Abstract
Background:
We previously reported that administration of bortezomib (BTZ) after 4 days of G-CSF significantly augments mobilization in mice. We hypothesized that administration of BTZ at peak G-CSF mobilization in patients with multiple myeloma (MM) would be safe, augment mobilization, and have an in vivo purging effect on circulating myeloma cells.
Methods:
This was a phase I study using 3 dose levels of BTZ. G-CSF was administered for five days. On the evening of the fourth day, a single dose of BTZ was administered. Peripheral blood was drawn 1–2 hours before and 15–18 hours following BTZ administration (prior to day 5 G-CSF administration) to analyze the mobilization effect of BTZ. Standard apheresis was then performed starting on day 5. Following mobilization, patients underwent autologous stem cell transplantation (ASCT) per institutional guidelines.
Results:
Ten patients were enrolled. There were no dose limiting toxicities. Median peripheral blood CD34+ cells at Day 4 prior to BTZ administration was 16/μL and 15 hours later was 32/μL suggesting that administration of BTZ at peak G-CSF mobilization augments the mobilization effect of G-CSF. The impact of BTZ on circulating MM cells was unclear. All patients had successful engraftment post-ASCT.
Conclusion:
Administration of one dose of BTZ at peak G-CSF mobilization was safe and well tolerated, enhanced stem cell mobilization, and did not impact graft viability. The mobilization effect of BTZ at peak G-CSF mobilization shown in this phase 1 study needs to be confirmed in a larger randomized trial.
MicroAbstract
G-CSF is the most frequently used agent for stem cell mobilization but it can result in suboptimal stem cell mobilization. We previously reported that administration of bortezomib after 4 days of G-CSF significantly augments mobilization in mice. Here we report the results of our institutional phase 1 clinical trial showing that administration of bortezomib at peak G-CSF mobilization is safe and potentially augments G-CSF mobilization.
INTRODUCTION
Over 2 decades ago, it was shown that treatment with high-dose chemotherapy (HDCT) and autologous stem cell transplant (ASCT) results in improved response, progression-free (PFS) and overall survival (OS) compared to standard dose chemotherapy for patients with multiple myeloma (MM). More recently, post-ASCT consolidation and maintenance has led to even deeper responses and better outcomes;1 however, virtually all patients still relapse. Relapse is generally thought to result from insufficient eradication of MM by HDCT, but reinfusion of myeloma cells with the peripheral blood stem cell (PBSC) autograft may be another potential cause of relapse. The consequence of reinfusing malignant cells remains unclear but small prospective and retrospective studies suggest that the presence of myeloma cells in the graft correlates with poorer outcomes after transplantation.2–7 Thus, in vivo or ex vivo purging of myeloma cells in autologous PBSC products may represent a valid strategy for reducing relapse.5
Granulocyte colony-stimulating factor (G-CSF) is the most frequently used agent for stem cell mobilization but it results in suboptimal stem cell yields in 5–30% of patients.8 Although other approaches for stem cell mobilization, such as the addition of plerixafor or chemomobilization, have been developed, the collection of both an optimal and minimum number of HSPCs for autologous HSCT remains a major problem.9 Therefore, new strategies are needed to optimize stem cell mobilization and collection.
We previously reported that in mice, administration of bortezomib (BTZ), after 4 days of G-CSF significantly augments mobilization with G-CSF by modulating VLA-4/VCAM-1 axis.10 We hypothesized that in patients with MM administration of BTZ at peak G-CSF mobilization would be safe, augment mobilization, and, due to its therapeutic activity in MM, have an in vivo purging effect on circulating myeloma cells. In this phase I study, we explored the safety and mobilization effects of BTZ when given at peak G-CSF mobilization in patients with MM.
METHODS
Study Design and Patients
This was a single-institution open-label phase I clinical trial of BTZ and G-CSF for autologous stem cell mobilization in patients with MM. The study was performed under a protocol approved by the Washington University School of Medicine Human Subjects Committee and all patients voluntarily provided informed consent for the trial. The study was registered at clinicaltrials.gov as NCT02220608.
