Summary
Background
The selective TRK inhibitor larotrectinib was approved for paediatric and adult patients with advanced TRK fusion-positive solid tumours based on a primary analysis set of 55 patients. The aim of our analysis was to explore the efficacy and long-term safety of larotrectinib in a larger population of patients with TRK fusion-positive solid tumours.
Methods
Patients were enrolled and treated in a phase 1 adult, a phase 1/2 paediatric, or a phase 2 adolescent and adult trial. Some eligibility criteria differed between these studies. For this pooled analysis, eligible patients were aged 1 month or older, with a locally advanced or metastatic non-CNS primary, TRK fusion-positive solid tumour, who had received standard therapy previously if available. This analysis set includes the 55 patients on which approval of larotrectinib was based. Larotrectinib was administered orally (capsule or liquid formulation), on a continuous 28-day schedule, to adults mostly at a dose of 100 mg twice daily, and to paediatric patients mostly at a dose of 100 mg/m2 (maximum of 100 mg) twice daily. The primary endpoint was objective response as assessed by local investigators in an intention-to-treat analysis. Contributing trials are registered with ClinicalTrials.gov, NCT02122913 (active not recruiting), NCT02637687 (recruiting), and NCT02576431 (recruiting).
Findings
Between May 1, 2014, and Feb 19, 2019, 159 patients with TRK fusion-positive cancer were enrolled and treated with larotrectinib. Ages ranged from less than 1 month to 84 years. The proportion of patients with an objective response according to investigator assessment was 121 (79%, 95% CI 72–85) of 153 evaluable patients, with 24 (16%) having complete responses. In a safety population of 260 patients treated regardless of TRK fusion status, the most common grade 3 or 4 larotrectinib-related adverse events were increased alanine aminotransferase (eight [3%] of 260 patients), anaemia (six, 2%), and decreased neutrophil count (five [2%]). The most common larotrectinib-related serious adverse events were increased alanine aminotransferase (two [<1%] of 260 patients), increased aspartate aminotransferase (two [<1%]), and nausea (two [<1%]). No treatment-related deaths occurred.
Interpretation
These data confirm that TRK fusions define a unique molecular subgroup of advanced solid tumours for which larotrectinib is highly active. Safety data indicate that long-term administration of larotrectinib is feasible.
Funding
Bayer and Loxo Oncology.
Introduction
The tropomyosin receptor kinase family of proteins TRKA (high-affinity nerve growth factor receptor), TRKB (BDNF/NT-3 growth factors receptor), and TRKC (NT-3 growth factor receptor) are encoded by the neurotrophic receptor tyrosine kinase genes (NTRK1, NTRK2, and NTRK3, respectively). TRK fusions (fusions between one of the NTRK genes and a 5′ partner gene) arise from intrachromosomal or interchromosomal rearrangements that collectively lead to the expression of a chimeric oncoprotein characterised by ligand-independent kinase activation, which consequently drives oncogenesis.1,2 TRK fusions are found in various adult and paediatric cancers. These cancers include rare tumours (eg, mammary analogue secretory carcinoma,3 secretory breast carcinoma,4 infantile fibrosarcoma,5 and cellular congenital mesoblastic nephroma6) in which TRK fusions are found in the majority of cases, and more common cancers (eg, lung, breast, and gastrointestinal carcinomas, melanomas, and sarcomas)7 in which TRK fusions are found at lower frequencies. TRK fusions can be identified by DNA-based or RNA-based next-generation sequencing, or other assays such as fluorescence in-situ hybridisation (FISH) or immunohistochemistry.1,8
We previously reported9 the activity and safety of larotrectinib, a highly selective and potent TRK inhibitor, in the first 55 consecutively enrolled adult and paediatric patients with TRK fusion-positive cancer who were treated across one of three clinical trials (primary analysis set). In this analysis, the proportion of patients with response according to independent review was 41 (75%) of 55 patients and 44 (80%) of 55 patients by investigator assessment. Based on these data, larotrectinib became the first tyrosine kinase inhibitor to be granted approval by the US Food and Drug Administration (FDA) and the European Medicines Agency for a tumour-agnostic indication; specifically, the treatment of adult and paediatric patients with advanced solid tumours that harbour an NTRK gene fusion. The multikinase inhibitor entrectinib10 has similarly been approved by the US FDA and the Japanese Ministry of Health, Labour, and Welfare for the treatment of patients with NTRK fusion-positive disease in a tumour-agnostic manner, and TRK inhibitors targeting acquired on-target resistance are in development.11,12
At the time of the previous prespecified analysis for larotrectinib, the median duration of response and progression-free survival had not been reached. Furthermore, little representation of several common cancer types including lung, melanoma, colon, and breast cancer in the primary analysis set made interpretation of efficacy challenging in these tumour types. Finally, long-term safety was unknown. To address these limitations, we report an expanded pooled efficacy analysis of 159 patients with TRK fusion-positive cancer treated with larotrectinib, and a safety analysis of 260 patients who received larotrectinib regardless of TRK fusion status.
Methods
Study design and participants
The protocols for the three individual clinical studies detailing full eligibility criteria and the statistical analysis plan for the integrated analysis have been previously reported.9 Briefly, eligible patients were aged 1 month or older, had a locally advanced or metastatic solid tumour, had received standard therapy previously (if available), and had adequate organ function. Eligibility criteria in relation to life expectancy, performance status, comorbidities and previous treatments not permitted or permitted (and washout periods), and laboratory test values required to assess eligibility varied across the contributing studies and are detailed in the individual trial protocols. Patients with both treated and untreated brain metastases were eligible, provided these metastases were not symptomatic. Patients with primary CNS tumours were eligible for the trials, but were excluded from the current analysis, which was focused on patients with Response Evaluation Criteria in Solid Tumours (RECIST)-measurable disease. TRK fusion positivity was determined by locally obtained molecular testing using either next-generation sequencing, FISH, or reverse transcriptase PCR. An implied TRK fusion based on ETV6 break-apart FISH positivity was considered acceptable for patients with infantile fibrosarcoma because NTRK3 was the only gene fusion partner reported with ETV6 in infantile fibrosarcoma and given the high frequency of ETV6–NTRK3 fusions in this disease.5
All studies were done in accordance with the standard of good clinical practice, the principles expressed in the Declaration of Helsinki, and all applicable country and local regulations. Protocols were approved by an institutional review board or independent ethics committee at each investigative site. All patients (or parents or guardians of minor patients) provided written informed consent before the initiation of any study-related procedures.
