Abstract
Background
The combination of vincristine, irinotecan, and temozolomide (VIT) is often used to treat children and adolescents with relapsed rhabdomyosarcoma (RMS); however, the outcome of these patients has not been previously described.
Procedures
We sought to determine the response rate (RR) and progression-free survival (PFS) for patients with relapsed RMS treated with VIT by retrospective review of patients treated at five tertiary care hospitals. Prior treatment with irinotecan was permitted.
Results
Among 19 patients with a median age of 8 years (range 2–17 years), 12 (63%) were males and 12 (63%) had embryonal histology. Median time to relapse from initial diagnosis was 16 months (range 2.8–45 months). VIT was used as first, second, third, or fourth line of therapy in four (21%), seven (37%), six (32%), and two (10%) patients, respectively. Four patients received VIT as adjuvant therapy following radiation and/or surgery. Therefore, among 15 evaluable patients, the best response to VIT was 0 (complete response, CR), 0 (partial response, PR), 4 (stable disease, SD), and 11 (progressive disease, PD) for an overall clinical benefit rate (CR + PR + SD) of 26.7% (95% CI: 7.8–55.1%). After a median follow-up of 8 months, 2 (10%) patients were alive without disease, 3 (16%) were alive with disease, and 14 (74%) patients died of PD. PFS at 3 months was 23% (95% CI: 5.7–46.7%).
Conclusions
VIT therapy in combination with adequate local control is associated with some disease control in patients with first relapse RMS and may be another reasonable option to offer patients as salvage therapy.
Keywords: chemotherapy, irinotecan, pediatric soft tissue sarcoma, rhabdomyosarcoma, relapse, temozolomide
1 |. INTRODUCTION
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma in children and adolescents with approximately 250 children diagnosed annually in the United States.1,2 Currently, more than 70% of children with localized RMS can be cured of their disease.2 Improvements in outcome have been attributed to the use of intensive combination chemotherapy, better staging, and more effective local therapy with surgery and radiation. However, relapsed disease is still extremely difficult to salvage with only 10% chance of patients surviving at 5 years.3
The combination of vincristine and irinotecan (VI) has been previously investigated in patients with first relapse RMS at high risk for poor outcome.4 Further, among patients with treatment naïve high-risk RMS, response rates (RRs) to VI combination and single agent irinotecan was 70% and 42%, respectively.5 Dose-limiting toxicities of VI are primarily gastrointestinal (diarrhea).4 Temozolomide as a single agent is of limited benefit in patients with RMS6 however, the addition of temozolomide to VI (vincristine, irinotecan, and temozolomide [VIT]) has previously been evaluated in patients with relapsed Ewing sarcoma, with an RR of 50–68%.7–9 Two small series of included patients with relapsed RMS, which documented an RR of 25% and 43% among four and seven patients, respectively10,11 The synergy of these two agents has shown to be due to temozolomide-induced methylation of DNA causing localization and enhancement of topoisomerase I cleavage complexes, allowing irinotecan to effectively stabilize the DNA-enzyme complex that leads to cytotoxicity of the tumor cells.12 Herein, we describe the progression-free survival (PFS) of patients with relapsed RMS in the largest series to date, compiling data on 19 patients from five tertiary care centers.
2 |. METHODS
We conducted a multicenter retrospective review from the following centers: Nationwide Children’s Hospital (Columbus, OH), Hospital for Sick Children (Toronto, Canada), Children’s Hospital of Philadelphia, Texas Children’s Hospital and Children’s Hospital of Los Angeles. Eligibility criteria for this study were as follows: Primary diagnosis of RMS from 2000 to 2013, which had received VIT at the time of first or subsequent relapse; patients who had previously received irinotecan were not excluded. The following data were collected: age at initial diagnosis, location of initial tumor, prior chemotherapy, number of relapses, time to recurrence after initial treatment, local control strategy used at relapse, status at last follow-up, and time to follow-up. RR was recorded as was assessed by the treating institution according to RECIST 1.1. Toxicity data were not collected. Approval from Institutional Review Board was obtained at each site prior to accrual of data. A RedCAP database13 was established to securely transfer data across institutions. Data were analyzed with descriptive statistics. PFS distributions were estimated using the Kaplan–Meier method14 and were compared using the log-rank test.15 Statistical significance was determined at the 0.05 level.