The primary objective of the study was to determine the maximum tolerated dose (MTD) of BTZ when administered at peak G-CSF mobilization. Secondary objectives included the efficacy of BTZ in augmenting stem cell mobilization, reducing myeloma cell contamination, and reducing relapse/prolonging remission post-transplantation. Secondary endpoints included proportion of patients collecting more than or equal to 6 × 106 CD34+ cells/kg, 4 × 106 CD34+ cells/kg (enough for two transplant), or 2 × 106 CD34+ cells/kg (enough for one transplant) in 4 or fewer apheresis. All analyses were descriptive in nature.
Eligible patients were 18 years of age or older with a diagnosis of MM undergoing autologous stem cell mobilization during front-line treatment. Patients with poor hematologic reserve (neutrophils < 1k/μL, hemoglobin < 8.0 g/dl, or platelets < 50k/μL), impaired liver or kidney function, or poor performance status were excluded as were patients with HIV, severe cardiac disease, or other contraindication to study treatment. Prior exposure to BTZ, G-CSF, radiotherapy, alkylating agents, or immunomodulatory drugs (IMiDs) was not exclusionary. Patients with prior stem cell collection or transplantation were excluded.
Treatment
To assess the kinetics of stem cell mobilization after BTZ alone in humans, all patients received a test dose of single-agent BTZ intravenously, at the cohort specified dose level, 4 days prior to beginning stem cell mobilization followed by serial blood collections (Figure 1). G-CSF was then administered in standard fashion; 10mcg/kg was administered in the morning (at approximately 08:00 AM) for five days. In the evening of day 4 at approximately 1800 hours, BTZ was administered intravenously. Three dose-levels of BTZ were tested: (−1) 0.7mg/m2, (1) 1.0mg/m2, (2) 1.3mg/m2 in a 3+3 design (Figure 1). At approximately 08:00 AM on day 5, blood was drawn for correlative studies including CD34+ enumeration, followed by day 5 G-CSF administration followed by apheresis. Apheresis began on day 5, 15–18 hours post-BTZ administration. Twenty liters of apheresis was performed with the COBE Spectra Apheresis System (Terumo BCT, Lakewood, CO). The target collection goal was 6.0×106 CD34+ cells/kg. If the goal was not meet after one apheresis, up to three additional days of G-CSF and apheresis were performed. No additional BTZ was administered past day 4 of mobilization. Following mobilization, patients proceeded to ASCT with melphalan conditioning. Patients underwent restaging at Day 100 post-ASCT and then were initiated on consolidation and/or maintenance treatment at the discretion of their treating physician. Dose limiting toxicities (DLTs) were defined as any of the following treatment emergent events within 28 days following mobilization: grade 4 neutropenia, thrombocytopenia, or anemia persisting > 7days; febrile neutropenia; or any grade 3 to 5 non-hematologic toxicities. The MTD was defined as the maximum dose level tested where no more than 1 patient of 6 treated experienced a DLT.
Figure 1:
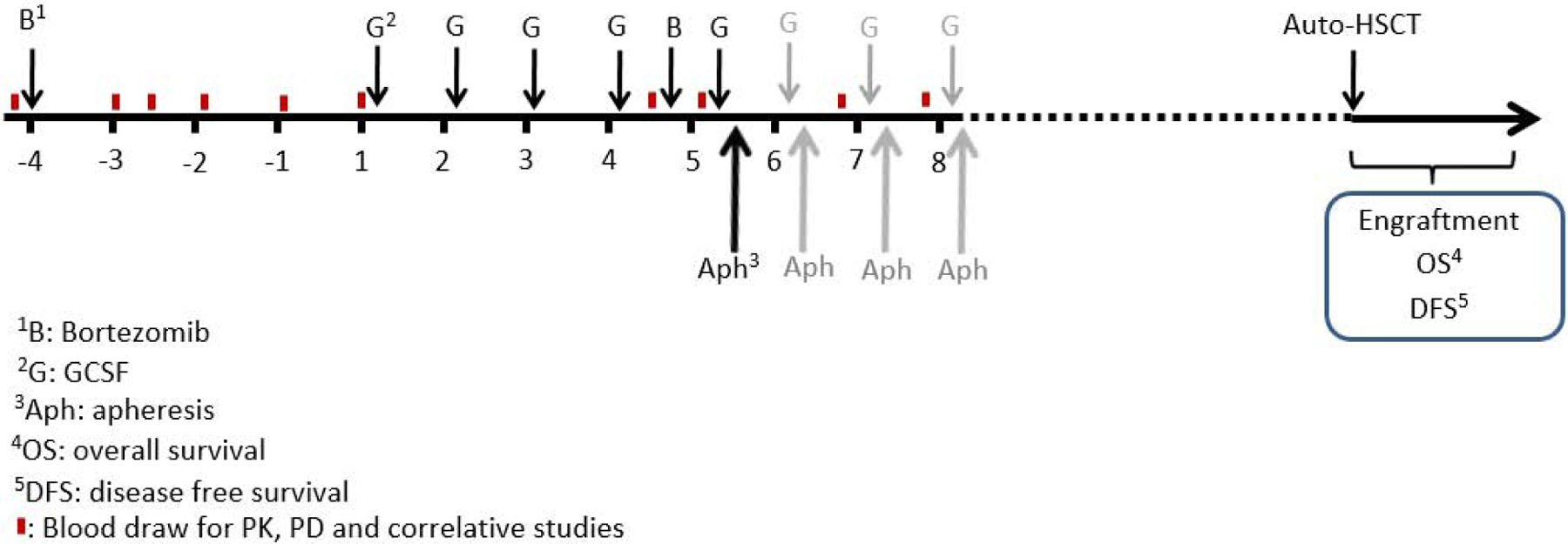
Study Schema.
Correlative Studies
