Abstract
Objective
In the present study, we investigated the most useful confirmatory test for reflecting the severity of primary aldosteronism (PA), by evaluating 24-hour blood pressure (BP), urine albumin, left ventricular mass (LVM), and intima media thickness (IMT).
Methods
This study included 113 patients (80 PA and 33 non-PA hypertensive patients) who were admitted to Oita University Hospital and evaluated using ambulatory blood pressure monitoring (ABPM). First, casual blood pressure (BP) and ABPM parameters were compared between PA and non-PA patients. Second, patients were divided into PA-positive and PA-negative groups based on confirmatory tests, including the saline infusion test (SIT), captopril challenge test (CCT), and oral salt loading test (OSLT), and casual BP and ABPM parameters were compared between the 2 groups. In addition, urine albumin excretion, LVM, and maximum IMT as markers of organ damage were compared between the 2 groups.
Results
The ABPM parameters but not casual BP, were higher in PA patients than in non-PA patients. Nocturnal and 24-hour systolic BP (SBP) in OSLT-positive patients were significantly higher than in OSLT-negative patients. ABPM parameters in other confirmatory tests were not different between the PA-positive and PA-negative groups. Urine albumin excretion in OSLT-positive patients was significantly higher than in the OSLT-negative patients. However, in other confirmatory tests, organ damage markers were not different between the 2 groups.
Conclusion
The OSLT is potentially useful not only for the diagnosis of PA but also for assessment of 24-hour SBP and organ damage, as indicated by urine albumin excretion.
Keywords: primary aldosteronism, blood pressure, ambulatory blood pressure monitoring, confirmatory test, oral salt loading test, urine albumin
Hypertension causes arteriosclerosis followed by myocardial infarction, stroke, and peripheral artery diseases [1, 2]. Reportedly, hypertension was the strongest risk factor for cerebrocardiovascular events in the Japanese population [3]. Ambulatory blood pressure monitoring (ABPM) that measures blood pressure (BP) during a 24-hour period is useful for evaluating hypertension and predicting cerebrocardiovascular events [4-9]. In particular, nocturnal systolic blood pressure (SBP) correlates with urine albumin [10-12], left ventricular mass (LVM) [13, 14], maximum intima media thickness (IMT) [14, 15], and cardiovascular death [16], therefore, nocturnal SBP is considered a prognostic factor for hypertension.
Primary aldosteronism (PA) is characterized by excessive aldosterone secretion, suppression of plasma renin activity, and hypertension. PA prevalence ranges from 3% to 10% among hypertensive patients [17, 18]. In previous studies, patients with PA were associated with a higher risk of cerebrocardiovascular events, atrial fibrillation, and chronic kidney disease compared with essential hypertension patients [19-21]. The Japan Primary Aldosteronism Study (JPAS) group reported that Japanese PA patients are at higher risk of cerebrocardiovascular events: 9.4% cardiovascular disease, 7.4% stroke, and 4.0% arrhythmia [22]. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH2019) recommends that PA diagnosis should be based on a screening test followed by confirmatory tests, such as saline infusion test (SIT), furosemide upright test (FUT), captopril challenge test (CCT), or oral salt loading test (OSLT). However, it is unknown which of these confirmatory tests most accurately reflects cardiovascular damage in PA patients. Furthermore, the use of ABPM for PA patients has been investigated in only a few studies, and correlation with confirmatory tests for PA when using ABPM was not discussed.
In the present study, we investigated which confirmatory test was most useful for reflecting the severity of PA, by evaluating 24-hour BP and measuring urine albumin, LVM, and IMT.
Patients and Methods
Subjects
In the present study, we recruited 113 patients with hypertension who were admitted to the Department of Endocrinology and Metabolism, Oita University Hospital, from January 2018 to October 2019 and who were examined using ABPM. Among 116 patients, 83 were diagnosed with PA based on confirmatory tests. Among the 83 PA patients, subjects diagnosed with atrial fibrillation, in whom BP could not be measured correctly, who had advanced diabetic nephropathy with overt albuminemia and estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2, or had subclinical Cushing syndrome, were excluded (n = 3), because these comorbidities might affect the BP or urine albumin results. In addition, 33 obese diabetic patients not diagnosed with PA (non-PA) and who received ABPM were used as controls. There were no suspected cases of other secondary hypertension, including renovascular hypertension, pheochromocytoma, and paraganglioma, using plasma renin and 24-hour urine fractionated metanephrine and normetanephrine excretion.
This study was approved by the Human Ethics Committee of Oita University and was performed in accordance with the Declaration of Helsinki.
BP measurement
Casual BP was measured early in the morning during hospitalization while the patient was at rest in a seated position. The ABPM (FB-270, Fukuda Denshi Co., Ltd., Tokyo, Japan) measured SBP and diastolic blood pressure (DBP) continuously every 30 minutes during the day (7 am to 10 pm) and every 60 minutes during the night (10 pm to 7 am).
