Abstract
Carbapenem resistance in Acinetobacter baumannii is due to bla OXA-23, which is endemic in India. Recently, the sporadic presence of bla OXA-58 as well as the occurrence of dual carbapenemases were observed. The mobility as well as the dissemination of these resistance genes were mainly mediated by various mobile genetic elements. The present study was aimed at characterizing the genetic arrangement of bla OXA-23, bla NDM-1 and bla OXA-58 identified in two complete genomes of carbapenem-resistant A. baumannii (CRAB). Complete genomes obtained using a hybrid-assembly approach revealed the accurate arrangement of Tn2006 with bla OXA-23, ISAba125 with bla NDM and ISAba3 with bla OXA-58. In addition, the association of IntI1 integrase with the bla CARB-2 gene and several virulence factors required for type-IV pili assembly, motility and biofilm formation have been identified. The current study provided deeper insight into the complete characterization of insertion sequences and transposons associated with the carbapenem-resistant genes using short reads of IonTorrent PGM and long reads of MinIon in A. baumannii .
Keywords: Acinetobacter baumannii, blaOXA-420, blaCARB-2, ISAba3, Int1
Introduction
Acinetobacter baumannii a nosocomial pathogen is of particular concern due to the global occurrence of multi-drug-resistant (MDR) and pan-drug-resistant (PDR) strains [1]. A. baumannii is a leading cause of various health-care-associated infections like blood-stream infections, ventilator-associated pneumonia, urinary tract infections and wound infections [2]. A. baumannii has acquired resistance to an array of antimicrobial agents belonging to different classes of antibiotics like cephalosporins, carbapenems, aminoglycosides, fluoroquinolones, chloramphenicol and tetracyclines [3]. Carbapenems belong to the β-lactam class of antimicrobials [4]. According to Ambler’s system of classification, β-lactamases are classified into four classes: A, B, C and D [4]. Further, classes A, C and D are termed as serine β-lactamases whereas class B is called metallo-β-lactamases [4].
Carbapenems are the last resort drugs to treat A. baumannii [4]. Therefore, the development of resistance to carbapenem is highly concerning [4]. Carbapenem resistance in A. baumannii is most predominantly mediated by oxacillinases (OXAs) like bla OXA-23 like, bla OXA-24 like, bla OXA-58 like and metallo-β-lactamases (MBLs) like bla NDM like, bla IMP like, bla VIM like and bla SIM like [5]. The sporadic presence of other carbapenemases like bla KPC like and bla GES like have also been reported [6, 7]. A recent study by Nordmann et al. reported region-wise carbapenem non-susceptibility rates in A. baumannii, which have ranges as follows : (i) Asia-Pacific – 55–91 %; Europe – 58–85 %; North America – 32–50%; and Latin America – 53–90 % [8]. In India, results from recent studies reported 40–75% carbapenem resistance in A. baumannii [9]. bla OXA-23 like is the most commonly reported carbapenemase followed by bla NDM like in A. baumannii strains [10]. In A. baumannii carbapenem-resistance genes can be disseminated by mobile genetic elements (MGEs) like insertion sequences, integrons and transposons [11].
Next-generation sequencing (NGS) has facilitated the complete characterization of whole-genome sequences from microbial species [12]. In addition, whole-genome sequencing (WGS) helps in studying the dynamics and genomic evolution of bacterial pathogens [13]. In this study, hybrid assembly was performed to obtain complete genomes using long reads from MinION and short reads from IonTorrent. With this, the main objective of this current study is (i) to identify genes encoding carbapenem resistance and (ii) to decipher the genetic arrangement of insertion sequences and integrons, which are involved in the mobilization and dissemination of resistance genes among CRAB.
Methods
Bacterial isolates and identification
Two clinical isolates, ACN21 from Sir Ganga Ram hospital, Delhi NCR and CIAT758 from Tata Medical Center, Kolkata collected as a part of Indian Council of Medical Research (ICMR) surveillance study in the year 2018 were included in this study. Both isolates were identified as A. baumannii-calcoaceticus complex (Acb complex) using standard biochemical methods. bla OXA-51 PCR was performed to confirm both isolates as A. baumannii [14].
Anti-microbial susceptibility testing (AST)
ACN21 and CIAT758 were subjected to AST to determine the MIC of imipenem and meropenem using broth micro-dilution (BMD) and interpreted as per The Clinical and Laboratory Standards Institute (CLSI) guidelines [15].
Multiplex PCR for anti-microbial resistance (AMR) genes
To determine the presence of AMR genes, multiplex PCR for class A, B and D carbapenemases was performed [5]. Known positive control for appropriate genes were included.
