Abstract
Stress negatively affects the gastrointestinal tract (GIT) barrier function, resulting in compromised animal health. A deeper understanding of how diet and stress impacts the GIT barrier function in feedlot cattle is needed. Aspirin decreases mucus production and mucosal repair in the GIT and could be used as a model for GIT barrier dysfunction research. The objective of this study was to evaluate the effectiveness of aspirin to induce GIT barrier dysfunction in beef cattle. In experiment 1, sixteen crossbred heifers (425.0 ± 8.6 kg) were allotted to 0, 50, 100, or 200 mg/kg body weight (BW) aspirin doses based on BW. Experiment 1 consisted of two periods separated by 4 wk where four heifers per treatment received the same aspirin dose during each period. Heifers were fed a 49.4% corn silage and 50.6% concentrate diet. The 200 mg/kg BW aspirin treatment was dosed as a 100 mg/kg BW aspirin oral bolus 36 and 24 h prior to Cr-ethylenediaminetetraacetic acid (EDTA) dosing (1 liter; 180 mM). The 50 and 100 mg/kg BW aspirin treatments were dosed as an oral bolus 24 h prior to Cr-EDTA dosing. Urine was collected every 3 h for 48 h and analyzed for Cr. Serum was collected at 0 and 48 h and analyzed for lipopolysaccharide-binding protein (LBP), interleukin-6, serum amyloid A (SAA), haptoglobin, and aspartate aminotransferase. In experiment 2, sixteen crossbred steers (576.0 ± 14.2 kg) fed a similar diet were allotted by BW to the 0 and 200 mg/kg BW aspirin treatments (eight steers/treatment) and were slaughtered 24 h after the last dose. Jejunal tissues were collected, and claudin (CLDN) 1, 2, and 3, occludin, and zonula occludens tight junction messenger ribonucleic acid (mRNA) expression was determined. Data were analyzed using the MIXED procedure of SAS. Urinary Cr excretion increased linearly at hours 3, 6, 9, and 12 (P ≤ 0.04) as aspirin dose increased from 0 to 200 mg/kg. Aspirin linearly increased Cr absorption (P = 0.02) and elimination (P = 0.04) rates and linearly decreased mean retention time of Cr (P = 0.02). Aspirin increased SAA (P = 0.04) and tended to increase LBP (P = 0.09) in serum but did not affect any other serum inflammatory marker (P ≥ 0.19). Aspirin tended to increase jejunal CLDN-1 mRNA expression (P = 0.10) but did not affect the mRNA expression of other genes regulating tight junction function (P ≥ 0.20). Results from this study indicate that aspirin disrupts the GIT barrier function in beef cattle and has a potential as a model in GIT permeability research.
Keywords: aspirin, beef feedlot, inflammation, leaky gut, tight junction
Introduction
There are a variety of circumstances in cattle in which the gastrointestinal tract (GIT) barrier function is compromised (i.e., leaky gut) and allows unwanted material to cross. Well-known examples include weaning (Moeser et al., 2007), transportation stress (Wan et al., 2014; Cooke, 2017), heat stress (Baumgard and Rhoads, 2013), feed restriction (Zhang et al., 2013), and acute or subacute ruminal acidosis (Khafipour et al., 2009; Minuti et al., 2014). Numerous studies have demonstrated that when pathogenic bacteria, endotoxins, or other antigens present in ruminant digesta cross the GIT barrier, they are linked to disorders, such as laminitis, liver abscesses, and respiratory disease (Garcia et al., 2017). Endotoxins are normally present in a large amount in the rumen and in the hindgut of ruminants. Bacterial lipopolysaccharide (LPS) will increase when ruminants are fed diets that are rich in easily digestible carbohydrates because Gram-negative bacteria experience growth benefits from such diets and thereby increased LPS shedding (Plaizier et al., 2012). High-grain feeding has been connected to increased amounts of free LPS in rumen fluid and acute-phase proteins in the peripheral circulation (Khafipour et al., 2009). Some of the negative effects of high-grain feeding can be mitigated with proper dietary transitions and careful management of feed intake; however, many stressors are challenging to mitigate with management alone. Thus, a deeper understanding of how diet and stress impact the GIT barrier function in feedlot cattle is needed.
GIT barrier function can be studied by imposing a stress, such as transport, feed restriction, or heat to induce leaky gut. However, imposing a stress causes unwanted side effects for the animal (sickness and decreased growth), and there is a large variation among animals in their response to stress. Indomethacin, a nonsteroidal anti-inflammatory drug (NSAID), is commonly used to induce leaky gut in laboratory animals and humans (Bjarnason et al., 1986) and also used in calves (Klein et al., 2007) in order to study leaky gut independent of other stressors. In addition, Kvidera et al. (2017) intentionally induced leaky gut in dairy cattle with gamma-secretase inhibitor (GSI), a drug that has the side effect of inducing goblet cell metaplasia and disrupting crypt cell differentiation. However, GSI and indomethacin are not FDA approved for animal use (FDA, 2020).