Procedures
Larotrectinib was administered orally (capsule or liquid formulation), continuously, on a 28-day schedule. In the phase 2 study, all adult and adolescent patients received the recommended starting dose of 100 mg twice daily. In the phase 1 studies, several patients were included from the dose escalation stage; the starting doses of larotrectinib are summarised in the appendix (p 1). The majority of paediatric patients (43 [83%] of 52) received 100 mg/m2 per dose (maximum of 100 mg) twice daily, with a minority (nine [16%] of 52 patients) receiving other doses (appendix p 1). Larotrectinib was administered until disease progression, withdrawal of the patient from the study, or the occurrence of an unacceptable level of adverse events. The criteria for removal of patients from therapy or assessment, and for dose modification, are specified in the individual trial protocols (appendix pp 22–743).
Patients with progressive disease could continue larotrectinib if, in the opinion of the site investigator, they continued to derive clinical benefit, and continuation of treatment was approved by the funder. For example, a patient with asymptomatic RECIST progression in a solitary site could receive radiotherapy or surgery as per investigator discretion if this particular lesion was amenable to local therapy; continued disease control at other sites would warrant larotrectinib continuation. In patients who had surgical resection for local control, if the surgery resulted in negative margins and study treatment was stopped, patients were permitted to restart larotrectinib if they had progressive disease, after discussion with the medical monitor.
Tumours were assessed radiographically by investigators using CT, PET, or MRI at baseline, every 8 weeks for 1 year, every 12 weeks thereafter, and for paediatric patients every 6 months after 2 years of treatment, in the absence of progressive disease. Tumour responses were confirmed by a second scan at least 28 days after the criteria for response were first met. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03, and were monitored throughout the studies. Laboratory monitoring was done throughout cycle 1 and on day 1 of each cycle thereafter. The safety assessment period was from the date informed consent was obtained until at least 28 days after the last dose of larotrectinib was administered.
Outcomes
The primary endpoint was objective response as assessed by local investigators according to RECIST, version 1.1,13 in the full analysis set, defined as patients with tumours harbouring a documented NTRK fusion, one or more measurable lesions at baseline, and who had received one or more doses of larotrectinib, analysed according to the intention-to-treat principle. Independent review committee assessment of the full analysis set was not done for patients included by the data cutoff date (ie, Feb 19, 2019). The proportion of patients with response was calculated as the total proportion of patients with a best overall response of complete or partial response. Secondary endpoints were duration of response, defined as the time from the start date of the initial response to the date of disease progression or death; progression-free survival, defined as the time from the date of the first dose of larotrectinib to the earliest date of documented disease progression or death; time to response, defined as the time from the date of the first dose of larotrectinib to the first documentation of an objective response that was subsequently confirmed by repeat imaging; and overall survival, defined as the time from the date of first dose of larotrectinib to death.
Statistical analysis
No formal sample size calculation was done for this pooled analysis. The efficacy population included all RECIST-evaluable patients with locally identified TRK fusion-positive, non-CNS primary tumours, who had received at least one dose of larotrectinib at the time of data cutoff (Feb 19, 2019; n=159). Patients who had not yet had at least one post-baseline response assessment, but were continuing on therapy (n=6), were not included in the overall response assessment, but were included in progression-free and overall survival calculations. Two-sided 95% exact binomial CIs for the proportion of patients with response were calculated using the Clopper-Pearson method. Patients who had surgical resection post-larotrectinib initiation who had no viable tumour cells and negative margins, in addition to having no remaining radiographic evidence of disease (defined as a pathological complete response), were considered to have had a complete response consistent with RECIST, version 1.1. Rates for time-to-event endpoints were summarised descriptively using the Kaplan-Meier method with 95% CIs calculated using Greenwood’s formula. The assessment of response in patients with brain metastases at baseline was a post-hoc exploratory subgroup analysis.
The previously reported analysis,9 which formed the basis of the initial approval of larotrectinib by the US FDA, included the first 55 consecutively enrolled patients across the three studies with TRK fusion-positive tumours that could be assessed according to RECIST, version 1.1, and who had received one or more doses of larotrectinib (primary analysis set). This updated integrated, pooled efficacy analysis included an additional 104 patients fulfilling these criteria (supplemental analysis set). To more fully explore the safety of larotrectinib, the safety population included all patients enrolled in one of the three clinical studies, who received at least one dose of larotrectinib, regardless of TRK fusion status (n=260).
Statistical analyses were done with SAS, version 9.4.
The clinical studies contributing patients to this pooled analysis are registered with ClinicalTrials.gov, NCT02122913 (active, not recruiting), NCT02637687 (recruiting), and NCT02576431 (recruiting).
Role of the funding source
As previously described,9 the phase 1 adult study was designed by the original funder, Loxo Oncology. The phase 1/2 paediatric study14 was designed by TWL, SGD, LM, ASP, and DSH, and the original funder. The phase 2 study involving adolescents and adults was designed by ADr, DMH, and the original funder. The current funders, Bayer, and Loxo Oncology, a wholly owned subsidiary of Eli Lilly and Company, collected the data, and analysed and interpreted these data in collaboration with the authors. The current funders commissioned medical writing services to support the drafting of this Article. ADr, DMH, and DSH had full access to all the data and had final responsibility for the decision to submit for publication.
Results
From May 1, 2014, to Feb 19, 2019, 159 patients with TRK fusion-positive, non-CNS solid tumours were enrolled and treated with larotrectinib (figure 1; appendix pp 2–3; the combined efficacy population included 55 patients from the primary analysis set and 104 patients from the supplemental analysis set). Baseline characteristics of all patients are summarised in table 1 and the appendix (pp 4–5, 16). Fusions involved NTRK1, NTRK2, and NTRK3. 29 distinct upstream fusion partners were identified (appendix p 5).
Figure 1: Trial profile.
