Abstract
Molecular differences in tumor locations may contribute to the sidedness-specific response to cetuximab in metastatic colorectal cancer (mCRC). We investigated genes associated with the response to cetuximab treatment depending on tumor sidedness. Our study included 77 patients with mCRC (13/63, right/left) with KRAS exon 2 wild-type tumors from phase II trials of first-line therapy with cetuximab. Expression levels of 2,551 genes were measured in tissue samples by HTG EdgeSeq Oncology Biomarker Panel. Univariate Cox regression analysis using log2 values of counts per million (CPM) was conducted in each sidedness to assess associations with clinical outcomes, and to define the optimal cut-off point for clinically significant genes. In addition, a gene set enrichment analysis (GSEA) was performed to identify significant gene pathways in each sidedness. Sixty-nine patients were assessable for gene expression data. Overexpression of BECN1 [log2(CPM) ≥ 6.8] was associated with favorable survival, regardless of tumor sidedness. High expression of NOTCH1 [log2(CPM) ≥ 7.5] predicted significantly longer progression-free survival (PFS; median 14.7 vs. 11.1 months, HR 0.43, P = 0.01) and overall survival (OS; median 42.8 vs. 26.5 months, HR 0.35, P = 0.01) in left side but not in right side. The GSEA showed that regulation of DNA replication gene set correlated with favorable survival in the left, whereas the subcellular component and leukocyte migration gene sets were associated with good survival in the right. In conclusion, genes contributing to the efficacy of cetuximab treatment may differ according to the sidedness in mCRC. NOTCH1 may potentially discriminate favorable responders tocetuximab inpatients with left-sided tumors.
Introduction
The location of the primary tumor has an impact on clinical behavior and has prognostic value in metastatic colorectal cancer (mCRC). Patients with mCRC who harbor right-sided tumors have been shown to have poorer outcomes than those who harbor left-sided tumors. This phenomenon may derive, in part, from higher frequency of BRAF mutations, increased microsatellite instability, and CpG island methylator phenotype, or higher incidences of mucinous differentiation and serrated pathway signature, which are more common in mCRC with right-sided primary tumors (1, 2). In contrast, amplification of EGFR and ERBB2, chromosomal instability, and TP53 gene mutations are more frequent in left-sided tumors (3). According to a sub-analysis of the CALGB80405 trial, primary tumor sidedness has been identified to be an independent prognostic marker in mCRC (4).
The molecular differences associated with sidedness in mCRC contribute, in part, to differences in the response to systemic treatment. Retrospective analyses of two first-line studies comparing chemotherapy plus cetuximab with chemotherapy plus bevacizumab reported better overall survival (OS) in the chemotherapy plus cetuximab group in patients with left-sided tumors. In contrast, patients with right-sided tumors appeared to receive more benefit from chemotherapy plus bevacizumab (5). Moreover, a recent meta-analysis suggested that tumor sidedness is a predictive marker of the response to anti-EGFR therapy in patients with RAS wild-type mCRC. Patients with left-sided tumors were shown to derive a greater benefit from chemotherapy plus anti-EGFR than from chemotherapy plus bevacizumab, whereas right-sided tumors were associated with trends toward detrimental effects of anti-EGFR therapy (6). On the basis of these results, the National Comprehensive Cancer Network (NCCN) guideline has recommended to consider the primary tumor site when deciding the first-line treatment for mCRC (7). Actually, anti-EGFR therapy with cetuximab or panitumumab is recommended for only RAS wild-type and left-sided tumors. In addition, the pan-Asian–adapted European Society for Medical Oncology (ESMO) consensus guidelines have proposed that tumor sidedness matters when we treat patients with RAS/BRAF wild-type tumors (8).
Although increasing evidence suggests that tumor sidedness is a predictor of the response to anti-EGFR antibodies, this does not mean that all patients with right-sided tumors should clinically avoid receiving anti-EGFR antibodies as an initial treatment. Data from prospective clinical trials have demonstrated that a few patients with right-sided tumors had a good depth of response and that even though a durable response was achieved in a larger proportion of patients with left-sided tumors than in patients with right-sided tumors, a few patients with right-sided tumors also had rapid and deep responses (9). Some patients with right-sided tumors may be responders who benefit from anti-EGFR antibodies and should receive anti-EGFR–based chemotherapy as an initial treatment. Therefore, there may be biomarkers to elucidate the subset of patients with mCRC who are likely to benefit from anti-EGFR treatment in each side.
We, therefore, performed a biomarker study to establish responders to anti-EGFR treatment using tissue samples obtained from patients enrolled in prospective clinical trials. The aim of this study was to investigate which genes are associated with the response to cetuximab treatment depending on tumor sidedness in patients with mCRC who received first-line cetuximab treatment.
Materials and Methods
Study design and patient population
We retrospectively collected tissue samples from two prospective clinical trials, which evaluated combination therapy with cetuximab and oxaliplatin-based chemotherapy as first-line treatment, the modified FOLFOX6 regimen (JACCRO CC-05: N = 57, UMIN000004197; ref. 10) and the SOX regimen (JACCRO CC-06: N = 67, UMIN000007022; ref. 11) for patients with mCRC with KRAS wild-type tumors (Supplementary Fig. S1). This biomarker study was conducted in accordance with the Declaration of Helsinki and was approved by the ethical committee of each participating institute. Written informed consent was obtained from the patients before enrollment. If the investigators could not obtain informed consent, the patient was eligible for enrollment under permission by the Institutional Review Board of each institute. Tissue samples at the time of biopsy or surgery before chemotherapy were collected.