Aspirin (acetylsalicylic acid) is an NSAID known to cause mucosal injury leading to increased gut permeability and tight junction damage in laboratory animals and humans and is used as a model to induce leaky gut (Oshima et al., 2008; Lambert et al., 2012). Aspirin covalently binds the active site of cyclooxygenase (COX)-1 and COX-2 isoenzymes (Simmons et al., 2004). COX is used for the production of prostaglandin from arachidonic acid, and prostaglandins are associated with producing pain and inflammation; however, prostaglandins also induce protective mucus production and provide mucosal repair (Takeuchi and Amagase, 2018). Frequent or heavy use of aspirin in humans is known to cause the GIT barrier dysfunction (Bjarnason et al., 1986; Lambert et al., 2012) and ulceration (Bjarnason et al., 1987). Bjarnason et al. (1987) discovered that in humans, inflammation from NSAID use can persist for up to 16 mo after discontinuation. GIT dysfunction and ulceration cause an immune response, which is signaled by inflammation. Thus, the use of NSAIDs can eventually cause small intestinal inflammation and associated blood and protein loss (Bjarnason et al., 1993). Aspirin is commonly used as an analgesic in cattle; the maximum recommended dosage is 100 mg/kg every 12 h (Gingerich et al., 1975) with a 24-h meat and milk withdrawal period (Smith et al., 2008). Based on the known mucosal injury in nonruminants, the objective of this study was to determine the optimal dosage of aspirin that causes GIT barrier dysfunction. We hypothesized that aspirin would cause mucosal damage, inflammation, and GIT dysfunction in beef cattle, resulting in increased GIT permeability.
Materials and Methods
All procedures performed in this study were approved by the Purdue University Animal Care and Use Committee and were in accordance with the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching (FASS, 2010). The experiment was conducted at the Purdue University Animal Sciences Research and Education Center (ASREC) in West Lafayette, IN.
Experiment 1
Animals and sampling
Sixteen Angus × Simmental heifers were allotted to 0, 50, 100, and 200 mg/kg body weight (BW) aspirin to determine the serum markers of inflammation and Cr appearance in urine as a measure of GIT leakiness. Experiment 1 consisted of two periods separated by 4 wk where four heifers per treatment received the same aspirin dose during each period. Heifers were weighed at the start of each period with scales (480 Legend, Rice Lake Weighing Systems, Rice Lake, WI) that weighed to the nearest 0.5 kg and were checked for accuracy at each weigh date. Heifers were allotted to treatments such that BW was equal among treatments. The mean BW at the start of each period was 401.0 ± 37.1 and 448.0 ± 33.7 kg, respectively, and heifers were approximately 10 mo old. The basal diet (Table 1) was formulated on a dry matter (DM) basis to meet or exceed National Academies of Sciences, Engineering, and Medicine (NASEM) (2016) requirements for crude protein (CP), vitamins, and minerals and consisted of 49.4% corn silage, 24.7% corn, 24.7% dried distillers grains with solubles (DDGS), and 1.2% vitamin/mineral supplement. Diets were fed for 6 wk before the first experimental period in an open-sided barn with straw-bedded pens (3.4 × 9.1 m) over a concrete floor. Feed was offered once daily at 0900 hours, and heifers were allowed ad libitum access to feed and water. Daily feed deliveries were adjusted using a 4-point bunk scoring system (Pritchard, 1993) to allow for ad libitum feed intake with little or no accumulation of unconsumed feed (score of ≤1). Aspirin (acetylsalicylic acid; 31.2 g boluses; Agri Laboratories, Ltd. St. Joseph, MO) was dosed orally using a balling gun. Aspirin boluses were trimmed to achieve the target dosage. Heifers receiving the 50 and 100 mg/kg treatments were dosed with aspirin 24 h prior to administering Cr-ethylenediaminetetraacetic acid (EDTA) and collecting urine. Because 100 mg/kg BW is the maximum recommended dosage allowed for cattle (Gingerich et al., 1975), heifers receiving the 200 mg/kg treatment were dosed with 100 mg/kg aspirin, 36 and 24 h prior to the urine collection procedure.
Table 1.
Basal diets
| Experiment 1 | Experiment 2 | |
|---|---|---|
| Corn silage | 49.4 | 50.0 |
| DDGS | 24.7 | 23.0 |
| Corn | 24.7 | 21.0 |
| Vitamin/mineral supplement | 1.21 | 6.02 |
| Diet composition, DM basis | ||
| CP3, % | 12.9 | 12.9 |
| NEm4, Mcal/kg | 2.12 | 2.07 |
| NEg4, Mcal/kg | 1.26 | 1.21 |
| Calcium3, % | 1.00 | 1.19 |
| Phosphorus3, % | 0.48 | 0.39 |
| Sulfur3, % | 0.26 | 0.24 |
1Vitamin/mineral supplement contained: 19.94% salt, 20.05% Ca, 11.97% Cl, 8.04% Na, 5.03% P, 3.00% Fe, 2.004% Mn, 2.003% Zn, 1.26% Mg, 0.803% Cu, 0.70% S, 0.26% K, 0.05% Co, 0.05% I, 0.01% Se, 678,000 IU vitamin A/kg, and 1,547 IU of vitamin E/kg of premix.
2Vitamin/mineral supplement contained (DM basis): 18.25% Ca, 1.32% K, 0.44% Mg, 0.18% S, 563.91 ppm Zn, 522.90 ppm Fe, 440.41 ppm Mn, 183.33 ppm Cu, 9.66 ppm I, 4.48 ppm Se, 3.43 ppm Co, 42.19 IU/g vitamin A, 4.98 IU/g vitamin D, 0.155 IU/g vitamin E, and 413.6 ppm monensin (176.4 g/kg, Elanco Animal Health, Indianapolis, IN).
3Analyzed at Cumberland Valley Analytical Services (Waynesboro, PA).
4Calculated based on NASEM (2016).