PA diagnosis
Patients were diagnosed with PA according to JSH2019 [23]. Screening tests were performed by measuring plasma renin activity (PRA) or active renin concentration (ARC) and plasma aldosterone concentration (PAC) from 8:00 to 10:00 am. The screening test was determined positive when the PAC (pg/mL)/PRA (ng/mL/h) ratio was >200 or the PAC/ARC (pg/mL) ratio was >40 plus basal PAC >120 pg/mL. Next, SIT, CCT, and OSLT were performed as confirmatory tests. SIT was performed by intravenous infusion of 2 L of saline over 4 hours, with the patient in a supine position. When PAC was >60 pg/mL after saline loading, the test was considered positive. CCT was performed by administration of 50 mg captopril tablets and blood was drawn after 90 minutes. When the PAC/PRA ratio was >200 or PAC/ARC ratio was >40 at 90 minutes after administration, the test was considered positive. OSLT was performed by 24-hour urine pooling after consumption of a high salt diet. The test was considered positive when the 24-hour urinary aldosterone and Na excretion were >8 μg/day and >170 mEq/day, respectively. When urine Na was <170 mEq/day, the data were excluded due to insufficient NaCl loading. Since angiotensin receptor blockers (ARBs), mineralocorticoid receptor antagonists (MRAs), beta-adrenergic blockers, and diuretics affect renin and aldosterone, these drugs were disncontinued or changed to calcium channel blockers (CCBs) or alpha-adrenergic blockers 2 weeks before the confirmatory test. Patients at risk of potassium depletion due to discontinuation of ARBs and MRAs were supplemented with potassium.
Comparison of BP, confirmatory tests, and hypertensive changes
First, SBP, DBP, and heart rate (HR) in casual, daytime, nocturnal, and continuous 24-hour ABPM were compared in PA and non-PA patients. Second, in each confirmatory test, patients were divided into PA-positive and PA-negative groups and the confirmatory test results and ABPM parameters were compared. Third, in each confirmatory test, urine albumin excretion, LVM, and maximum IMT were compared between the PA-positive and PA-negative groups.
Laboratory tests
Blood was sampled early in the morning after fasting. PAC was measured using the Chemiluminescent Enzyme Immunoassay (CLEIA) Accuraseed Aldosterone Kit (FUJIFILM Wako Pure Chemical Corporation, Osaka), ARC was measured only at the baseline hormone level using the CLEIA Accuraseed Renin Kit (FUJIFILM Wako Pure Chemical Corporation), PRA was measured only at CCT using the Radio Immunoassay (RIA) Renin FR Kit (FUJIREBIO Inc., Tokyo), and urine aldosterone was measured using the RIA Spac S Aldosterone Kit (TFB Corporation, Tokyo).
Statistical analysis
Values are expressed as means ± standard error (SE). For statistical analysis, the unpaired t test in Microsoft Excel 2019 was performed for comparison of the 2 groups, and analysis of variance was performed by GraphPad prism 7 (GraphPad Software Inc., La Jolla, CA, USA) for comparison of the 3 groups. P < 0.05 was considered statistically significant.
Results
Background of subjects
As shown in Table 1, PA patients presented with lower serum K and ARC but higher PAC and aldosterone-to-renin ratio (ARR) than non-PA patients. Non-PA patients showed higher body mass index (BMI), glycated hemoglobin A1c (HbA1c), and maximum IMT than PA patients. The proportion of antihypertensive drugs in PA and non-PA patients was as follows: CCBs, 82.7% and 33.3%; ARBs, 13.3% and 33.3%; MRAs, 8.0% and 9.1%; diuretics, 2.7% and 27.3%; beta-adrenergic blockers, 16.0% and 12.0%; alpha-adrenergic blockers, 9.3% and 6.1%, respectively. Among the 80 PA patients, 48 underwent subtype testing using adrenal vein sampling (39 bilateral and 9 unilateral PA). The lateral PA diagnosis was unknown in the remaining 32 PA patients because adrenal vein sampling failed or was not conducted.
Table 1.