Assessment of efficacy
The endpoints of this biomarker study were progression-free survival (PFS) and OS. The JACCRO trials included the same secondary endpoints of OS and PFS based on disease progression detected by external review or death from any cause. Disease progression was evaluated according to RECIST, version 1.1 by the investigators and was then validated by an external review board.
RNA isolation and gene expression analysis
Formalin-fixed, paraffin-embedded (FFPE) tumor specimens from primary tumor site were cut into sections with a thickness of 3 or 10 µm. A pathologist stained one 3-µm slide with hematoxylin and eosin and then evaluated for tumor content and marked for areas with dominant tumor foci for the preparation of macrodissection. Macrodissection was performed by scraping the marked areas with a blade to ensure that as many tumor cells as possible were dissected. Total RNA was extracted from FFPE tissue of the tumor samples using an miRNeasy FFPE Kit (QIAGEN KK) according to the manufacturer’s protocol.
Gene expression levels were measured by the HTG EdgeSeq Oncology Biomarker Panel, with probes targeting 2,551 genes implicated in numbers of pathways, using next-generation sequencing for quantitative analysis of targeted genes (https://www.htgmolecular.com/assays/obp).
This study was conducted in accordance with the Reporting Recommendations for Tumor Marker prognostic studies (REMARK; ref. 12). Tissue analyses were performed blindly to the clinical dataset at HTG Molecular, Inc. after approval in the Institutional Review Board of each institution that participated in the JACCRO CC-05/06AR trials (UMIN000010635).
Statistical analysis
The R statistical software (version 3.3.2, https://www.r-project.org/) was used for the survival analyses of the gene expression profiles. Univariate Cox regression analysis using log2 values of counts per million (CPM) was conducted for all genes that passed quality control filtering in each side to assess the association with clinical outcomes. P < 0.05 was considered as statistically significant. Further univariate Cox regression analysis was performed to define an optimal cut-off point for significant genes. In addition, a gene set enrichment analysis (GSEA, http://software.broadinstitute.org/gsea) was performed to identify classes of genes associated with outcomes in each side by the GSEA preranked analysis based on the HR of each gene calculated during the univariate Cox regression analysis. The biological process entries in Gene Ontology terms (cate-gory c5.bp) were used for the target gene sets for GSEA. The gene sets satisfying both P < 0.05 and FDR < 0.25 were considered statistically significant.
SAS 9.0.3 software (SAS Institute) was used to perform all analyses unless specified otherwise. All tests were two-sided with a significance level of 0.05.
Results
Patient characteristics
A total of 77 patients were studied. Sixty-three (82%) of the patients had left-sided tumors, and 13 (17%) had right-sided tumors. The patient characteristics are summarized in Supplementary Table S1. There were no statistically significant differences in characteristics between patients with left-sided tumors and those with right-sided tumors. In the enrolled patients, the objective response rate (ORR) was 73%. The median PFS and OS were 10 months [95% confidence interval (CI), 8.8–11.8 months] and 33.9 months (95% CI, 26.5–not reached), respectively. Univariate Cox regression analysis included 69 patient samples that passed the internal quality control metrics of HTG EdgeSeq Oncology Biomarker Panel.
Identified significant genes in both sides
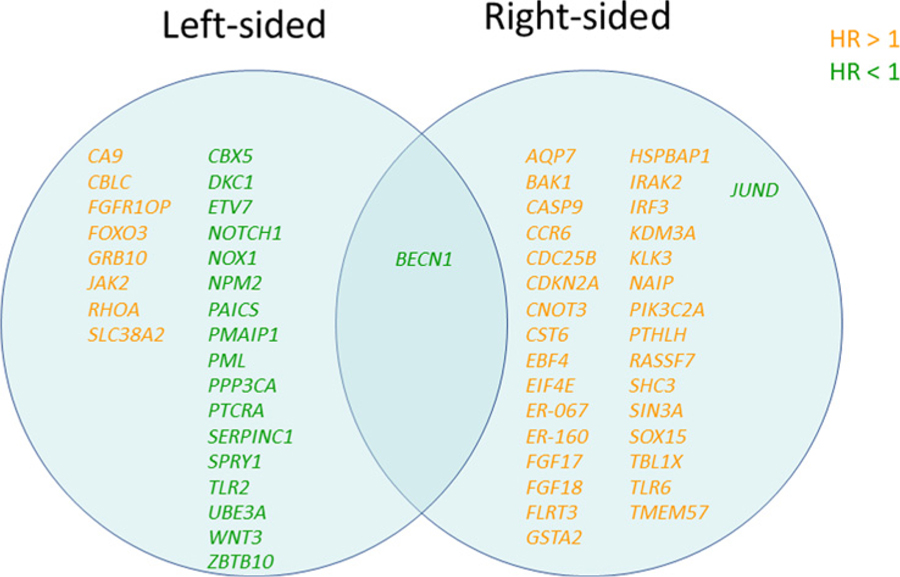
Overexpression of BECN1 [log2(CPM) ≥ 6.8] was associated with favorable OS for both left-sided tumors and right-sided tumors. On the other hand, there was no gene that correlated with bad OS in both left-sided tumors and right-sided tumors (Fig. 1). In the analysis of PFS, no gene was significantly associated with PFS in both left-sided tumors and right-sided tumors (Fig. 2).