3 |. RESULTS
Demographics
The median age of 19 enrolled patients was 8 years (range 2–17 years) at primary diagnosis. Twelve (63%) were males and 12 (63%) had embryonal histology. Metastatic disease was present in three (16%) patients at initial diagnosis. Median time to relapse from initial diagnosis was 16 months (range 2.8–45 months). VIT was used as first, second, third, or fourth line of therapy in four (21%), seven (37%), six (32%), and two (10%) patients, respectively. Sites of relapse were as follows: seven (37%), local; nine (47%), distant, and three (16%), combined.
Treatment
Therapy at initial diagnosis included doxorubicin-based 4 (20%) and vincristine, dactinomycin, and cyclophosphamide 15 (75%). Of these, three (57%) received an irinotecan-based regimen, in whom VIT was used as first, second, and fourth line after relapse. At relapse, VIT was administered every 21 days as follows: vincristine, 1.5 mg/m2 intravenously (IV) on day 1; irinotecan, 50 mg/m2 IV or 70–100 mg/m2 orally, days 1–5; temozolomide 100–150 mg/m2 orally, days 1–5. Local control was administered to 11 (58%) patients and included radiation for 8 (42%), and surgery for 5 (26%) patients (Table 1).
TABLE 1.
Demographics and treatment data
| Age at pri. dx (yrs) | Sex | Histologic subtype (fusion status) [pri. site] | Metastatic disease at pri. diagnosis | Primary chemo regimen | Time to relapse (mo) | Site of relapse | VIT line of tx | VIT regimen useda | Cycles of VITb | Local control | Reason for stopping chemo | Best response to therapy | Length of follow-up from date of relapse/start of VIT (mo) | Status at last follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | M | Emb [GU] | No | VAC | 12 | Local | 4 | 1–50 mg/m2 IV × 5 days T-150 mg/m2 × 5 days |
2 | RT | PD | PD | 12/2.4 | DOD |
| 2 | 5 | F | Emb [HN non-PM] | No | VAC | 9 | Local | 2 | 1–50 mg/m2 IV × 5 days T-100 mg/m2 × 5 days |
1 | None | PD | PD | 3/2.7 | DOD |
| 3 | 14 | F | Alv(+) [trunk] | No | VAC | 16 | Distant (bone) | 2 | 1–50 mg/m2 IV × 5 days T-100 mg/m2 × 5 days |
1 | RT | Toxicity | PD | 4/1.2 | DOD |
| 4 | 17 | F | Emb [GU non-B/P] | No | VAC, VA | 46 | Distant (brainstem) | 2 | 1–50 mg/m2 IV × 5 days T-100 mg/m2 × 5 days |
6 | RT | End of therapy | SD | 17/16.6 | DOD |
| 5 | 10 | F | Emb [GU non-B/P] | No | VAC | 3 | Local | 3 | 1–100 mg/m2 PO × 10 days T-100 mg/m2 × 5 days |
2 | None | PD | PD | 28/11.0 | DOD |
| 6 | 8 | M | Emb [orbit-PM] | No | VAC | 12 | Local | 3 | 1–50 mg/m2 IV × 5 days T-100 mg/m2 × 5 days |
2 | None | PD | PD | 7/4.9 | DOD |
| 7 | 9 | M | Emb [HN non-PM] | No | VAC | 27 | Local | 2 | 1–50 mg/m2 IV × 5 days T-100 mg/m2 × 5 days |