Clinical Features of Patients With Primary Aldosteronism (PA) and non-PA
| PA | non-PA | P value | |
|---|---|---|---|
| Sex (male / female) | 32 / 48 | 19 / 14 | |
| Age | 54.2 ± 1.6 | 57.1 ± 3.2 | 0.412 |
| BMI (kg / m2) | 25.0 ± 4.7* | 29.6 ± 7.3 | 0.002 |
| Number of antihypertensive drugs | 1.2 ± 0.1 | 1.2 ± 0.2 | 0.999 |
| eGFR (mL / min / 1.73m2) | 71.8 ± 1.8 | 76.9 ± 4.9 | 0.339 |
| HbA1c (%) | 5.9 ± 0.1* | 8.5 ± 0.4 | < 0.001 |
| Na (mEq/L) | 140.4 ± 0.3 | 139.5 ± 0.5 | 0.082 |
| K (mEq/L) | 3.8 ± 0.1* | 4.2 ± 0.1 | < 0.001 |
| ARC (pg/mL) | 3.9 ± 0.5* | 16.5 ± 3.3 | < 0.001 |
| PAC (pg/mL) | 213.7 ± 9.7* | 135.8 ± 8.2 | < 0.001 |
| ARR | 140.4 ± 18.6* | 16.9 ± 2.3 | < 0.001 |
| Adrenal tumor (n) | 29 / 80 | 0 / 33 | |
| Urine albumin (mg/day) | 38.5 ± 13.2 | 46.3 ± 25.9 | 0.765 |
| LVM (g) | 157.5 ± 5.5 | 157.1 ± 11.4 | 0.972 |
| max IMT (mm) | 1.18 ± 0.09* | 1.53 ± 0.13 | 0.025 |
Data are presented as means ± SE.
Abbreviations: ARC, active renin concentration; ARR, aldosterone-to-renin ratio; BMI, body mass index; CCT, captopril challenge test; eGFR, estimated glomerular filtration rate; IMT, intima media thickness; LVM, left ventricular mass; PA, primary aldosteronism; PAC, plasma aldosterone concentration; SE, standard error.
* Compared with non-PA patients (P < 0.05).
Comparison of casual BP and ABPM parameters between PA and non-PA patients
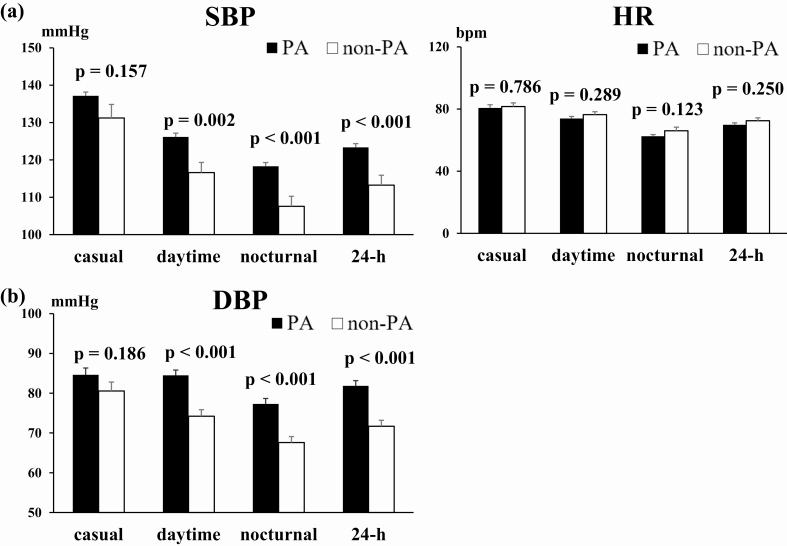
Casual BP and ABPM parameters were compared between PA and non-PA patients (Fig. 1). Significant differences in casual SBP and DBP were not observed between PA and non-PA patients (casual SBP: 137.2 ± 2.2 mmHg and 131.2 ± 3.6 mmHg, P = 0.157; casual DBP: 84.6 ± 1.7 mmHg and 80.5 ± 2.3 mmHg, P = 0.186, respectively). In contrast, mean daytime, nocturnal, and 24-hour SBP were significantly higher in PA than non-PA patients (daytime SBP: 126.2 ± 1.7 mmHg and 116.6 ± 2.7 mmHg, P = 0.002; nocturnal SBP: 118.3 ± 1.6 mmHg and 107.6 ± 2.7 mmHg, P < 0.001; 24-hour SBP: 123.4 ± 1.6 mmHg, 113.2 ± 2.6 mmHg, P < 0.001, respectively). Similarly, mean daytime, nocturnal, and 24-hour DBP were also significantly higher in PA than in non-PA patients (daytime DBP: 84.5 ± 1.3 mmHg and 74.2 ± 1.7 mmHg, P < 0.001; nocturnal DBP: 77.3 ± 1.3 mmHg and 67.6 ± 1.5 mmHg, P < 0.001; 24-hour DBP: 81.9 ± 1.3 mmHg and 71.7 ± 1.5 mmHg, P < 0.001, respectively). Casual, daytime, nocturnal, and 24-hour HR were not significantly different between the 2 groups.
Figure 1.
Comparison of systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) in primary aldosteronism (PA) and non-PA patients BP was compared between PA and non-PA patients. Casual, daytime, nocturnal, and 24-hour (a) SBP, (b) DBP) and (c) HR. Bar shows standard error.