Figure 1.
Identified significant genes associated with OS by tumor sidedness.
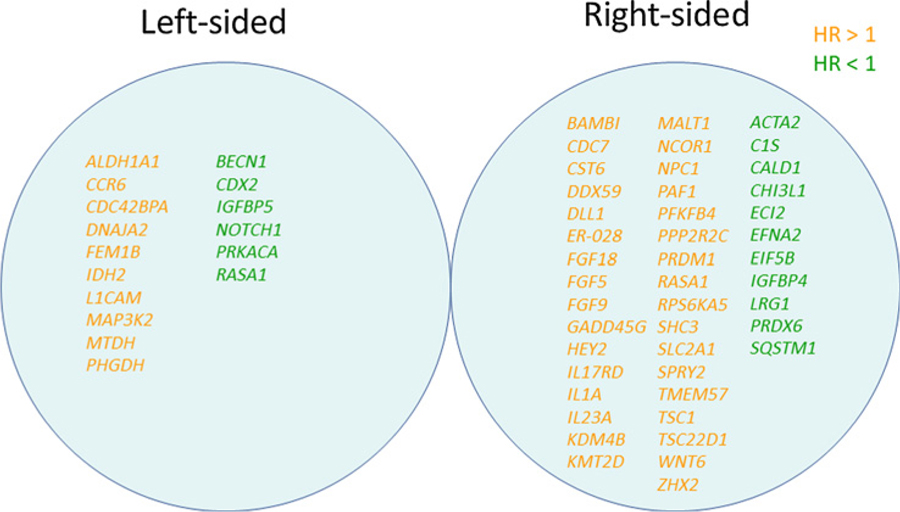
Figure 2.
Identified significant genes associated with PFS by tumor sidedness.
Promising significant genes to predict prognosis in each side
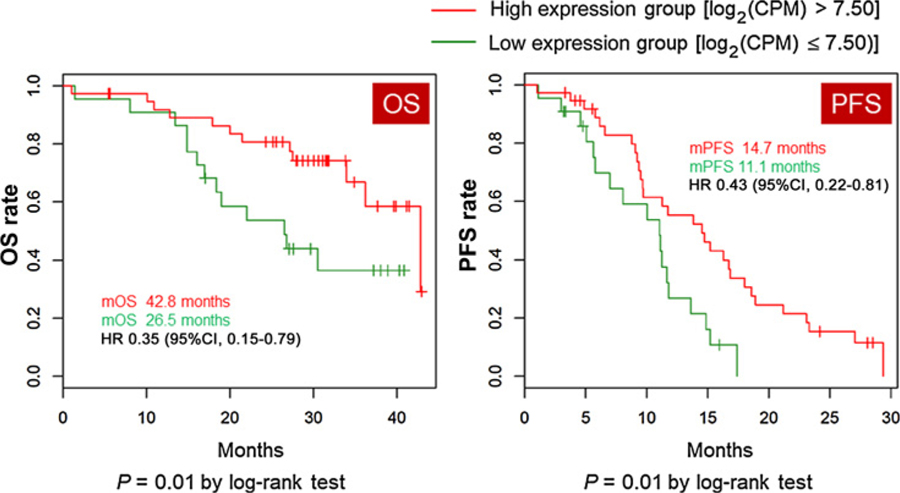
In the left-sided tumor group (n = 60), the Cox regression analysis identified 16 genes associated with clinical outcomes for PFS and 26 genes associated with clinical outcomes for OS (P < 0.01). Six of the 16 genes associated with PFS and 18 of the 26 genes associated with OS were associated with favorable survival. BECN1 and NOTCH1 genes were identified to be positively associated with both better PFS and better OS. When the cut-off point was defined as log2(CPM) = 7.5, patients with NOTCH1-high expression had significantly longer PFS (median 14.7 months vs. 11.1 months, HR 0.43, 95% CI 0.22–0.81, P = 0.01) and OS (median 42.8 months vs. 26.5 months, HR 0.35, 95% CI 0.15–0.79, P = 0.01) compared with those with NOTCH1-low expression in the left-sided tumor group, but not in the right-sided tumor group (Fig. 3).
Figure 3.
Kaplan–Meier curves of OS and PFS by NOTCH1 expression level in patients with left-sided primary tumors. CI, confidence interval.
In the right-sided tumor group (n = 9), the Cox regression analysis showed that 44 genes were associated with clinical outcomes for PFS, and 33 genes were associated with clinical outcomes for OS (P < 0.01). Eleven of the 44 genes associated with PFS and 2 of the 33 genes associated with OS were associated with favorable survival. Moreover, the analysis identified 4 genes (CST6, FGF18, SHC3, and TMEM57) that were associated with both worse PFS and worse OS. There was no gene significantly associated with both better PFS and OS.
GSEA identified significant pathways associated with outcomes of tumors on each side
In the GSEA, we identified gene sets of left-sided tumors and right-sided tumors that were associated with PFS or OS and had P values of less than 5% and FDR of less than 25% (Table 1 and Table 2).