Next-generation sequencing
Short-read sequencing using IonTorrent PGM
DNA was isolated from pure cultures using Qiagen DNA Mini Kit (QIAGEN, Hilden, Germany). Whole-genome shotgun sequencing was performed using Ion Torrent PGM TM platform with 400 bp chemistry (Life Technologies, Carlsbad, CA, USA) as per the manufacturer’s instructions.
Long-read sequencing using MinIon
Long-read sequencing was performed using MinIon Oxford Nanopore sequencing. DNA library preparation was constructed using SQK- LSK108 Kit R9 version (Oxford Nanopore Technologies, Oxford, UK) using the 1D sequencing method for sequencing of genomes according to the manufacturer’s protocol (https://nanoporetech.com/resource-centre/protocols). FLO-MIN106 R9 flow cell in a MinION Mk 1B sequencer was utilized for sequencing. The sequencing was conducted upto 48 h and raw fast5 files were generated using MinKNOW software ver. 1.15.1.
De novo assembly of genomes
Short-read assembly
AssemblerSPAdes version 5.0.0.0 embedded in Torrent Suite Server v.5.0.3 was employed for short-read error correction and assembly. Subsequently, the quality metrics of resulting fragmented genome with multiple contigs were analysed.
Long-read assembly
Reads processing and base calling
The raw fast5 files generated from sequencing were subjected to basecalling using ONT Albacore Sequencing Pipeline Software version 2.0.2 (https://nanoporetech.com/about-us/news/new-basecaller-now-performs-raw-basecalling-improved-sequencing-accuracy). Further, the genomes were separated according to the barcodes and resulting fastq files were merged for subsequent analysis.
Canu assembly and polishing
Low-quality reads often lead to mis-assemblies and frame-shifted INDELS. The low-quality reads were removed using Nanofilt v2.5 (https://github.com/wdecoster/nanofilt). The filtered fastq files are then error corrected and trimmed with canu v1.7 [16] to further increase the quality of reads. Further, the trimmed reads were assembled using canu assembly option incorporated in canu v1.7. The long-read assembly generally contains a significant amount of errors and INDELS. Hence, long-read assemblies were polished using Nanopolish version 0.10.1 (https://github.com/jts/nanopolish) to reduce the inconsistency of the assembly. Initially, the genomes were split into fragments 50 kb each and individually polished in parallel. Then the polished fragments were merged to obtain complete genome assembly.
Hybrid assembly and quality assessment
Hybrid assembly incorporating both IonTorrent and MinION reads was performed using Unicycler (v0.4.6) [17]. Initially, the short reads were error corrected with different k-mers using SPAdes [18] to filter out low-quality reads. In addition, long-read assembly and polishing was performed using Miniasm v0.3 [19] and Racon v1.3.3 [20], respectively. Further, both long-read and short-read assemblies were bridged to generate genome assembly. Finally, repeated short-read polishing steps with Pilon [21] were performed to generate a complete and accurate genome assembly. The assembly statistics and other quality metrics were analysed using Quast [22] to verify the quality, completeness and contiguity of the genomes.
Genome annotation
Genome annotation was performed using NCBI Prokaryotic Genome Annotation Pipeline (PGAP) (www.ncbi.nlm.nih.gov/genome/annotation_prok/) and the PATRIC database (http://www.patricbrc.or g) [23]. Antimicrobial resistance genes were identified using ResFinder v3.0 (https://cge.cbs.dtu.dk//services/ResFinder/) [24]. Virulence factors were detected using the Virulence Factors Database, VFDB [25]. Insertion sequences and the presence of prophage-related sequences were screened with IS Finder (https://www-is.biotoul.fr/blast.php) and the PHAST tool, respectively [26, 27]. Genomic Island was detected using Island Viewer 4 with the SIGI-HMM algorithm [28]. Sequence types of the isolates were identified with MLST 2.0 tool (multi-locus sequence typing) [29].
Results and discussion
Both isolates were confirmed as A. baumannii by the presence of intrinsic, bla OXA-51-like gene. Both phenotypically and genotypically, ACN21 and CIAT758 were confirmed as carbapenem resistant. However, they differ only in the type of carbapenem resistance gene they harbour. The MIC of imipenem and meropenem were determined as 128 µg ml−1 and 256 µg ml−1 for ACN21, respectively, whereas for CIAT758 the MICs for both were 128 µg ml−1. Multiplex PCR revealed the presence of bla NDM like and bla OXA-58 like in ACN21 and bla OXA-23 like and bla OXA-58 like in CIAT758.
Hybrid assembly of ACN21 revealed the presence of one chromosome of size 3 827 138 bp with eight plasmids ranging from 116 047–5734 bp size whereas CIAT758 had a single chromosome with 4 017 696 bp size and three plasmids of 78 125, 47 417 and 29 128 bp size. The genome features of the isolates were mentioned in Table 1.