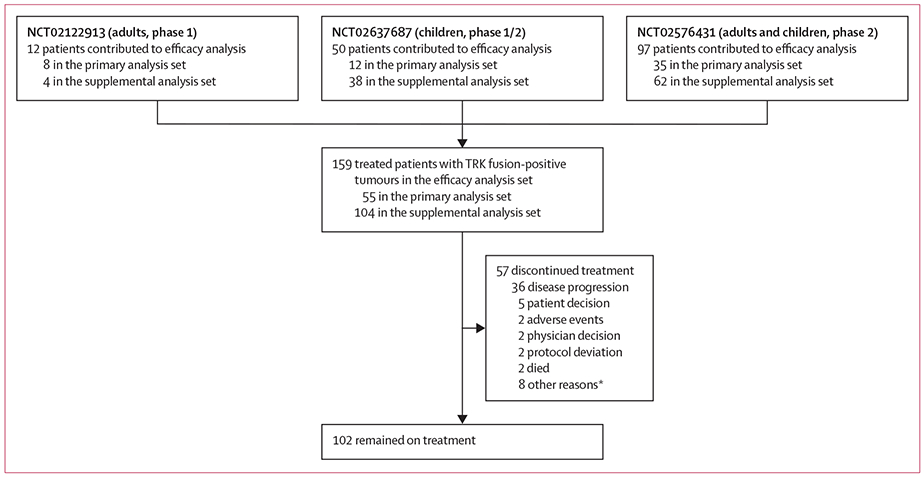
Three trials contributed patients to this analysis. The contributing trials did not have a prescreening component with central molecular testing; data on the number of patients screened for TRK fusions by local testing are consequently not available. The primary analysis set comprises those original patients included in the primary report with longer follow-up. The supplemental analysis set comprises the additional patients included in this Article. *Seven patients stopped treatment after surgery with curative intent.
Table 1:
Patient characteristics at baseline
| All patients (n=159) | |
|---|---|
| Study* | |
| Adult phase 1 | 12 (8%) |
| Paediatric phase 1/2 | 50 (31%) |
| Adolescents and adult phase 2 basket study | 97 (61%) |
| Sex | |
| Male | 77 (48%) |
| Female | 82 (52%) |
| Age | |
| Median, years | 43·0 (6·5–61) |
| Range | <1 month to 84 years |
| Age group distribution, years | |
| <1 | 24 (15%) |
| 1 to <18 | 28 (18%) |
| 18 to <65 | 77 (48%) |
| ≥65 | 30 (19%) |
| ECOG or equivalent Lansky performance status score | |
| 0 | 76 (48%) |
| 1 | 61 (38%) |
| 2 | 19 (12%) |
| 3 | 3 (2%) |
| Number of previous systemic treatment regimens | |
| 0 | 35 (22%) |
| 1 | 48 (30%) |
| 2 | 34 (21%) |
| ≥3 | 42 (26%) |
| Known brain metastasis at enrolment | |
| Yes | 13 (8%) |
| No | 146 (92%) |
| Tumour type | |
| Soft tissue sarcoma | |
| Infantile fibrosarcoma | 29 (18%) |
| Gastrointestinal stromal tumour | 4 (3%) |
| Other | 36 (23%) |
| Thyroid | 26 (16%) |
| Salivary gland | 21 (13%) |
| Lung | 12 (8%) |
| Colon | 8 (5%) |
| Melanoma | 7 (4%) |
| Breast | 5 (3%) |
| Bone sarcoma | 2 (1%) |
| Cholangiocarcinoma | 2 (1%) |
| Pancreas | 2 (1%) |
| Appendix | 1 (<1%) |
| Congenital mesoblastic nephroma | 1 (<1%) |
| Hepatocellular | 1 (<1%) |
| Prostate | 1 (<1%) |
| Unknown primary | 1 (<1%) |
| NTRK gene | |
| NTRK1 | 64 (40%) |
| NTRK2 | 4 (3%) |
| NTRK3† | 88 (55%) |
| Not confirmed‡ | 3 (2%) |
Data are n (%) or median (IQR), unless otherwise stated. ECOG=Eastern Cooperative Oncology Group.
Clinical trials NCT02122913, NCT02637687, and NCT02576431.
Directly shown or inferred (eight of 88 patients) by ETV6 break apart fluorescence in-situ hybridisation in patients with infantile fibrosarcoma.
Molecular profiling test not done in a Clinical Laboratory Improvement Amendments-certified (or other similar accrediting body) environment.
Durations of treatment ranged from 0·03 to 47·2 months, with treatment ongoing. At the time of data analysis, 102 (64%) of 159 patients with TRK fusion-positive cancer remained on treatment (figure 1; appendix pp 1, 17). 23 (14%) of 159 patients received treatment beyond progression because, in the opinion of the site investigator, they continued to derive clinical benefit.
At data cutoff, of the 159 patients with TRK-fusion positive tumours, 153 were evaluable for response, with the remaining six awaiting an initial response assessment while continuing on study (table 2). 121 (79%, 95% CI 72–85) of 153 patients had an objective response. 24 (16%) of 153 patients had a complete response, and 97 (63%) had a partial response. 19 (12%) had stable disease, and nine (6%) had progressive disease. Response was not determined in four (3%) patients because of withdrawal for clinical deterioration before an initial response assessment. Excluding patients with unconfirmed responses, the proportion of evaluable patients with a confirmed response was 108 (77%) of 140. Responses were observed in adult and paediatric patients—74 (73%) of 102 and 47 (92%) of 51 evaluable patients, respectively— with a wide range of tumor types (figure 2; appendix pp 6, 18) and regardless of NTRK fusion gene (appendix p 19). The proportion of patients with responses and duration of response by individual tumour type are shown in table 3. The median time to response was 1·8 months (IQR 1·7–1·9; range 0·9–6·1), consistent with the first protocol-mandated response assessment at 8 weeks (time to response for individual patients, appendix p 17). The proportions of patients with a response in the primary and supplemental analysis sets were similar—44 (80%) of 55 patients and 77 (79%) of 98 patients, respectively—according to investigator assessment (appendix p 7).
Table 2:
Response
| Investigator assessment (n=159) | |
|---|---|
| Evaluable patients | 153* |
| Patients with an objective response | 121 (79%, 72–85) |
| Best response | |
| Complete response† | 24 (16%) |
| Partial response | 97 (63%) |
| Stable disease | 19 (12%) |
| Progressive disease | 9 (6%) |
| Not determined | 4 (3%) |
Data are n; n (%, 95% CI); or n (%). Data cutoff Feb 19, 2019.
Evaluable patients include 13 patients with unconfirmed partial responses pending confirmation (deemed to be responses), but does not include six patients continuing on study and awaiting an initial response assessment. Best response percentages are calculated from the evaluable patient population (n=153).
Including three patients with pathological complete response; two patients had complete responses pending confirmation after an earlier assessment of partial response (ie, classified overall as confirmed responses).
Figure 2: Waterfall plot of the maximum percentage change in tumour size according to tumour type.
