Abstract
Background & Aims
Chronic liver disease (CLD) represents a major global health burden. We undertook this study to identify the factors associated with adverse outcomes in patients with CLD who acquire the novel coronavirus-2019 (COVID-19).
Methods
We conducted a multi-center, observational cohort study across 21 institutions in the United States (US) of adult patients with CLD and laboratory-confirmed diagnosis of COVID-19 between March 1, 2020 and May 30, 2020. We performed survival analysis to identify independent predictors of all-cause mortality and COVID-19 related mortality, and multivariate logistic regression to determine the risk of severe COVID-19 in patients with CLD.
Results
Of the 978 patients in our cohort, 867 patients (mean age 56.9 ± 14.5 years, 55% male) met inclusion criteria. The overall all-cause mortality was 14.0% (n = 121), and 61.7% (n = 535) had severe COVID-19. Patients presenting with diarrhea or nausea/vomiting were more likely to have severe COVID-19. The liver-specific factors associated with independent risk of higher overall mortality were alcohol-related liver disease (ALD) (hazard ratio [HR] 2.42, 95% confidence interval [CI] 1.29–4.55), decompensated cirrhosis (HR 2.91 [1.70–5.00]) and hepatocellular carcinoma (HCC) (HR 3.31 [1.53–7.16]). Other factors were increasing age, diabetes, hypertension, chronic obstructive pulmonary disease and current smoker. Hispanic ethnicity (odds ratio [OR] 2.33 [1.47–3.70]) and decompensated cirrhosis (OR 2.50 [1.20–5.21]) were independently associated with risk for severe COVID-19.
Conclusions
The risk factors which predict higher overall mortality among patients with CLD and COVID-19 are ALD, decompensated cirrhosis and HCC. Hispanic ethnicity and decompensated cirrhosis are associated with severe COVID-19. Our results will enable risk stratification and personalization of the management of patients with CLD and COVID-19. Clinicaltrials.gov number NCT04439084
Keywords: COVID-19, Cirrhosis, Alcohol, Mortality
Abbreviations used in this paper: ALD, alcohol-related liver disease; CI, confidence interval; CLD, chronic liver disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; HCC, hepatocellular carcinoma; ICD, International Classification of Diseases; ICU, intensive care unit; HR, hazard ratio; IQR, interquartile range; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
What You Need to Know.
Background
The clinical outcomes of patients with chronic liver disease (CLD) and the novel coronavirus disease 2019 (COVID-19) are not well-defined. Also, it is not clear which patients with CLD are most vulnerable to adverse outcomes from COVID-19.
Findings
In this large study of 867 patients from 21 centers across the US with CLD with COVID-19 we determine that patients with alcohol-related liver disease (ALD), decompensated cirrhosis, and hepatocellular carcinoma have a high risk for all-cause mortality from COVID-19. Lack of adequate COVID-19 testing during the early phase of the pandemic could have led to decreased representation of patients with CLD and mild COVID-19 in our cohort.
Implications for patient care
Our findings will enable risk stratification and personalized management of patients with CLD who acquire COVID-19. Moreover, the association between ALD and poor outcomes with COVID-19 has broad public health implications because of recent concerns about increased alcohol consumption during the pandemic.
Chronic liver disease (CLD) is a major international public health concern, and its prevalence has been increasing over the past 2 decades.1 , 2 Around 1.5 billion people have CLD worldwide, and it causes more than 2 million deaths per year.3 , 4 With the rapid spread of the global pandemic of coronavirus disease 2019 (COVID-19), there has been significant concern that patients with CLD represent a vulnerable population at higher risk for complications.
Initial concerns were based on the observation that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, is genetically related to SARS-CoV and Middle East respiratory syndrome coronavirus, both of which impair liver function.5 , 6 These concerns appear to have been substantiated, with early studies reporting elevations in liver enzymes in up to 50% of patients with COVID-19, with higher prevalence in those with worse prognosis.7 , 8 Preliminary studies from the United States (US) and Europe also suggest that patients with CLD who acquire COVID-19 have high rates of hospitalization and mortality.9, 10, 11 Although these reports raise the alarm, it is not known whether all patients with CLD are affected equally or whether there are specific subgroups at higher risk for COVID-19 related mortality and morbidity.
Identifying predictors of mortality will allow for risk stratification of patients with CLD affected by COVID-19 and help improve healthcare delivery. To comprehensively characterize the clinical outcomes of COVID-19 in patients with CLD, we undertook a multicenter, observational study of patients with CLD who were diagnosed with COVID-19 in 21 centers across the US.
Methods
Study Design
This is a multicenter observational cohort study. The consortium of investigators to study COVID-19 in chronic liver disease (COLD) study was formed on April 14, 2020, and accrual of data started immediately (registered Clinicaltrials.gov NCT04439084). A total of 21 centers from the US participated in the study (Supplementary Table 1). The institutional review board of each participating center reviewed and approved the study protocol. Inclusion criteria constituted age older than 18 years, laboratory-confirmed diagnosis of COVID-19, and presence of preexisting CLD (according to predefined International Classification of Diseases [ICD]-10 codes listed in Supplementary Table 2 and confirmed by manual chart review). Patients who had undergone liver transplantation were excluded. Patients with COVID-19 diagnosis based on clinical suspicion were excluded. All participating institutions independently identified patients meeting inclusion criteria by searching their electronic medical records and collected data as per the previously established data accrual plan. The study retrospectively identified cases diagnosed between March 1 and April 14, and subsequent cases diagnosed with COVID-19 between April 15 and May 30, 2020 were identified prospectively. All data were collected until death or date of last follow-up. Death was attributed to COVID-19 if it was clinically related to COVID-19 illness, and there were no other unrelated causes of death.12
Data Collection
We collected de-identified data using 170 structured and text variables in 10 different categories. Complete details on the data collection tool are available in Supplementary Table 3. Diagnosis of cirrhosis was confirmed by documentation of fibrosis by magnetic resonance elastography, fibroscan, Fibrosis-4, or biopsy, which was available in 75% of patients (655/867). Diagnosis of cirrhosis was ascertained in other patients by detailed chart review for clinical, radiologic, or biochemical evidence of liver cirrhosis. Alcohol use was defined as no drinking, social drinking (2 drinks/day for men and up to 1 drink/day for women), or current daily drinking (drinking more than social drinking limits on a daily basis).13 Data on decompensation were collected from chart review for clinical events. The presence and severity of ascites, encephalopathy, variceal bleeding, and other major decompensating events at baseline and during COVID-19 were collected. If patients developed acute worsening of ascites, hepatic encephalopathy, or variceal bleeding during COVID-19, they were deemed to have decompensated during COVID-19.
Statistical Analysis
A predefined statistical data analysis plan was followed. Continuous variables are expressed as medians and interquartile ranges (IQRs) or mean and standard deviation, as appropriate. Categorical variables are summarized as counts and percentages. The statistical significance of differences between groups was evaluated by using the independent t test or the Mann-Whitney U test for continuous variables and the χ2 test for categorical variables. No imputation was made for missing data. The primary outcome studied was overall survival. The secondary outcomes were COVID-19 related mortality and a composite endpoint for severe COVID-19 (death, hospitalization, oxygen requirement, intensive care unit [ICU] admission, requirement of vasopressors, or mechanical ventilation).14
To determine the independent risk factors for the outcome, we performed univariate Cox proportional hazards analysis. Variables were selected for inclusion in the models on the basis of clinical plausibility, statistical significance in the univariate model, and availability in more than 90% of the patients. Multivariate analysis was performed by using Cox proportional hazards analysis for outcomes regarding all-cause mortality and deaths due to COVID-19. To investigate the independent determining factors for mortality among patients with and without cirrhosis, analyses were performed by using backward stepwise logistic regression (probability to enter = 0.05 and probability to remove = 0.1) because of insufficient outcome events. All analyses were performed by using STATA 15.1 (StataCorp, College Station, TX). Two-sided P values were used and considered statistically significant if P ≤ .05. All authors had access to the study data and reviewed and approved the final manuscript.
Results
Demographic and Clinical Features of the Study Cohort
We collected data from 21 institutions across 13 states representing all 5 regions of the United States (Supplementary Table 1). Data were collected from a total of 978 patients with CLD, of whom 867 patients met the inclusion criteria (Supplementary Figure 1). The largest proportion of the cases were from the Northeast (41.8%) and Southeast (28.4%) regions of the US. The overall all-cause mortality in the cohort was 14.0% (n = 121), and 61.7% (n = 535) patients experienced the composite endpoint of severe COVID-19. Table 1 shows the demographic and clinical characteristics of the patients in the overall cohort and also their proportional distribution based on clinical outcomes. The mean age at the time of COVID-19 diagnosis was 56.9 ± 14.5 years, and 271 patients (31.3%) were ≥65 years (Supplementary Figure 2). Patient ethnicity was relatively evenly distributed: non-Hispanic white (268, 30.9%), non-Hispanic black (267, 30.8%), or Hispanic (219, 25.3%) (Supplementary Figure 3). The overall median follow-up of patients was 38 days (interquartile range [IQR], 15–94). Most patients (776, 89.5%) had at least 1 comorbid medical condition in addition to CLD, whereas 261 (30.1%) had more than 3 nonhepatic comorbidities. The most common comorbidities were hypertension (492, 56.8%), diabetes mellitus (372, 42.9%), obesity (365, 42.1%), and hyperlipidemia (335, 38.6%) (Supplementary Figure 4).
Supplementary Figure 1.
Patient study cohort. The flowchart shows how the study cohort was selected. CLD, chronic liver disease; COVID-19, coronavirus disease 2019.
Table 1.