Table 1.
Genomic features and resistance genes identified in A. baumannii strains ACN21 and CIAT758
|
Strain name |
ACN21 |
CIAT758 |
||
|---|---|---|---|---|
|
Genomic size |
3 827 138 bp |
4 017 696 bp |
||
|
Total coding sequences |
3822 |
4040 |
||
|
Total pseudo genes |
183 |
277 |
||
|
GC content |
38.88 |
39.02 |
||
|
Anti-microbial resistance genes |
Gene |
Location |
Gene |
Location |
|
NDM-1* |
Chromosome |
OXA-68 |
Chromosome |
|
|
ADC-25 |
Chromosome |
OXA-23* |
Chromosome |
|
|
OXA-94 |
Chromosome |
ADC-25 |
Chromosome |
|
|
mph(E) |
Chromosome |
OXA-58* |
Plasmid 1 |
|
|
msr(E) |
Chromosome |
msr(E) |
Plasmid 1 |
|
|
Sul1 |
Plasmid 2 |
mph(E) |
Plasmid 1 |
|
|
armA |
Plasmid 2 |
tet(39) |
Plasmid 1 |
|
|
CARB-2* |
Plasmid 2 |
aac(3)-IId |
Plasmid 1 |
|
|
OXA-420 |
Plasmid 2 |
Sul1 |
Plasmid 2 |
|
|
mph(E) |
Plasmid 2 |
aph(3’)-VIa |
Plasmid 2 |
|
|
msr(E) |
Plasmid 2 |
PER-7 |
Plasmid 2 |
|
*Genetic arrangement of these resistance genes were shown in Figs 1 and 2.
Ion Torrent short-read assembly produced accurate genome with multiple fragments. In contrast, MinIon Nanopore long-read assembly generated a single chromosomal contig but with higher frame-shifted INDELS (>30 %) even after repeated long-read polishing that makes downstream analysis difficult. Further, the repeated polishing with short reads generated a highly accurate single chromosomal contig with reduced frame-shifted INDELS (<10 %), which enabled accurate analysis of the AMR and other MGEs.
Long-read-sequencing technologies such as Pacific Biosciences (PacBio) and Oxford Nanopore will be helpful in generating longer reads, which in turn will allow the gaps to be covered [30]. MinIon only reads may be inclined to errors, however that can be efficiently overcome by combining with short-read sequencers like IonTorrent and Illumina [30].
The genome of ACN21 has been deposited in GenBank with accession number CP038644 for chromosome and CP038645–CP038652 for plasmids 1–8. For CIAT758, the chromosome and three plasmids were deposited with accession numbers CP038500 and CP038501, CP038502, CP038503, respectively.
Resistance determinants
Multiple resistant genes conferring resistance to various antimicrobial classes were identified in both ACN21 and CIAT758 genomes. The genes that may contribute resistance to aminoglycosides, beta lactams, macrolides, lincosamide streptogramin B, sulphonamide and tetracycline are listed in Table 1.
In A. baumannii , carbapenem resistance is predominantly due to acquired class-D bla OXA-23 followed by other oxacillinases and metallo-beta lactamases [1]. The genome ACN21 was devoid of bla OXA-23, while the presence of bla NDM-1 and bla OXA-420 could be encoding for carbapenem resistance. In ACN21, bla NDM-1 resides in chromosome and bla OXA-420 in plasmids whereas previous studies reported the co-presence of bla NDM and bla OXA-58 on the same plasmid [31–34]. In CIAT 758, bla OXA-58 was carried on the plasmid (Table 1). Presence of bla OXA-23 and bla OXA-58 in six CRAB isolates was previously reported by Principe et al. [35]. El Bannah et al. reported that 86 % of carbapenem resistance could be due to the presence of either bla OXA-23 alone or bla OXA-23 together with bla KPC [36]. However, in this study bla OXA-23, bla OXA-58 and bla NDM-1 was present, but bla KPC was not observed.
Mobile genetic elements
The main characteristic feature of A. baumannii is its ability to acquire, retain and disseminate multiple resistance mechanisms by combining resistance genes with an array of MGEs like insertion sequences, resistance islands and bacteriophages, which mediate the exchange of genetic material, rearrange bacterial genomes and provides an endless source of genetic adaptability [11]. Increased resistance to carbapenems due to upstream insertion of bla OXA-51 and bla OXA-23 with ISAba1, bla OXA-58 with ISAba3 and bla NDM with ISAba125 were reported from previous studies [37–39]. In this study, IS Finder analysis revealed the presence of various insertion sequences belonging to IS family IS1, IS3, IS4, IS5 and IS30 in both ACN21 and CIAT758 genomes. Phage analysis identified three phage regions (one intact, one incomplete and one questionable) in the ACN21 genome and one intact phage region in CIAT758 genome. Further analysis using Island viewer revealed the presence of six and nine genomic islands in ACN21 and CIAT758 genomes, respectively.