Twenty-four hours after the final aspirin dose, 16 heifers were restrained in a working chute and given 1 liter of a 180-mM Cr-EDTA solution using an esophageal tube. Chromium-EDTA was prepared according to Binnerts et al. (1968). A latex Foley urinary catheter (C. R. Bard, Inc., Covington, GA) was inserted into the bladder; heifers were moved to individual stalls (1.1 × 2.1 m), tied to the front of the stall, and the urinary catheter line was attached to 4-liters drainage bags (Medline, Northfield, IL), which was fixed to the stall using string. Urine weight was recorded, and a 45-mL subsample was collected every 3 h and frozen at −20 °C for subsequent analysis of Cr. The urine that was not subsampled was discarded to allow fresh urine to be collected for the next time period. During the sampling period, grass hay and water were provided ad libitum for all animals.
Chromium analysis
Approximately, 2.5 mL of urine was digested in duplicate with 5 mL of nitric and 3 mL of perchloric acid at a temperature of 175 °C (Sandell, 1959). Each sample was allowed to cool to room temperature, diluted to 30 mL with nanopure water, and analyzed for Cr by atomic absorption spectroscopy at 425.4 nm (SpectrAA 220 FS, Varian, Inc., Palo Alto, CA) according to the procedures of Vicente et al. (2004). Urinary Cr content at 3 h each time point was calculated as urine amount × Cr concentration. Absorption and elimination rates were determined with the 3 h urine samples using a one-compartment model described by Atkins (1969). Elimination rate (k1, h−1) of Cr was calculated by fitting a linear regression to the natural logarithm of Cr concentration in the urine (mg/L) against sampling time (h) during the terminal phase, after maximum concentration was achieved. Absorption rate (k2, h−1) of Cr was calculated by fitting a linear regression to the natural logarithm of the residual of Cr concentration in urine against sampling time (h) prior to the terminal phase. The mean retention time (MRT) was estimated according to Grovum and Phillips (1973):
where A1 and A2 are intercept values for Cr concentration in urine and k1 and k2 are defined above.
Blood analysis
Approximately, 10 mL of blood was collected from the jugular vein at time 0 and 48 h after Cr-EDTA dosing into tubes (BD Vacutainer, Becton Dickinson, Franklin Lakes, NJ). Blood samples were centrifuged at 1,250 × g for 20 min, and serum was collected in three aliquots within 2 h of collection. Serum was stored in 5 mL polystyrene tubes and frozen at −20 °C until analysis. Serum concentrations of interleukin-6 (IL-6; Abcam, ab205280, Cambridge, UK), lipopolysaccharide-binding protein (LBP; LSBio, LS-F7412, Seattle, WA), serum amyloid A (SAA; Tridelta Development Ltd., TP 802, Maynooth, County Kildare, IE), and haptoglobin (ICL, E-10HPT, Inc., Portland, OR) were analyzed using enzyme-linked immunosorbent assays (ELISA) kits at 450 nm on a Spark 10M plate reader (Tecan Life Sciences, Männedorf, Zürich, Switzerland). ELISA kits were bovine specific (IL-6, LBP, and haptoglobin) or designed to be used with multiple species (SAA) including bovine. Bovine aspartate aminotransferase concentrations in serum were determined using a colorimetric kit (Sigma-Aldrich, MAK055, St. Louis, MO) at 450 nm with the previously mentioned plate reader. Intra-assay coefficient of variations (CVs) for LBP, IL-6, haptoglobin, SAA, and aspartate aminotransferase (AST) were 5.70, 6.04, 3.47, 4.66, and 5.24, respectively, and inter-assay CVs for LBP, IL-6, haptoglobin, SAA, and AST were 18.97, 12.31, 12.81, 8.97, and 3.42, respectively.
Statistical analysis
Data were analyzed as a completely randomized design using the MIXED procedure of SAS (SAS Institute Inc., Cary, NC) with individual animal considered the experimental unit. The Shapiro–Wilk test was performed to test for normality. Serum metabolite concentrations and urine Cr concentration were analyzed as repeated measures over time. Serum IL-6, SAA, and haptoglobin were not normally distributed and were log10 transformed for statistical analysis. The model included the random effect of individual and the fixed effect of treatment, time, and the treatment × time interaction. Urine Cr amount was analyzed as repeated measures between periods and over time. The model included the random effect of individual and the fixed effect of period, treatment, time, and the treatment × time interaction. The treatment × period interaction was not significant and was removed from the model. Five covariance structures (autoregressive order one, heterogenous autoregressive order one, compound symmetry, heterogeneous compound symmetry, and variance components) were compared for each variable and the structure that yielded the lowest Bayesian Information Criterion was used for all results. The SLICE function of SAS was used to determine the simple effect of aspirin within time. Urine Cr kinetics were analyzed using the MIXED procedure of SAS without repeated measures. The model included the fixed effect of treatment. Linear and quadratic coefficients were generated with the IML procedure of SAS and used to determine the dose-dependent effect of aspirin inclusion. Breed composition was used as a covariate in all of the statistical analyses. Treatment comparisons were made using Fisher’s protected least significant difference, and the least square means statement was used to calculate adjusted means. Differences were considered significant when P ≤ 0.05 and 0.05 < P ≤ 0.10 was considered a tendency.