Clinical Characteristics of Patients With Chronic Liver Disease and Clinical Outcome of COVID-19
| Total (n = 867) | All-cause mortality status (n = 817) |
P value | Severe COVID-19 (n = 857) |
P value | |||
|---|---|---|---|---|---|---|---|
| Alive (n = 696) | Died (n = 121) | No (n = 322) | Yes (n = 535) | ||||
| Demographic factors | |||||||
| Age (y) | 56.9 ± 14.5 | 55.7 ± 14.4 | 65.4 ± 12.7 | <.001 | 52.1 ± 13.7 | 59.8 ± 14.3 | <.001 |
| <65 | 596 (68.7) | 497 (71.4) | 62 (51.2) | <.001 | 260 (80.8) | 330 (61.7) | <.001 |
| ≥65 | 271 (31.3) | 199 (28.6) | 59 (48.8) | 62 (19.3) | 205 (38.3) | ||
| Gender (male, %) | 473 (54.7) | 377 (54.3) | 68 (56.2) | .702 | 159 (49.5) | 308 (57.6) | .022 |
| Race/ethnicity | .431 | .020 | |||||
| Non-Hispanic white | 268 (30.9) | 204 (29.3) | 46 (38.0) | 107 (33.2) | 156 (29.2) | ||
| Non-Hispanic black | 267 (30.8) | 217 (31.2) | 37 (30.6) | 112 (34.8) | 152 (28.4) | ||
| Hispanic | 219 (25.3) | 183 (26.3) | 25 (20.7) | 69 (21.4) | 148 (27.7) | ||
| Non-Hispanic Asian | 43 (5.0) | 31 (4.5) | 6 (5.0) | 14 (4.3) | 29 (5.7) | ||
| Other | 38 (4.4) | 32 (4.6) | 5 (4.15) | 8 (2.5) | 30 (5.4) | ||
| Missing | 32 (3.6) | 29 (4.2) | 2 (1.7) | 12 (3.7) | 20 (3.7) | ||
| Liver-related factors | |||||||
| Etiology | <.001 | <.001 | |||||
| HCV | 190 (21.9) | 143 (20.6) | 34 (28.1) | 56 (17.4) | 130 (24.3) | ||
| HBV | 62 (7.2) | 49 (7.0) | 5 (4.1) | 25 (7.8) | 37 (6.9) | ||
| NAFLD | 456 (52.6) | 394 (56.6) | 46 (38.0) | 199 (61.8) | 256 (47.9) | ||
| ALD | 94 (10.8) | 58 (8.3) | 28 (23.1) | 18 (5.6) | 72 (13.5) | ||
| Other | 65 (7.5) | 52 (7.5) | 8 (6.6) | 24 (7.5) | 40 (7.5) | ||
| Missing | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Cirrhosis | <.001 | <.001 | |||||
| No cirrhosis | 620 (71.5) | 529 (76.0) | 62 (51.2) | 254 (78.9) | 363 (67.9) | ||
| Compensated cirrhosis | 134 (15.4) | 107 (15.4) | 19 (15.7) | 48 (14.9) | 83 (15.5) | ||
| Decompensated cirrhosis | 93 (10.7) | 48 (6.9) | 38 (31.4) | 14 (4.3) | 77 (14.4) | ||
| Missing | 20 (2.3) | 12 (1.7) | 2 (1.7) | 6 (1.9) | 12 (2.2) | ||
| Hepatocellular carcinoma | 22 (2.5) | 10 (1.4) | 9 (7.4) | <.001 | 3 (0.9) | 18 (3.4) | .026 |
| Comorbidities | |||||||
| Diabetes | 372 (42.9) | 294 (42.2) | 66 (54.5) | .012 | 110 (34.2) | 259 (48.4) | <.001 |
| Hypertension | 492 (56.8) | 387 (55.6) | 83 (68.6) | .008 | 165 (51.2) | 321 (60.0) | .012 |
| Obesity | 365 (42.1) | 305 (43.8) | 47 (38.8) | .307 | 150 (46.6) | 213 (39.8) | .052 |
| Hyperlipidemia | 335 (38.6) | 273 (39.2) | 53 (43.0) | .419 | 113 (35.1) | 218 (40.8) | .100 |
| Cardiovascular disease | 150 (17.3) | 111 (16.0) | 33 (27.3) | .003 | 32 (9.9) | 116 (21.7) | <.001 |
| HIV | 24 (2.8) | 21 (3.0) | 1 (0.8) | .169 | 8 (2.5) | 16 (3.0) | .664 |
| COPD | 77 (8.9) | 54 (7.8) | 20 (16.5) | .002 | 15 (4.7) | 62 (11.6) | .001 |
| Asthma | 91 (10.5) | 78 (11.2) | 10 (8.3) | .335 | 29 (9.0) | 61 (11.4) | .268 |
| Other cancer | 68 (7.8) | 48 (6.9) | 15 (12.4) | .036 | 21 (6.5) | 45 (8.4) | .315 |
| Behavioral factors | |||||||
| Alcohol use | <.001 | .001 | |||||
| Current daily drinking | 107 (12.3) | 75 (10.8) | 25 (20.7) | 34 (10.6) | 70 (13.1) | ||
| Social drinking | 532 (61.3) | 424 (60.9) | 81 (66.9) | 183 (56.8) | 345 (64.5) | ||
| Do not drink currently | 172 (19.8) | 153 (22.0) | 10 (8.3) | 85 (26.4) | 85 (15.9) | ||
| Missing | 56 (6.5) | 44 (6.3) | 5 (4.1) | 20 (6.2) | 35 (6.5) | ||
| Smoking | |||||||
| Current smoker | 95 (10.9) | 70 (10.1) | 19 (15.7) | <.001 | 35 (10.9) | 59 (11.0) | .032 |
| Past smoker | 259 (29.8) | 195 (28.0) | 50 (41.3) | 82 (25.5) | 175 (32.7) | ||
| Never smoker | 482 (55.6) | 414 (59.5) | 46 (38.0) | 199 (61.8) | 278 (52.0) | ||
| Missing | 31 (3.6) | 24 (3.4) | 6 (4.9) | 6 (1.9) | 23 (4.3) | ||
| Opioid use | 31 (3.6) | 23 (3.3) | 2 (1.7) | .330 | 8 (2.5) | 22 (4.1) | .209 |
| Marijuana use | 24 (2.8) | 17 (2.4) | 5 (4.1) | .548 | 10 (3.1) | 13 (2.4) | .553 |
| Treatment | |||||||
| Remdesivir | 39 (4.5) | 31 (4.5) | 5 (4.1) | .874 | 0 (0.0) | 39 (7.3) | <.001 |
| Steroids | 54 (6.2) | 44 (6.3) | 10 (8.3) | .427 | 4 (1.2) | 50 (9.4) | <.001 |
| Hydroxychloroquine | 87 (10.0) | 69 (9.9) | 12 (9.9) | .999 | 4 (1.2) | 83 (15.5) | <.001 |
| Azithromycin | 101 (11.7) | 78 (11.2) | 21 (17.4) | .056 | 25 (7.8) | 76 (14.2) | .005 |
| Hydroxychloroquine + azithromycin | 135 (15.6) | 95 (13.7) | 38 (31.4) | <.001 | 4 (1.2) | 131 (24.5) | <.001 |
NOTE. Data are expressed as mean ± standard deviation or number (proportion). Boldface indicates statistical significance.
ALD, alcohol-related liver disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; HBV, hepatitis B virus infection; HCV, hepatitis C virus infection; HIV, human immunodeficiency virus; NAFLD, nonalcoholic fatty liver disease.
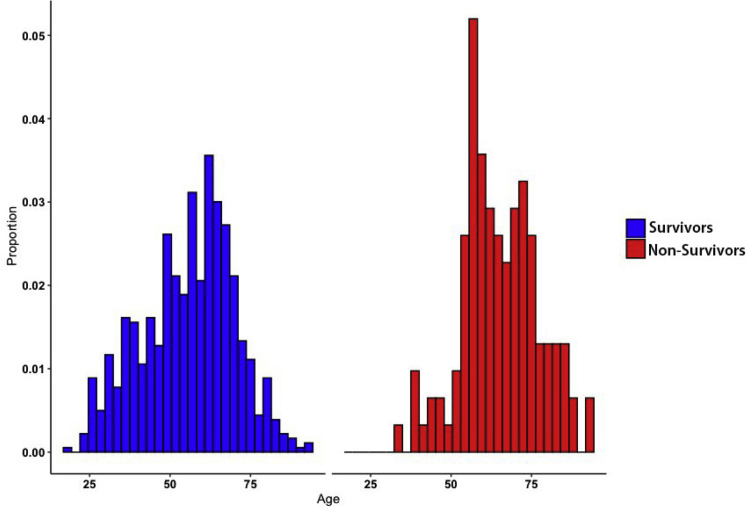
Supplementary Figure 2.
Age at time of diagnosis of COVID-19 in patients with CLD stratified by overall mortality. Histogram shows distribution of age (years) in the entire patient cohort compared with deceased patients. CLD, chronic liver disease; COVID-19, coronavirus disease 2019.
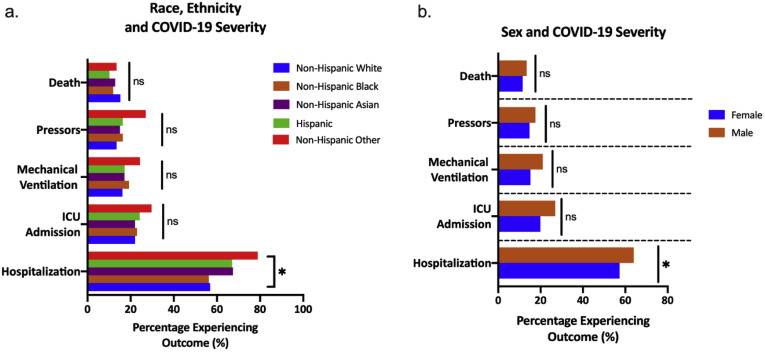
Supplementary Figure 3.
Patient demographics stratified by clinical outcomes. (A) Clinical outcomes of patients with CLD and COVID-19 stratified by race and ethnicity. (B) Clinical outcomes of patients with CLD and COVID-19 stratified by sex. CLD, chronic liver disease; COVID-19, coronavirus disease 2019; ICU, intensive care unit; ns, not significant.
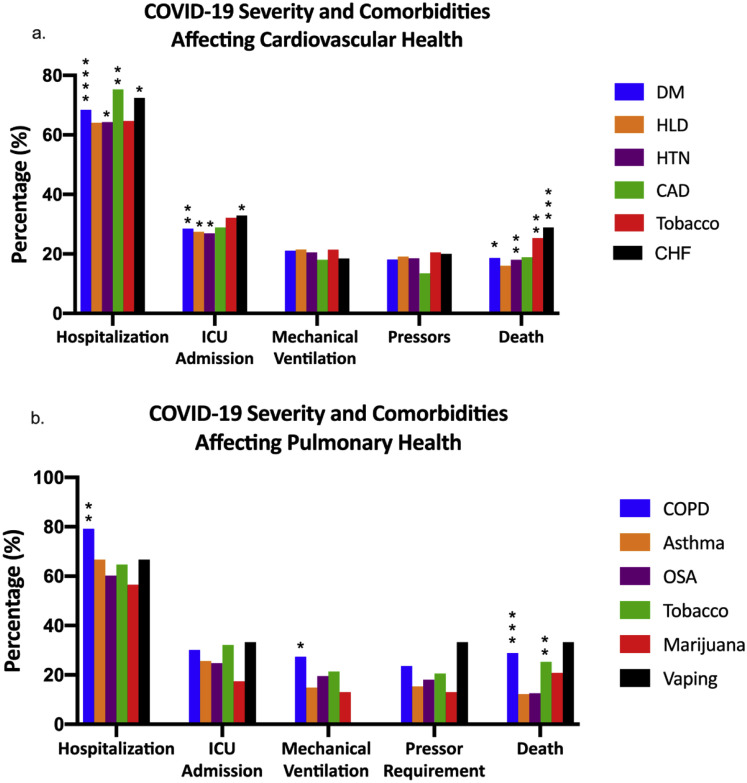
Supplementary Figure 4.
Comorbidities in patients with CLD and COVID-19. (A) Clinical severity of patients with CLD and COVID-19 CLD stratified by comorbidities that affect cardiovascular health. (B) Clinical severity of patients with CLD and COVID-19 stratified by comorbidities that affect pulmonary health. Graph shows the percentage of patients with a specific comorbidity who had these outcomes. Significance determined by comparing clinical outcomes in patients with (shown) vs those without (not shown) the specific comorbidity. CAD, coronary artery disease; CHF, congestive heart failure; CLD, chronic liver disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; DM, diabetes mellitus; HLD, hyperlipidemia; HTN, hypertension; ICU, intensive care unit; OSA, obstructive sleep apnea. ∗indicates a significantly higher proportion. ∗P < .05; ∗∗P < 0.01; ∗∗∗P < .001; ∗∗∗∗P < .0001; ∗∗∗∗∗P < .00001.