Comparison of the confirmatory test results and BP levels
The SBP, DBP, and HR were compared between test-positive and test-negative patients in each confirmatory test (Table 2). Nocturnal and 24-hour SBP were significantly higher in OSLT-positive patients than in OSLT-negative patients; however, the differences were not observed between SIT- or CCT-positive and -negative patients. Clinical features were not significantly different between test-positive and test-negative patients in each confirmatory test (Table 3). Analysis of variance for absolute PAC values of OSLT-positive, SIT-positive, and CCT-positive cases showed no significant difference (OSLT vs CCT, P = 0.363; OSLT vs SIT, P = 0.292; and CCT vs SIT, P = 0.991 , respectively).
Table 2.
Comparison of Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), and Heart Rate (HR) Between Confirmatory Test-Positive and -Negative Patients of Primary Aldosteronism
| OSLT | p value | SIT | p value | CCT | p value | ||||
|---|---|---|---|---|---|---|---|---|---|
| positive | negative | positive | negative | positive | negative | ||||
| SBP | |||||||||
| Casual | 138.6 ± 5.3 | 136.9 ± 4.9 | 0.825 | 138.8 ± 2.7 | 141.3 ± 13.0 | 0.821 | 136.3 ± 3.1 | 139.3 ± 5.0 | 0.626 |
| Daytime | 131.8 ± 3.4 | 122.7 ± 3.0 | 0.053 | 125.7 ± 1.8 | 133.3 ± 7.1 | 0.300 | 125.8 ± 2.0 | 125.1 ± 2.8 | 0.858 |
| Nocturnal | 124.3 ± 3.2* | 114.4 ± 3.2 | 0.036 | 117.8 ± 1.8 | 125.3 ± 9.1 | 0.334 | 119.3 ± 2.1 | 115.3 ± 4.0 | 0.375 |
| 24-hour | 129.2 ± 3.3* | 119.9 ± 3.0 | 0.044 | 119.7 ± 1.7 | 130.3 ± 7.6 | 0.310 | 123.3 ± 1.9 | 121.7 ± 3.1 | 0.675 |
| DBP | |||||||||
| Casual | 87.6 ± 3.7 | 85.6 ± 3.8 | 0.711 | 86.4 ± 2.0 | 79.0 ± 12.3 | 0.401 | 83.7 ± 2.3 | 83.8 ± 4.0 | 0.969 |
| Daytime | 88.2 ± 2.7 | 82.4 ± 2.4 | 0.118 | 84.6 ± 1.6 | 84.7 ± 0.9 | 0.987 | 83.9 ± 1.7 | 83.7 ± 1.7 | 0.955 |
| Nocturnal | 80.8 ± 2.3 | 76.2 ± 3.0 | 0.231 | 77.3 ± 1.6 | 60.3 ± 5.4 | 0.840 | 76.9 ± 1.8 | 78.6 ± 2.5 | 0.637 |
| 24-hour | 85.8 ± 2.6 | 80.1 ± 2.5 | 0.123 | 72.7 ± 1.5 | 81.7 ± 1.5 | 0.975 | 81.2 ± 1.6 | 81.7 ± 1.9 | 0.878 |
| HR | |||||||||
| Casual | 77.6 ± 4.2 | 81.6 ± 4.8 | 0.539 | 81.9 ± 2.2 | 81.7 ± 14.7 | 0.977 | 78.7 ± 2.1 | 81.9 ± 5.6 | 0.511 |
| Daytime | 72.8 ± 2.2 | 72.4 ± 3.1 | 0.915 | 74.5 ± 1.5 | 84.0 ± 10.5 | 0.139 | 74.5 ± 1.6 | 74.1 ± 3.7 | 0.920 |
| Nocturnal | 61.4 ± 1.7 | 61.8 ± 2.6 | 0.453 | 63.6 ± 1.2 | 76.0 ± 3.6 | 0.082 | 63.3 ± 1.2 | 62.9 ± 2.7 | 0.902 |
| 24-h | 68.9 ± 2.0 | 68.9 ± 2.8 | 0.498 | 73.0 ± 1.3 | 76.3 ± 9.2 | 0.305 | 70.5 ± 1.4 | 70.0 ± 3.1 | 0.886 |
Data are presented as means ± SE.
Abbreviations: BP, blood pressure; CCT, captopril challenge test; DBP, diastolic blood pressure; HR, heart rate; OSLT, oral salt loading test; PA, primary aldosteronism; SBP. systolic blood pressure; SE, standard error; SIT, saline infusion test.
*BP comparison between confirmatory test-positive and -negative PA patients (P < 0.05).
Table 3.