Experiment 2
Animals and diets
Sixteen crossbred steers (566 ± 29.7 kg) purchased from a commercial auction market were used to determine the effect of aspirin on tight junction gene expression in the jejunum. The 200 mg/kg BW aspirin dose was used in experiment 2 because it exhibited the greatest response in experiment 1. Eight steers per treatment were allotted to either 0 or 200 mg/kg of BW aspirin such that BW was equal between treatments. BW was determined the day prior to dosing aspirin using scales (480 Legend, Rice Lake Weighing Systems, Rice Lake, WI) that weighed to the nearest 0.5 kg and were checked for accuracy. Steers were housed in a slatted-floor finishing barn in 6.1 × 3.7 m pens with 0.55 m of bunk space and adjustable curtains. Cattle were fed a basal diet (DM basis) that consisted of 50% corn silage, 21% corn, 23% DDGS, and 6% vitamin/mineral premix for 6 wk before slaughter. The basal diet met or exceeded NASEM (2016) requirements for protein, vitamins, and minerals. Feed was offered once daily at 0900 hours, and steers were allowed ad libitum access to feed and water. Daily feed deliveries were adjusted using a 4-point bunk scoring system (Pritchard, 1993) to allow for ad libitum feed intake with little or no accumulation of unconsumed feed (score of ≤1). Because 100 mg/kg BW is the prescribed maximum dosage for cattle (Gingerich et al., 1975), steers given aspirin received two doses of 100 mg/kg of BW at 36 and 24 h prior to slaughter. Aspirin boluses (Agri Laboratories, Ltd. St. Joseph, MO) contained 31.2 g of aspirin and were dosed orally using a balling gun. Aspirin boluses were trimmed to achieve the target dosage.
Sample collection
Cattle were slaughtered at the Purdue Animal Science meat laboratory, and samples were collected from the jejunum within 30 min after exsanguination according to the procedures described by Lindholm-Perry et al. (2016). Jejunal tissues were sectioned, rinsed with phosphate-buffered saline, inspected, and appeared normal at harvest. The mucosal epithelial tissue layer of the jejunum was removed from the wall of the small intestine by scraping off the mucosal layers with a sterile scalpel blade. Approximately, 0.5 g of tissue was flash-frozen in liquid nitrogen, then stored at −80 °C for evaluation of tight junction messenger ribonucleic acid (mRNA) expression using quantitative real-time polymerase chain reaction (PCR). Tissue was homogenized with a TissueRuptor rotor-stator homogenizer (Qiagen; Valencia, CA), and total mRNA was isolated using the RNeasy Mini kit (Qiagen) according to the manufacturer’s protocol. A NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA) was used to quantify ribonucleic acid (RNA) concentration and an RNA integrity number (RIN) was obtained using an Agilent Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA). All samples had a RIN > 6. Genes evaluated were occludin (OCLN), zonula occludens-1 (TJP1), claudin (CLDN) 1, CLDN 2, and CLDN 3. Primers and assay kits were ordered from Thermo Fisher Scientific (TaqMan) with accession numbers of NM_001082433.2, AJ313183.1, NM_001001854.2, NM_205781.2, and NM_205801.2 for OCLN, TJP1, CLDN 1, CLDN 2, CLDN 3, respectively. A total of 300 ng of RNA was used to synthesize complementary deoxyribonucleic acid (cDNA), and each sample and was diluted 1:10 for RT-PCR analysis. Each reaction contained 5 ul of diluted cDNA, 4 ul of water, 1 ul primer, and 10 ul TaqMan Fast Advanced Master Mix. The qPCR was performed in triplicate using a 7500 Real-time PCR system machine (Applied Biosystem), according to the manufacturer’s instructions. Gene expression was calculated relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (accession number NM_001034034.2, Thermo Fisher Scientific) as a housekeeping gene. Fold expression was calculated using the delta–delta Ct method.
Statistical analysis
Data were analyzed as a completely randomized design, using the MIXED procedure of SAS (SAS Institute Inc., Cary, NC) with individual animal considered the experimental unit. The model included the fixed effect of treatment. BW was used as a covariate in the statistical analysis. Treatment comparisons were made using Fisher’s protected least significant difference, and the least square means statement was used to calculate adjusted means. Differences were considered significant when P ≤ 0.05 and 0.05 < P ≤ 0.10 was considered a tendency.
Results and Discussion
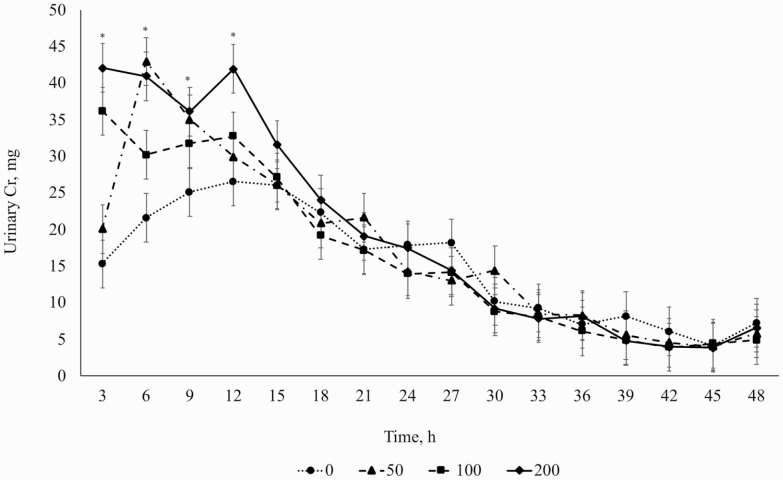
There was an overall treatment × time effect (P = 0.005) and overall linear effect (P = 0.03) of aspirin on urinary Cr excretion. Urinary Cr excretion increased linearly at hours 3, 6, 9, and 12 (P ≤ 0.04) as aspirin dose increased from 0 to 200 mg/kg (Figure 1). No differences were detected at any other time point (P ≥ 0.16). Absorption rate (%/h) and elimination rate (%/h) increased linearly (P = 0.02), and MRT decreased linearly (P = 0.02) as aspirin dosage increased (Table 2). Dosing with Cr-EDTA is a well-established technique for measuring the GIT barrier function in vivo in nonruminants (Bjarnason, 1986) and ruminants (Schweigel et al., 2005; Zhang et al., 2013). Chromium-EDTA is not digested or metabolized, has a high renal clearance rate (Bjarnason et al., 1986), permeates the GIT through the paracellular pathway, and appears in the urine (García-Lafuente et al., 2001; Schweigel et al., 2005; Ten Bruggencate et al., 2006). In healthy ruminants, approximately 2.5% of ruminally dosed Cr-EDTA is absorbed and excreted through urine (Shingfield et al., 2008). The mass of an endotoxin is 2 to 70 kDa (Magalhães et al., 2007), whereas Cr-EDTA has the mass of 340 Da and the size of approximately 10 Å (García-Lafuente et al., 2001). Thus, Cr-EDTA is an indicator of gut barrier dysfunction, not necessarily of bacteria or endotoxin translocation. Increased absorption and elimination rates of Cr-EDTA caused by aspirin, as shown in the present study, indicate that aspirin may cause mucosal damage that results in GIT leakiness.