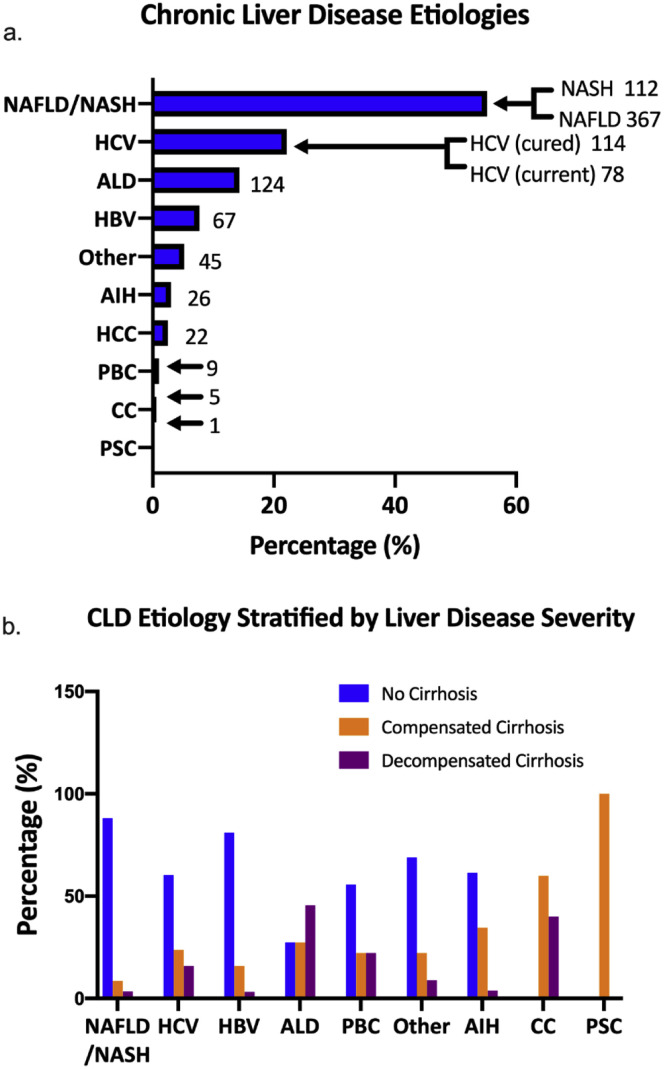
The most common cause of CLD was nonalcoholic fatty liver disease (456, 52.6%), followed by hepatitis C virus infection (190, 21.9%), alcohol-related liver disease (ALD) (94, 10.8%), and hepatitis B virus infection (62, 7.2%) (Supplementary Figures 5 and 6). The majority of patients had non-cirrhotic stage disease (620, 71.5%); 227 patients (26.2%) had a diagnosis of cirrhosis. Most patients with cirrhosis were well-compensated at the time of inclusion (134, 59.1%), with 93 patients (40.9%) having decompensated cirrhosis before diagnosis with COVID-19. Among patients with decompensated cirrhosis, 71 (76.3%) had ascites, 51 (54.8%) had hepatic encephalopathy, 24 (25.8%) had history of variceal bleeding, and 10 (10.8%) had other decompensating events. Among the patients with preexisting hepatocellular carcinoma (HCC) (22, 2.5%), 8 (36.4%) of them had received locoregional therapy, 2 (9.1%) had received immunotherapy, and none of them were on tyrosine kinase inhibitors.
Supplementary Figure 5.
Etiology of CLD among patients with COVID-19. (A) Prevalence of different etiologies of CLD in patients with COVID-19. (B) Stage of CLD in patients with COVID-19. AIH, autoimmune hepatitis; ALD, alcohol-related liver disease; CC, cholangiocarcinoma; CLD, chronic liver disease; COVID-19, coronavirus disease 2019; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis.
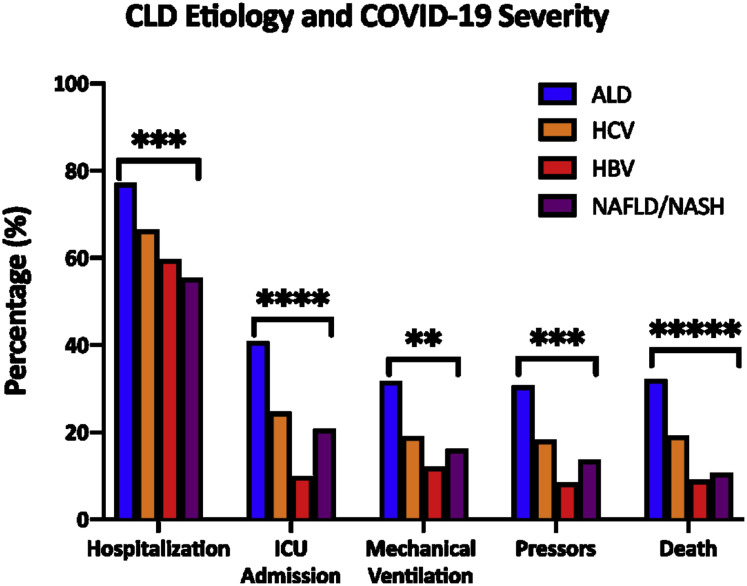
Supplementary Figure 6.
Etiology of CLD and severity of COVID-19. Comparing the proportion of patients with different etiologies of CLD requiring hospitalization, ICU admission, mechanical ventilation, vasopressors or mortality. ALD, alcohol-related liver disease; CLD, chronic liver disease; COVID-19, coronavirus disease 2019; HBV, hepatitis B virus; HCV, hepatitis C virus; ICU, intensive care unit; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; ∗∗∗∗∗P < .00001.
Clinical Course of Coronavirus Disease 2019 in Patients With Chronic Liver Disease
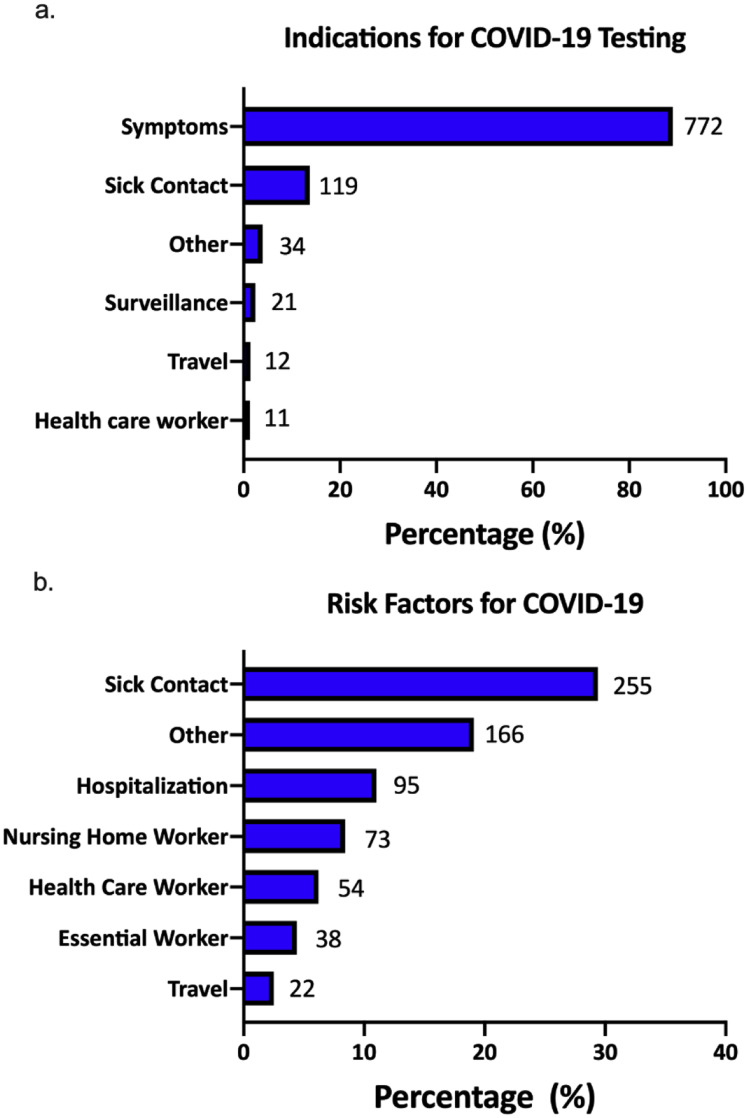
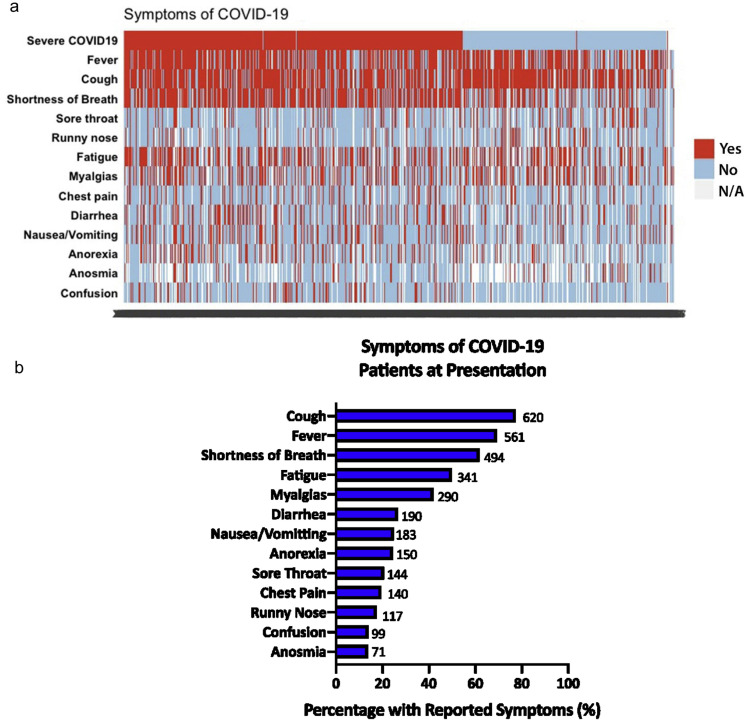
The majority of patients were tested for COVID-19 because they presented with symptoms (772, 89%) (Supplementary Figure 7). The top 3 risk factors for acquiring COVID-19 were exposure to sick contacts (255, 29.4%), recent visit to a healthcare facility (95, 11.0%), or nursing home stay (73, 8.4%). The most common presenting symptom was cough (620, 77.4%), followed by fever (561, 69.3%), shortness of breath (494, 61.8%), fatigue (341, 49.9%), and diarrhea (190, 26.6%) (Table 2 , Supplementary Figure 8). Patients presenting with gastrointestinal symptoms of diarrhea (odds ratio [OR], 1.89; 95% confidence interval [CI], 1.30–2.74) or nausea/vomiting (OR, 1.84; 95% CI, 1.27–2.68) were more likely to have severe COVID-19 than patients without gastrointestinal symptoms (Table 2). Also, patients presenting with respiratory symptoms such as shortness of breath, sore throat, runny nose, or confusion were at higher risk for both mortality and severe COVID-19.