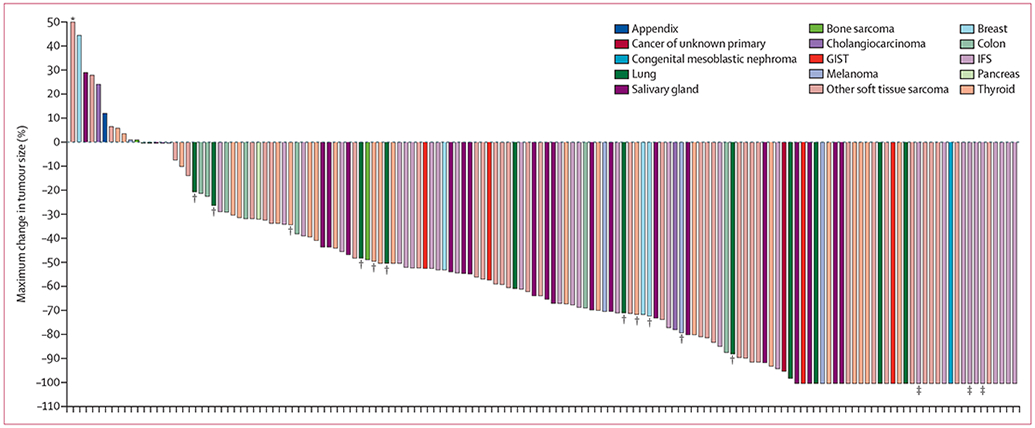
The waterfall plot excludes four patients who had clinical deterioration before an initial response assessment and six patients who were not evaluable due to insufficient time on therapy. GIST=gastrointestinal stromal tumour. IFS=infantile fibrosarcoma. *Maximum change in tumour size of 93% tumour growth. †Patients with brain metastases. ‡Patients with a pathological complete response.
Table 3:
Proportion of patients with response and duration of responses by tumour type according to investigator assessment
| Patients | Patients with response | Median duration of response, months* |
|
|---|---|---|---|
| Overall | 153 | 121 (79%, 72–85) | 35·2 (22·8–NE) |
| Soft tissue sarcoma | |||
| Infantile fibrosarcoma | 28 | 27 (96%, 82–100) | NE (NE–NE) |
| Gastrointestinal stromal tumour | 4 | 4 (100%, 40–100) | 26·3 (7·6–26·3) |
| Other | 36 | 29 (81%, 64–92) | NE (10·1–NE) |
| Thyroid | 24 | 19 (79%, 58–93) | NE (14·8–NE) |
| Salivary gland | 20 | 18 (90%, 68–99) | 35·2 (13·3–NE) |
| Lung | 12 | 9 (75%, 43–95) | NE (NE–NE) |
| Colon | 8 | 4 (50%, 16–84) | 3·7 (3·7–NE) |
| Melanoma | 7 | 3 (43%, 10–82) | NE (3·7–NE) |
| Breast | 4 | 3 (75%, 19–99) | NE (NE–NE) |
| Bone sarcoma | 2 | 1 (50%, 1–99) | 7·7 (NE–NE) |
| Cholangiocarcinoma | 2 | 1 (50%, 1–99) | 7·3 (NE–NE) |
| Pancreas | 2 | 1 (50%, 1–99) | 3·5 (NE–NE) |
| Appendix | 1 | 0 (NC) | |
| Congenital mesoblastic nephroma | 1 | 1 (100%, 3–100) | NE (NE–NE) |
| Hepatocellular | 1 | 0 (NC) | |
| Unknown primary | 1 | 1 (100%, 3–100) | NE (NE–NE) |
Data are n, n (%, 95% CI), or median (95% CI). NC=not calculable. NE=not estimable.
In patients with confirmed responses (n=108).
Although baseline brain imaging in asymptomatic patients was not required as per the protocol, 13 (8%) of 159 patients were known to have brain metastases before enrolment. Tumour types in these patients included lung cancer (n=6), thyroid cancer (n=4; one non-evaluable because of insufficient time on study), melanoma (n=2), and breast cancer (n=1). Including all sites of disease, in a post-hoc exploratory analysis of evaluable patients with brain metastases, the proportion of patients with a response was nine (75%) of 12 patients (appendix p 8). Only three of 12 patients with evaluable intracranial disease had measurable intracranial disease at baseline; in these patients, best intracranial responses included one complete response, one partial response (46% reduction in target tumour size; RECIST, version 1.1), and one stable disease (14% reduction in target tumour size). One patient with non-target intracranial disease at baseline progressed in the brain after 2 months of treatment.
In the overall population, at a median follow-up of 12·9 months (IQR 5·7–23·1), 25 progression events occurred in 108 (23%) patients with confirmed responses, and the median duration of response was 35·2 months (95% CI 22–8-not estimable [NE]; figure 3). At 12 months, an estimated 80% (95% CI 71–89) of responses were ongoing. The longest duration of response (44·2 months and ongoing) was in the first patient with a TRK fusion-positive sarcoma who was treated with larotrectinib.15 With 47 events (30%) in 159 patients, the median progression-free survival was 28·3 months (95% CI 22–1-NE; figure 3) with a median follow-up of 11–1 months (IQR 5·5–22·1). The estimated proportion of patients who were progression-free at 12 months was 67% (95% CI 58–76). At a median follow-up of 13·9 months (IQR 6·5–24·9), 23 (14%) of 159 patients had died (due to disease progression in 17, bleeding from stoma in one, bowel perforation secondary to colon cancer progression in one, and with reason not reported or missing in four), and the median overall survival was 44·4 months (95% CI 36·5–NE; appendix p 20).The estimated proportion of patients surviving at 12 months was 88% (95% CI 83–94).
Figure 3: Kaplan-Meier plots of duration of response and progression-free survival.
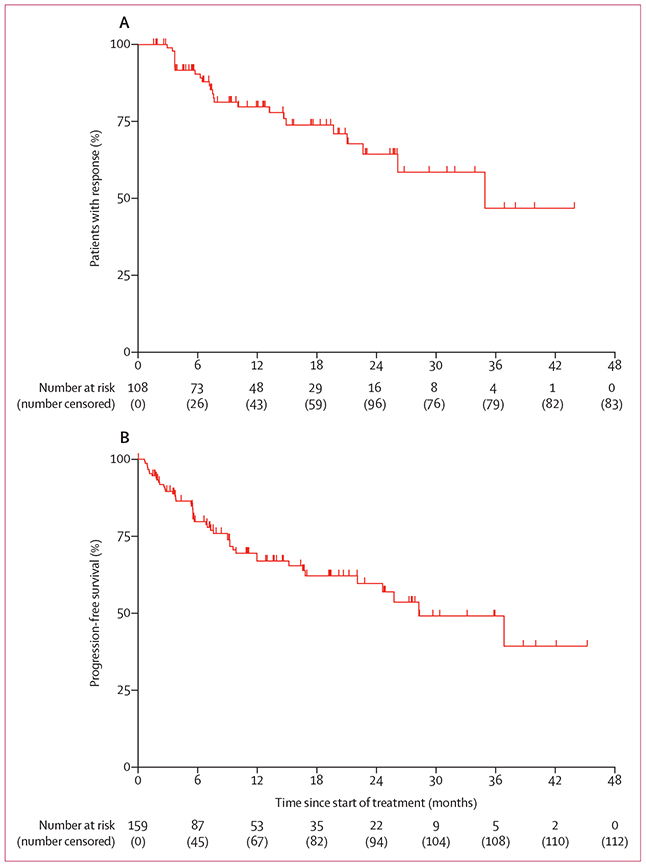
(A) Duration of response in 108 patients with confirmed responses in the combined primary and supplemental analysis sets. Two patients had complete responses pending confirmation following an earlier assessment of partial response (ie, classified overall as confirmed responses). (B) Progression-free survival in all 159 patients in the combined primary and supplemental analysis sets. Tick marks indicate censored patients.