Genetic context of resistance genes
ACN21
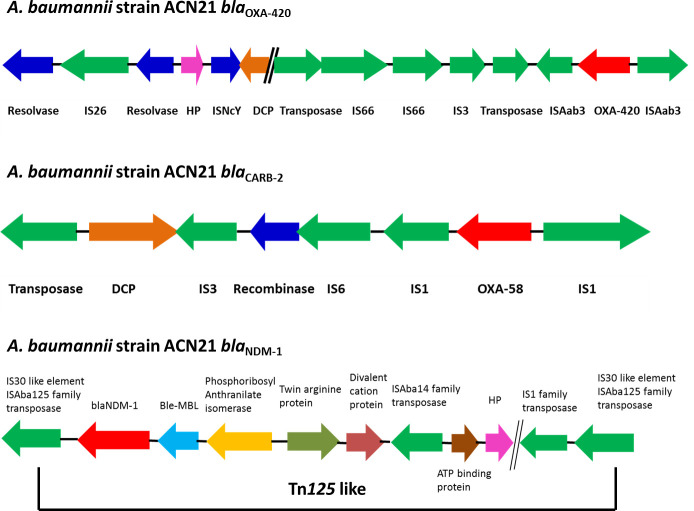
In the ACN21 genome, the variant of the bla OXA-58 gene, bla OXA-420 is present. The upstream of the plasmid-carried bla OXA-420 gene has truncated ISAba3 belonging to the family IS1. Immediately downstream from the bla OXA-420 gene, another truncated ISAba3 element was present. The bla OXA-420 gene is bracketed by two ISAba3 elements thereby forming a composite transposon (Fig. 1). Earlier studies report on the association of bla OXA-58 with ISAba3 [40, 41].
Fig. 1.
Graphical representation of genetic arrangement of bla OXA-420, bla CARB-2 and bla NDM-1 in A. baumannii strain ACN21. The direction of arrows indicates the orientation of ORFs.
An acquired extended spectrum beta lactamase unusual in A. baumannii , bla CARB-2 (carbenicillinase gene) has been identified in this genome, which has been initially reported in GenBank from China but unpublished. The bla CARB-2 gene was first reported in a plasmid from P. aeruginosa in 1991 by Huovinen and Jacoby [42] and known as Pseudomonas -specific enzyme-1 (PSE-1). Variants of the bla CARB gene, bla CARB - 4, 5, 8 and 10 have been reported earlier in A. baumannii . Kamolvit et al. reported the presence of the bla CARB-2 gene in A. pittii with upstream presence of truncated class 1 integrase gene (intI1) [43]. In the ACN21 genome, though the bla CARB-2 gene has upstream presence of intI1, it is not truncated (Fig. 1).
The ACN21 genome has the bla NDM-1 gene with downstream ISAba125, upstream ble-MBL gene and bracketed by two copies of ISAba125. However, an additional insertion sequence, ISAba14 was identified upstream ATP binding protein thereby forming a Tn125-like composite transposon, which could be a novel finding. This is similar to the report documented by Poirel et al. and Jones et al. [44, 45]. The genetic arrangement of bla NDM-1 gene is shown in Fig. 1.
CIAT758
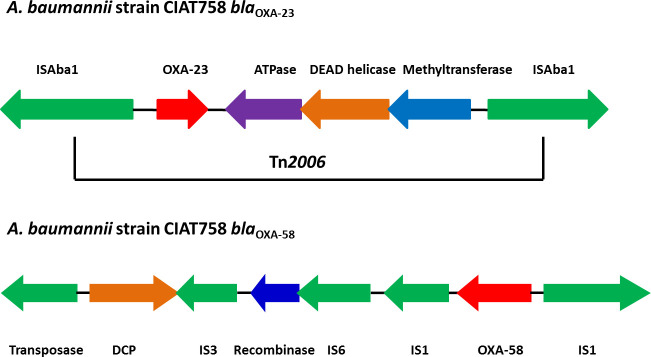
The CIAT758 genome harbours bla OXA-23 on the chromosome and bracketed by two copies of the IS4 family insertion element, ISAba1 (Fig. 2). A similar scenario was documented by several other studies [1, 5, 46].
Fig. 2.
Graphical representation of genetic arrangement of bla OXA-23 and bla OXA-58 in A. baumannii strain CIAT758. The direction of arrows indicates the orientation of ORFs.