Clinical Features of Patients Undergoing Ambulatory Blood Pressure Monitoring (ABPM) and Divided Into Confirmatory Test-Positive or -Negative Groups
| OSLT | P value | SIT | P value | CCT | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| positive | negative | positive | negative | positive | negative | ||||
| N | 21 | 21 | 50 | 3 | 40 | 14 | |||
| BMI (kg/m 2) | 25.4 ± 1.2 | 23.3 ± 0.8 | 0.157 | 25.0 ± 0.7 | 22.4 ± 0.7 | 0.397 | 25.1 ± 0.8 | 23.9 ± 0.9 | 0.451 |
| Antihypertensive drugs (n) | 1.6 ± 0.4 | 0.8 ± 0.1 | 0.074 | 1.0 ± 0.1 | 1.0 ± 0.0 | 0.941 | 1.2 ± 0.2 | 1.1 ± 0.1 | 0.821 |
|
eGFR
(mL / min /1.73m2) |
76.6 ± 3.7 | 75.7 ± 3.5 | 0.851 | 72.4 ± 2.3 | 79.1 ± 11.4 | 0.490 | 73.2 ± 2.4 | 70.6 ± 5.0 | 0.609 |
| K (mEq/L) | 3.7 ± 0.1 | 3.9 ± 0.1 | 0.292 | 3.8 ± 0.1 | 3.8 ± 0.2 | 0.880 | 3.8 ± 0.1 | 3.8 ± 0.1 | 0.617 |
|
Basal ARC
(pg/mL) |
3.8 ± 1.4 | 1.9 ± 0.4 | 0.243 | 3.6 ± 0.7 | 8.8 ± 6.3 | 0.100 | 3.1 ± .0.7 | 4.2 ± 0.7 | 0.390 |
|
Basal PAC
(pg/mL) |
259.2 ± 27.2 | 199.5 ± 21.4 | 0.100 | 218.4 ± 13.4 | 162.9 ± 33.4 | 0.324 | 221.0 ± 15.0 | 195.3 ± 20.1 | 0.364 |
| Basal ARR | 192.1 ± 51.0 | 193.2 ± 52.7 | 0.988 | 156.4 ± 26.8 | 96.2 ± 72.7 | 0.591 | 186.0 ± 31.9 | 95.2 ± 30.0 | 0.117 |
Data are presented as means ± SE.
Abbreviations: ARC, active renin concentration; ARR, aldosterone-to-renin ratio; BMI, body mass index; CCT, captopril challenge test; eGFR, estimated glomerular filtration rate; OSLT, oral salt loading test; PAC, plasma aldosterone concentration; SE, standard error; SIT, saline infusion test; SE, standard error; SIT, saline infusion test.
Relationship between confirmatory test result and organ damage markers
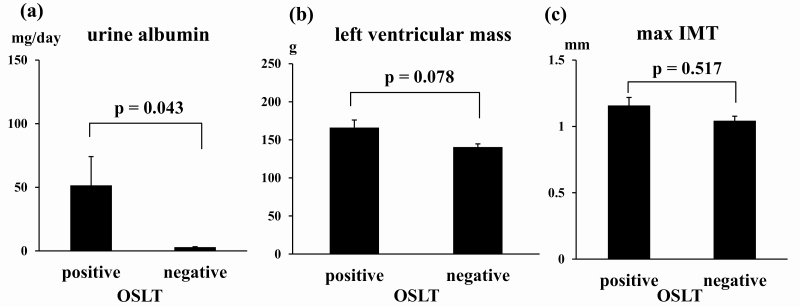
The OSLT was performed in 52 PA patients and 10 of 52 patients were excluded due to insufficient NaCl loading, based on urine Na <170 mEq/day. Consequently, the numbers of OSLT-positive and OSLT-negative patients were 21 and 21, respectively. The prevalence of diabetes or impaired glucose tolerance, duration of diabetes, HbA1c, and eGFR were not significantly different between OSLT-positive and OSLT-negative patients (prevalence of diabetes or impaired glucose tolerance: 23.8% and 28.6%; duration of diabetes: 14.0 ± 6.7 years and 6.9 ± 3.8 years, P = 0.393; HbA1c: 5.8 ± 0.1% and 6.0 ± 0.2%, P = 0.447; eGFR: 74.5 ± 3.7 mL/min/1.73m2 and 74.6 ± 3.1 mL/min/1.73 m2, respectively). Urine albumin, LVM, and IMT were considered as organ damage markers and compared (Fig. 2). Urine albumin excretion in OSLT-positive patients was significantly higher than in OSLT-negative patients (51.6 ± 22.5 mg/day and 3.0 ± 0.3 mg/day, respectively, P = 0.043). The LVM in OSLT-positive and OSLT-negative patients was similar (123.0 ± 1.7 g and 124.5 ± 8.5 g, respectively, P = 0.87). The maximum IMT in OSLT-positive and OSLT-negative patients was also not statistically different (1.16 ± 0.13 mm and 1.04 ± 0.12 mm, respectively, P = 0.517). Based on the results showing OSLT is associated with urine albumin excretion, OSLT may be useful for assessing 24-hour SBP and organ damage.