Minimal residual disease (MRD) detection via next generation sequencing of immunoglobulin gene variable regions (Adaptive clonoSEQ, Seattle WA) was performed to quantify the number of circulating MM prior to and 15 hours post-BTZ administration (days 4 and 5 of G-CSF). In this test, amplification and sequencing of immunoglobulin gene segments at diagnosis (“ID” sample) allows for quantification of the patient specific cancer molecules in later assessments with an assay sensitivity of 1 cell per million.11 Next generation sequencing techniques such as Adaptive clonoSEQ have been increasingly utilized for minimal residual disease testing in patients with MM, but in addition have been used to assess MM cell contamination in ASCT products.12
RESULTS
Patients
Ten patients were enrolled over a 12 month period. The median age of the study population was 63.5 years (range 56–71) and 60% were male. The median number of prior cycles of anti-MM therapy was 4 (range 4–18). All patients received BTZ-based induction regimens; 6 received combination BTZ-lenalidomide-dexamethasone, 3 received BTZ-cyclophosphamide-dexamethasone, and 1 received BTZ-dexamethasone. Patient characteristics are summarized in Table I.
Table 1:
Patient Characteristics
| Bortezomib | Mobilization | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | Age | Sex | Induction | Resting CD34+ (cells/uL) | Peak CD34+ (cells/uL) | Day 4 CD34+ (cells/uL) | Day 5 CD34+ (cells/uL) | Day 5 Yield (106 cells/kg) | Apheresis Days | Total Yield (106 cells/kg) |
| 1 | 67 | Male | BD × 6 cycles | 2 | 2 | 16 | 47 | 8.2 | 1 | 8.2 |
| 2 | 68 | Male | BCD × 4 cycles | 1 | 2 | 47 | 84 | 8.5 | 1 | 8.5 |
| 3 | 62 | Male | RVD × 4 cycles | ND | 2 | ND | 22 | 3.1 | 3 | 7.2 |
| 4 | 60 | Female | BCD × 4 cycles | 2 | 8 | 59 | 53 | 5.9 | 1 | 5.9 |
| 5 | 56 | Female | RVD × 4 cycles | 1 | 1 | 29 | 42 | 7.9 | 1 | 7.9 |
| 6 | 56 | Male | RVD × 4 cycles | 2 | 6 | 49 | 72 | 7.9 | 1 | 7.9 |
| 7 | 65 | Female | RVD × 18 cycles | 0 | 0 | 1 | 2 | 0.4 | 2 | 0.6 |
| 8 | 71 | Male | RVD × 7 cycles | 1 | 1 | 8 | 12 | 1.8 | 4 | 3.7 |
| 9 | 68 | Male | RVD × 4 cycles | 3 | 2 | 2 | 12 | 0.5 | 4 | 2.2 |
| 10 | 59 | Female | BCD × 4 cycles | 2 | 1 | 12 | 20 | 2.1 | 4 | 5.7 |
Abbreviations: BD- Bortezomib/Dexamethasone; BCD- Bortezomib/Cyclophosphamide/Dexamethasone; RVD- Lenalidomide/Bortezomib/Dexamethasone; ND - No Data
MTD Determination and Toxicity
No DLTs were observed in any of the dose levels tested, and thus, an MTD was not established. One patient received BTZ at dose level −1 (0.7mg/m2), 3 patients were enrolled at dose level 1 (1.mg/m2), and 6 patients were enrolled at dose level 2 (1.3mg/m2). Subject that received dose level −1 (0.7 mg/m2) was originally enrolled in dose level 1, but inadvertently received BTZ at dose level −1. This subject was replaced in cohort 1 but was included in safety and mobilization efficacy analysis. There were 2 serious adverse events during the study follow-up period, one patient was admitted for diarrhea on Day 3 of G-CSF, but the symptoms had started prior to his start of study treatment, and one was admitted after developing pneumonia 1 week following mobilization. Neither of these events were considered related to study treatment.