In addition, CIAT758 harbours the bla OXA-58 gene with two copies of ISAba3 with one copy present downstream being truncated. Downstream of ISAba3 possess another insertion element, IS1008 belonging to IS6 family transposase (Fig. 2). Chen et al. reported the presence of bla OXA-58 with truncated ISAba3 and IS1008, which provides two independent promoters for the transcription of bla OXA-58 gene [47]. Further, the deletion of promoters provided by IS1008 results in decreased transcription of the bla OXA-58 gene and was not noticed in this genome [47].
Virulence factors
Several virulence factors required for pathogenesis have been identified in A. baumannii [12]. Both the sequenced genomes harbour genes required for assembly of type-IV pili (pilB and pilF), for twitching motility (pilT and pilU), surface polysaccharides (pgaA/B/C/D), which is involved in the synthesis of poly β-(1-6)-N-acetyl glucosamine for biofilm formation and protects the bacteria against innate host defenses [48, 49], outer membrane protein (OmpA) involved in pathogenesis and signal processing [50], phospholipase C, an important factor in cellular damage [51] and phospholipase D, important for human serum resistance and epithelial cell invasion, siderophores (bauA and basD), encodes for acinetobactin transport and biosynthesis, respectively [11]. Additionally, ACN21 possess the csuE gene, which belongs to the CsuA/BABCDE chaperone usher complex responsible for pili production and biofilm formation [52] whereas CIAT758 possesses the biofilm-associated protein (Bap) required for the development of mature biofilm structures [53].
Multi-locus sequence typing (MLST)
Currently, in A. baumannii , epidemiological characterization of clinical isolates has been described with eight international clonal lineages (IC-1 to IC-8). Among these, IC-1, IC-2 and IC-3 were the most common clonal lineages reported from various countries [54]. The majority of outbreaks due to CRAB were reported to be associated with isolates belonging to the IC-2 lineage [10].
In silico analysis of MLST using Oxford scheme identified ACN21 with ST1089. Uwingabiye et al. reported the presence of ST1089 and also mentioned that this ST was first reported in India in 2015 from PGIMER, Chandigarh [55]. Unlike other STs, ST195, which was widely reported from Asian countries and European Nations, ST1089 is very rare. While in CIAT758, ST585 was identified and belongs to International Clone 8 (IC-8). ST585 is the single loci variant (SLV) of ST391, which was first reported in India by Rynga et al. [56]. Another study from Egypt showed that the study isolates belonging to ST391 carries bla OXA-23 alone or bla NDM alone or bla OXA-23 with bla KPC, which is in contrast to the current study where CIAT758 belongs to ST589 and carries bla OXA-23 with bla OXA-58 [36]. Such findings indicate that despite diverse sequence types, bla OXA-23 is the major contributor of carbapenem resistance in A. baumannii worldwide. As discussed by El Bannah et al., certain STs are endemic in particular countries whereas others belong to a worldwide clonal complex and disseminate globally [36]. Similarly, a previous study by the same authors reported ST208 as the endemic sequence type, which is a SLV of globally disseminated ST92 [57].
Novel findings using hybrid assembly
In A. baumannii , MGEs like insertion sequences and integrons play a major role in the dissemination of anti-microbial resistance genes. Short reads from Ion Torrent sequencing results in a greater number of contigs, which leads to difficulty in deciphering the appropriate arrangement of MGEs. Hybrid assembly provides the complete genome, which is helpful in identifying the association of insertion sequences/integrons with resistance genes. In ACN21, the association of IntI1 integrase with the bla CARB-2 gene was observed in the complete genome using a hybrid-assembly approach, whereas this association was completely missing in short-read genome assembly. Similarly, in the CIAT758 genome the presence of insertion element, ISAba1, was observed in duplicates, whereas the hybrid genome assembly approach enabled the complete and accurate structural arrangement to be revealed.
Limitations of the study
Only two complete genomes of CRAB were characterized in this study. Characterization of the clinical isolates of more complete genomes of A. baumannii with different variants of the above mentioned resistance genes will be helpful in the comprehensive understanding of the variations in their genetic arrangement.
Future recommendations
Whole-genome sequencing of A. baumannii using the hybrid-assembly approach in the upcoming studies are necessary. Such studies will be helpful to gain thorough knowledge regarding various antibiotic resistance genes and the role of respective MGEs that are involved in the dissemination of the same.