Figure 2.
Comparison of urine albumin, left ventricular mass (LVM), and maximum intima media thickness (IMT) between oral salt loading test (OSLT)-positive and OSLT-negative patients with primary aldosteronism (PA) Patients were divided into OSLT-positive and OSLT-negative patients, and urine albumin, left ventricular mass (LVM), and maximum IMT were evaluated. (a) Urine albumin excretion, (b) LVM, and (c) maximum IMT. Bar shows standard error.
Discussion
In the present study, which confirmatory test was most useful for reflecting the PA severity was determined by evaluating 24-hour BP and organ damage markers. Thus, 24-hour BP and organ damage markers were investigated in patients diagnosed with PA based on confirmatory tests: SIT, CCT, and OSLT. The results showed that OSLT, but not SIT or CCT, was positively correlated with nocturnal and 24-hour SBP. Furthermore, OSLT-positive patients showed higher urine albumin excretion than OSLT-negative patients.
First, ABPM parameters including daytime, nocturnal, and 24-hour BP were higher in PA patients than in non-PA patients, although higher proportions of obesity and diabetes were observed in non-PA patients. Differences in casual BP were not found between the 2 groups. The results indicated 24-hour BP measurement using ABPM is more important for accurately evaluating BP than casual BP measurement. The present study results are consistent with several previous reports demonstrating that nocturnal SBP in PA patients was higher than essential hypertension [24, 25]. Multiple mechanisms may play crucial roles in severe and resistant hypertension in PA patients due to excessive aldosterone-mediated mineralocorticoid receptor activation followed by increased effective circulatory blood volumes, sympathetic nervous system activity, salt sensitivity, cardiovascular inflammation, and complication of obstructive sleep apnea syndrome. We did not investigate whether efficacies of antihypertensive drugs are different between PA and non-PA subjects. Since the numbers of antihypertensive drugs to control BP were not significantly different between PA and non-PA patients (Table 1), it is not likely that proportion of resistant hypertension is different between these 2 groups.
Second, whether one PA confirmatory test was superior to others was not proven. The clinical practice guideline for PA recommends that at least one test should be positive to confirm PA diagnosis. However, a positive confirmatory test could indicate not only a PA diagnosis but also clinical or biochemical PA severity. In previous reports, the SIT results correlated with cerebrocardiovascular events [26]. In another report, the number of positive confirmatory tests correlated with cerebrocardiovascular events [27]. However, the comparison between confirmatory tests and ABPM or organ damage markers has not been reported in previous studies. In the present study, OSLT, but not SIT or CCT, was associated with 24-hour SBP and urine albumin. The positive result associated with 24-hour BP and urine albumin in OSLT, but not SIT or CCT, was presumedly because 24-hour urine collection was required for OSLT. We assumed that a reason why 24-hour urine collection explain the positive result with the 24-hour BP monitoring may be attributable to different methods of sample collection by each confirmatory test. The OSLT uses 24-hour urine aldosterone during 24-hour salt-loading period. In contrast, both SIT and CCT utilize 1 PAC value, at 4 hours and 90 minutes, respectively, after aldosterone suppression. Moreover, the positive rate with SIT (94%) and CCT (74%) was higher than that with OSLT (50%) in this study. Since patients who were tested with each confirmatory test were partially overlapped, but not exactly same, it is not possible to determine the superiority or inferiority of the performance of each confirmatory test. The different cutoff values for each confirmatory test by JSH2019 compared with other guidelines [28] and previous reports [29] may be attributable to different positive rates.
Third, OSLT-positive patients excreted significantly more urine albumin than OSLT-negative patients and tended to have a higher LVM than OSLT-negative patients. According to numerous reports, PA caused kidney damage and increased urinary albumin excretion [30-33]. Possible mechanisms include increased renal fibrosis and impaired reabsorption of albumin in renal tubules. Ribstein et al also confirmed that albumin levels decrease after PA treatment [30]. Based on the data, the relationship between PA and urinary albumin excretion is evident. The OSLT-positive patients had higher urine albumin excretion than OSLT-negative patients, possibly due to long-standing higher 24-hour SBP. As shown in the results, because significant differences in HbA1c and the number of diabetic patients were not found between OSLT-positive and OSLT-negative patients, the possibility of a higher proportion of diabetic nephropathy cases in OSLT-positive patients was eliminated. Similar to urinary albumin excretion, PA reportedly affected LVM and IMT [32, 33]. In the present study, LVM and IMT were not significantly different between OSLT-positive and OSLT-negative patients; however, OSLT-positive patients tended to have higher LVM than OSLT-negative patients. Other organ damage markers, such as LVM and maximum IMT, may be statistically different between PA-positive and PA-negative patients if a larger number of PA patients is investigated.