Supplementary Figure 7.
Indications for testing and risk factors for COVID-19 and in patients with CLD. (A) Indications of COVID-19 testing in patients with CLD. (B) Risk factors for acquiring COVID-19 in patients with CLD. CLD, chronic liver disease; COVID-19, coronavirus disease 2019.
Table 2.
Clinical Presentation of Patients With Chronic Liver Disease and COVID-19 and Clinical Outcomes
| Total (n = 867) | All-cause mortality status (n = 817) |
P value | Severe COVID-19 (n = 857) |
P value | |||
|---|---|---|---|---|---|---|---|
| Alive | Died | No | Yes | ||||
| General symptom | |||||||
| Fever (n = 810) | 561 (69.3) | 463 (69.9) | 76 (69.1) | .858 | 189 (64.3) | 371 (72.2) | .019 |
| Cough (n = 801) | 620 (77.4) | 524 (79.3) | 68 (66.7) | .004 | 236 (80.0) | 383 (76.0) | .191 |
| Shortness of breath (n = 799) | 494 (61.8) | 388 (59.2) | 86 (78.9) | <.001 | 122 (42.8) | 371 (72.6) | <.001 |
| Sore throat (n = 699) | 144 (20.6) | 136 (23.0) | 7 (8.8) | .004 | 71 (27.1) | 73 (16.8) | .001 |
| Runny nose (n = 667) | 117 (17.5) | 105 (18.9) | 9 (10.8) | .073 | 56 (22.5) | 61 (14.7) | .010 |
| Fatigue (n = 684) | 341 (49.9) | 277 (49.8) | 54 (55.7) | .288 | 109 (45.6) | 231 (52.1) | .103 |
| Myalgia (n = 692) | 290 (41.9) | 249 (43.4) | 28 (31.8) | .039 | 106 (43.0) | 182 (41.0) | .592 |
| Chest pain (n = 719) | 140 (19.5) | 118 (19.7) | 17 (19.3) | .933 | 45 (17.2) | 95 (20.8) | .249 |
| Confusion (n = 711) | 99 (13.9) | 51 (8.8) | 44 (43.1) | <.001 | 7 (2.7) | 92 (20.4) | <.001 |
| Gastrointestinal symptom | |||||||
| Diarrhea (n = 715) | 190 (26.6) | 158 (26.7) | 23 (25.0) | .733 | 48 (19.0) | 141 (30.7) | .001 |
| Nausea/vomiting (n = 738) | 183 (24.8) | 153 (25.0) | 22 (23.7) | .773 | 47 (17.7) | 134 (28.5) | .001 |
| Anorexia (n = 614) | 150 (24.4) | 120 (23.8) | 25 (30.9) | .169 | 30 (14.2) | 119 (29.7) | <.001 |
| Anosmia (n = 517) | 71 (13.7) | 62 (14.5) | 6 (9.4) | .269 | 33 (19.1) | 38 (11.1) | .013 |
NOTE. Data are expressed as the number (proportion among patients with reported symptoms).
COVID-19, coronavirus disease 2019.
Supplementary Figure 8.
Presenting symptoms of COVID-19 among patients with CLD. (A) Tiled heatmap of symptoms of COVID-19 stratified by severity of COVID-19. Each vertical bar represents a single patient. (B) Frequency of different COVID-19 symptoms in patients with CLD. CLD, chronic liver disease; COVID-19, coronavirus disease 2019.
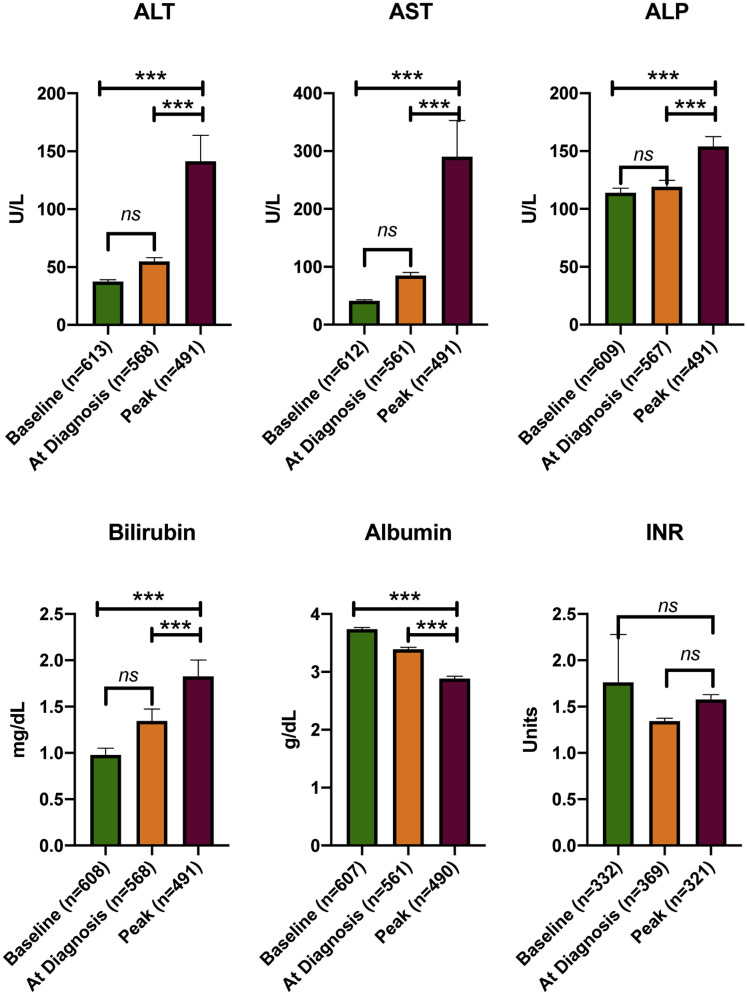
Among patients with CLD and COVID-19, 60.4% (n = 524) were hospitalized, 49.9% (n = 433) required supplemental oxygen, 23.0% (n = 199) were admitted to the ICU, 15.7% (n = 136) received vasopressors, and 17.8% (n = 154) required mechanical ventilation. The majority of the deaths were due to COVID-19 (86.7%, n = 105). Sixteen patients had non–COVID-19 related mortality, and the cause of death was available in 37.5% of these patients (n = 6). Two of them died of cardiac failure, 2 of acute liver failure due to acute alcoholic hepatitis, 1 of bleeding complications due to coagulopathy, and 1 of septic shock in the setting of acute cholecystitis. New or worsening hepatic decompensation during COVID-19 was noted in 67 patients (7.7%); 23 patients (34.3%) had severe hepatic encephalopathy, 11 (16.4%) had severe ascites, and 7 (10.4%) had variceal bleed during the clinical course of COVID-19. Median baseline liver tests before COVID-19 were aspartate aminotransferase, 28.0 IU/L (IQR 25); alanine aminotransferase 27.0 IU/L (IQR 27); alkaline phosphatase 88 IU/L (IQR 59); and bilirubin 0.5 mg/dL (IQR 0.5). As shown in previous studies,9 peak values of all liver tests were significantly elevated during COVID-19 (Supplementary Figure 9).
Supplementary Figure 9.
Liver tests during COVID-19. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio.
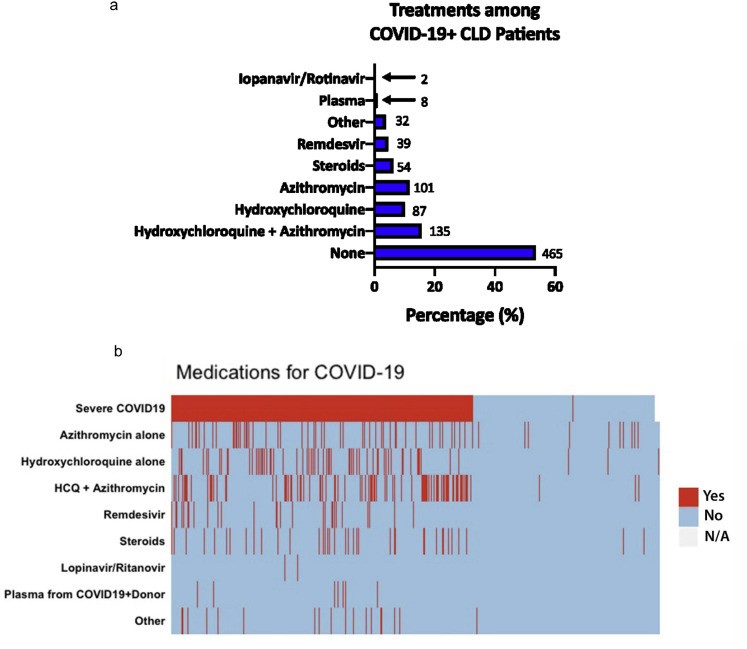
The combination of azithromycin and hydroxychloroquine (135, 15.6%), azithromycin alone (101, 11.6%), and hydroxychloroquine alone (87, 10.0%) were the most commonly used medications for COVID-19. A higher proportion of patients who received medications directed against COVID-19 had more severe disease (Supplementary Figure 10).
Supplementary Figure 10.
Treatment for COVID-19 among patients with CLD. (A) Frequency of COVID-19 treatments in patients with CLD. (B) Tiled heatmap of treatment of COVID-19 stratified by severity of disease. Each horizontal bar represents a single patient. CLD, chronic liver disease; COVID-19, coronavirus disease 2019; HCQ, hydroxychloroquine.
Predictors of All-Cause Mortality and Coronavirus Disease 2019–Related Mortality in Patients With Chronic Liver Disease
To identify the predictors of all-cause mortality and COVID-19 related mortality, we performed univariate and multivariate survival analysis (Table 3 ). The multivariate model for all-cause mortality was adjusted for age, sex, race/ethnicity, etiology of CLD, cirrhosis, hepatic decompensation, HCC, diabetes, hypertension, cardiovascular disease, chronic obstructive pulmonary disease (COPD), smoking status, and alcohol consumption, all of which were statistically significant in the univariate model and are plausibly clinically relevant.
Table 3.