The median duration of follow-up in the 44 patients with confirmed responses in the primary analysis set of 55 patients was 25· 9 months (IQR 21·0–32·1). With progression events in 17 (39%) of the 44 patients, the median duration of response in the primary analysis set was similar to that of the overall efficacy population at 35·2 months (95% CI 21·2-NE, appendix p 20) in an exploratory analysis. The median progression-free survival in the primary analysis set was also similar to the overall efficacy population at 25·8 months (95% CI 9·9–NE, appendix p 21), with a median follow-up of 27·6 months (IQR 22·8–35·8), as was the median overall survival (median 44·4 months, 95% CI 36·5–NE; median follow-up 28·5 months [IQR 24·8–35·0]; appendix p 21) in an exploratory analysis.
In the expanded safety population of 260 patients, no new larotrectinib safety signals were identified (appendix pp 9–11). This was also the case in the subset of paediatric patients with TRK fusion-positive tumours (appendix p 12). Adverse events were primarily grade 1 and 2 (table 4) and the pattern and frequency were similar across adult and paediactric age groups (appendix pp 9–12). In total, 101 (39%) and 17 (7%) of 260 patients had grade 3 or 4 treatment-emergent adverse events, respectively. The most common grade 3 or worse treatment-emergent adverse events (regardless of attribution) were anaemia (25 [10%] of 260 patients) and decreased neutrophil count (14 [5%]; table 4). The most common treatment-emergent serious adverse events were pneumonia (six [2%] of 260 patients), pyrexia (six [2%]), abdominal pain (five [2%]), and diarrhoea (five [2%]; appendix p 13–14).
Table 4:
Adverse events
| Adverse events, regardless of attribution |
Treatment-related adverse events |
||||
|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 4 | Grade 3 | Grade 4 | |
| Fatigue | 79 (30%) | 6 (2%) | 0 | 1 (<1%) | 0 |
| Alanine aminotransferase increased | 64 (25%) | 7 (3%) | 2 (<1%) | 7 (3%) | 1 (<1%) |
| Cough | 71 (27%) | 1 (<1%) | 0 | 0 | 0 |
| Constipation | 69 (27%) | 1 (<1%) | 0 | 0 | 0 |
| Anaemia | 44 (17%) | 25 (10%) | 0 | 6 (2%) | 0 |
| Aspartate aminotransferase increased | 62 (24%) | 6 (2%) | 1 (<1%) | 2 (<1%) | 0 |
| Dizziness | 64 (25%) | 2 (<1%) | 0 | 1 (<1%) | 0 |
| Nausea | 62 (24%) | 2 (<1%) | 0 | 2 (<1%) | 0 |
| Vomiting | 62 (24%) | 2 (<1%) | 0 | 0 | 0 |
| Diarrhoea | 59 (23%) | 3 (1%) | 0 | 0 | 0 |
| Pyrexia | 50 (19%) | 2 (<1%) | 1 (<1%) | 0 | 0 |
| Dyspnoea | 35 (13%) | 6 (2%) | 0 | 0 | 0 |
| Myalgia | 38 (15%) | 3 (1%) | 0 | 2 (<1%) | 0 |
| Peripheral oedema | 40 (15%) | 1 (<1%) | 0 | 0 | 0 |
| Headache | 38 (15%) | 1 (<1%) | 0 | 1 (<1%) | 0 |
| Neutrophil count decreased | 18 (7%) | 12 (5%) | 2 (<1%) | 4 (2%) | 1 (<1%) |
| Lymphocyte count decreased | 22 (8%) | 7 (3%) | 2 (<1%) | 2 (<1%) | 0 |
| Hypokalaemia | 12 (5%) | 8 (3%) | 1 (<1%) | 0 | 0 |
| Hypophosphataemia | 5 (2%) | 9 (3%) | 0 | 0 | 0 |
Data are n (%). n=260. The adverse events listed here are those that occurred at any grade in at least 15% of patients, or at grade 3 or worse in at least 3% of patients, regardless of attribution. Treatment-emergent adverse events occurring regardless of attribution in 10% or more of patients at grade 1 or 2 in severity, and all grade 3–5 events are presented in the appendix (pp 9–11).
Grade 3 or 4 larotrectinib-related adverse events were infrequent, being reported in 33 (13%) and two (<1%) of 260 patients, respectively (table 4). The most common were increased alanine aminotransferase (eight [3%] of 260 patients), anaemia (six [2%]), and decreased neutrophil count (five [2%]). 13 (5%) of 260 patients had serious adverse events related to larotrectinib (appendix p 15). The most common were increased alanine aminotransferase (two [<1%] of 260 patients), increased aspartate aminotransferase (two [<1%]), and nausea (two [<1%]). Treatment-emergent adverse events associated with death occurred in 14 (5%) of 260 patients overall (appendix pp 9–11) and in six (4%) of 159 of patients in the efficacy population with TRK fusion-positive cancer. These were predominantly secondary to disease progression and none were deemed to be related to larotrectinib.
Doses were reduced because of adverse events in 22 (8%) of 260 patients overall and in 13 (8%) of 159 patients with TRK fusion-positive cancer. Dose discontinuation because of treatment-related adverse events occurred in six (2%) of 260 patients (alanine aminotransferase increased, amylase increased, aspartate aminotransferase increased, electrocardiogram QT prolonged, enterocutaneous fistula, lipase increased, muscular weakness, and nausea, recognising that more than one cause could be listed per patient); two of the six patients who discontinued had TRK fusion-positive cancer.