Figure 1.
The effect of aspirin on chromium (mg) appearance in the urine. Treatment × hour effect (P = 0.005) and overall linear treatment effect (P = 0.03). *Indicates a linear effect as aspirin dose increased (P ≤ 0.05). 0 = no aspirin; 50 = 50 mg/kg of BW of aspirin; 100 = 100 mg/kg of BW of aspirin; 200 = 200 mg/kg of BW of aspirin.
Table 2.
Kinetics of chromium appearance in urine, 24 h after an aspirin dose
| Aspirin dose, mg/kg of BW1 | P-value | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 200 | SEM | Linear | Quadratic | |
| Absorption rate, k2, %/h | 0.227 | 0.256 | 0.264 | 0.289 | 0.0161 | 0.02 | 0.62 |
| Elimination rate, k1, %/h | 0.063 | 0.064 | 0.077 | 0.081 | 0.0082 | 0.02 | 0.67 |
| MRT, h | 24.71 | 21.16 | 18.73 | 17.33 | 2.02 | 0.02 | 0.34 |
10, no aspirin; 50, 50 mg/kg of BW of aspirin; 100, 100 mg/kg of BW of aspirin; 200, 200 mg/kg of BW of aspirin.
LBP tended to increase (P = 0.09) and SAA increased (P = 0.04) in cattle dosed with 200 mg/kg aspirin compared with cattle dosed with no aspirin (Table 3), indicating that LPS may have entered into circulation due to compromised gut integrity and initiated an inflammatory response. However, IL-6 (a pro- and anti-inflammatory cytokine), haptoglobin (acute phase protein), and AST (a marker of liver stress) did not differ between the 0 and 200 mg/kg treatments (P ≥ 0.21). The role of LBP is to directly bind with LPS or lipoteichoic acid (LTA) to further signal for opsonization by phagocytes (Ceciliani et al., 2012). Both LPS (Gram-negative) and LTA (Gram-positive) are components of bacterial cell walls that are recognized as endotoxins, meaning that they have toxic effects on the host after they are shed from lysed bacteria. Endotoxins entering the lymphatic system or portal vein will be targeted by local macrophages, initiating a cascade of immune signaling that results in inflammation and the production of acute-phase proteins in the liver (Ceciliani et al., 2012). Kvidera et al. (2017) correlated increased circulating LBP to decreased jejunum villus height-to-crypt depth ratio in lactating dairy cows, suggesting that an increase in LBP is correlated with greater intestinal damage and decreased barrier function. Thus, greater LBP in cattle fed aspirin in the current study suggests that aspirin induced GIT damage. Serum albumin A opsonizes Gram-negative and Gram-positive bacteria, removes phagocytosed cell membranes at inflammatory sites, and is a chemoattractant that mediates the migration, adhesion, and tissue infiltration of monocytes and neutrophils (Ceciliani et al., 2012). Intestinal mucins, whose function is to protect the intestine from endotoxins in periods of stress, increase in the presence of SAA (Larson et al., 2003; Mack et al., 2003; Ceciliani et al., 2012). An increase in SAA in the present study because of aspirin further supports that a greater amount of bacterial endotoxin leaked across the GIT barrier. When Kvidera et al. (2017) intentionally induced GIT barrier dysfunction with GSI, they did not see a difference in SAA or haptoglobin. Acute-phase protein production in the liver is a secondary (nonlocal) response to toxic stimuli and can be an indicator of systemic inflammation (Ceciliani et al., 2012). Haptoglobin suppresses endotoxin-induced inflammatory effects by promoting the production of anti-inflammatory cytokines and binds with iron that pathogens need to grow (Ceciliani et al., 2012). However, haptoglobin responds to a greater number of inflammatory stimuli compared with SAA and LBP (Ceciliani et al., 2012), which could explain why haptoglobin in animals fed aspirin did not differ from control animals in the current study.
Table 3.
The effect of aspirin on serum inflammatory markers
| Treatment1 | P-value | ||||
|---|---|---|---|---|---|
| 0 mg/kg | 200 mg/kg | SEM | TRT | TRT × time | |
| LBP, ug/mL | 22.7 | 37.3 | 4.22 | 0.09 | 0.49 |
| IL-6, pg/mL | 46.5 | 36.6 | 7.98 | 0.84 | 0.65 |
| Haptoglobin, ug/mL | 4.19 | 8.15 | 4.729 | 0.21 | 0.19 |
| SAA, ug/mL | 45.8 | 86.7 | 10.40 | 0.04 | 0.10 |
| Aspartate aminotransferase, mU/mL2 | 15.6 | 15.2 | 1.26 | 0.86 | 0.96 |
10, no aspirin; 200, 200 mg/kg of BW of aspirin.