Univariate and Multivariate Analyses: Overall Survival in Patients With Chronic Liver Disease and COVID-19
| Univariate model for all-cause mortality |
Multivariate model for all-cause mortality (events = 121) |
Multivariate model for mortality due to COVID-19 (events = 105) |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Demographic factors | ||||||
| Age (per 10 year) | 1.55 (1.35–1.77) | <.001 | 1.44 (1.21–1.71) | <.001 | 1.52 (1.27–1.82) | <.001 |
| Male | 1.16 (0.81–1.66) | .416 | 1.16 (0.77–1.75) | .472 | 1.23 (0.79–1.91) | .359 |
| Race/ethnicity | ||||||
| Non-Hispanic white | 1 | 1 | 1 | |||
| Non-Hispanic black | 0.75 (0.48–1.15) | .186 | 0.81 (0.50–1.32) | .400 | 0.84 (0.50–1.43) | .524 |
| Hispanic | 0.73 (0.45–1.20) | .216 | 0.94 (0.56–1.60) | .830 | 1.20 (0.69–2.09) | .522 |
| Non-Hispanic Asian | 1.03 (0.44–2.42) | .941 | 1.60 (0.54–4.70) | .395 | 1.93 (0.64–5.77) | .244 |
| Other | 0.77 (0.31–1.94) | .580 | 0.60 (0.18–1.96) | .393 | 0.80 (0.24–2.66) | .711 |
| Liver-related factors | ||||||
| Etiology of liver disease | ||||||
| HCV | 1 | 1 | 1 | |||
| ALD | 1.75 (1.06–2.89) | .028 | 2.42 (1.29–4.55) | .006 | 2.69 (1.44–5.02) | .002 |
| NAFLD | 0.48 (0.31–0.75) | .001 | 1.05 (0.59–1.87) | .872 | 1.08 (0.59–1.97) | .804 |
| HBV | 0.57 (0.22–1.47) | .247 | 0.80 (0.23–2.74) | .718 | 0.81 (0.23–2.83) | .746 |
| Other | 0.69 (0.32–1.49) | .344 | 1.66 (0.72–3.81) | .236 | 1.15 (0.42–3.13) | .782 |
| Presence of cirrhosis | ||||||
| No | 1 | 1 | 1 | |||
| Compensated cirrhosis | 1.45 (0.87–2.42) | .158 | 0.83 (0.46–1.49) | .532 | 0.90 (0.49–1.65) | .743 |
| Decompensated cirrhosis | 5.26 (3.51–7.89) | <.001 | 2.91 (1.70–5.00) | <.001 | 2.41 (1.34–4.32) | .003 |
| Presence of HCC | 4.91 (2.48–9.70) | <.001 | 3.31 (1.53–7.16) | .002 | 3.96 (1.74–8.98) | .001 |
| Comorbidities | ||||||
| Diabetes | 1.49 (1.04–2.13) | .028 | 1.59 (1.02–2.46) | .040 | 1.82 (1.15–2.89) | .011 |
| Hypertension | 1.55 (1.05–2.27) | .003 | 1.77 (1.11–2.81) | .016 | 1.69 (1.04–2.76) | .034 |
| Cardiovascular disease | 1.70 (1.14–2.53) | .010 | 1.10 (0.70–1.74) | .667 | 0.86 (0.53–1.42) | .564 |
| COPD | 2.01 (1.25–3.26) | .004 | 1.77 (1.03–3.05) | .040 | 2.29 (1.32–3.96) | .003 |
| Behavioral factors | ||||||
| Smoking status | ||||||
| No | 1 | 1 | 1 | |||
| Past smoker | 2.18 (1.46–3.25) | <.001 | 1.30 (0.82–2.05) | .263 | 1.39 (0.86–2.26) | .179 |
| Current smoker | 2.67 (1.56–4.56) | <.001 | 2.48 (1.30–4.73) | .006 | 2.99 (1.56–5.72) | .001 |
| Alcohol consumption | ||||||
| Do not drink currently | 1 | 1 | ||||
| Social drinking | 0.35 (0.18–0.67) | .002 | 0.61 (0.31–1.22) | .160 | ||
| Current daily drinking | 1.63 (1.04–2.56) | .032 | 1.37 (0.77–2.46) | .287 | ||
NOTE. Multivariate model for all-cause mortality was adjusted for age, gender, race/ethnicity, etiology of chronic liver disease, cirrhosis, HCC, diabetes, hypertension, cardiovascular disease, COPD, smoking status, and alcohol consumption. Multivariate model for death due to COVID-19 was adjusted for age, gender, race/ethnicity, etiology of chronic liver disease, cirrhosis, HCC, diabetes, hypertension, obesity, cardiovascular disease, COPD, and smoking status. Boldface indicates statistical significance.
ALD, alcohol-related liver disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; HBV, hepatitis B virus infection; HCC, hepatocellular carcinoma; HCV, hepatitis C virus infection; HR, hazard ratio; NAFLD, nonalcoholic fatty liver disease.
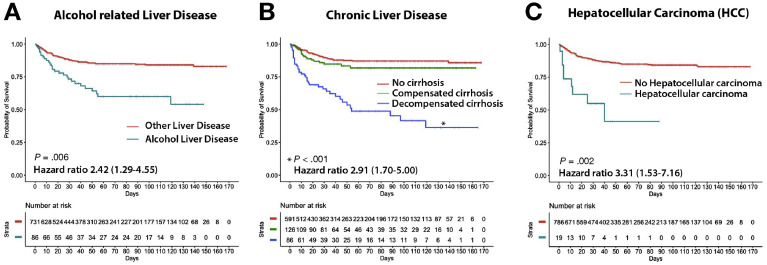
The liver-specific predictors of all-cause mortality were ALD (hazard ratio [HR], 2.42; 95% CI, 1.29–4.55), presence of hepatic decompensation at baseline (HR, 2.91; 95% CI, 1.70–5.00), and HCC (HR, 3.31; 95% CI, 1.53–7.16) (Figure 1 ). Other independent predictors of all-cause mortality were increasing age (HR, 1.44; 95% CI, 1.21–1.71 per 10 years), presence of diabetes (HR, 1.59; 95% CI, 1.02–2.46), hypertension (HR, 1.77; 95% CI, 1.11–2.81), COPD (HR, 1.77; 95% CI, 1.03–3.05), and history of current smoking (HR, 2.48; 95% CI, 1.30–4.73). For the secondary outcome of deaths due to COVID-19 (Table 3), the results were largely identical. Furthermore, we did not find significant interactions between ALD and decompensated CLD or HCC for overall survival on multivariate analysis (test of interaction P > .2) (Supplementary Table 4).
Figure 1.
Liver-specific factors predicting overall survival in patients with chronic liver disease and COVID-19. (A) Overall survival from time of diagnosis of COVID-19 in patients with alcohol-related liver disease (ALD) compared with other liver disease etiologies. (B) Overall survival in patients with liver disease stratified into those with no cirrhosis vs compensated cirrhosis vs decompensated cirrhosis. Significant and hazard ratios are derived from comparison of decompensated cirrhosis vs no cirrhosis. (C) Overall survival from time of diagnosis of COVID-19 in patients with underlying hepatocellular carcinoma (HCC). COVID-19, coronavirus disease 2019.
Next we performed a subgroup survival analysis in patients with cirrhosis and COVID-19 (Table 4 ). The liver-specific factors associated with higher all-cause mortality in patients with cirrhosis were prior hepatic decompensation (HR, 3.89; 95% CI, 2.18–6.95) and HCC (HR, 3.66; 95% CI, 1.67–8.01). In the subgroup of patients with non-cirrhotic CLD, ALD was associated with higher all-cause mortality (HR, 4.72; 95% CI, 2.05–10.85) and higher COVID-19 related mortality (HR, 7.39; 95% CI, 2.96–18.46) (Supplementary Table 5).
Table 4.
Univariate and Multivariate Analyses of Risk for Survival in Patients With Cirrhosis and COVID-19 (n = 212)
| Univariate model for all-cause mortality |
Multivariate model for all-cause mortality (events = 57) |
Multivariate model for mortality due to COVID-19 (events = 45) |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Demographic factors | ||||||
| Age (per 10 year) | 1.20 (0.97–1.50) | .095 | ||||
| Male | 0.77 (0.46–1.30) | .329 | ||||
| Race/ethnicity | ||||||
| Non-Hispanic white | 1 | |||||
| Non-Hispanic black | 0.84 (0.46–1.58) | .609 | ||||
| Hispanic | 0.66 (0.33–1.34) | .249 | ||||
| Non-Hispanic Asian | — | — | ||||
| Other | 1.43 (0.49–4.15) | .592 | ||||
| Liver-related factors | ||||||
| Etiology of liver disease | ||||||
| HCV | 1 | |||||
| ALD | 1.64 (0.85–3.14) | .138 | ||||
| NAFLD | 1.08 (0.53–2.22) | .829 | ||||
| HBV | — | — | ||||
| Other | 1.22 (0.48–3.12) | .679 | ||||
| Decompensated cirrhosis | 3.67 (2.11–6.37) | <.001 | 3.89 (2.18–6.95) | <.001 | 3.12 (1.68–5.79) | <.001 |
| Presence of HCC | 3.26 (1.52–6.97) | .002 | 3.66 (1.67–8.01) | .001 | 3.61 (1.58–8.25) | .002 |
| Comorbidities | ||||||
| Diabetes | 0.96 (0.57–1.62) | .888 | ||||
| Hypertension | 0.88 (0.53–1.49) | .652 | ||||
| Cardiovascular disease | 1.15 (0.64–2.04) | .646 | ||||
| COPD | 1.60 (0.76–3.38) | .217 | 3.12 (1.68–5.79) | <.001 | ||
| Behavioral factors | ||||||
| Smoking status | ||||||
| No | 1 | |||||
| Past smoker | 1.42 (0.79–2.58) | .244 | ||||
| Current smoker | 2.16 (1.03–4.53) | .042 | ||||
| Alcohol consumption | ||||||
| Do not drink currently | 1 | |||||
| Social drinking | 0.26 (0.04–1.91) | .187 | ||||
| Current daily drinking | 2.44 (1.38–4.30) | .002 | 2.34 (1.27–4.30) | .006 | ||
NOTE. To identify candidate risk factors of mortality, we performed a stepwise backward logistic regression analysis (probability to enter = 0.05 and probability to remove = 0.1) using all variables in the univariate model. Boldface indicates statistical significance.
ALD, alcohol-related liver disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; HBV, hepatitis B virus infection; HCC, hepatocellular carcinoma; HCV, hepatitis C virus infection; HR, hazard ratio; NAFLD, nonalcoholic fatty liver disease.
Serial liver-related lab results were available in a majority of the hospitalized patients but not in the majority of those managed as outpatient. We performed a subgroup analysis in hospitalized patients in whom serial lab values were available for analysis. Peak values of aspartate aminotransferase, bilirubin, alkaline phosphatase, and Model for End-Stage Liver Disease score were observed to predict mortality (Supplementary Table 6).
Predictors of Severe Coronavirus Disease 2019 in Patients With Chronic Liver Disease
Overall, 535 patients with CLD met criteria for the composite endpoint of severe COVID-19. As shown in Table 5 , multivariate analysis showed that a history of hepatic decompensation (OR, 2.50; 95% CI, 1.20–5.21) predicted severe COVID-19. Other independent predictors were increasing age (OR, 1.43; 95% CI, 1.25–1.65), Hispanic ethnicity (OR, 2.33; 95% CI, 1.47–3.70), diabetes (OR, 1.51; 95% CI, 1.04–2.19), cardiovascular disease (OR, 1.85; 95% CI, 1.09–3.13), and COPD (OR, 2.26; 95% CI, 1.15–4.45).
Table 5.