Discussion
In an expanded population of 159 adult and paediatric patients with TRK fusion-positive solid tumours, we confirmed the tumour-agnostic activity of larotrectinib, as shown by the high proportion of patients with an objective response, consistent with our previous analysis.9 This population is nearly three times the size of that previously reported and provides additional efficacy data in several common tumour types that were underrepresented in the initial dataset.
With longer follow-up than our previous report, this analysis has more fully characterised the durability of disease control with larotrectinib. In the overall population, the median duration of response was 35·2 months and the median progression-free survival was 28·3 months. Despite the majority of patients having received previous therapy and the underlying heterogeneity of tumour types treated, this durability of disease control compares favourably with that achieved by many other tyrosine kinase inhibitors in oncogene-addicted cancers,16-18 and is even broadly similar to outcomes achieved with third-generation ALK inhibitors in a more homogenous population of treatment-naive patients with ALK fusion-positive non-small-cell lung cancer.19
Although the majority of tumour types that harbour TRK fusions do not typically have tropism to CNS metastasis, a minority do, including lung cancer, thyroid cancer, and melanoma. For patients with such tumours, we present data suggesting that larotrectinib achieved similar overall treatment outcomes in patients with preexisting CNS metastases as in those without. These data, combined with the reported intracranial responses,20,21 suggest that larotrectinib is active within the CNS. The accrual of patients with primary CNS tumours is ongoing and outcome data will be published separately.
The expanded safety analysis in 260 patients treated with larotrectinib confirms the favourable tolerability profile of this agent, including in individual patients with longer treatment durations compared with our previous report. The proportion of patients with TRK fusion-positive cancer who had a dose reduction because of adverse events was 8%, which is lower than the originally reported 15% in the primary analysis set. Moreover, treatment discontinuation because of a drug-related adverse event occurred in only 2% of the overall population. No new safety signals were identified and treatment-emergent adverse events potentially attributable to TRK inhibition (eg, weight gain and dizziness) were not common and mostly of low grade (grade 1 or 2). Characterising a safety profile with more protracted larotrectinib use and identifying any unique delayed consequences, or lack thereof, in paediatric patients are areas of ongoing monitoring.22
In terms of resistance to larotrectinib, we previously reported possible contributory factors in patients with primary progressive disease.9 We also identified mechanisms of acquired resistance in patients with larotrectinib-treated TRK fusion-positive tumours, including the emergence of NTRK kinase domain mutations or bypass tract activation.9,23 Early clinical data suggest that particular on-target resistance mechanisms might be overcome by next-generation TRK inhibitors, such as selitrectinib and repotrectinib.11,12
A limitation of the current study is that patients were derived from single-group trials, with no comparators. However, the activity of larotrectinib in patients with TRK fusion-positive cancer with no other effective treatment options would mean that randomised controlled trials in disease-specific settings would probably be unethical. An additional limitation is that for many tumour types, relatively small numbers of patients have been treated with larotrectinib. The ongoing NCT02576431 and NCT02637687 trials will further increase the number of adult and paediatric patients studied in disease-specific settings.
In conclusion, this expanded analysis confirms that TRK fusions define a unique molecular subgroup of advanced solid tumours in children and adults for which larotrectinib is highly active and amenable to long-term administration. Testing for TRK fusions in the clinic remains crucial to identify patients likely to benefit from treatment with this agent.8,24,25
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed and major congress abstracts (including those from meetings of the American Society of Clinical Oncology and the American Association for Cancer Research) using the terms “TRK or NTRK”, “fusion or rearrangement”, “cancer” and “treatment”. We did not restrict our search by publication date or language. The results of this search showed that TRK inhibition with the first-generation TRK inhibitors larotrectinib or entrectinib was active in TRK fusion-positive cancers of various histologies. Both drugs had been approved by multiple regulatory agencies for the treatment of TRK fusion-positive cancers in both adult and paediatric populations.
The next-generation TRK inhibitors selitrectinib or repotrectinib showed preliminary activity in small series that included patients with resistance to first-generation TRK inhibitors.
Added value of this study
In the initial analysis of the first 55 consecutively enrolled adult and paediatric patients with TRK fusion cancer who were treated with larotrectinib, the durability of benefit and long-term safety had not been fully characterised.
This expanded efficacy analysis includes data on the durability of disease control in a combined efficacy population almost three times larger than previously reported. Additionally, our expanded safety analysis in 260 patients confirms the favourable tolerability profile of larotrectinib, including in patients with longer treatment durations.
Implications of all the available evidence
TRK fusions define a unique molecular subgroup of advanced solid tumours for which the activity of larotrectinib is highly durable. Long-term safety is favourable. Our findings underscore the need to test for TRK fusions in the clinic with assays that are best poised to identify these alterations.
Acknowledgments
This study was funded by Bayer and Loxo Oncology, a wholly owned subsidiary of Eli Lilly and Company. We thank the patients and their families and the study teams at the participating centres. We also thank the study teams from Bayer and Loxo Oncology, including Ariadna Holynskyj, Nicoletta Brega, Patricia Maeda, Steve Almond, John Reeves, Florian Hiemeyer, Michael Cox, and Nora Ku for their support. Medical writing support was provided by Jim Heighway of Cancer Communications and Consultancy (Knutsford, UK) with funding from Bayer and Loxo Oncology.