Table 1.
Results of gene set enrichment analysis for PFS
| Left |
Right |
|||||
|---|---|---|---|---|---|---|
| NES | FDR | NES | FDR | |||
| Good prognosis | Vasculature development | −2.644 | 0.022 | Organonitrogen compound biosynthetic process | −2.914 | 0.002 |
| Extracellular structure organization | −2.617 | 0.022 | Translational initiation | −2.713 | 0.011 | |
| Blood vessel morphogenesis | −2.664 | 0.028 | Leukocyte migration | −2.419 | 0.054 | |
| Chromatin modification | −2.735 | 0.029 | Amide biosynthetic process | −2.421 | 0.067 | |
| Chromatin organization | −2.489 | 0.046 | Peptide metabolic process | −2.444 | 0.075 | |
| Angiogenesis | −2.451 | 0.050 | Cellular amide metabolic process | −2.328 | 0.083 | |
| Regulation of vasculature development | −2.386 | 0.066 | Dicarboxylic acid metabolic process | −2.224 | 0.115 | |
| Skeletal muscle organ development | −2.342 | 0.079 | Acute inflammatory response | −2.211 | 0.115 | |
| Multicellular organism metabolic process | −2.297 | 0.097 | Small-molecule metabolic process | −2.229 | 0.125 | |
| Multicellular organismal macromolecule metabolic process | −2.193 | 0.146 | Organonitrogen compound metabolic process | −2.235 | 0.137 | |
| Regulation of blood circulation | −2.193 | 0.159 | Response to prostaglandin | −2.132 | 0.158 | |
| Chromatin remodeling | −2.203 | 0.165 | G protein–coupled receptor signaling pathway | −2.114 | 0.164 | |
| Regulation of cellular component movement | −2.137 | 0.170 | Cell chemotaxis | −2.136 | 0.169 | |
| Circulatory system development | −2.120 | 0.177 | ||||
| Regulation of gene expression epigenetic | −2.111 | 0.177 | ||||
| Inflammatory response | −2.139 | 0.179 | ||||
| Bad prognosis | Cell-cycle phase transition | 2.725 | 0.026 | |||
| Nucleoside monophosphate biosynthetic process | 2.500 | 0.068 | ||||
| Cell-cycle G1–S phase transition | 2.242 | 0.201 | ||||
| Protein dephosphorylation | 2.269 | 0.225 | ||||
Abbreviation: NES, normalized enrichment score.
Table 2.
Results of gene set enrichment analysis for OS
| Left |
Right |
|||||
|---|---|---|---|---|---|---|
| NES | FDR | NES | FDR | |||
| Good prognosis | Regulation of DNA replication | −2.265 | 0.235 | Movement of cell or subcellular component | −2.722 | 0.034 |
| Leukocyte migration | −2.603 | 0.042 | ||||
| Small-molecule metabolic process | −2.402 | 0.060 | ||||
| Taxis | −2.352 | 0.064 | ||||
| Cell chemotaxis | −2.403 | 0.071 | ||||
| Regulation of protein maturation | −2.354 | 0.072 | ||||
| Positive regulation of transcription from RNA polymerase II promoter in response to stress | −2.295 | 0.078 | ||||
| Ion homeostasis | −2.302 | 0.083 | ||||
| Cell motility | −2.409 | 0.086 | ||||
| Response to hormone | −2.230 | 0.091 | ||||
| Bad prognosis | DNA metabolic process | 2.694 | 0.015 | |||
| DNA repair | 2.700 | 0.030 | ||||
| Cellular response to DNA damage stimulus | 2.472 | 0.047 | ||||
| Mitotic cell cycle | 2.489 | 0.055 | ||||
| Cell-cycle process | 2.339 | 0.066 | ||||
| Negative regulation of chromosome segregation | 2.348 | 0.069 | ||||
| Protein modification by small protein conjugation or removal | 2.353 | 0.077 | ||||
| Negative regulation of cellular protein catabolic process | 2.369 | 0.083 | ||||
| Protein phosphorylation | 2.280 | 0.084 | ||||
| Peptidyl amino acid modification | 2.248 | 0.092 | ||||
Abbreviation: NES, normalized enrichment score.
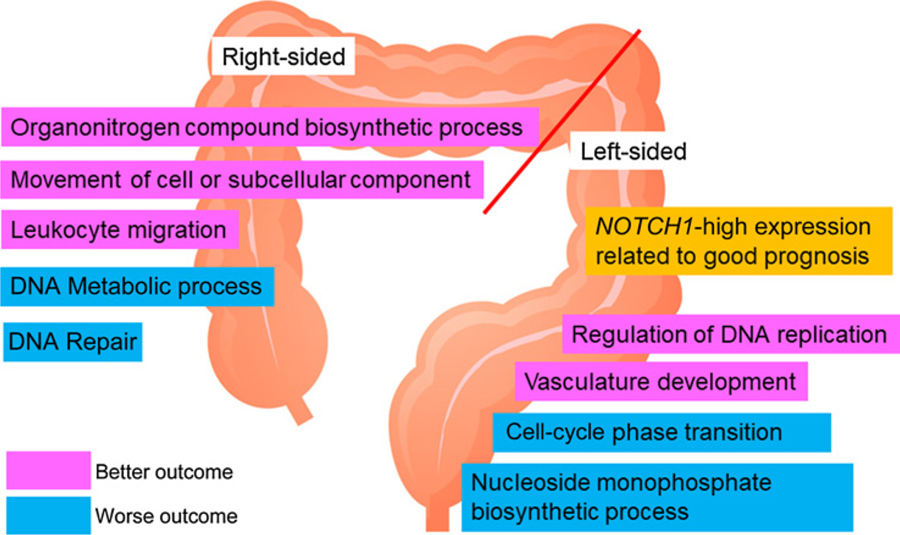
In the left-sided tumor group, one gene set regarding regulation of DNA replication was significantly associated with better OS. Sixteen gene sets correlated with better PFS, whereas 4 gene sets were associated with worse PFS. Among 16 gene sets, 3 gene sets related to angiogenesis, extracellular structure, or chromatin organization were strongly associated with favorable PFS (FDR < 0.05).