Hematologic toxicity was mild and similar to those seen with standard G-CSF mobilization. Seven patients developed new or worsened thrombocytopenia, 7 worsened anemia. Two patients reported new or worsened neuropathy. The only other non-hematologic toxicities reported to be related to study treatment were GI upset and bone pain.
Mobilization
There was little CD34+ mobilization seen with BTZ alone. After the test-dose of single agent BTZ, serial blood collections were performed at 15, 18, 24, 40, and 88 hours post. Mean peripheral blood CD34+ cells before and after single agent BTZ were 2 CD34+ cells/μL (range: 0–3 CD34+ cells/μL) and 2 CD34+ cells/μL (range: 0–8 CD34+ cells/μL) respectively. Increased circulating CD34+ counts were only observed in 2 patients. Patient 4, who was treated with 1.0mg/m2, had a resting CD34+ of 2/μL which increased to 8/μL at 15 and 18 hours post-dose. Patient 6, who was treated at 1.3mg/m2, had a resting CD34+ of 2/μL which increased to 4/μL at 15 hours and to 6/μL at 18 hours.
Following 4 days of G-CSF, and prior to Day 4 BTZ dose, the median circulating CD34+ was 16/μL (range 1–59). On Day 5, 15–18 hours post-day 4 BTZ (and before day 5 G-CSF administration), the median circulating CD34+ was 32/μL (range 2–84). The median increase was 67% (range: 10%−194%). Only one patient had a reduction in circulating CD34+ following BTZ. The CD34+ count of patient 4 who was treated with 1.0mg/m2 went from 59/μL to 53/μL. Figure 2 and table 1 summarize CD34+ mobilization following G-CSF and BTZ.
Figure 2:
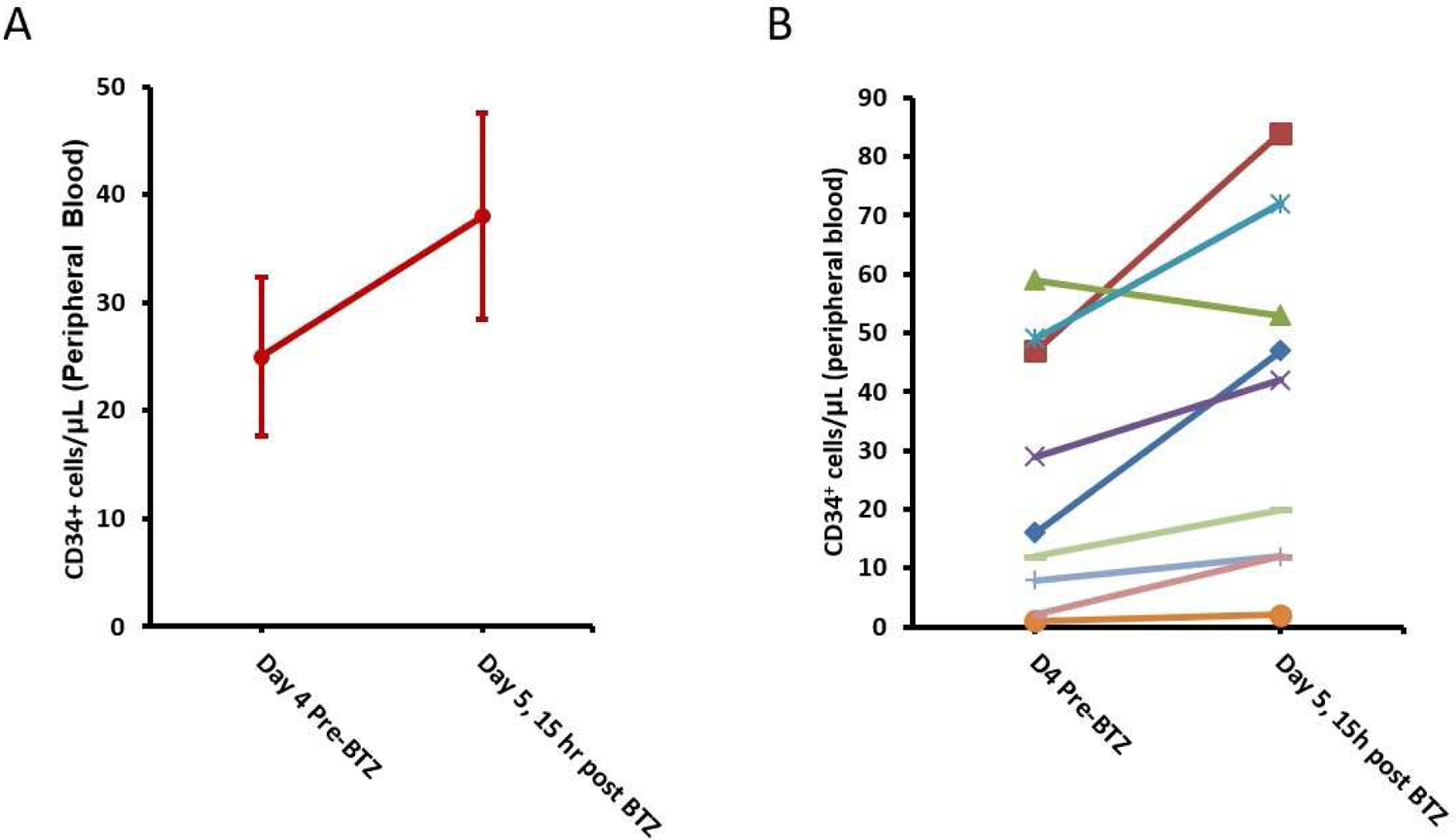
Kinetics of Stem Cell Mobilization with Bortezomib (BTZ) given on day 4 at peak G-CSF mobilization. (A) Mean +/− standard error before and after BTZ. (B) Peripheral blood CD34+ cells before and after BTZ in each patient.