18 | S + RT | End of therapy | Not evaluablec | 34/33.5 | Alive, on hospice care |
| 8 | 9 | M | Alv(unk) [extremity] | Yes | ARST0431 | 10 | Lung | 4 | 1–50 mg/m2 IV × 5 days T-100 mg/m2 × 5 days |
2 | None | PD | PD | 11/7.4 | DOD |
| 9 | 4 | M | Emb [cheek, PM] | Yes | ARST08P1 | 10 | Lung | 1 | 1–50 mg/m2 IV × 5 days T-100 mg/m2 × 5 days |
2 | RT | End of therapy | PD | 2/12.6 | DOD |
| 10 | 14 | F | Alv(unk) [trunk] | No | VAC | 13 | Distant (lung, bone) | 2 | 1–50 mg/m2 IV × 5 days T-100 mg/m2 × 5 days |
1 | RT | Toxicity | Not evaluablec | 2/7.1 | DOD |
| 11 | 7 | M | Emb [GU non-B/P] | No | VAC | 13 | LN | 1 | 1–50 mg/m2 IV × 5 days T-100 mg/m2 × 5 days |
1 | S + RT | PD | PD | 23/23.0 | DOD |
| 12 | 1.75 | F | Alv (+) [HN non-PM] | No | VAC | 16 | Local | 1 | 1–90 mg/m2 PO × 5 days T-100 mg/m2 × 5 days |
12 | S | Physician preference | Not evaluablec | 31/31.0 | Alive without disease |
| 13 | 7 | M | Emb [pelvis] | No | VAC | 23 | Distant (liver) | 3 | 1–20 mg/m2 IV × 5 days T-100 mg/m2 × 5 days |
9 | S | PD | SD | 27/9.4 | DOD |
| 14 | 9 | F | Alv (unk) [extremity] | No | VAC | 33 | Lung | 3 | 1–50 mg/m2 IV × 5 days T-100 mg/m2 × 5 days |
1 | None | PD | PD | 9/2.0 | DOD |
| 15 | 4 | M | Emb [orbit-PM] | No | VAC | 6 | Local | 3 | 1–50 mg/m2 IV × 5 days T-100 mg/m2 × 5 days |
2 | None | PD | PD | 6/2.7 | DOD |
| 16 | 16 | M | Alv (+) [cervical with LN] | Yes | ARST0431 | 22 | Max sinus | 2 | 1–90 mg/m2 PO × 5 days T-150 mg/m2 × 5 days |
6 | RT | PD | SD | 17/8.6 | Alive with disease |
| 17 | 13 | M | Emb [GU non-B/P] | No | VAC | 35 | Distant (lung, bone, LN, abdomen) | 2 | 1–70 mg/m2 PO × 5 days T-125 mg/m2 × 5 days |
2 | None | PD | PD | 10/2.6 | DOD |
| 18 | 4 | M | Emb [GU non-B/P] | No | VA | 18 | Abdomen | 1 | 1–50 mg/m2 IV × 5 days T-100 mg/m2 × 5 days |
6 | S | Last follow-up | Not evaluablec | 4/4.0 | Alive without disease |
| 19 | 4 | M | Alv (+) [cheek/mandible-PM] | No | VAC | 17 | Local, LN | 3 | 1–90 mg/m2 PO × 5 days T-150 mg/m2 × 5 days |
7 | None | PD | SD | 20/14.8 | Alive with disease |
Yrs, years, mo, months; pri. Dx, primary diagnosis; Pri. Site, primary site; tx, treatment; M, male; F, female; Emb, embryonal; Alv, alveolar; unk, unknown; pos, positive; GU, genitourinary; HN non-PM, head and neck nonparameningeal; PM, parameningeal; LN, lymph node; VAC, vincristine, actinomycin, cyclophosphamide; VA, vincristine, actinomycin; I, irinotecan; T, temozolomide; IV, intravenous; PO, per oral; S, surgery; RT, radiotherapy; CR, complete response; SD, stable disease; PD, progressive disease; DOD, died of disease.