In the right-sided tumor group, 13 gene sets correlated with better PFS. Ten gene sets were associated with better OS, whereas 10 other gene sets correlated with worse OS. In particular, gene sets of organonitrogen compound biosynthetic process and translational initiation were strongly associated with favorable PFS. Subcellular component and leukocyte migration genes correlated with better OS, whereas gene sets of DNA metabolic process, DNA repair, and cellular response to DNA damage stimulus were strongly associated with worse OS (FDR < 0.05; Fig. 4).
Figure 4.
Enriched genes and gene pathways for outcome of cetuximab treatment in each side.
Discussion
Our study demonstrated that there were significant differences in gene expression levels that were associated with clinical outcomes between primary tumor sidedness in patients with mCRC treated with first-line cetuximab-based chemotherapy. A pooled analysis of 6 randomized trials evaluating the prognostic and predictive values of primary tumor sidedness in patients with RAS wild-type mCRC confirmed that adding an anti-EGFR drug had a greater effect than adding bevacizumab; this effect was greatest in patients with left-sided tumors (6). However, it has been reported that some patients with right-sided tumors respond to chemotherapy combined with anti-EGFR antibody (9, 13). In this study, we found 16 genes in the left-sided tumor group and 44 significant genes in the right-sided tumor group that were significantly associated with PFS. There were no common genes that were significantly associated with PFS in both tumor side groups. Moreover, in the GSEA, gene sets associated with PFS differed between the left-sided and right-sided tumor groups. This finding suggests that gene expression signatures may explain differences in cetuximab efficacy dependent on tumor sidedness.
Patients with left-sided tumors are more likely to respond to anti-EGFR antibodies, leading to survival benefit. However, some patients do not respond to anti-EGFR therapy even if they have left-sided primary tumors, indicating that RAS and sidedness may be not a sufficient predictor of the response to anti-EGFR antibodies. There is an urgent need for predictive biomarkers for anti-EGFR therapy to identify certain responders among patients with mCRC with left-sided primary tumors. Our findings suggest that NOTCH1 gene expression is significantly associated with survival in the left-sided tumor group, but not in the right-sided group. A family of membrane-bound receptors related to the NOTCH proteins (NOTCH1, NOTCH2, NOTCH3, and NOTCH4) contributes to regulate tumor cell proliferation, differentiation, apoptosis, and migration (14, 15). Several biomarker studies have reported that increased NOTCH1 expression is associated with lymph node metastasis in colorectal cancer (16). Moreover, NOTCH1-high expression had multivariable associations with poor outcomes in CRC (16–19). NOTCH1 mediates the resistant effects of regorafenib in colorectal cancer cells (20). It has also been shown that NOTCH1 expression is a detrimental prognostic factor in patients with mCRC who receive chemotherapy plus the anti-VEGF antibody bevacizumab (21). NOTCH1 expression may contribute to tumor resistance to bevacizumab by inducing angiogenesis, which generates large vessels that increase the tumor blood supply and diminish the sensitivity to bevacizumab (22). In our study, high expression of the NOTCH1 gene was associated with favorable clinical outcomes in patients with left-sided tumors who received cetuximab-based chemotherapy. This finding indicates that patients with mCRC harboring NOTCH1-high tumors may receive more benefit from cetuximab and that the gene expression might be a useful marker for identifying patients who are likely to benefit from anti-EGFR antibody or bevacizumab as first-line treatment for RAS wild-type mCRC.