The median CD34+ collection yield after one apheresis was 4.5×106 cells/kg (range 0.4–8.17 ×106 cells/kg). The proportion of patients collected more or equal to 6 × 106 CD34+ cells/kg, 4 × 106 CD34+ cells/kg, or 2 × 106 CD34+ cells/kg in 4 or fewer apheresis were 5/10 (50%), 7/10 (70%) and 9/10 (90%) respectively. Five of the patients met the collection goal of 6 × 106 CD34+ cells/μL in one apheresis. The other 5 underwent additional days of G-CSF and apheresis. One reached the collection goal after the third day of apheresis. Three underwent 4 days of apheresis, the maximum allowed, but failed to reach the collection goal. In one patient apheresis was abandoned following the second day due to poor yields.
Among the 4 patients who did not meet the protocol specified collection goal of 6×106 CD34+ cells/kg, two were able to collect 4.0×106 CD34+ cells/kg, the institutional standard for transplantation for MM, and proceeded to ASCT. The other two patients underwent an additional stem cell mobilization with G-CSF and plerixafor off protocol after a few weeks of rest. Both patients had superior yields following G-CSF and plerixafor (day 1 collection yields of 2.0×106 and 5.4 × 106 CD34+ cells/kg) and were able to complete stem cell collections.
MM Cell Contamination
Seven patients met sample requirements for MRD detection via next generation sequencing. The other 3 patients did not have the required “ID” sample available. Patient 2 had 14 MM cells per million cells in circulation prior to BTZ. Post-BTZ no circulating MM cells were observed. Patient 4 had 35 MM cells per million cells in circulation prior to BTZ and 436 post. Patient 10 had 41 MM cells per million pre and 66 post. The other four patients had no circulating MM cells detected at either the pre- or post-BTZ time points.