All except patient 8 received vincristine 0.5 mg/m2.
Number.
VIT used as adjuvant therapy, response not evaluable.
Outcome
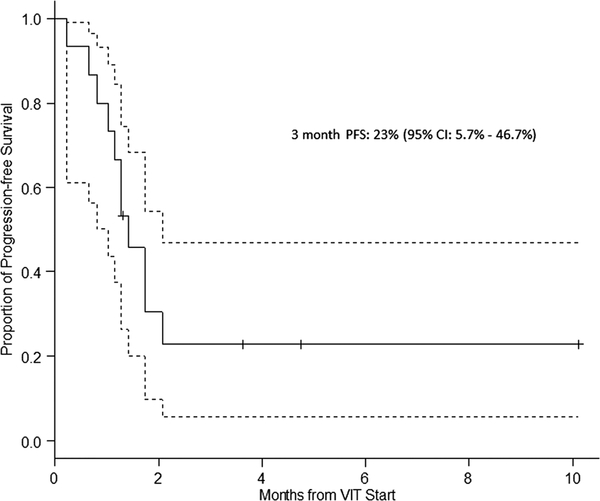
Four patients who received adjuvant VIT chemotherapy postsurgery for local control are not included in the response analysis. Among the 15 evaluable patients, the best response to VIT was as follows: 0 (complete response, CR), 0 (partial response, PR), 4 (stable disease, SD), and 11 (progressive disease, PD) for an overall clinical benefit rate (CR + PR + SD) of 26.7% (95% CI: 7.8–55.1%). One patient with SD had received prior irinotecan. Two (13%) patients remain alive with disease at 8.6 and 14.8 months. Thirteen (87%) patients had died of PD at a median time of 4.9 months (range: 1.2–23 months). PFS at 3 months was 23% (95% CI: 5.7–46.7%; Fig. 1). All three patients who are alive without disease had had only local recurrence, had local therapy with surgery, RT or both, and received adjuvant VIT chemo.
FIGURE 1.
Proportion of progression-free survival Three-month PFS 23% (95% CI: 5.7–46.7%).
4 |. DISCUSSION
Collecting information on disease outcome outside a clinical trial is challenging despite multicenter involvement. Moreover, retrospective analyses such as this one are inherently limited by quality of information documented and collected, with no central radiology or pathology review and interpatient variability in dosing of chemotherapy agents. In the current series, toxicity information was not collected. Nonetheless, herein, we describe the largest series to date of children and adolescents treated with VIT for relapsed RMS. Best response to VIT was SD, however, this was achieved in a quarter of patients. Notably, all patients alive at last follow-up had only a local recurrence and received adjuvant VIT.
Almost half of the patients in our series had local relapses, and local control was offered at the time of relapse with surgery, radiation, or both to over half of all patients. This study was not equipped to analyze the overall impact of local control in addition to chemotherapy on outcome, although surgery has been previously demonstrated to play an important role in patients with relapsed RMS.16 In our dataset, the three patients who were alive at last follow-up underwent aggressive local control with surgery and/or radiation therapy.
Irinotecan has been incorporated into front-line therapy of patients with newly diagnosed RMS in the two most recently closed studies through the Children’s Oncology Group (COG). ARST0431 utilized a dose-intensive multiagent regimen that includes vincristine, doxorubicin, cyclophosphamide/ifosfamide and etoposide alternating with vincristine, actinomycin, cyclophosphamide/VI (VAC/VI) for the treatment of high-risk RMS.17 Unfortunately, the reported 3-year eventfree survival of 38% was similar to prior studies, and so it remains unclear whether VI will be used in future studies of high-risk patients. Conversely, when patients with intermediate-risk disease enrolled on ARST0531 were randomized to receiving either VAC alone or alternating with VI, patients on both arms had similar favorable outcome.18 Due to the decreased toxicity associated with VAC alternating with VI, this has been chosen to be the new standard backbone for the current study in patients with intermediate-risk RMS. Thus, moving forward, many patients will likely have received irinotecan in upfront therapy. In the current study, one of the four who derived clinical benefit from VIT received prior irinotecan.