Limitations
The present study had several limitations. First, the non-PA patient group was not completely uniform and included a substantial number of diabetic and obese patients. Second, PA patients often have sleep apnea syndrome; however, the presence of sleep apnea syndrome was not investigated in the present study. Third, urine albumin, LVM, and IMT were investigated as organ damage markers; however, actual long-term prognosis, such as cerebrocardiovascular events and mortality were not investigated. These data should be investigated in future studies.
Conclusion
In the present study, the OSLT results were positively correlated with 24-hour SBP, and OSLT-positive patients had higher urine albumin excretion than OSLT-negative patients.
OSLT is potentially useful not only for PA diagnosis but also for assessment of 24-hour SBP and organ damage, as indicated by urine albumin excretion.
Acknowledgments
Clinical Trial Information: This clinical study was approved by the Oita University School of Medicine Ethics Committee on June 12, 2017 as Registration No. 909.
Glossary
Abbreviations
- ABPM
ambulatory blood pressure monitoring
- ARB
angiotensin receptor blocker
- ARC
active renin concentration
- ARR
aldosterone-to-renin ratio
- BP
blood pressure
- BMI
body mass index
- CCT
captopril challenge test
- DBP
diastolic blood pressure
- eGFR
estimated glomerular filtration rate
- FUT
furosemide upright test
- HbA1c
glycated hemoglobin A1c
- HR
heart rate
- IMT
intima media thickness
- LVM
left ventricular mass
- MRA
mineralocorticoid receptor antagonist
- OSLT
oral salt loading test
- PA
primary aldosteronism
- PAC
plasma aldosterone concentration
- PRA
plasma renin activity
- SBP
systolic blood pressure
- SIT
saline infusion test
Additional Information
Disclosure Summary: The authors have nothing to disclose. All study participants provided informed consent, and the study design was approved by an ethics review board. All the authors have read the manuscript and have approved this submission.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Chobanian AV, Bakris GL, Black HR, et al. ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. [DOI] [PubMed] [Google Scholar]
- 2. Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335(8693): 827-838. [DOI] [PubMed] [Google Scholar]
- 3. Ikeda N, Inoue M, Iso H, et al. Adult mortality attributable to preventable risk factors for non-communicable diseases and injuries in Japan: a comparative risk assessment. Plos Med. 2012;9(1):e1001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ohkubo T, Hozawa A, Nagai K, et al. Prediction of stroke by ambulatory blood pressure monitoring versus screening blood pressure measurements in a general population: the Ohasama study. J Hypertens. 2000;18(7):847-854. [DOI] [PubMed] [Google Scholar]
- 5. Suzuki Y, Kuwajima I, Aono T, et al. Prognostic value of nighttime blood pressure in the elderly: a prospective study of 24-hour blood pressure. Hypertens Res. 2000;23(4):323-330. [DOI] [PubMed] [Google Scholar]
- 6. Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111(14):1777-1783. [DOI] [PubMed] [Google Scholar]
- 7. Kikuya M, Ohkubo T, Asayama K, et al. Ambulatory blood pressure and 10-year risk of cardiovascular and noncardiovascular mortality: the Ohasama study. Hypertension. 2005;45(2):240-245. [DOI] [PubMed] [Google Scholar]
- 8. Verdecchia P, Reboldi GP, Angeli F, et al. Short- and long-term incidence of stroke in white-coat hypertension. Hypertension. 2005;45(2):203-208. [DOI] [PubMed] [Google Scholar]
- 9. Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46(1):156-161. [DOI] [PubMed] [Google Scholar]
- 10. Kario K, Matsuo T, Kobayashi H, Imiya M, Matsuo M, Shimada K. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients. Advanced silent cerebrovascular damage in extreme dippers. Hypertension. 1996;27(1):130-135. [DOI] [PubMed] [Google Scholar]
- 11. Bianchi S, Bigazzi R, Baldari G, Sgherri G, Campese VM. Diurnal variations of blood pressure and microalbuminuria in essential hypertension. Am J Hypertens. 1994;7(1):23-29. [DOI] [PubMed] [Google Scholar]
- 12. Tsioufis C, Antoniadis D, Stefanadis C, et al. Relationships between new risk factors and circadian blood pressure variation in untreated subjects with essential hypertension. Am J Hypertens. 2002;15(7 Pt 1):600-604. [DOI] [PubMed] [Google Scholar]