Transplantation Outcomes
All 10 patients underwent melphalan conditioning and ASCT with median interval from start of mobilization and ASCT of 22 days; however, 2 patients received CD34+ cells collected with G-CSF and plerixafor and, thus, were considered non-evaluable for the transplant outcomes. In the 8 evaluable patients, the median CD34+ cells infused was 4×106 CD34+ cells/kg (range 2.8–5.4 × 106 CD34+ cells/kg). Post-ASCT, engraftment was rapid; the median times to neutrophil and platelet engraftment were 12 days (range 11–13) and 19 days (range 17–27), respectively. The complete response rate at Day 100 post-ASCT was 25%. At the time of manuscript preparation, 2 patient have had disease progression after a median follow-up of 27 months (range 20–33).
DISCUSSION
Approximately 5,000 autologous transplants for MM are performed every year in US.13 Recent advances have improved mobilization success making ASCT feasible for more patients and maintenance regimens have improved long term outcomes. Yet a number of patients still fail to collect the requisite stem cell graft and virtually all patients will inevitably relapse. Incorporating BTZ into mobilization regimens has the potential to improve both of these limitations.
In this study single agent bortezomib did not mobilize CD34+ cells significantly but resulted in a modest increase in CD34+ cells when was given at peak G-CSGF mobilization. We found that BTZ administration at peak mobilization with G-CSF is safe, well tolerated, and augments stem cell mobilization. Circulating CD34+ cells increased by nearly 70% on average following BTZ. Collection success rates in this study were similar to G-CSF only arm in phase 3 trial of G-CSF versus GCSF plus plerixafor in patients with MM.14 In that study, the proportion of patients collecting more or equal to 6 × 106 CD34+ cells/kg or 2 × 106 CD34+ cells/kg in 4 or fewer apheresis were 51.3% and 88.3%, respectively. In our phase 1 trial, the proportion of patients collecting more or equal to 6 × 106 CD34+ cells/kg or 2 × 106 CD34+ cells/kg in 4 or fewer apheresis were 5/10 (50%) and 9/10 (90%), respectively.
Two out of 10 patients were still unable to collect the institutional minimum for ASCT of 4 × 106/kg. One of these two patients had extended administration of lenalidomide (18 cycles) which likely impaired mobilization. While the patient subsequently underwent a successful mobilization with G-CSF and plerixafor, the yield, 2.0 × 106/kg, was low compared to our institutional norm. The other did not have any overt risks for poor stem cell mobilization and had a relatively normal CD34+ cell yield with subsequent G-CSF and plerixafor mobilization.
The impact of BTZ on circulating MM cells during mobilization is unclear. We found that 42% of patients (3/7) had measurable MM cells in circulation. This is similar to what was observed by Takamatsu and colleagues.12 Following BTZ administration, 1 patient had resolution of circulating MM cells, while the other 2 were relatively stable. Lack of consistent drop in circulating myeloma cells in this study could be related to low bortezomib dose or lower number of doses of bortezomib administered at peak G-CSF mobilization.
Previous studies have shown that ex vivo purging of myeloma cells in autologous PBSC products is feasible. Stewart et al. conducted a phase III randomized trial using CD34+-selected versus standard autograft products for ASCT in MM.7 There was a 3 log decrease in MM cell contamination in the MM product following selection; however, there was no difference in PFS or OS between the two cohorts. Another prospective study by Gertz et al. suggested that the presence of myeloma cells in PBSC grafts correlates with poor outcome after transplantation.2 Separate retrospective analyses of the European and North American transplant registries showed that patients who received syngeneic transplant from a twin had fewer relapses and improved event-free survival compared with matched patients who received an autograft.3, 4 Additional studies are needed to further define the efficacy of in vivo or ex vivo purging of MM cells in reducing relapse.
There were no dose limiting toxicities in this study. One limitation of this study is that we did not add another cohort with higher dose or higher number of doses of bortezomib to achieve better mobilization or neoplastic cell purging. Another limitation of this study is that we did not have a G-CSF only control group; therefore the increase in circulating CD34+ cells after bortezomib could have been secondary to the chance or timing.