Conclusion
In this study, complete genomes of two CRAB, ACN21 and CIAT758 were characterized using the hybrid-assembly approach. This study deciphers the genetic arrangement of bla OXA-23 with Tn2006, bla NDM-1 with a Tn125-like transposon, bla OXA-420 with ISAba3 and bla CARB-2 with class 1 integron. A novel Tn125-like transposon carrying bla NDM-1 was identified in the complete genome of ACN21. One limitation with this study is that only two complete genomes were characterized. However, such findings using complete genomes will be helpful in studying the genetic backbone of significant resistance genes and also in exploration of associated novel MGEs.
Funding information
The study has been funded by Indian Council of Medical Research, New Delhi, India (ref. no AMR/TF/54/13ECDHIII dated 23/10/2013).
Author contributions
B.V., S.A., S.V., K.V., C.W., J.K.O., S.B. and K.W. contributed to the design of the study; S.V. collected and analyzed the data; K.V. performed bioinformatic analysis; B.V., S.A. and S.V. wrote the manuscript. The manuscript was revised, read and approved by all authors.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: bla, Beta lactamase; CRAB, Carbapenem resistant Acinetobacter baumannii; MBL, Metallo beta lactamase; OXA, Oxacillinase.
This complete genome project has been deposited at GenBank: ACN21 with accession number CP038644 for chromosome and CP038645–CP038652 for plasmids 1–8. CIAT758, with accession number CP038500 for chromosome and CP038501–CP038503 for plasmids 1–3.
References
- 1.Poirel L, Nordmann P. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect. 2006;12:826–836. doi: 10.1111/j.1469-0691.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- 2.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii . Nat Rev Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 3.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tooke CL, Hinchliffe P, Bragginton EC, Colenso CK, Hirvonen VH, et al. β-Lactamases and β-lactamase inhibitors in the 21st century. J Mol Biol. 2019;18:3472–3500. doi: 10.1016/j.jmb.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijayakumar S, Gopi R, Gunasekaran P, Bharathy M, Walia K, et al. Molecular characterization of invasive carbapenem-resistant Acinetobacter baumannii from a tertiary care hospital in South India. Infect Dis Ther. 2016;5:379–387. doi: 10.1007/s40121-016-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez T, Martinez I, Vazquez GJ, Aquino EE, Robledo IE. Genetic environment of the KPC gene in Acinetobacter baumannii ST2 clone from Puerto Rico and genomic insights into its drug resistance. J Med Microbiol. 2016;65:784–792. doi: 10.1099/jmm.0.000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammoudi D, Moubareck CA, Hakime N, Houmani M, Barakat A, et al. Spread of imipenem-resistant Acinetobacter baumannii co-expressing OXA-23 and GES-11 carbapenemases in Lebanon. Int J Infect Dis. 2015;36:56–61. doi: 10.1016/j.ijid.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Nordmann P, Poirel L. Epidemiology and diagnostics of carbapenem resistance in gram-negative bacteria. Clin Infect Dis. 2019;69:S521–S528. doi: 10.1093/cid/ciz824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu L-Y, Apisarnthanarak A, Khan E, Suwantarat N, Ghafur A, et al. Carbapenem-resistant Acinetobacter baumannii and Enterobacteriaceae in South and Southeast Asia. Clin Microbiol Rev. 2017;30:1–22. doi: 10.1128/CMR.00042-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vijayakumar S, Anandan S, Ms DP, Kanthan K, Vijayabaskar S, et al. Insertion sequences and sequence types profile of clinical isolates of carbapenem-resistant A. baumannii collected across India over four year period. J Infect Public Health. 2019;19:30361–30362. doi: 10.1016/j.jiph.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Roca I, Espinal P, Vila-Farrés X, Vila J, Subir R., I The Acinetobacter baumannii oxymoron: commensal Hospital dweller turned pan-drug-resistant menace. Front Microbiol. 2012;3:148. doi: 10.3389/fmicb.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metzker ML. Sequencing technologies - the next generation. Nat Rev Genet. 2010;11:31. doi: 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]
- 13.Bentley SD, Parkhill J. Genomic perspectives on the evolution and spread of bacterial pathogens. Proc Biol Sci. 2015;282:20150488. doi: 10.1098/rspb.2015.0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turton JF, Woodford N, Glover J, Yarde S, Kaufmann ME, et al. Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J Clin Microbiol. 2006;44:2974–2976. doi: 10.1128/JCM.01021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing: Twenty second Informational Supplement M100-S28. USA: CLSI, Wayne, PA; 2018. [Google Scholar]