Another commonly used treatment regimen for relapsed RMS includes cyclophosphamide and topotecan for which a window phase II study demonstrated an RR of 47% in patients with newly diagnosed RMS.19 In 15 patients with relapsed RMS, PR and SD was 67% and 20%, respectively.20 However, this study limited inclusion to those who had ≤2 prior lines of therapy. Our data highlight the importance of number of prior lines of therapy when evaluating response to novel treatments. Due to the small sample size, there was no significant statistical difference when comparing patients who received VIT after varied number of prior cycles of therapy. The outcome of those who received VIT as second-line therapy was poorer than when VIT was administered as first-line therapy. Despite these limitations, the role of VIT at first relapse in patients previously exposed to irinotecan deserves consideration.
The clinical trial sponsored by Centre Oscar Lambret randomizing patients to VI versus VIT has completed accrual, and although prior irinotecan was not permitted, there was no restriction to prior lines of therapy.21 Most early-phase studies within pediatric oncology also do not limit number of prior lines of therapy, which may impact overall responses when evaluating new agents. Interestingly, the most recently completed COG trial ARST0921 enrolled patients with first relapse RMS, with no prior therapy for relapsed disease. This study was informative and actually demonstrated a difference in PFS in those receiving a combination of vinorelbine, cyclophosphamide, and temsirolimus over those receiving the same backbone chemotherapy and bevacizumab.18 The results of this study have helped with the inclusion of temsirolimus in the currently open study for intermediate-risk RMS.22 In conclusion, VIT may be considered in patients with relapsed RMS; however, overall outcome is likely driven by disease biology and type of recurrence.
Abbreviations
- CR
complete response
- PD
progressive disease
- PFS
progression-free survival
- PR
partial response
- RR
response rate
- RMS
rhabdomyosarcoma
- SD
stable disease
- VAC/VI
vincristine, actinomycin, cyclophosphamide/vincristine, irinotecan
- VIT
vincristine, irinotecan, and temozolomide
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- 1.Gurney JGYJ, Roffers SD, et al. Soft tissue sarcomas In: Ries LAG, Smith MA, Gurney JG, et al., eds. Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program; 1999:1975–1995. [Google Scholar]
- 2.Pappo AS, Shapiro DN, Crist WM, Maurer HM, et al. Biology and therapy of pediatric rhabdomyosarcoma. J Clin Oncol. 1995;13(8):2123–2139. [DOI] [PubMed] [Google Scholar]
- 3.Pappo AS, Anderson JR, Crist WM, et al. Survival after relapse in children and adolescents with rhabdomyosarcoma: a report from the Intergroup Rhabdomyosarcoma Study Group. J Clin Oncol. 1999;17(11):3487–3493. [DOI] [PubMed] [Google Scholar]
- 4.Mascarenhas L, Lyden ER, Breitfield PP, et al. Randomized phase II window trial of two schedules of irinotecan with vincristine in patients with first relapse or progression of rhabdomyosarcoma: a report from the Children’s Oncology Group. J Clin Oncol. 2010;28(30):4658–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pappo AS, Lyden E, Breitfeld P, et al. Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: the Children’s Oncology Group. J Clin Oncol. 2007;25(4):362–369. [DOI] [PubMed] [Google Scholar]