The GSEA indicated that gene sets associated with cell-cycle phase transition and nucleoside monophosphate biosynthesis were related to poor PFS in patients with left-sided tumors who received cetuximab plus chemotherapy. The cell phase transition is the cell-cycle process by which a cell commits to enter the next phase of the cell cycle. Cell-cycle progression is related to cancer cell proliferative activity, whereas cell-cycle arrest promotes apoptosis and autophagy of colon tumor cells. Epithelial growth factor (EGF), via its receptor ((EGFR), elicits proliferation in many human cancers (23). Cetuximab occludes the binding sites of EGFR, and EGFR signaling is one of the main drivers of colon cancer growth. Previous studies have reported that cetuximab obstructs the cell cycle in G1 phase (24). Conversely, the impairment of the EGF/EGFR system induced by cetuximab might be reduced by activation of the cell-cycle transit phase pathway. In addition, in our study, the most significant gene contributing to poor outcomes in the left-sided tumor group was the ALDH1A1 gene (P = 0.0022). It has also been reported in colorectal cancer that aldehyde dehydrogenase 1A1 (ALDH1A1) is an immuno-histologic biomarker of various solid tumors (25). Several studies have suggested that ALDH1A1 may be a biomarker of cancer stem cells and can be used as a prognostic predictor of colorectal cancer (26). A biomarker study of colorectal cancer indicated that the ALDH1A1 expression was not related to differences in survival time (27); however, it has been shown that nuclear expression of ALDH1A1 is significantly associated with shorter OS and that the ratio of the ALDH1A1 level in adjacent mucosa to that in tumor tissue is closely related to invasion, metastasis, and prognosis in colorectal cancer (25, 28). A previous molecular subtyping study demonstrated that a subtype that is stem-like and includes upregulation of genes involved in matrix remodeling and epithelial– mesenchymal transition carries a very poor prognosis and, moreover, is refractory to EGFR-targeted therapy (29). Therefore, the ALDH1A1 gene may be a prognostic factor but may be also a predictor of a poor response to cetuximab in mCRC.
In the right-sided tumor group, the CST6, FGF18, SHC3, and TMEM57 genes were associated with worse PFS and OS. Cystatin 6 (CST6) has been considered to be a tumor-suppressor in breast tissue (30), reducing breast cancer cell proliferation, adhesion to endothelial cells, Matrigel invasion, and migration (31), but this has not been reported in colorectal cancer. Loss of CST6 gene expression has ascribed promoting hypermethylation in breast cancer (30). Cystatin M, a protein coded by the CST6 gene, which controls the activity of legumain, is found to be an oncogene and an indicator of a poor prognosis in colorectal and breast cancers, but also to be overexpressed in the majority of human solid tumors (32). FGFR3 has been reported to negatively regulate bone growth (33) and also to be involved in carcinogenesis. Upregulation of FGF18, one of ligands of FGFR3, was shown to have oncogenic impact (34) and to lead malignant cell growth and survival in human colorectal cancer cell lines (35). The FGF-receptor splice variant FGFR3-IIIc mediates FGF18-dependent signaling. In colon adenoma cells, an FGF18/FGFR3-IIIc autocrine growth and survival loop is upregulated in a Wnt-dependent manner and controls tumor cell growth (36). Several types of genes associated with tumor growth may contribute to poorer outcomes in mCRC with right-sided primary tumors.
The BECN1-high expression was found to be significantly associated with favorable survival in both tumor sidedness groups of our study. BECN1 has an important role in canonical autophagy, to regulate autophagic phosphatidylinositol 3-phosphate generation and recruit additional ATG proteins for autophago-some formation (37). Autophagy-related genes are overregulated or downregulated in cancers, but also significantly correlate with poor prognoses, suggesting the complex biological role of autophagy in cancer (38, 39). Monoallelic loss of the BECN1 gene causes susceptibility to metabolic stress and promotes tumorigenesis (40). A retrospective review from clinicopathologic and IHC data indicated that the absence of autophagy-related protein expression correlated with poor prognosis in colorectal cancer; therefore, suggested that these proteins may be novel prognostic markers (41). Our results suggest that BECN1 may be a promising gene for predicting favorable outcomes in mCRC.
Our study had several limitations that must be taken into account when interpreting the results. A major limitation was that our study population may include patients with non-exon 2 KRAS and NRAS mutations. An extended RAS test is now recommended for patient selection for anti-EGFR therapy as it is known that patients with non-exon 2 KRAS and NRAS mutations do not derive benefit from anti-EGFR antibodies (42, 43). These patients should be excluded from the analysis; however, it was not able to check RAS status due to lack of the remaining tissue samples. The sample size of patients with right-sided primary tumors (n = 13) was very small in this study; therefore, it may be not statistically reliable. It is difficult to exactly assess the impact of candidate genes on the effectiveness of cetuximab because the patient cohort comprised only patients who received cetuximab, meaning that we are unable to evaluate the genes as predictive markers. We found that there were no common genes and pathways that were significantly associated with PFS in both the left-sided and right-sided tumor groups; however, this may have resulted from the small study group. To resolve these limitations, our findings will be validated using data of an ongoing randomized trial, the DEEPER (NCT02515734), which evaluates triplet-regimen plus cetuximab or bevacizumab as first-line treatment for RAS wildtype mCRC.
In conclusion, our data suggest that genes contributing to the response or resistance to cetuximab treatment may differ between right-sided tumors and left-sided tumors in patients with mCRC. NOTCH1 may potentially discriminate certain responders to cetuximab in patients with left-sided primary tumors, although several genes may contribute resistance to cetuximab in patients with right-sided primary tumors. These findings need to be confirmed in studies using larger cohorts with patients with RAS wild-type mCRC.
Supplementary Material
Acknowledgments
We thank the patients, their families, and the investigators who participated in the JACCRO CC-05 and CC-06 trials. We also thank Toshifusa Nakajima for study support, Peter Star (Medical Network K.K.) for English editorial support, John Luecke, Debrah Thompson, and Bonnie LaFleur (HTG Molecular Diagnostics, Inc.) for gene expression analysis, and Atsushi Kakimoto and Nahoko Hirabayashi (SRL, Inc.) for genetic testing. This study was supported by the Japan Clinical Cancer Research Organization (JACCRO).