- 16.Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, et al. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017;27:722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H. Minimap and miniasm: fast mapping and de novo assembly for noisy long sequences. Bioinformatics. 2016;32:2103–2110. doi: 10.1093/bioinformatics/btw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaser R, Sović I, Nagarajan N. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res. 2017;27:737–746. doi: 10.1101/gr.214270.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 2014;9:e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurevich A, Saveliev V, Vyahhi N, Tesler G. QUAST: quality assessment tool for genome assemblies. Bioinformatics. 2013;29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wattam AR, Abraham D, Dalay O, Disz TL, Driscoll T, et al. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res. 2014;42:D581–D591. doi: 10.1093/nar/gkt1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Yang J, Yu J, Yao Z, Sun L, et al. VFDB: a reference database for bacterial virulence factors. Nucleic Acids Res. 2005;33:D325–D328. doi: 10.1093/nar/gki008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siguier P, Pérochon J, Lestrade L, Mahillon J, Chandler M. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006;34:D32–D36. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, Liang Y, Lynch KH, Dennis JJ, Wishart DS. PHAST: a fast phage search tool. Nucleic Acids Res. 2011;39:W347–W352. doi: 10.1093/nar/gkr485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertelli C, Laird MR, Williams KP, Lau BY, et al. Simon Fraser University Research Computing Group IslandViewer 4: expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017;45:W30–W35. doi: 10.1093/nar/gkx343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, et al. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol. 2012;50:1355–1361. doi: 10.1128/JCM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamidian M, Wick RR, Hartstein RM, Judd LM, Holt KE, et al. Insights from the revised complete genome sequences of Acinetobacter baumannii strains AB307-0294 and ACICU belonging to global clones 1 and 2. Microb Genom. 2019;5 doi: 10.1099/mgen.0.000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramoul A, Loucif L, Bakour S, Amiri S, Dekhil M, et al. Co-Occurrence of blaNDM-1 with blaOXA-23 or blaOXA-58 in clinical multidrug-resistant Acinetobacter baumannii isolates in Algeria. J Glob Antimicrob Resist. 2016;6:136–141. doi: 10.1016/j.jgar.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Regeen H, Al-Sharafa-Kittaneh D, Kattan R, Al-Dawodi R, Marzouqa H, et al. First report of blaNDM and blaOXA-58 coexistence in Acinetobacter junii . J Clin Microbiol. 2014;52:3492–3493. doi: 10.1128/JCM.01152-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou S, Chen X, Meng X, Zhang G, Wang J, et al. "Roar" of blaNDM-1 and "silence" of blaOXA-58 co-exist in Acinetobacter pittii . Sci Rep. 2015;5:8976. doi: 10.1038/srep08976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Guo P, Huang H, Huang Y, Wu Z, et al. Detection of co-harboring OXA-58 and NDM-1 carbapenemase producing genes resided on a same plasmid from an Acinetobacter pittii clinical isolate in China. Iran J Basic Med Sci. 2019;22:106–111. doi: 10.22038/ijbms.2018.28934.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Principe L, Piazza A, Giani T, Bracco S, Caltagirone MS, et al. Epidemic diffusion of OXA-23-producing Acinetobacter baumannii isolates in Italy: results of the first cross-sectional countrywide survey. J Clin Microbiol. 2014;52:3004–3010. doi: 10.1128/JCM.00291-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Bannah AMS, Nawar NN, Hassan RMM, Salem STB. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii in a tertiary care hospital in Egypt: clonal spread of bla OXA-23. Microb Drug Resist. 2018;24:269–277. doi: 10.1089/mdr.2017.0057. [DOI] [PubMed] [Google Scholar]
- 37.Turton JF, Ward ME, Woodford N, Kaufmann ME, Pike R, et al. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii . FEMS Microbiol Lett. 2006;258:72–77. doi: 10.1111/j.1574-6968.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- 38.Poirel L, Lebessi E, Héritier C, Patsoura A, Foustoukou M, et al. Nosocomial spread of OXA-58-positive carbapenem-resistant Acinetobacter baumannii isolates in a paediatric hospital in Greece. Clin Microbiol Infect. 2006;12:1138–1141. doi: 10.1111/j.1469-0691.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 39.Lopes BS, Evans BA, Amyes SGB. Disruption of the blaOXA-51-like gene by ISAba16 and activation of the blaOXA-58 gene leading to carbapenem resistance in Acinetobacter baumannii Ab244. J Antimicrob Chemother. 2012;67:59–63. doi: 10.1093/jac/dkr415. [DOI] [PubMed] [Google Scholar]