In conclusion, administration of BTZ at peak G-CSF mobilization was safe and well tolerated, enhanced stem cell mobilization, and did not impact graft function. The combination of BTZ and G-CSF for stem cell mobilization may warrant further study in randomized placebo controlled trials.
Clinical Practice Points.
G-CSF can result in suboptimal hematopoietic stem cell mobilization in 5–30% of patients. Additionally, mobilized product in patients with multiple myeloma are usually contaminated with small number of multiple myeloma cells. Although the consequence of reinfusing malignant cells remains controversial, several studies suggest that the presence of myeloma cells in the mobilized product correlates with poorer outcomes after transplantation.
In preclinical studies, Bortezomib augmented G-CSF mobilization when it was given at peak GCSF mobilization. In this phase 1 study, we gave bortezomib on day 4 of G-CSF mobilization with the goal of augmenting G-CSF mobilization and decreasing multiple myeloma contamination.
This phase 1 study showed that giving bortezomib at peak G-CSF mobilization is safe and is not negatively affecting engraftment, has modest effect in increasing hematopoietic stem cell mobilization and my decrease the number of circulating myeloma cells in some patients.
Acknowledgments
A.G. was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2 TR002346 (principle investigator: Victoria J. Fraser). Takeda provided bortezomib.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
REFERENCES
- 1.Al-Ani F, Louzada M. Post-transplant consolidation plus lenalidomide maintenance vs lenalidomide maintenance alone in multiple myeloma: A systematic review. Eur J Haematol. 2017;99:479–488. [DOI] [PubMed] [Google Scholar]
- 2.Gertz MA, Witzig TE, Pineda AA, Greipp PR, Kyle RA, Litzow MR. Monoclonal plasma cells in the blood stem cell harvest from patients with multiple myeloma are associated with shortened relapse-free survival after transplantation. Bone Marrow Transpl. 1997;19:337–342. [DOI] [PubMed] [Google Scholar]
- 3.Bashey A, Perez WS, Zhang MJ, et al. Comparison of twin and autologous transplants for multiple myeloma. Biol Blood Marrow Tr. 2008;14:1118–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gahrton G, Svensson H, Bjorkstrand B, et al. Syngeneic transplantation in multiple myeloma - a case-matched comparison with autologous and allogeneic transplantation. Bone Marrow Transpl. 1999;24:741–745. [DOI] [PubMed] [Google Scholar]
- 5.Yang H, Robinson SN, Nieto Y, et al. Ex Vivo Graft Purging and Expansion of Autologous Blood Progenitor Cell Products from Patients with Multiple Myeloma. Cancer Res. 2011;71:5040–5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogel W, Kopp HG, Kanz L, Einsele H. Myeloma cell contamination of peripheral blood stem-cell grafts can predict the outcome in multiple myeloma patients after high-dose chemotherapy and autologous stem-cell transplantation. J Cancer Res Clin. 2005;131:214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart AK, Vescio R, Schiller G, et al. Purging of autologous peripheral-blood stem cells using CD34 selection does not improve overall or progression-free survival after high-dose chemotherapy for multiple myeloma: Results of a multicenter randomized controlled trial. J Clin Oncol. 2001;19:3771–3779. [DOI] [PubMed] [Google Scholar]
- 8.Rettig MP, Ansstas G, DiPersio JF. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia. 2012;26:34–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiPersio JF, Uy GL, Yasothan U, Kirkpatrick P. Fresh from the Pipeline Plerixafor. Nat Rev Drug Discov. 2009;8:105–106. [DOI] [PubMed] [Google Scholar]
- 10.Ghobadi A, Rettig MP, Cooper ML, et al. Bortezomib is a rapid mobilizer of hematopoietic stem cells in mice via modulation of the VCAM-1/VLA-4 axis. Blood. 2014;124:2752–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faham M, Zheng JB, Moorhead M, et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2012;120:5173–5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takamatsu H, Takezako N, Zheng J, et al. Prognostic value of sequencing-based minimal residual disease detection in patients with multiple myeloma who underwent autologous stem-cell transplantation. Ann Oncol. 2017;28:2503–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasquini MC WZCuaoohsctCSS, 2012. Available at: http://www.cibmtr.org.
- 14.DiPersio JF, Stadtmauer EA, Nademanee A, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113:5720–5726. [DOI] [PubMed] [Google Scholar]