2One milliunit (mU) of aspartate aminotransferase is defined as the amount of enzyme that will generate 1.0 μmol of glutamate per minute at pH 8.0 and 37 °C.
In the present study, CLDN-1 expression tended (P = 0.10) to increase in the jejunum in cattle dosed with 200 mg/kg aspirin compared with cattle not dosed with aspirin (Table 4). However, OCLN, zonula occludens, CLDN-1, and CLDN-2 expression in the jejunum did not change (P ≥ 0.51). Tight junction proteins regulate paracellular movement and protect against luminal bacteria and endotoxins. Each tight junction protein has a specific role in the intestine. CLDNs 1, 3, 4, 5, 8, 9, 11, and 14 decrease paracellular permeability, whereas CLDNs 2, 7, 12, and 15 increase paracellular permeability (Robinson et al., 2015). In a human gastric epithelial cell line, aspirin increased permeability and downregulated CLDN 7 (Oshima et al., 2008) and in mice, aspirin downregulated ZO-1 and OCLN expression (Lai et al., 2015). Increased CLDN-1 mRNA expression in the current study indicates that tight junction proteins may have been damaged and were potentially being replaced. In dairy cattle, ruminal CLDN 1 and 4 mRNA expression were upregulated 24 h after acidosis was induced (McCann et al., 2016). Pederzolli et al. (2018) restricted feed intake to 25% of DM intake for 4 d in Holstein steers and observed that CLDN 1, OCLN, ZO-1, ZO-2, and toll-like receptor 4 expression in the rumen and jejunum increased. Acidosis and feed restriction cause intestinal lining deterioration, which is indicated by the upregulation in tight junction proteins (McCann et al., 2016; Pederzolli et al., 2018). Although the deterioration of the intestinal lining during acidosis and feed restriction seem to be caused by acidic conditions (Emmanuel et al., 2007) or the presence of LPS (Chin et al., 2006), similar GIT damage in the current study when dosing feedlot cattle with aspirin indicates that feeding aspirin may be a good model to study the diet-induced leaky gut.
Table 4.
Effect of aspirin on fold-change mRNA expression of tight junction proteins in the jejunum
| Treatment1 | ||||
|---|---|---|---|---|
| Item | 0 mg/kg | 200 mg/kg | SEM | P-value |
| OCLN | 1.00 | 0.82 | 0.274 | 0.64 |
| Zonula occludens | 0.99 | 0.85 | 0.173 | 0.97 |
| CLDN 1 | 0.98 | 1.47 | 0.270 | 0.10 |
| CLDN 2 | 0.97 | 1.29 | 0.342 | 0.51 |
| CLDN 3 | 1.00 | 0.76 | 0.270 | 0.56 |
10, no aspirin; 200, 200 mg/kg of BW of aspirin.
Administration of two doses of 100 mg/kg BW of aspirin 12 h apart induced the greatest amount of GIT barrier dysfunction in the current study based on the amount of Cr excreted in urine and the absorption and elimination kinetics of Cr. Increased serum SAA and tendencies for increased serum concentration of LBP and upregulation of CLDN 1 in the jejunum further support the idea that aspirin can induce leaky gut and be used as a model to further study leaky gut in healthy beef cattle.
Acknowledgment
Appreciation is extended to the employees of the Purdue University Beef Research and Educational Center for assistance in the conduction of this research.
Glossary
Abbreviations
- AST
aspartate aminotransferase
- BW
body weight
- cDNA
complementary deoxyribonucleic acid
- CLDN
claudin
- COX
cyclooxygenase
- CP
crude protein
- CV
coefficient of variation
- DDGS
dried distillers grains with solubles
- DM
dry matter
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbent assays
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GIT
gastrointestinal tract
- GSI
gamma-secretase inhibitor
- IL-6
interleukin-6
- LBP
lipopolysaccharide-binding protein
- LPS
lipopolysaccharide
- LTA
lipoteichoic acid
- mRNA
messenger ribonucleic acid
- MRT
mean retention time
- NSAID
nonsteroidal anti-inflammatory drug
- OCLN
occludin
- PCR
polymerase chain reaction
- RIN
RNA integrity number
- RNA
ribonucleic acid
- SAA
serum amyloid A
Conflict of interest statement
The authors report no conflicts of interest.