Univariate and Multivariate Analyses: Risk of Severe COVID-19 (Composite Endpoint) Among Patients With Chronic Liver Disease and COVID-19
| Univariate model for severe COVID-19 |
Multivariate model for severe COVID-19 |
|||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Demographic factors | ||||
| Age (per 10 year) | 1.46 (1.32–1.62) | <.001 | 1.43 (1.25–1.65) | <.001 |
| Male | 1.38 (1.05–1.83) | .022 | 1.28 (0.90–1.81) | .172 |
| Race/ethnicity | ||||
| Non-Hispanic white | 1 | 1 | ||
| Non-Hispanic black | 0.93 (0.66–1.32) | .685 | 0.83 (0.54–1.28) | .406 |
| Hispanic | 1.47 (1.01–2.14) | .045 | 2.33 (1.47–3.70) | <.001 |
| Non-Hispanic Asian | 1.42 (0.72–2.81) | 314 | 1.90 (0.85–4.27) | .124 |
| Other | 2.57 (1.14–5.83) | .024 | 3.40 (1.31–8.81) | .012 |
| Liver-related factors | ||||
| Etiology of liver disease | ||||
| HCV | 1 | 1 | ||
| ALD | 1.72 (0.94–3.15) | .077 | 2.08 (0.97–4.45) | .059 |
| NAFLD | 0.55 (0.39–0.80) | .001 | 0.68 (0.41–1.13) | .137 |
| HBV | 0.64 (0.35–1.15) | .139 | 0.99 (0.46–2.13) | .973 |
| Other | 0.72 (0.40–1.30) | .275 | 1.27 (0.60–2.70) | .536 |
| Presence of cirrhosis | ||||
| No | 1 | 1 | ||
| Compensated cirrhosis | 1.21 (0.82–1.79) | .338 | 0.70 (0.43–1.14) | .152 |
| Decompensated cirrhosis | 3.85 (2.13–6.95) | <.001 | 2.50 (1.20–5.21) | .015 |
| Presence of HCC | 3.70 (1.08–12.67) | .037 | 2.99 (0.62–14.36) | .171 |
| Comorbidities | ||||
| Diabetes | 1.81 (1.36–2.41) | <.001 | 1.51 (1.04–2.19) | .029 |
| Hypertension | 1.43 (1.08–1.89) | .012 | 1.16 (0.80–1.68) | .434 |
| Obesity | 0.76 (0.57–1.00) | .052 | 1.21 (0.84–1.76) | .302 |
| Cardiovascular disease | 2.51 (1.65–3.81) | <.001 | 1.85 (1.09–3.13) | .022 |
| COPD | 2.68 (1.49–4.80) | .001 | 2.26 (1.15–4.45) | .019 |
| Behavioral factors | ||||
| Smoking status | ||||
| No | 1 | 1 | ||
| Past smoker | 1.53 (1.11–2.10) | .009 | 0.96 (0.65–1.43) | .855 |
| Current smoker | 1.21 (0.76–1.90) | .419 | 1.00 (0.54–1.83) | .990 |
| Alcohol consumption | ||||
| Do not drink currently | 1 | 1 | ||
| Social drinking | 0.53 (0.37–0.75) | <.001 | 0.84 (0.55–1.26) | .390 |
| Current daily drinking | 1.09 (0.70–1.71) | .699 | 0.98 (0.53–1.83) | .953 |
NOTE. Multivariate model for all-cause mortality was adjusted for age, gender, race/ethnicity, etiology of chronic liver disease, cirrhosis, HCC, diabetes, hypertension, obesity, cardiovascular disease, COPD, smoking status, and alcohol consumption. Boldface indicates statistical significance.
ALD, alcohol-related liver disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; HBV, hepatitis B virus infection; HCC, hepatocellular carcinoma; HCV, hepatitis C virus infection; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio.
Discussion
According to the Centers for Disease Control, patients with CLD might be at increased risk for severe illness with COVID-19.15 CLD represents a clinical spectrum ranging from mild asymptomatic disease to severe decompensated cirrhosis. It is not clear which subgroups of patients with CLD are more vulnerable to adverse outcomes with COVID-19. In this multicenter study, we investigated predictors of mortality and COVID-19 disease severity in patients with CLD and SARS-CoV-2 infection. Among the 867 patients with CLD from 21 centers across the US, we observed an all-cause mortality of 14.0%; 60.4% were hospitalized, and 23% were admitted to the ICU. New or worsening hepatic decompensation during COVID-19 was noted in 7.7% of patients. We identified the liver-specific factors ALD, hepatic decompensation, and HCC as predictors of adverse outcomes from COVID-19, apart from established factors such as older age, hypertension, diabetes, and COPD. In addition, we found that patients of Hispanic ethnicity had a higher risk for severe COVID-19. Thus, our large multicenter study identifies specific subgroups of patients with CLD who have higher mortality with COVID-19.
Because COVID-19 is a novel pandemic, our knowledge of its impact on patients with CLD is still evolving. Singh et al9 recently identified 250 patients with COVID-19 who had an underlying CLD by using a de-identified research network database and reported a hospitalization rate of 52% and mortality of 12%, similar rates to our study. However, preliminary data from an international registry of 152 patients with CLD reported a higher overall mortality rate of 31% and a hospitalization rate of 95% for patients with cirrhosis.11 The higher mortality rates in this clinician-reported registry study may have been due to selection bias. Around 90% of the patients with CLD and COVID-19 in our cohort had mild liver disease with either non-cirrhotic stage disease or compensated cirrhosis at baseline, and they had relatively favorable outcomes. Patients with decompensated cirrhosis were disproportionately adversely affected by COVID-19, with an all-cause mortality rate of 31.4% in this subgroup. These findings are in line with the higher morbidity and mortality in patients with decompensated cirrhosis and influenza pneumonia.16 , 17 We posit the less favorable outcomes noted in patients with decompensated cirrhosis may be due to cirrhosis-associated immune dysfunction and fragile physiological buffers, likely increasing susceptibility to severe COVID-19.18 Our findings highlight the challenges in taking extra precautions to minimize the risk of exposure to SARS-CoV-2 in the vulnerable patients with decompensated cirrhosis, while continuing to optimally manage their decompensating events.
In our study, ALD was independently associated with a higher risk of poor survival and COVID-19 related mortality. This is a novel association and one that has significant implications for patients with CLD. Patients with ALD are known to be at higher risk for infections because of the underlying dysregulation of the immune system.19 ALD is associated with a sterile inflammatory state induced by damage-associated molecular patterns, which leads to the systemic production of proinflammatory cytokines by various immune cells.20 , 21 We hypothesize that the superimposed cytokine storm triggered by SARS-CoV-2 could exacerbate the heightened inflammatory state in patients with ALD, thus leading to worse outcomes.22 Moreover, there has been significant concern about increased alcohol use during the COVID-19 pandemic, highlighting the importance of this association.23 , 24 In our study, up to one third of patients with CLD and an alarming 50% of patients with ALD reported daily alcohol consumption, which was disconcertingly associated with poor outcomes in patients with cirrhosis and COVID-19. These findings emphasize the need to implement an aggressive remote care plan for patients with ALD to manage their alcohol use disorder, while simultaneously minimizing the risk of exposure to COVID-19. Future studies will be needed to analyze specific subgroups within the spectrum of ALD who are at higher risk for adverse outcomes with COVID-19.
Another subgroup in our study that was found to be at significantly high risk for mortality was that of patients with HCC. The all-cause mortality rate in this subgroup was 52.4% (n = 11), almost 7-fold higher than in patients without HCC; however, the number of patients is small. Patients with cancer, in general, have worse clinical outcomes after COVID-19.14 , 25 Patients with HCC may be uniquely susceptible to COVID-19 related complications because of a constellation of active malignancy, presence of cirrhosis, as well as the presence of an active underlying liver disease that led to that cirrhosis, all resulting in compromised immune function, which may be further complicated by HCC-directed treatment.
Our cohort includes a racially and ethnically diverse population that is 31% non-Hispanic white, 31% non-Hispanic black, and 25% Hispanic. We found that patients of Hispanic ethnicity had a higher risk of developing severe COVID-19 compared with non-Hispanic whites, even after adjusting for age, comorbidities, and hepatic decompensation. These findings are in line with recent reports showing higher age-adjusted rates of hospitalization in Hispanic patients.26 , 27
The strengths of our study include large sample size, broad geographical distribution of sites across the US, as well as the granularity of the collected data. We have included patients treated as outpatients or inpatients and also patients with non-cirrhotic or cirrhotic stage CLD, thus making our findings generalizable. Limitations of our study include the retrospective-prospective timeline, which was used mainly because of the rapidly evolving nature of the pandemic. Another limitation of our study is the restriction of SARS-CoV-2 testing during the earlier phase of the pandemic, likely leading to decreased representation of mild COVID-19. Also, we could have enrollment bias because not all patients with CLD have a documented ICD-9/10 code in their electronic health records. Also, despite our best efforts, it is possible that not all patients with CLD and COVID19 were identified from the participating centers. Last, the majority of the contributing centers are tertiary medical health systems, potentially introducing referral bias. However, our cohort represents an ethnically diverse population with varying stages of liver disease. Larger and longer-term studies will be needed to confirm these findings.
To date, this is the largest study on COVID-19 among patients with CLD in the United States. Our cohort of 867 patients with CLD had substantial rates of all-cause mortality (14.0%), hospitalization (60.4%), and ICU admission (23%). We identify decompensated cirrhosis, ALD, and HCC to be determinants of mortality in patients with CLD and also show that Hispanic ethnicity is independently associated with severe COVID-19. These findings can be used to prospectively design protective measures for these vulnerable populations, such as continuing the emphasis on telemedicine, prioritizing them for future vaccinations, as well as actively including these patients in prospective COVID-19 surveillance studies and drug trials.
Acknowledgments
The authors thank the following individuals for their expertise and assistance throughout all aspects of our study: Zoe Reinus, Michael Daidone, Julia Sjoquist, Faruq Pradhan, Mohanad Al-Qaisi, Nael Haddad, Nicholas Blackstone, Katherine Marx, Susan McDermott, Alyson Kaplan, Mallori Ianelli, Julia Speiser, Angela Wong, Dhuha Alhankawi, Sunny Sandhu, Sameeha Khalid, Aalam Sohal, Jennifer Smart, and Neil Marimoto. They also thank all of the first-line responders who are working tirelessly and with dedication to care for patients and their families during the COVID-19 crisis.
CRediT Authorship Contributions
Donghee Kim, MD, PhD (Formal analysis: Equal; Methodology: Equal; Writing – original draft: Supporting; Writing – review & editing: Equal)
Nia Adeniji, MEng (Conceptualization: Equal; Data curation: Equal; Formal analysis: Equal; Investigation: Equal; Methodology: Equal; Writing – original draft: Equal; Writing – review & editing: Equal)
Nyan Latt, MD (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Sonal Kumar, MD (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Patricia P. Bloom, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Elizabeth S. Aby, MD (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Ponni Perumalswami, MD (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Marina Roytman, MD, FACP (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Michael Li, MD (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Alexander S. Vogel, MD (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Andreea M. Catana, MD (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Kara Wegermann, MD (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Rotonya M. Carr, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Costica Aloman, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Vincent Chen, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Atoosa Rabiee, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Brett Sadowski, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Veronica Nguyen, MD (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Winston Dunn, MD (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Kenneth Chavin, MD, PhD (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Kali Zhou, MD (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Blanca Lizaola-Mayo, MD (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Akshata Moghe (Conceptualization: Supporting; Writing – review & editing: Supporting)
José Debes, MD, MS (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Tzu-Hao Lee, MD (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Andrea Branch, PhD (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Kathleen Viveiros, MD (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Walter Chan, MD, MPH (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
David Chascsa, MD (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Paul Kwo, MD (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Supporting)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2020.09.027.