Footnotes
Note: Supplementary data for this article are available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
Y. Sunakawa has received speakers bureau honoraria from Taiho Pharmaceutical, Chugai Pharma, Yakult Honsha, Takeda, Merck Serono, Bayer Yakuhin, Eli Lilly Japan, and Sanofi. H.-J. Lenz is a consultant/advisory board member for BMS, Merck Serono, Roche, and Bayer. A. Tsuji has received speakers bureau honoraria from Merck Serono, Daiichi Sankyo, Taiho Pharmaceutical, Chugai Pharma, Takeda Pharmaceutical, and Bristol-Myers Squibb Japan. T. Denda has received speakers bureau honoraria from Yakult Honsha and Taiho Pharmaceutical. K. Shimada reports receiving other commercial research support from Yakult Honsha and Taiho Pharmaceutical. M. Nakamura has received speakers bureau honoraria from Merck Serono, Taiho Pharmaceutical, and Yakult Honsha. M. Kotaka has received speakers bureau honoraria from Yakult Honsha and Chugai Pharma. Y. Segawa reports receiving a commercial research grant from Taiho Pharmaceutical, PAREXEL International, Bayer, Daiichi Sankyo, Eisai, Novartis Pharma, GlaxoSmithKline, Chugai Pharma, and AstraZeneca; and has received speakers bureau honoraria from Taiho Pharmaceutical, Novartis Pharma, and Mochida Pharmaceutical. W. Ichikawa reports receiving a commercial research grant from Chugai Pharma, Takeda Pharmaceutical, and Taiho Pharmaceutical; and has received speakers bureau honoraria from Chugai Pharma, Merck Serono, Takeda Pharmaceutical, and Taiho Pharmaceutical. No potential conflicts of interest were disclosed by other authors.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, Nishihara R, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut 2012;61:847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee GH, Malietzis G, Askari A, Bernardo D, Al-Hassi HO, Clark SK. Is right-sided colon cancer different to left-sided colorectal cancer? - a systematic review. Eur J Surg Oncol 2015;41:300–8. [DOI] [PubMed] [Google Scholar]
- 3.Missiaglia E, Jacobs B, D’Ario G, Di Narzo AF, Soneson C, Budinska E, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol 2014;25: 1995–2001. [DOI] [PubMed] [Google Scholar]
- 4.Venook AP, Ou FS, Lenz HJ, Kabbarah O, Qu X, Niedzwiecki D, et al. Primary (1°) tumor location as an independent prognostic marker from molecular features for overall survival (OS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol 2017;35:3503. [Google Scholar]
- 5.Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol 2016. October 10 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 6.Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 2017;28: 1713–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Cancer Net 2018;16:359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol 2018;29: 44–70. [DOI] [PubMed] [Google Scholar]
- 9.Sunakawa Y, Tsuji A, Fujii M, Ichikawa W. No benefit from the addition of anti-EGFR antibody in all right-sided metastatic colorectal cancer? Ann Oncol 2017;28:2030–1. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji A, Sunakawa Y, Ichikawa W, Nakamura M, Kochi M, Denda T, et al. Early tumor shrinkage and depth of response as predictors of favorable treatment outcomes in patients with metastatic colorectal cancer treated with FOLFOX plus cetuximab (JACCRO CC-05). Target Oncol 2016;11: 799–806. [DOI] [PubMed] [Google Scholar]
- 11.Sunakawa Y, Ichikawa W, Tsuji A, Denda T, Segawa Y, Negoro Y, et al. Prognostic impact of primary tumor location on clinical outcomes of metastatic colorectal cancer treated with cetuximab plus oxaliplatin-based chemotherapy: a subgroup analysis of the JACCRO CC-05/06 trials. Clin Colorectal Cancer 2017;16:e171–e180. [DOI] [PubMed] [Google Scholar]
- 12.Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. PLoS Med 2012;9:e1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stintzing S, Miller-Phillips L, Modest DP, Fischer von Weikersthal L, Decker T, Kiani A, et al. Impact of BRAF and RAS mutations on first-line efficacy of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab: analysis of the FIRE-3 (AIO KRK-0306) study. Eur J Cancer 2017;79:50–60. [DOI] [PubMed] [Google Scholar]
- 14.Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer 2011;11:338–51. [DOI] [PubMed] [Google Scholar]
- 15.Suman S, Das TP, Ankem MK, Damodaran C. Targeting notch signaling in colorectal cancer. Curr Colorectal Cancer Rep 2014;10:411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park HS, Jung CK, Lee SH, Chae BJ, Lim DJ, Park WC, et al. Notch1 receptor as a marker of lymph node metastases in papillary thyroid cancer. Cancer Sci 2012;103:305–9. [DOI] [PubMed] [Google Scholar]
- 17.Huang R, Tang Q, You Q, Liu Z, Wang G, Chen Y, et al. Disparity expression of Notch1 in benign and malignant colorectal diseases. PLoS One 2013;8: e81005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu D, Li Y, Wang W, Zhao Q, Li J, Lu Y, et al. High level of Notch1 protein is associated with poor overall survival in colorectal cancer. Ann Surg Oncol 2010;17:1337–42. [DOI] [PubMed] [Google Scholar]