- 6.De Sio L, Milano GM, Castellano A, et al. Temozolomide in resistant or relapsed pediatric solid tumors. Pediatr Blood Cancer. 2006;47(1):30–36. [DOI] [PubMed] [Google Scholar]
- 7.Casey DA, Wexler LH, Merchant MS, et al. Irinotecan and temozolomide for Ewing sarcoma: the Memorial Sloan-Kettering experience. Pediatr Blood Cancer. 2009;53(6):1029–1034. [DOI] [PubMed] [Google Scholar]
- 8.Raciborska A, Bilska K, Drabko K, et al. Vincristine, irinotecan, and temozolomide in patients with relapsed and refractory Ewing sarcoma. Pediatr Blood Cancer. 2013;60(10):1621–1625. [DOI] [PubMed] [Google Scholar]
- 9.Wagner LM, McAllister N, Goldsby RE, et al. Temozolomide and intravenous irinotecan for treatment of advanced Ewing sarcoma. Pediatr Blood Cancer. 2007;48(2):132–139. [DOI] [PubMed] [Google Scholar]
- 10.Mixon BA, Eckrich MJ, Lowas S, Engel ME, et al. Vincristine, irinotecan, and temozolomide for treatment of relapsed alveolar rhabdomyosarcoma. J Pediatr Hematol Oncol. 2013;35(4):e163–e166. [DOI] [PubMed] [Google Scholar]
- 11.Winter S, Fasola S, Brisse H, Mosseri V, Orbach D, et al. Relapse after localized rhabdomyosarcoma: Evaluation of the efficacy of second-line chemotherapy. Pediatr Blood Cancer. 2015;62(11):1935–1941. [DOI] [PubMed] [Google Scholar]
- 12.Pourquier P, Waltman JL, Urasaki Y, et al. Topoisomerase I-mediated cytotoxicity of N-methyl-N’-nitro-N-nitrosoguanidine: trapping of topoisomerase I by the O6-methylguanine. Cancer Res. 2001;61(1):53–58. [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer. 1977;35(1):1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Corti F, Bisogno G, Dall’lgna P, et al. Does surgery have a role in the treatment of local relapses of non-metastatic rhabdomyosarcoma? Pediatr Blood Cancer. 2011;57(7):1261–1265. [DOI] [PubMed] [Google Scholar]
- 17.Weigel BJ, Lyden E, Anderson JR, et al. Intensive multiagent therapy, including dose-compressed cycles of ifosfamide/etoposide and vincristine/doxorubicin/cyclophosphamide, irinotecan, and radiation, in patients with high-risk rhabdomyosarcoma: a report from the Children’s Oncology Group. J Clin Oncol. 2016;34(2):117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mascarenhas L, Lyden E, Rodeberg DA, et al. Randomized Phase 2Trial of Bevacizumab and Temsirolimus in combination with Vinorelbine (V) and Cyclophosphamide (C) for First Relapse/Disease Progression of Rhabdomyosarcoma (RMS): A Report from the Children’s Oncology Group. Am Soc Clin Oncol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walterhouse DO, Lyden ER, Breitfeld PP, Qualman SJ, Wharam MD, Meyer WH, et al. Efficacy of topotecan and cyclophosphamide given in a phase II window trial in children with newly diagnosed metastatic rhabdomyosarcoma: a Children’s Oncology Group study. J Clin Oncol. 2004;22(8):1398–1403. [DOI] [PubMed] [Google Scholar]
- 20.Saylors RL 3rd, Stine KC, Sullivan J, et al. Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: a Pediatric Oncology Group phase II study. J Clin Oncol. 2001;19(15):3463–3469. [DOI] [PubMed] [Google Scholar]
- 21.Vincristine and Irinotecan With or without Temozolomide in Children and Adults With Refractory or Relapsed Rhabdomyosarcoma: International Randomized Trial (VIT-0910). https://clinicaltrials.gov/ct2/show/NCT01355445 Accessed June 6 2017.
- 22.Combination Chemotherapy With or Without Temsirolimus in Treating Patients With Intermediate Risk Rhabdomyosarcoma. https://clinicaltrials.gov/ct2/show/NCT02567435 Accessed June 6 2017.