Supplementary Material
Supplementary Table 1.
List of Participating Institutions and Investigators From Each Site
| Institution | Principal investigator | |
|---|---|---|
| 1 | Ochsner Medical Center, LA | Nyann Latt |
| 2 | Massachusetts General Hospital, MA | Patricia P. Bloom |
| 3 | Mount Sinai School of Medicine, NY | Ponni Perumalswami |
| 4 | University of California San Francisco, Fresno, CA | Marina Roytman |
| 5 | Hennepin County Medical Center, MN | Elizabeth Aby, Jose Debes |
| 6 | Brigham and Women’s Hospital, MA | Kathleen Viveiros, Walter Chan |
| 7 | Duke University, NC | Kara Wegermann, Tzu-Hao Lee |
| 8 | Beth Israel Deaconess Medical Center, MA | Maria Andreea Catana |
| 9 | Stanford University, CA | Donghee Kim, Nia Adeniji, Paul Kwo, Renumathy Dhanasekaran |
| 10 | University of Pennsylvania, PA | Rotonya Carr |
| 11 | Rush University Medical Center, IL | Costica Aloman |
| 12 | University of Michigan, MI | Vincent Chen |
| 13 | Veterans Administration (VA) Medical Center, Washington, DC | Atoosa Rabiee |
| 14 | University of Minnesota, MN | Elizabeth Aby |
| 15 | Georgetown University, Washington DC | Brett Sadowski |
| 16 | University of Arizona/Banner Health, AZ | Veronica Nguyen |
| 17 | The University of Kansas Medical Center, KS | Winston Dunn |
| 18 | University Hospitals Cleveland Medical Center, OH | Kenneth Chavin |
| 19 | University of Southern California, CA | Kali Zhou |
| 20 | Mayo Clinic, AZ | Blanca Lizaola-Mayo |
| 21 | Weill Cornell Medicine, NY | Sonal Kumar |
Supplementary Table 2.
List of International Classification of Diseases-10 Codes for Chronic Liver Disease and COVID-19
| COVID-19 | SARS-CoV-2 Lab Code |
|---|---|
| U07.1 COVID-19 | LAB9309 |
| NASH/NAFLD | Unspecified chronic liver disease |
| K75.81 Nonalcoholic steatohepatitis (NASH) | K73 Chronic hepatitis |
| K76.0 NAFLD (nonalcoholic fatty liver disease) | K73.0 Chronic persistent hepatitis |
| Alcohol-related liver disease | K73.1 Chronic lobular hepatitis |
| K70 Alcoholic liver disease | K73.2 Chronic active hepatitis |
| K70.1 Alcoholic hepatitis | K73.8 Other chronic hepatitis, Recurrent hepatitis |
| K70.10 …… without ascites | K73.9 Chronic hepatitis, unspecified |
| K70.11 …… with ascites | K74 Fibrosis and cirrhosis of liver |
| K70.2 Alcoholic fibrosis and sclerosis of liver | K74.0 Hepatic fibrosis |
| K70.3 Alcoholic cirrhosis of liver | K74.1 Hepatic sclerosis |
| K70.30 …… without ascites | K74.2 Hepatic fibrosis with hepatic sclerosis |
| K70.31 …… with ascites | K74.4 Secondary biliary cirrhosis |
| K70.4 Alcoholic hepatic failure | K74.5 Biliary cirrhosis, unspecified |
| K70.40 …… without coma | K74.6 Other and unspecified cirrhosis of liver |
| K70.41 …… with coma | K74.60 Unspecified cirrhosis of liver |
| K70.9 Alcoholic liver disease, unspecified | K74.69 Other cirrhosis of liver |
| Chronic Hepatitis C/Hepatitis B | K71.7 Toxic liver disease with fibrosis and cirrhosis |
| B18.2 Chronic hepatitis C | K71.3 Toxic liver disease with chronic hepatitis |
| K74.6 Chronic hepatitis C with cirrhosis | K71.4 Toxic liver disease with chronic lobular hepatitis |
| K74.69, B19.2 cirrhosis to HCV | K71.5 Toxic liver disease with chronic active hepatitis |
| B18.1 Chronic hepatitis B | K71.50 …… without ascites |
| K74.6, B19.1 Chronic hepatitis B with cirrhosis | K71.51 …… with ascites |
| PBC/PSC/Autoimmune hepatitis | K76.6 Portal hypertension |
| K74.3 Primary biliary cirrhosis (PBC) | K76.7 Hepatorenal syndrome |
| K74.3 Cirrhosis due to primary sclerosing cholangitis (PSC) | K76.81 Hepatopulmonary syndrome |
| K83.01 Primary sclerosing cholangitis | Decompensated cirrhosis |
| K75.4 Autoimmune hepatitis | K72.9 Decompensated hepatic cirrhosis |
| K74.69 Decompensated liver disease |
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Supplementary Table 3.
Data Elements of the COLD Study Data Collection Forms
| Variable | Category |
|---|---|
| Center-specific record ID | Identifier |
| Center name | |
| Age (at diagnosis of COVID) | |
| Gender | Male Female Other |
| Race | White African American Asian American Indian Other |
| Ethnicity | Hispanic or Latino Non-Hispanic |
| Date of data collection | |
| Home Address Zip Code | |
| Residence | Home: apartment Home: single family home Skilled nursing home Long-term care facility Assisted living facility Other Do not know None/Shelter |
| Number of people at home | |
| Insurance | Medicare/Medicaid Private insurance Uninsured Do not know |
| Date of COVID-19 diagnosis | |
| Mode of diagnosis of COVID-19 | Positive PCR test Positive serologic test Not clear Other |
| Risk factors for COVID-19 | Travel to high risk region Sick contacts Hospitalization within the past month Healthcare worker Essential worker Nursing home resident Other |
| Indication of testing | Travel to high risk region Contacts who tested positive for COVID-19 Symptoms Healthcare worker surveillance Surveillance Other |
| Hospitalization for COVID-19 | Yes No |
| Use of supplemental oxygen | Yes No |
| Use of vasopressors | Yes No |
| Number of pressors used | |
| ICU admission | Yes No |
| Mechanical ventilation | Yes No |
| Noninvasive positive pressure ventilation | Yes No |
| Length of hospital stay (days) | |
| Death related to COVID-19 | Yes No |
| Symptoms | Cough, shortness of breath, sore throat, runny nose, fatigue, myalgias, chest pain, diarrhea, nausea/vomiting, anorexia, anosmia, confusion |
| Number of years since the patient has a known diagnosis of chronic liver disease | |
| Etiology | Hepatitis C: current Hepatitis C: cured Hepatitis B Nonalcoholic fatty liver disease: hepatic steatosis alone Nonalcoholic steatohepatitis Alcoholic liver disease Cryptogenic cirrhosis Primary biliary cholangitis Primary sclerosing cholangitis Autoimmune hepatitis Hepatocellular carcinoma (HCC) Other |
| Cirrhosis | Yes No |
| Fibroscan | F0-1 F2 F3 F4 |
| Fibrosis-4 | F0-1 F2 F3 F4 |
| NAFLD fibrosis score | F0-1 F2 F3 F4 |
| MR elastography | F0-1 F2 F3 F4 |
| US elastography | F0-1 F2 F3 F4 |
| Biopsy | F0-1 F2 F3 F4 |
| Other | F0-1 F2 F3 F4 |
| Comorbidities | Diabetes Hypertension Hyperlipidemia Obesity Tobacco smoking Coronary artery disease Congestive heart Failure HIV positive COPD Asthma Non-liver malignancy Opioid use disorder Obstructive sleep apnea Chronic lung disease: non-asthma, non-COPD Vaping Marijuana use |
| Tobacco smoking status | Never smoker Former smoker (smoked at least 100 cigarettes in lifetime) Current smoker |
| Alcohol use | Do not drink currently Social drinking Daily drinking |
| Has the patient received a liver transplantation? | Yes No |
| Date of transplant | |
| Type of immunosuppression at the time of COVID-19 diagnosis | Tacrolimus Cyclosporine Prednisone (<20 mg/day) Prednisone (>21 mg/day) Mycophenolate Azathioprine mTOR inhibitors (Sirolimus, Everlolimus) |
| Did the patient receive any of these within 6 months of COVID-19 diagnosis? | Intravenous methyprednisolone Anti-thymocyte globulin (ATG) Basiliximab Rituximab |
| Other immunosuppression | |
| Was immunosuppression modified during COVID-19? | Yes No |
| How was immunosuppression modified? | |
| Did the patient experience acute rejection during COVID-19? | Yes No |
| Laboratory data (before COVID-19, at diagnosis of COVID-19, peak during COVID-19, after COVID-19) | ALT, AST, Alkaline Phosphatase, GGT, Total Bilirubin, Albumin, Creatinine, Sodium, INR, Platelets, Ferritin, WBC, Lymphocytes, Neutrophils, Triglycerides, LDL, HbA1c, CK |
| Decompensation before COVID-19 | None Ascites Variceal bleed Hepatic encephalopathy Other |
| Did the patient decompensate during COVID-19? | Yes No |
| Ascites before COVID-19 (field annotation: 1–6 months before COVID-19) | None Mild-Moderate Severe |
| Encephalopathy before COVID-19 (field annotation: 1–6 months before COVID-19) | None Mild-Moderate Severe |
| Ascites during COVID-19 | None Mild-Moderate Severe |
| Encephalopathy during COVID-19 | None Mild-Moderate Severe |
| Ascites after COVID-19 | None Mild-Moderate Severe |
| Encephalopathy after COVID-19 | None Mild-Moderate Severe |
| Did the patient develop variceal bleeding during COVID-19? | Yes No |
| Were any of the following delayed or cancelled due to diagnosis of COVID-19? | Endoscopy for esophageal varices surveillance Imaging for HCC surveillance Paracentesis for symptomatic ascites Hepatitis C treatment Hepatitis B treatment Liver transplant evaluation Liver transplantation Other |
| Were ambulatory clinic visits to hepatology delayed or canceled due to COVID-19? | Yes No |
| Were ambulatory clinic visits to primary care or other specialties delayed or cancelled due to COVID-19? | Yes No |
| Were medical procedures not related to liver disease delayed or cancelled? | Yes No |
| Was care impacted not directly by COVID-19 but because of health system overload? | Yes No |
| Did the patient have a telehealth visit during COVID-19? | Yes No |
| COVID-19 treatment | Remdesivir Chloroquine Hydroxychloroquine Azithromycin Prednisone or Methylprednisolone Lopinavir/ritonavir Donor plasma Other None |
| Did the patient receive any of the following antibiotics during COVID-19? | Amoxicillin/Clavulanate Cephalosporins Aminoglycosides Macrolides Minocycline Anti-tuberculosis drugs Fluoroquinolones Azithromycin None |
| Did the patient receive any of the following hepatotoxic medications for more than 3 days during COVID-19 infection? | Acetaminophen >2 g per day NSAIDs Anticonvulsants: Phenytoin, Valproic acid, Carbamazepine Azoles Amiodarone Anesthetics: Halothane, Isoflurane Statins Other hepatotoxic medications None |
| Was the patient chronically on any of the following medications before acquiring COVID-19 infection? | Proton pump inhibitors ACE inhibitors Angiotensin receptor blockers |
| If the patient had HCC, had they received any of the following? | Transarterial therapy Ablative therapy Tyrosine kinase inhibitors Immunotherapy None |
| Date of last follow-up | |
| Status | Alive, fully recovered Alive, still impacted by COVID-19 Death from COVID-19 Death from other causes |
ACE, angiotensin-converting enzyme; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; COLD, COVID-19 in chronic liver disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; GGT, gamma-glutamyl transferase; HbA1c, glycosylated hemoglobin; HIV, human immunodeficiency virus; ICU, intensive care unit; INR, international normalized ratio; LDL, low-density lipoprotein; MR, magnetic resonance; NAFLD, nonalcoholic fatty liver disease; NSAIDs, nonsteroidal anti-inflammatory drugs; PCR, polymerase chain reaction; US, ultrasound; WBC, white blood cell count.