- 40.Bertini A, Poirel L, Bernabeu S, Fortini D, Villa L, et al. Multicopy blaOXA-58 gene as a source of high-level resistance to carbapenems in Acinetobacter baumannii . Antimicrob Agents Chemother. 2007;51:2324–2328. doi: 10.1128/AAC.01502-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravasi P, Limansky AS, Rodriguez RE, Viale AM, Mussi MA. ISAba825, a functional insertion sequence modulating genomic plasticity and bla(OXA-58) expression in Acinetobacter baumannii . Antimicrob Agents Chemother. 2011;55:917–920. doi: 10.1128/AAC.00491-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huovinen P, Jacoby GA. Sequence of the PSE-1 -Lactamase Gene. Antimicrob Agents Chemother. 1991;35:2428–2430. doi: 10.1128/AAC.35.11.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamolvit W, Derrington P, Paterson DL, Sidjabat HE. A case of IMP-4-, OXA-421-, OXA-96-, and CARB-2-producing Acinetobacter pittii sequence type 119 in Australia. J Clin Microbiol. 2015;53:727–730. doi: 10.1128/JCM.02726-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poirel L, Bonnin RA, Boulanger A, Schrenzel J, Kaase M, et al. Tn125-related acquisition of blaNDM-like genes in Acinetobacter baumannii . Antimicrob Agents Chemother. 2012;56:1087–1089. doi: 10.1128/AAC.05620-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones LS, Toleman MA, Weeks JL, Howe RA, Walsh TR, et al. Plasmid carriage of bla NDM-1 in clinical Acinetobacter baumannii isolates from India. Antimicrob Agents Chemother. 2014;58:4211–4213. doi: 10.1128/AAC.02500-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mugnier PD, Poirel L, Nordmann P. Functional analysis of insertion sequence ISAba1, responsible for genomic plasticity of Acinetobacter baumannii . J Bacteriol. 2009;191:2414–2418. doi: 10.1128/JB.01258-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen T-L, Wu RC-C, Shaio M-F, Fung C-P, Cho W-L. Acquisition of a plasmid-borne blaOXA-58 gene with an upstream IS1008 insertion conferring a high level of carbapenem resistance to Acinetobacter baumannii . Antimicrob Agents Chemother. 2008;52:2573–2580. doi: 10.1128/AAC.00393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi AHK, Slamti L, Avci FY, Pier GB, Maira-Litrán T. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-beta-1-6-N-acetylglucosamine, which is critical for biofilm formation. J Bacteriol. 2009;191:5953–5963. doi: 10.1128/JB.00647-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cerqueira GM, Peleg AY. Insights into Acinetobacter baumannii pathogenicity. IUBMB Life. 2011;63:1055–1060. doi: 10.1002/iub.533. [DOI] [PubMed] [Google Scholar]
- 50.Perez F, Ponce-Terashima R, Adams MD, Bonomo RA. Are we closing in on an "elusive enemy"? The current status of our battle with Acinetobacter baumannii . Virulence. 2011;2:86–90. doi: 10.4161/viru.2.2.15748. [DOI] [PubMed] [Google Scholar]
- 51.Camarena L, Bruno V, Euskirchen G, Poggio S, Snyder M. Molecular mechanisms of ethanol-induced pathogenesis revealed by RNA-sequencing. PLoS Pathog. 2010;6:e1000834. doi: 10.1371/journal.ppat.1000834. p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sauer FG, Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. Bacterial pili: molecular mechanisms of pathogenesis. Curr Opin Microbiol. 2000;3:65–72. doi: 10.1016/S1369-5274(99)00053-3. [DOI] [PubMed] [Google Scholar]
- 53.Loehfelm TW, Luke NR, Campagnari AA. Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J Bacteriol. 2008;190:1036–1044. doi: 10.1128/JB.01416-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saffari F, Monsen T, Karmostaji A, Azimabad FB, Widerström M. Significant spread of extensively drug-resistant Acinetobacter baumannii genotypes of clonal complex 92 among intensive care unit patients in a university hospital in southern Iran. J Med Microbiol. 2017;66:1656–1662. doi: 10.1099/jmm.0.000619. [DOI] [PubMed] [Google Scholar]
- 55.Uwingabiye J, Lemnouer A, Roca I, Alouane T, Frikh M, et al. Clonal diversity and detection of carbapenem resistance encoding genes among multidrug-resistant Acinetobacter baumannii isolates recovered from patients and environment in two intensive care units in a Moroccan Hospital. Antimicrob Resist Infect Control. 2017;26: 6:99. doi: 10.1186/s13756-017-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rynga D, Shariff M, Deb M. Phenotypic and molecular characterization of clinical isolates of Acinetobacter baumannii isolated from Delhi, India. Ann Clin Microbiol Antimicrob. 2015;14:40. doi: 10.1186/s12941-015-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vijayakumar S, Mathur P, Kapil A, Das BK, Ray P, et al. Molecular characterization & epidemiology of carbapenem-resistant Acinetobacter baumannii collected across India. Indian J Med Res. 2019;2:240–246. doi: 10.4103/ijmr.IJMR_2085_17. [DOI] [PMC free article] [PubMed] [Google Scholar]