Literature Cited
- Atkins G L. 1969. Multicompartment models for biological systems. London (UK):Methuen & Co. [Google Scholar]
- Baumgard L H, and Rhoads R P Jr. 2013. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 1:311–337. doi: 10.1146/annurev-animal-031412-103644 [DOI] [PubMed] [Google Scholar]
- Binnerts W, Van′t Klooster A T, and Frens A. . 1968. Soluble chromium indicator measured by atomic absorption in digestion experiments. Vet. Rec. 82:470. [Google Scholar]
- Bjarnason I, Hayllar J, MacPherson A J, and Russell A S. . 1993. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology 104:1832–1847. doi: 10.1016/0016-5085(93)90667-2 [DOI] [PubMed] [Google Scholar]
- Bjarnason I, Williams P, Smethurst P, Peters T J, and Levi A J. . 1986. Effect of non-steroidal anti-inflammatory drugs and prostaglandins on the permeability of the human small intestine. Gut 27:1292–1297. doi: 10.1136/gut.27.11.1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I, Zanelli G, Smith T, Prouse P, Williams P, Smethurst P, Delacey G, Gumpel M J, and Levi A J. . 1987. Nonsteroidal anti-inflammatory drug-induced intestinal inflammation in humans. Gastroenterology 93:480–489. doi: 10.5555/uri:pii:0016508587909097 [DOI] [PubMed] [Google Scholar]
- Ceciliani F, Ceron J J, Eckersall P D, and Sauerwein H. . 2012. Acute phase proteins in ruminants. J. Proteomics 75:4207–4231. doi: 10.1016/j.jprot.2012.04.004 [DOI] [PubMed] [Google Scholar]
- Chin A C, Flynn A N, Fedwick J P, and Buret A G. . 2006. The role of caspase-3 in lipopolysaccharide-mediated disruption of intestinal epithelial tight junctions. Can. J. Physiol. Pharmacol. 84:1043–1050. doi: 10.1139/y06-056 [DOI] [PubMed] [Google Scholar]
- Cooke R F. 2017. Nutritional and management considerations for beef cattle experiencing stress-induced inflammation. Prof. Anim. Sci. 33:1–11. doi: 10.15232/pas.2016-01573 [DOI] [Google Scholar]
- Emmanuel D G, Madsen K L, Churchill T A, Dunn S M, and Ametaj B N. . 2007. Acidosis and lipopolysaccharide from Escherichia coli B:055 cause hyperpermeability of rumen and colon tissues. J. Dairy Sci. 90:5552–5557. doi: 10.3168/jds.2007-0257 [DOI] [PubMed] [Google Scholar]
- FASS. 2010. Guide for the care and use of agricultural animals in agricultural research and teaching. 3rd ed. Consortium for developing a guide for the care and use of agricultural animals in agricultural research and teaching. Champaign (IL): Federation of Animal Science Societies (FASS). [Google Scholar]
- FDA. 2020. Green book report section 2: active ingredients. Silver Spring: (MD):FDA. [Google Scholar]
- Garcia M, Bradford B J, and Nagaraja T G. . 2017. Invited Review: Ruminal microbes, microbial products, and systemic inflammation. Prof. Anim. Sci. 33:635–650. doi: 10.15232/pas.2017-01663 [DOI] [Google Scholar]
- García-Lafuente A, Antolín M, Guarner F, Crespo E, and Malagelada J R. . 2001. Modulation of colonic barrier function by the composition of the commensal flora in the rat. Gut 48:503–507. doi: 10.1136/gut.48.4.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingerich D A, Baggot J D, and Yeary R A. . 1975. Pharmacokinetics and dosage of aspirin in cattle. J. Am. Vet. Med. Assoc. 167:945–948. [PubMed] [Google Scholar]
- Grovum W L, and Phillips G D. . 1973. Rate of passage of digesta in sheep. 5. Theoretical considerations based on a physical model and computer simulation. Br. J. Nutr. 30:377–390. doi: 10.1079/bjn19730042 [DOI] [PubMed] [Google Scholar]
- Khafipour E, Krause D O, and Plaizier J C. . 2009. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J. Dairy Sci. 92:1060–1070. doi: 10.3168/jds.2008-1389 [DOI] [PubMed] [Google Scholar]
- Klein P, Moravcová J, Kleinová T, Volek Z, and Skrivanova V. . 2007. Assessment of intestinal permeability in pre-ruminant calves by lactulose/mannitol test. J. Anim. Feed Sci. 16:43–52. doi: 10.22358/jafs/66725/2007 [DOI] [Google Scholar]
- Kvidera S K, Dickson M J, Abuajamieh M, Snider D B, Fernandez M V S, Johnson J S, Keating A F, Gorden P J, Green H B, Schoenberg K M, . et al. 2017. Intentionally induced intestinal barrier dysfunction causes inflammation, affects metabolism, and reduces productivity in lactating Holstein cows. J. Dairy Sci. 100:4113–4127. doi: 10.3168/jds.2016-12349 [DOI] [PubMed] [Google Scholar]
- Lai Y, Zhong W, Yu T, Xia Z S, Li J Y, Ouyang H, Shan TD, Yang H S, and Chen Q K. . 2015. Rebamipide promotes the regeneration of aspirin-induced small-intestine mucosal injury through accumulation of β-Catenin. PLoS One. 10:e0132031. doi: 10.1371/journal.pone.0132031 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lambert G P, Schmidt A, Schwarzkopf K, and Lanspa S. . 2012. Effect of aspirin dose on gastrointestinal permeability. Int. J. Sports Med. 33:421–425. doi: 10.1055/s-0032-1301892 [DOI] [PubMed] [Google Scholar]
- Larson M A, Wei S H, Weber A, Mack D R, and McDonald T L. . 2003. Human serum amyloid A3 peptide enhances intestinal MUC3 expression and inhibits EPEC adherence. Biochem. Biophys. Res. Commun. 300:531–540. doi: 10.1016/s0006-291x(02)02901-7 [DOI] [PubMed] [Google Scholar]
- Lindholm-Perry A K, Butler A R, Kern R J, Hill R, Kuehn L A, Wells J E, Oliver W T, Hales K E, Foote A P, and Freetly H C. . 2016. Differential gene expression in the duodenum, jejunum and ileum among crossbred beef steers with divergent gain and feed intake phenotypes. Anim. Genet. 47:408–427. doi: 10.1111/age.12440 [DOI] [PubMed] [Google Scholar]