Supplementary Table 4.
Interaction of Alcoholic Liver Disease and Decompensated Cirrhosis/HCC With the Risk for All-Cause Mortality/Mortality due to COVID-19
| Univariate model |
Multivariable model |
|
|---|---|---|
| P for interaction | P for interaction | |
| All-cause mortality | ||
| Alcoholic liver disease∗decompensated cirrhosis | .033 | .278 |
| Alcoholic liver disease∗HCC | .976 | .771 |
| Mortality due to COVID-19 | ||
| Alcoholic liver disease∗decompensated cirrhosis | .044 | .225 |
| Alcoholic liver disease∗HCC | .640 | .531 |
NOTE. Multivariate model for all-cause mortality was adjusted for age, gender, race/ethnicity, etiology of chronic liver disease, decompensated cirrhosis, HCC, diabetes, hypertension, cardiovascular disease, COPD, smoking status, alcohol consumption, and the interaction term (alcoholic liver disease∗decompensated cirrhosis or alcoholic liver disease∗HCC). Multivariate model for mortality due to COVID-19 was adjusted for age, gender, race/ethnicity, etiology of chronic liver disease, decompensated cirrhosis, HCC, diabetes, hypertension, cardiovascular disease, COPD, smoking status, and the interaction term (alcoholic liver disease∗decompensated cirrhosis or alcoholic liver disease∗HCC).
COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; HCC, hepatocellular carcinoma.
Supplementary Table 5.
Univariate and Multivariate Survival Analyses in Patients With Non-Cirrhotic Chronic Liver Disease and COVID-19
| Univariate model for all-cause mortality |
Multivariate model for all-cause mortality (events = 62) |
Multivariate model for mortality due to COVID-19 (events = 59) |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Demographic factors | ||||||
| Age (per 10 year) | 1.76 (1.46–2.12) | <.001 | 1.72 (1.40–2.12) | <.001 | 1.66 (1.34–2.04) | <.001 |
| Male | 1.63 (0.97–2.73) | .064 | ||||
| Race/ethnicity | ||||||
| Non-Hispanic white | 1 | |||||
| Non-Hispanic black | 0.73 (0.40–1.34) | .310 | ||||
| Hispanic | 0.80 (0.41–1.59) | .532 | ||||
| Non-Hispanic Asian | 1.52 (0.58–4.01) | .395 | ||||
| Other | 0.29 (0.04–2.16) | .227 | ||||
| Liver-related factors | ||||||
| Etiology of liver disease | ||||||
| HCV | 1 | |||||
| ALD | 1.48 (0.62–3.55) | .376 | 4.72 (2.05–10.85) | <.001 | 7.39 (2.96–18.46) | <.001 |
| NAFLD | 0.43 (0.24–0.76) | .004 | ||||
| HBV | 0.67 (0.23–1.99) | .472 | ||||
| Other | 0.30 (0.07–1.31) | .110 | ||||
| Comorbidities | ||||||
| Diabetes | 2.15 (1.30–3.61) | .003 | 1.87 (1.08–3.23) | .025 | ||
| Hypertension | 3.15 (1.64–6.05) | .001 | 2.04 (1.00–4.15) | .049 | 2.36 (1.14–4.91) | .021 |
| Cardiovascular disease | 2.02 (1.16–3.53) | .014 | ||||
| COPD | 2.20 (1.15–4.22) | .018 | 2.01 (1.00–4.04) | .050 | ||
| Behavioral factors | ||||||
| Smoking status | ||||||
| No | 1 | |||||
| Past smoker | 2.30 (1.33–3.97) | .003 | ||||
| Current smoker | 2.43 (1.10–5.38) | .028 | 3.46 (1.52–7.84) | .003 | 2.97 (1.24–7.13) | .014 |
| Alcohol consumption | ||||||
| Do not drink currently | 1 | |||||
| Social drinking | 0.43 (0.21–0.88) | .021 | ||||
| Current daily drinking | 0.74 (0.32–1.74) | .489 | ||||
NOTE. Boldface indicates statistical significance.
ALD, alcohol-related liver disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; HBV, hepatitis B virus infection; HCC, hepatocellular carcinoma; HCV, hepatitis C virus infection; HR, hazard ratio; NAFLD, nonalcoholic fatty liver disease.
Supplementary Table 6.
Laboratory Characteristics Among Hospitalized Patients With Chronic Liver Disease and COVID-19 (n = 524)
| All-cause mortality status |
P value | ||
|---|---|---|---|
| Alive | Died | ||
| ALT | |||
| Before COVID-19 (n = 374) | 27 (18–40) | 21 (15–33) | .075 |
| At COVID-19 diagnosis (n = 467) | 35 (22–63) | 31.5 (24–57) | .220 |
| Peak (n = 428) | 50 (28–104) | 40.5 (23–88) | .307 |
| Delta-ALT (n = 410) | 0 (0–27.5) | 3 (0–22.5) | .278 |
| AST | |||
| Before COVID-19 (n = 375) | 27 (20–40) | 32 (21–65) | .178 |
| At COVID-19 diagnosis (n = 463) | 50 (30–81.5) | 63.5 (38–63.5) | .021 |
| Peak (n = 428) | 65 (39–120) | 92.5 (51.5–225) | .001 |
| Delta-AST (n = 406) | 5 (0–35) | 22 (0–123) | .075 |
| ALP | |||
| Before COVID-19 (n = 373) | 89 (69–128) | 109 (75–152) | .032 |
| At COVID-19 diagnosis (n = 467) | 83 (63–119) | 91.5 (63–91.5) | .374 |
| Peak (n = 427) | 99 (70–158.5) | 131 (79–235) | .001 |
| Delta-ALP (n = 413) | 8.5 (0–48) | 5.0 (0–87) | .330 |
| Bilirubin | |||
| Before COVID-19 (n = 372) | 0.5 (0.4–0.8) | 0.7 (0.5–1.7) | .004 |
| At COVID-19 diagnosis (n = 467) | 0.6 (0.4–0.9) | 0.8 (0.5–2.2) | <.001 |
| Peak (n = 428) | 0.7 (0.4–1.2) | 1.5 (0.7–4.0) | <.001 |
| Delta-bilirubin (n = 409) | 0.1 (0–0.5) | 0.2 (0–1.6) | .037 |
| MELD score | |||
| Before COVID-19 (n = 276) | 10.0 (7–14) | 11.0 (8–21) | <.001 |
| At COVID-19 diagnosis (n = 375) | 10.5 (7–18) | 16.5 (11–24) | <.001 |
| Peak (n = 291) | 14.0 (8–21) | 21.5 (13–32) | <.001 |
| Delta-MELD (n = 254) | 1.0 (0–4) | 5.0 (0–12.5) | <.001 |
NOTE. Data are expressed as median (interquartile range). Mann-Whitney U test was performed for comparison between groups.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; COVID-19, coronavirus disease 2019.
References
- 1.Hirode G., Saab S., Wong R.J. Trends in the burden of chronic liver disease among hospitalized US adults. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Cirrhosis Collaborators The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245–266. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asrani S.K., Devarbhavi H., Eaton J. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chau T.-N., Lee K.-C., Yao H. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alsaad K.O., Hajeer A.H., Al Balwi M. Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection: clinicopathological and ultrastructural study. Histopathology. 2018;72:516–524. doi: 10.1111/his.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan Z., Chen L., Li J. Clinical features of COVID-19-related liver damage. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh S., Khan A. Clinical characteristics and outcomes of COVID-19 among patients with pre-existing liver disease in United States: a multi-center research network study. Gastroenterology. 2020;159:768–771.e3. doi: 10.1053/j.gastro.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iavarone M., D'Ambrosio R., Soria A. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063–1071. doi: 10.1016/j.jhep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon A.M., Webb G.J., Aloman C. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol. 2020;73:705–708. doi: 10.1016/j.jhep.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidance for certifying deaths due to coronavirus disease 2019 (COVID-19). National Center for Health Statistics. https://www.cdc.gov/nchs/data/nvss/vsrg/vsrg03-508.pdf Available at: Accessed May 15, 2020.
- 13.Zakhari S., Li T.-K. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46:2032–2039. doi: 10.1002/hep.22010. [DOI] [PubMed] [Google Scholar]
- 14.Kuderer N.M., Choueiri T.K., Shah D.P. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coronavirus disease 2019: people with certain medical conditions. CDC. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fneed-extra-precautions%2Fgroups-at-higher-risk.html Available at: Accessed September 1, 2020.
- 16.Duchini A., Viernes M.E., Nyberg L.M. Hepatic decompensation in patients with cirrhosis during infection with influenza A. Arch Intern Med. 2000;160:113–115. doi: 10.1001/archinte.160.1.113. [DOI] [PubMed] [Google Scholar]
- 17.Schütte A., Ciesek S., Wedemeyer H. Influenza virus infection as precipitating event of acute-on-chronic liver failure. J Hepatology. 2019;70:797–799. doi: 10.1016/j.jhep.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Albillos A., Lario M., Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385–1396. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Chan C., Levitsky J. Infection and alcoholic liver disease. Clin Liver Dis. 2016;20:595–606. doi: 10.1016/j.cld.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Shim Y.-R., Jeong W.-I. Recent advances of sterile inflammation and inter-organ cross-talk in alcoholic liver disease. Exp Mol Med. 2020;52:772–780. doi: 10.1038/s12276-020-0438-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szabo G. Gut–liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8:e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Da B.L., Im G.Y., Schiano T.D. COVID-19 hangover: a rising tide of alcohol use disorder and alcohol-associated liver disease. Hepatology. 2020;72:1102–1108. doi: 10.1002/hep.31307. [DOI] [PubMed] [Google Scholar]
- 24.Clay J.M., Parker M.O. Alcohol use and misuse during the COVID-19 pandemic: a potential public health crisis? Lancet Public Health. 2020;5:e259. doi: 10.1016/S2468-2667(20)30088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tenforde M.W., Rose E.B., Lindsell C.J. Characteristics of adult outpatients and inpatients with COVID-19: 11 academic medical centers, United States, March–May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:841–846. doi: 10.15585/mmwr.mm6926e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.COVID-19 in racial and ethnic minority groups Atlanta, GA: US Department of Health and Human Services, CDC. 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/racialethnic-minorities.html Available at: Accessed September 1, 2020.