Footnotes
Declaration of interests
DSH reports grant funding, personal fees, and non-financial support from Bayer, Genmab, Loxo Oncology, and MiRNA; grant funding and personal fees from AbbVie, Adaptimmune, Amgen, Eisai, Genentech, Infinity, Kyowa, Eli Lilly, Merrimack, Pfizer, Seattle Genetics, and Takeda; grant funding from AstraZeneca, Bristol-Myers Squibb, Daiichi-Sankyo, Fate Therapeutics, Ignyta, Kite, Medimmune, Merck, Mirati, Molecular Templates, Mologen, NCI-CTEP, Novartis, Aldi-Norte, CPRIT, and Turning Point Therapeutics; personal fees from Axiom, Baxter, GLG, Group H, Guidepoint Global, Janssen, Medscape, Numab, Trieza Therapeutics, Alpha Insights, Acuta Capital, Prime Oncology, and WebMD; non-financial support from ASCO, AACR, and SITC; and other support in relation to roles as a founder and advisor for OncoResponse, and an advisor for Molecular Match and Presagia. SGD reports personal fees and travel expenses for advisory board attendance from Loxo Oncology, and travel expenses for advisory board attendance from Roche. SK reports personal fees from Loxo Oncology in relation to advisory board participation. AFF reports grant funding and personal fees from Bayer, Loxo Oncology, AbbVie, PharmaMar, Genentech, AstraZeneca, Roche, Bristol-Myers Squibb, and Merck; grant funding from Amgen and Ignyta; and personal fees from Boehringer Ingelheim. KSR reports grant funding and non-financial support from Loxo Oncology; grants, non-financial support, and travel and accommodation expenses from Roche; grant funding and non-financial support from Bayer, Orion Pharma, Pfizer, PUMA, Cantargia, Genmab, Novartis, Incyte, Symphogen, AstraZeneca, Alligator, Merck, Bristol-Myers Squibb, and Eli Lilly; and travel and accommodation expenses from Sanofi. CMvT reports personal fees from Bayer and Novartis in relation to advisory boards. JDB reports personal fees from Bayer and research support from Bayer and Loxo Oncology; personal fees and research support from Taiho, FivePrime, and LSK Pharmaceuticals; personal fees from Celgene, Arno, AstraZeneca, Eisai, Seattle Genetics, Clovis, and Ipsen; research support from Novartis (Array), AbbVie, Immunomedics, Genentech/Roche, Eli Lilly, Incyte, Pharmacyclics, EMD Serono, Boston Biomedical, PsiOxus, Macrogenics, Symphogen, and Pfizer; participation in data and safety monitoring boards for AstraZeneca, Pancreatic Cancer Action Network, and Novocure; and a relationship with NCI (IDSC work). NF reports consulting and speaker’s bureau honoraria from Bayer. LM reports personal fees from Bayer in relation to speaker’s bureaus, institutional support for a paediatric phase 1 trial from Loxo Oncology, and travel and accommodation expenses from Thermo Fisher Scientific in relation to clinical gene sequencing; consulting fees relating to clinical trial development from Lilly Oncology and drug supply and financial support for an investigator-initiated trial from AstraZeneca, in both cases paid to his institution. ADo reports grant funding to his institution in relation to clinical trial support from Loxo Oncology, EMD Serono, Tesaro, Roche, Vertex, Regeneron, Eli Lilly, Ipsen, United Therapeutics, Mirati, Bristol-Myers Squibb, and Incuron; grant funding to his institution in relation to clinical trial support and personal fees for consultancy from AbbVie, AstraZeneca, Millenium, Takeda, and Seattle Genetics; and personal fees for consultancy from Ariad. ASP reports personal fees from Loxo Oncology, Bayer, Array Pharma, Eusa, and Roche. SB reports non-financial support from Loxo Oncology and Bayer; and personal fees in relation to advisory boards or consultancy from Bayer, Clinigen, Ipsen, Isofol, Eli Lilly, Novartis, Roche, and Sensorion. FD reports investigator fees from Loxo Oncology and Bayer; investigator fees and other support in relation to boards and travel from Bristol-Myers Squibb; investigator fees from Boehringer Ingelheim and Novartis; investigator fees and other support in relation to boards from Roche; other support in relation to boards from Tesaro; and other support in relation to consulting from Servier. RMcD reports clinical trial-related grant funding from Loxo Oncology. JDP reports personal fees from AbbVie, AstraZeneca, and Takeda in relation to advisory roles.
RJS reports financial support covering clinical trial costs from Loxo Oncology; and personal fees in relation to consultancies from Incyte, Immunogen, and Flatiron; and chairmanship of an independent data and safety monitoring board from Celsion. MT reports grant funding and personal fees from Bayer, Loxo Oncology, Ono Pharmaceutical, AstraZeneca, Pfizer, Rakuten Medical, and Bristol-Myers Squibb; grant funding from Novartis; and personal fees from Merck Serono, Eisai, Celgene, and Amgen. ML reports grant funding from Loxo Oncology to support expanded clinical testing for NTRK fusions; personal fees from Bayer, AstraZeneca, Bristol-Myers Squibb, and Takeda for ad hoc advisory boards; personal fees from Merck for a global advisory board; and grant funding from Helsinn Therapeutics for preclinical drug studies. ERR reports other support to her institution as compensation for time spent participating on advisory boards for Loxo Oncology and Bayer. SN and BHC are salaried employees of Bayer HealthCare Pharmaceuticals. TWL reports grant funding, personal fees, and non-financial support from Loxo Oncology; and grant funding, and non-financial support from Bayer; grant funding, personal fees, and non-financial support from Novartis; personal fees and non-financial support from Eli Lilly; and grant funding from Pfizer. DMH reports research grant funding from Loxo Oncology and Puma Biotechnology; research grant funding and personal fees for consulting from Bayer and AstraZeneca; personal fees for consulting from Chugai, Boehringer Ingelheim, Pfizer and Genentech/Roche; and personal fees for scientific advisory board participation from Fount Therapeutics. ADr reports honoraria for advisory boards from Loxo Oncology and Bayer; honoraria for advisory boards from Ignyta/Genentech/Roche, Lilly, Takeda/Ariad/Millenium, TP Therapeutics, AstraZeneca, Pfizer, Blueprint, Helsinn, Beigene, BergenBio, Hengrui, Exelixis, Tyra, Verastem, MORE Health, and AbbVie; and other relationships including associated research paid to his institution from Pfizer, Exelixis, GlaxoSmithKlein, Teva, Taiho, PharmaMar, research (Foundation Medicine), royalties (Wolters Kluwer), other (Merck/Puma), and continuing medical education honoraria from Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, Research to Practice, and Oncology. CMA, RN, BG, SMF, and DW declare no competing interests.
Data sharing
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing”. This pertains to scope, timepoint, and process of data access. As such, Bayer commits to sharing on request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the USA and EU as necessary for doing legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after Jan 1, 2014. Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymised patient-level data and supporting documents from clinical studies to do further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the study sponsors section of the portal. Data will be available no later than within 1 year of study completion. Data access will be granted to anonymised patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
Contributor Information
David S Hong, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Steven G DuBois, Dana-Farber/Boston Children’s Cancer and Blood Disorders Center and Harvard Medical School, Boston, MA, USA.
Shivaani Kummar, Stanford University School of Medicine, Stanford University, Palo Alto, CA, USA.
Anna F Farago, Massachusetts General Hospital, Boston, MA, USA.
Catherine M Albert, Seattle Children’s Hospital, University of Washington, Seattle, WA, USA.
Kristoffer S Rohrberg, Rigshospitalet, Copenhagen University, Copenhagen, Denmark.
Cornells M van Tilburg, Hopp Children’s Cancer Center Heidelberg, Heidelberg University Hospital, Heidelberg, Germany; German Cancer Research Center, Heidelberg, Germany.