- 19.Chu D, Zhou Y, Zhang Z, Li Y, Li J, Zheng J, et al. Notch1 expression, which is related to p65 status, is an independent predictor of prognosis in colorectal cancer. Clin Cancer Res 2011;17:5686–94. [DOI] [PubMed] [Google Scholar]
- 20.Mirone G, Perna S, Shukla A, Marfe G. Involvement of Notch-1 in resistance to regorafenib in colon cancer cells. J Cell Physiol 2016;231: 1097–105. [DOI] [PubMed] [Google Scholar]
- 21.Paiva TF Jr, de Jesus VH, Marques RA, da Costa AA, de Macedo MP, Peresi PM, et al. Angiogenesis-related protein expression in bevacizumab-treated metastatic colorectal cancer: NOTCH1 detrimental to overall survival. BMC Cancer 2015;15:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res 2008;14:6371–5. [DOI] [PubMed] [Google Scholar]
- 23.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006;366:2–16. [DOI] [PubMed] [Google Scholar]
- 24.Corona G, Deiana M, Incani A, Vauzour D, Dessi MA, Spencer JP. Hydroxytyrosol inhibits the proliferation of human colon adenocarcinoma cells through inhibition of ERK1/2 and cyclin D1. Mol Nutr Food Res 2009;53:897–903. [DOI] [PubMed] [Google Scholar]
- 25.Xu SL, Zeng DZ, Dong WG, Ding YQ, Rao J, Duan JJ, et al. Distinct patterns of ALDH1A1 expression predict metastasis and poor outcome of colorectal carcinoma. Int J Clin Exp Pathol 2014;7:2976–86. [PMC free article] [PubMed] [Google Scholar]
- 26.Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res 2009;69:3382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lugli A, Iezzi G, Hostettler I, Muraro MG, Mele V, Tornillo L, et al. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br J Cancer 2010;103:382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baratti D, Kusamura S, Cabras AD, Deraco M. Cytoreductive surgery with selective versus complete parietal peritonectomy followed by hyperthermic intraperitoneal chemotherapy in patients with diffuse malignant peritoneal mesothelioma: a controlled study. Ann Surg Oncol 2012;19:1416–24. [DOI] [PubMed] [Google Scholar]
- 29.De Sousa E Melo F, Wang X, Jansen M, Fessler E, Trinh A, de Rooij LP, et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med 2013;19:614–8. [DOI] [PubMed] [Google Scholar]
- 30.Ai L, Kim WJ, Kim TY, Fields CR, Massoll NA, Robertson KD, et al. Epigenetic silencing of the tumor suppressor cystatin M occurs during breast cancer progression. Cancer Res 2006;66:7899–909. [DOI] [PubMed] [Google Scholar]
- 31.Shridhar R, Zhang J, Song J, Booth BA, Kevil CG, Sotiropoulou G, et al. Cystatin M suppresses the malignant phenotype of human MDA-MB-435S cells. Oncogene 2004;23:2206–15. [DOI] [PubMed] [Google Scholar]
- 32.Murthy RV, Arbman G, Gao J, Roodman GD, Sun XF. Legumain expression in relation to clinicopathologic and biological variables in colorectal cancer. Clin Cancer Res 2005;11:2293–9. [DOI] [PubMed] [Google Scholar]
- 33.L’Hote CG, Knowles MA. Cell responses to FGFR3 signalling: growth, differentiation and apoptosis. Exp Cell Res 2005;304:417–31. [DOI] [PubMed] [Google Scholar]
- 34.Shimokawa T, Furukawa Y, Sakai M, Li M, Miwa N, Lin YM, et al. Involvement of the FGF18 gene in colorectal carcinogenesis, as a novel downstream target of the beta-catenin/T-cell factor complex. Cancer Res 2003;63:6116–20. [PubMed] [Google Scholar]
- 35.Sonvilla G, Allerstorfer S, Stattner S, Karner J, Klimpfinger M, Fischer H, et al. FGF18 in colorectal tumour cells: autocrine and paracrine effects. Carcinogenesis 2008;29:15–24. [DOI] [PubMed] [Google Scholar]
- 36.Koneczny I, Schulenburg A, Hudec X, Knofler M, Holzmann K, Piazza G, et al. Autocrine fibroblast growth factor 18 signaling mediates Wnt-dependent stimulation of CD44-positive human colorectal adenoma cells. Mol Carcinog 2015;54:789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wirawan E, Lippens S, Vanden Berghe T, Romagnoli A, Fimia GM, Piacentini M, et al. Beclin1: a role in membrane dynamics and beyond. Autophagy 2012;8:6–17. [DOI] [PubMed] [Google Scholar]
- 38.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A 2003;100:15077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu XD, Yao J, Tripathi DN, Ding Z, Xu Y, Sun M, et al. Autophagy mediates HIF2alpha degradation and suppresses renal tumorigenesis. Oncogene 2015;34:2450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, et al. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev 2007;21:1367–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi JH, Cho YS, Ko YH, Hong SU, Park JH, Lee MA. Absence of autophagy-related proteins expression is associated with poor prognosis in patients with colorectal adenocarcinoma. Gastroenterol Res Pract 2014;2014: 179586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Cutsem E, Lenz HJ, Kohne CH, Heinemann V, Tejpar S, Melezinek I, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 2015;33:692–700. [DOI] [PubMed] [Google Scholar]
- 43.Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.