Gram DX, Knudsen SM, Nielsen FS, Thygesen P, Reedtz-Runge S, Kruse T, Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide, J. Med. Chem 58 (2015) 7370–7380. 10.1021/acs.jmedchem.5b00726. [DOI] [PubMed] [Google Scholar]
- [52].Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ, Structure-based programming of lymph-node targeting in molecular vaccines, Nature. 507 (2014) 519–522. 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sarett SM, Werfel TA, Lee L, Jackson MA, Kilchrist KV, Brantley-Sieders D, Duvall CL, Lipophilic siRNA targets albumin in situ and promotes bioavailability, tumor penetration, and carrier-free gene silencing, Proc. Natl. Acad. Sci 114 (2017) E6490–E6497. 10.1073/pnas.1621240114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sindhwani S, Syed AM, Ngai J, Kingston BR, Maiorino L, Rothschild J, MacMillan P, Zhang Y, Rajesh NU, Hoang T, Wu JLY, Wilhelm S, Zilman A, Gadde S, Sulaiman A, Ouyang B, Lin Z, Wang L, Egeblad M, Chan WCW, The entry of nanoparticles into solid tumours, Nat. Mater 19 (2020) 566–575. 10.1038/s41563-019-0566-2. [DOI] [PubMed] [Google Scholar]
- [55].Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, Rabinowitz JD, Metallo CM, Heiden MGV, Bar-Sagi D, Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells, Nature. 497 (2013) 633–637. 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].van Vliet EA, da Costa Araújo S, Redeker S, van Schaik R, Aronica E, Gorter JA, Blood–brain barrier leakage may lead to progression of temporal lobe epilepsy, Brain. 130 (2007) 521–534. 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]