- Mack D R, McDonald T L, Larson M A, Wei S, and Weber A. . 2003. The conserved TFLK motif of mammary-associated serum amyloid A3 is responsible for up-regulation of intestinal MUC3 mucin expression in vitro. Pediatr. Res. 53:137–142. doi: 10.1203/00006450-200301000-00023 [DOI] [PubMed] [Google Scholar]
- Magalhães P O, Lopes A M, Mazzola P G, Rangel-Yagui C, Penna T C, and Pessoa A Jr. 2007. Methods of endotoxin removal from biological preparations: a review. J. Pharm. Pharm. Sci. 10:388–404. [PubMed] [Google Scholar]
- McCann J C, Luan S, Cardoso F C, Derakhshani H, Khafipour E, and Loor J J. . 2016. Induction of subacute ruminal acidosis affects the ruminal microbiome and epithelium. Front. Microbiol. 7:701. doi: 10.3389/fmicb.2016.00701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minuti A, Ahmed S, Trevisi E, Piccioli-Cappelli F, Bertoni G, Jahan N, and Bani P. . 2014. Experimental acute rumen acidosis in sheep: consequences on clinical, rumen, and gastrointestinal permeability conditions and blood chemistry. J. Anim. Sci. 92:3966–3977. doi: 10.2527/jas.2014-7594 [DOI] [PubMed] [Google Scholar]
- Moeser A J, Ryan K A, Nighot P K, and Blikslager A T. . 2007. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 293:G413–G421. doi: 10.1152/ajpgi.00304.2006 [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (NASEM). 2016. Nutrient requirements of beef cattle. 8th rev. ed. Washington (DC):The National Academies Press. [Google Scholar]
- Oshima T, Miwa H, and Joh T. . 2008. Aspirin induces gastric epithelial barrier dysfunction by activating p38 MAPK via claudin-7. Am. J. Physiol. Cell Physiol. 295:C800–C806. doi: 10.1152/ajpcell.00157.2008 [DOI] [PubMed] [Google Scholar]
- Pederzolli R A, Van Kessel A G, Campbell J, Hendrick S, Wood K M, and Penner G B. . 2018. Effect of ruminal acidosis and short-term low feed intake on indicators of gastrointestinal barrier function in Holstein steers. J. Anim. Sci. 96:108–125. doi: 10.1093/jas/skx049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaizier J, Khafipour E, Li S, Gozho G, and Krause D. . 2012. Subacute ruminal acidosis (SARA), endotoxins and health consequences. Anim. Feed Sci. Technol. 172:9–21. doi: 10.1016/j.anifeedsci.2011.12.004 [DOI] [Google Scholar]
- Pritchard R H. 1993. Bunk management. In: Land O’Lakes Beef Seminar, Cedar Rapids, IA, Columbus, NE, and Storm Lake, IA; March 1–4 St. Paul (MN):Land O’Lakes Inc; p. 4–15. [Google Scholar]
- Robinson K, Deng Z, Hou Y, and Zhang G. . 2015. Regulation of the intestinal barrier function by host defense peptides. Front. Vet. Sci. 2:57. doi: 10.3389/fvets.2015.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell E B. 1959. Colorimetric determination of traces of metals. 3rd ed. New York (NY):Interscience. [Google Scholar]
- Schweigel M, Freyer M, Leclercq S, Etschmann B, Lodemann U, Böttcher A, and Martens H. . 2005. Luminal hyperosmolarity decreases Na transport and impairs barrier function of sheep rumen epithelium. J. Comp. Physiol. B. 175:575–591. doi: 10.1007/s00360-005-0021-3 [DOI] [PubMed] [Google Scholar]
- Shingfield K J, Arölä A, Ahvenjärvi S, Vanhatalo A, Toivonen V, Griinari J M, and Huhtanen P. . 2008. Ruminal infusions of cobalt-EDTA reduce mammary delta9-desaturase index and alter milk fatty acid composition in lactating cows. J. Nutr. 138:710–717. doi: 10.1093/jn/138.4.710 [DOI] [PubMed] [Google Scholar]
- Simmons D L, Botting R M, and Hla T. . 2004. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 56:387–437. doi: 10.1124/pr.56.3.3 [DOI] [PubMed] [Google Scholar]
- Smith G W, Davis J L, Tell L A, Webb A I, and Riviere J E. . 2008. Extralabel use of nonsteroidal anti-inflammatory drugs in cattle. J. Am. Vet. Med. Assoc. 232:697–701. doi: 10.2460/javma.232.5.697 [DOI] [PubMed] [Google Scholar]
- Takeuchi K, and Amagase K. . 2018. Roles of cyclooxygenase, prostaglandin E2 and EP receptors in mucosal protection and ulcer healing in the gastrointestinal tract. Curr. Pharm. Des. 24:2002–2011. doi: 10.2174/1381612824666180629111227 [DOI] [PubMed] [Google Scholar]
- Ten Bruggencate S J M, Bovee-Oudenhoven I M J, Lettink-Wissink M L G, Katan M B, and van der Meer R. . 2006. Dietary fructooligosaccharides affect intestinal barrier function in healthy men. J. Nutr. 136:70–74. doi: 10.1093/jn/136.1.70 [DOI] [PubMed] [Google Scholar]
- Vicente F., Sarraseca A., de Vega A., and Guada J. A.. 2004. Performance of several Cr and Yb analytical techniques applied to samples of different biological origin (digesta or faeces). J. Sci. Food Agric. 84:2035–2040. doi: 10.1002/jsfa.1908 [DOI] [Google Scholar]
- Wan C, Yin P, Xu X, Liu M, He S, Song S, Liu F, and Xu J. . 2014. Effect of simulated transport stress on the rat small intestine: a morphological and gene expression study. Res. Vet. Sci. 96:355–364. doi: 10.1016/j.rvsc.2014.01.008 [DOI] [PubMed] [Google Scholar]
- Zhang S, Albornoz R I, Aschenbach J R, Barreda D R, and Penner G B. . 2013. Short-term feed restriction impairs the absorptive function of the reticulo-rumen and total tract barrier function in beef cattle. J. Anim. Sci. 91:1685–1695. doi: 10.2527/jas.2012-5669 [DOI] [PubMed] [Google Scholar]