- 13. Cicconetti P, Morelli S, Ottaviani L, et al. Blunted nocturnal fall in blood pressure and left ventricular mass in elderly individuals with recently diagnosed isolated systolic hypertension. Am J Hypertens. 2003;16(11 Pt 1):900-905. [DOI] [PubMed] [Google Scholar]
- 14. Cuspidi C, Macca G, Sampieri L, et al. Target organ damage and non-dipping pattern defined by two sessions of ambulatory blood pressure monitoring in recently diagnosed essential hypertensive patients. J Hypertens. 2001;19(9):1539-1545. [DOI] [PubMed] [Google Scholar]
- 15. Pierdomenico SD, Lapenna D, Guglielmi MD, et al. Arterial disease in dipper and nondipper hypertensive patients. Am J Hypertens. 1997;10(5 Pt 1):511-518. [DOI] [PubMed] [Google Scholar]
- 16. Garimella PS, Uhlig K. Current issues in the management and monitoring of hypertension in chronic kidney disease. Curr Opin Nephrol Hypertens. 2013;22(6):599-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Funder JW, Carey RM, Fardella C, et al. ; Endocrine Society Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(9):3266-3281. [DOI] [PubMed] [Google Scholar]
- 18. Conn JW. The evolution of primary aldosteronism: 1954-1967. Harvey Lect. 1966;62:257-291. [PubMed] [Google Scholar]
- 19. Takeda R, Matsubara T, Miyamori I, Hatakeyama H, Morise T. Vascular complications in patients with aldosterone producing adenoma in Japan: comparative study with essential hypertension. The Research Committee of Disorders of Adrenal Hormones in Japan. J Endocrinol Invest. 1995;18(5):370-373. [DOI] [PubMed] [Google Scholar]
- 20. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8):1243-1248. [DOI] [PubMed] [Google Scholar]
- 21. Nishimura M, Uzu T, Fujii T, et al. Cardiovascular complications in patients with primary aldosteronism. Am J Kidney Dis. 1999;33(2):261-266. [DOI] [PubMed] [Google Scholar]
- 22. Ohno Y, Sone M, Inagaki N, et al. ; Nagahama Study; JPAS Study Group Prevalence of cardiovascular disease and its risk factors in primary aldosteronism: A Multicenter Study in Japan. Hypertension. 2018;71(3):530-537. [DOI] [PubMed] [Google Scholar]
- 23. Umemura S, Arima H, Arima S, et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42(9):1235-1481. [DOI] [PubMed] [Google Scholar]
- 24. Mansoor GA, White WB. Circadian blood pressure variation in hypertensive patients with primary hyperaldosteronism. Hypertension. 1998;31(3):843-847. [DOI] [PubMed] [Google Scholar]
- 25. Uzu T, Nishimura M, Fujii T, et al. Changes in the circadian rhythm of blood pressure in primary aldosteronism in response to dietary sodium restriction and adrenalectomy. J Hypertens. 1998;16(12 Pt 1):1745-1748. [DOI] [PubMed] [Google Scholar]
- 26. Hayashi R, Tamada D, Murata M, et al. Saline infusion test highly associated with the incidence of cardio- and cerebrovascular events in primary aldosteronism. Endocr J. 2017;64(5):507-513. [DOI] [PubMed] [Google Scholar]
- 27. Saiki A, Tamada D, Hayashi R, et al. The number of positive confirmatory tests is associated with the clinical presentation and incidence of cardiovascular and cerebrovascular events in primary aldosteronism. Hypertens Res. 2019;42(8):1186-1191. [DOI] [PubMed] [Google Scholar]
- 28. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889-1916. [DOI] [PubMed] [Google Scholar]
- 29. Meng X, Li Y, Wang X, Li J, Liu Y, Yu Y. Evaluation of the saline infusion test and the Captopril challenge test in Chinese patients with primary aldosteronism. J Clin Endocrinol Metab. 2018;103(3):853-860. [DOI] [PubMed] [Google Scholar]
- 30. Ribstein J, Du Cailar G, Fesler P, Mimran A. Relative glomerular hyperfiltration in primary aldosteronism. J Am Soc Nephrol. 2005;16(5):1320-1325. [DOI] [PubMed] [Google Scholar]
- 31. Sechi LA, Novello M, Lapenna R, et al. Long-term renal outcomes in patients with primary aldosteronism. Jama. 2006;295(22):2638-2645. [DOI] [PubMed] [Google Scholar]
- 32. Blumenfeld JD, Sealey JE, Schlussel Y, et al. Diagnosis and treatment of primary hyperaldosteronism. Ann Intern Med. 1994;121(11):877-885. [DOI] [PubMed] [Google Scholar]
- 33. Halimi JM, Mimran A. Albuminuria in untreated patients with primary aldosteronism or essential hypertension. J Hypertens. 1995;13(12 Pt 2):1801-1802. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.