Ramamoorthy Nagasubramanian, Nemours Children’s Hospital, Orlando, FL, USA.
Jordan D Berlin, Vanderbilt University Medical Center, Nashville, TN, USA.
Noah Federman, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, USA.
Leo Mascarenhas, Saban Research Institute, Children’s Hospital Los Angeles, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Birgit Geoerger, Gustave Roussy Cancer Center, Department of Pediatric and Adolescent Oncology, Université Paris-Saclay, Villejuif, France.
Afshin Dowlati, University Hospitals Cleveland Medical Center, Cleveland, OH, USA.
Alberto S Pappo, St Jude Children’s Research Hospital, Memphis, TN, USA.
Stefan Bielack, Klinikum Stuttgart—Olgahospital, Stuttgart Cancer Center, Pediatrics 5 (Oncology, Hematology, Immunology), Stuttgart, Germany.
Francois Doz, SIREDO Center Care, Innovation, Research In Pediatric, Adolescent and Young Adult Oncology, Institut Curie and Paris Descartes University, Paris, France.
Ray McDermott, St Vincent’s University Hospital and Cancer Trials Ireland, Dublin, Ireland.
Jyoti D Patel, University of Chicago, Chicago, IL, USA.
Russell J Schilder, Sidney Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA, USA.
Makoto Tahara, National Cancer Center Hospital East, Kashiwa, Japan.
Stefan M Pfister, Hopp Children’s Cancer Center Heidelberg, Heidelberg University Hospital, Heidelberg, Germany; German Cancer Research Center, Heidelberg, Germany; German Cancer Network, Heidelberg, Germany.
Olaf Witt, Hopp Children’s Cancer Center Heidelberg, Heidelberg University Hospital, Heidelberg, Germany; German Cancer Research Center, Heidelberg, Germany.
Marc Ladanyi, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Erin R Rudzinski, Seattle Children’s Hospital, Seattle, WA, USA.
Shivani Nanda, Bayer HealthCare Pharmaceuticals, Whippany, NJ, USA.
Barrett H Childs, Bayer HealthCare Pharmaceuticals, Whippany, NJ, USA.
Theodore W Laetsch, University of Texas Southwestern Medical Center/Children’s Health, Dallas, TX, USA.
David M Hyman, Memorial Sloan Kettering Cancer Center, New York, NY, USA; Weill Cornell Medical College, New York, NY, USA.
Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY, USA; Weill Cornell Medical College, New York, NY, USA.
References
- 1.Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol 2018; 15: 731–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kummar S, Lassen U. TRK inhibition—a new tumor-agnostic treatment strategy. Target Oncol 2018; 13: 545–56. [DOI] [PubMed] [Google Scholar]
- 3.Skalova A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol 2010; 34: 599–608. [DOI] [PubMed] [Google Scholar]
- 4.Tognon C, Knezevich SR, Huntsman D, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell 2002; 2: 367–76. [DOI] [PubMed] [Google Scholar]
- 5.Bourgeois JM, Knezevich SR, Mathers JA, Sorensen PH. Molecular detection of the ETV6-NTRK3 gene fusion differentiates congenital fibrosarcoma from other childhood spindle cell tumors. Am J Surg Pathol 2000; 24: 937–46. [DOI] [PubMed] [Google Scholar]
- 6.El Demellawy D, Cundiff CA, Nasr A, et al. Congenital mesoblastic nephroma: a study of 19 cases using immunohistochemistry and ETV6-NTRK3 fusion gene rearrangement. Pathology 2016; 48: 47–50. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Yang L, Kucherlapati M, et al. A pan-cancer compendium of genes deregulated by somatic genomic rearrangement across more than 1,400 cases. Cell Rep 2018; 24: 515–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchiò C, Scaltriti M, Ladanyi M, et al. ESMO recommendations on the standard methods to detect NTRK fusions in daily practice and clinical research. Ann Oncol 2019; 30: 1417–27. [DOI] [PubMed] [Google Scholar]
- 9.Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med 2018; 378: 731–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drilon A, Siena S, Ou SI, et al. Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK Inhibitor entrectinib: combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov 2017; 7: 400–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drilon A, Nagasubramanian R, Blake JF, et al. A next-generation TRK kinase inhibitor overcomes acquired resistance to prior TRK kinase inhibition in patients with TRK fusion-positive solid tumors. Cancer Discov 2017; 7: 963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drilon A, Ou SI, Cho BC, et al. Repotrectinib (TPX-0005) Is a next-generation ROS1/TRK/ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent-front mutations. Cancer Discov 2018; 8: 1227–36. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47 [DOI] [PubMed] [Google Scholar]
- 14.Laetsch TW, DuBois SG, Mascarenhas L, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol 2018; 19: 705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doebele RC, Davis LE, Vaishnavi A, et al. An oncogenic NTRK fusion in a patient with soft-tissue sarcoma with response to the tropomyosin-related kinase inhibitor LOXO-101. Cancer Discov 2015; 5: 1049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018; 378: 113–25. [DOI] [PubMed] [Google Scholar]
- 17.Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014; 371: 1963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002; 347: 472–80. [DOI] [PubMed] [Google Scholar]
- 19.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 2017; 377: 829–38. [DOI] [PubMed] [Google Scholar]
- 20.Rosen E, Schram A, Young R, et al. Larotrectinib demonstrates CNS efficacy in TRK fusion-positive solid tumors. JCO Precis Oncol 2019; published online May 16 DOI: 10.1200/PO.19.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drilon A, DuBois S, Farago A, et al. Activity of larotrectinib in TRK fusion cancer patients with brain metastases or primary central nervous system tumors. J Clin Oncol 2019. 37: 15; 2006. [Google Scholar]
- 22.Geoerger B, vanTilburg C, DuBois S, et al. Larotrectinib efficacy and safety in pediatric patients with TRK fusion cancer. Pediatr Blood Cancer 2019; 66: S4; FP114 SIOP19-0869. [Google Scholar]
- 23.Cocco E, Schram AM, Kulick A, et al. Resistance to TRK inhibition mediated by convergent MAPK pathway activation. Nat Med 2019; 25: 1422–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penault-Llorca F, Rudzinski ER, Sepulveda AR. Testing algorithm for identification of patients with TRK fusion cancer. J Clin Pathol 2019; 72: 460–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cocco E, Benhamida J, Middha S, et al. Colorectal carcinomas containing hypermethylated MLH1 promoter and wild-type BRAF/KRAS are enriched for targetable kinase fusions. Cancer Res 2019; 79: 1047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


