Abstract
Introduction
Efficient execution of image-guided percutaneous biopsy is a procedural competency milestone in radiology training. Despite the importance of achieving such mastery, literature on successful execution by residents is limited. The purpose of this study was to evaluate resident performance as measured by nondiagnostic biopsy and major complication percentages, on CT-guided transthoracic core needle biopsies (TTNB) of lung and mediastinal lesions.
Methods
A 12-year retrospective cohort study was conducted using charts from an academic hospital, 2006 – 2018, to evaluate TTNBs. Inclusion criteria were ≥ 18 years of age and ≥ 1 follow-up CT scan and chest x-ray. Bivariable associations by outcome(s) were evaluated.
Results
Of 1,191 biopsies conducted, case distribution was 41%, 26%, 18%, and 15% for postgraduate years (PGY) 2 – 5, respectively. Results from biopsies were 139 (11.7%) nondiagnostic, 218 (18.3%) benign, and 834 (70.0%) malignant cases. Resident year by nondiagnostic outcome was not significant; p = 0.430. There were 148 major complications. Complication rate by PGY 2 – 5 was 13.0%, 13.3%, 12.9%, and 9.2%, respectively; differences were not significant, p = 0.488. Of the 139 nondiagnostic cases, 42 were re-biopsied during the study period with 81% re-classified as malignant; no repeat biopsy was observed for the remaining 97 nondiagnostic cases.
Conclusion
Of 1,191 lung/mediastinal biopsies analyzed, nearly 12% were nondiagnostic and over 12% had major complications; neither associated with resident level of experience. Outcomes were not affected significantly by level of training. Residency programs may benefit from affording opportunities for newer PGY classes to participate in procedures. Nondiagnostic cases may benefit from timely, repeat biopsies.
Keywords: internship and residency, clinical competence, image-guided biopsy, large-core needle, lung neoplasms
INTRODUCTION
Computed tomography (CT) guided transthoracic needle biopsy (TTNB) is a minimally invasive diagnostic procedure for tissue diagnosis of peripheral lung nodules.1 Since the first use of a needle to diagnose lung pathology in 1883,2 percutaneous TTNB has evolved with the fields of radiology and cytopathology to become an everyday tool in safely evaluating indeterminate pulmonary nodules or inflammatory processes.3
TTNB is less invasive and associated with lower morbidity and mortality than an open, surgical biopsy, yet it is not without complications. 4,5 Pneumothorax and hemorrhage are a common occurrence. One large meta-analysis found the overall complication rates reached 38%, with major complication rates as high as 5.7%.6 Further, there is always the risk of unsuccessful sampling. Expert consensus guidelines on quality improvement have set the risk of obtaining a nondiagnostic biopsy as high as 25% in CT-guided TTNBs.4
The successful and efficient execution of image-guided biopsies has become a U.S. competency milestone in both diagnostic and interventional radiology training.7,8 Despite the importance of achieving mastery in this technique, research is lacking for evaluating the acquisition of CT-guided TTNB procedural skills during residency training. With the adoption of the United States Preventive Services Task Force’s 2013 recommendations for annual lung cancer screening, it is important to assess this core competency for residents.9 Thus, the purpose of this study was to measure diagnostic success and complication rates of CT-guided TTNB when performed by resident physicians of varying experience levels.
METHODS
This project was approved by the institutional review board at the Wichita Medical Research & Education Foundation. Study data were collected and managed using Research Electronic Data Capture (REDCap®) electronic data capture tools hosted at the University of Kansas Medical Center.10
Study Population
A retrospective cohort study to evaluate TTNBs was conducted at an academic hospital in the heartland of the USA from July 2006 to July 2018. Inclusion criteria were individuals, either inpatient or outpatient, 18 years of age or older, who received a transthoracic, core-needle biopsy performed by resident, and with a follow-up CT scan and at least one follow-up chest x-ray to allow for adequate monitoring of post-procedural complications. Exclusion criteria were patients whose procedures were performed without a resident physician, without a follow-up CT scan, without a finalized pathology report, and those enrolled in a concurrent study aimed at prevention of pneumothorax.
Outcomes
Primary and secondary outcomes were diagnostic yield and major complication rates of TTNBs performed by residents. Diagnostic yield was dichotomized as diagnostic versus nondiagnostic, with diagnostic defined as procurement of sufficient material to establish a pathologic diagnosis or guide patient management.4 Biopsy specimens were classified as benign or malignant. Nondiagnostic biopsies were identified as such in the pathology report or if the specimen did not allow the formulation of a definitive plan for treatment. Updated diagnostic classifications from repeat biopsies also were evaluated on a subsample.
Complications were classified as major or minor. Major complications were defined as pneumothorax requiring intervention (chest tube placement or manual aspiration), hemothorax, air embolism, and needle tract seeding. Minor complications were defined as pneumothorax without need for intervention, pulmonary hemorrhage, hematoma, and transient hemoptysis.
Other patient variables of interest included age, sex, height, and weight. Because height and weight were missing from many of the patient records, a surrogate measure for body mass index and chest wall thickness, was reported. Information collected regarding the biopsy procedure included region, patient position, approach, needle size, number of cores collected, if the lesion was abutting the pleura, and total tissue traversed (measured by the entry point of the chest wall to the lesion). Lesion size was measured along its longest plane.
Because resident level of experience was important to evaluate, a bias assessment by experience was conducted utilizing all variables of interest. Resident postgraduate year was calculated using the date of the biopsy and the start date of residency based on alumni records provided by the residency program. Level of experience was classified as PGY 2, PGY 3, PGY 4, and PGY 5.
Procedure
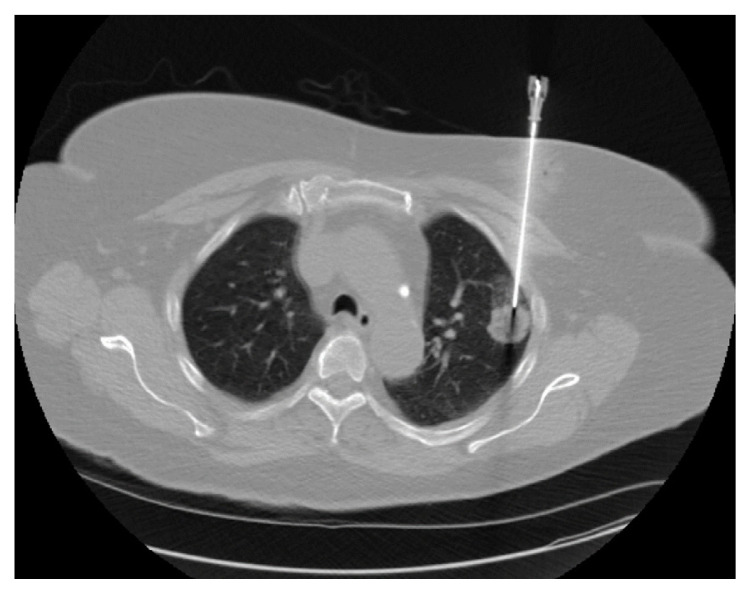
All TTNBs were performed with the patient placed in a CT scanner. The biopsy site was marked and anesthetized, and a coaxial needle was inserted percutaneously under fluoroscopic guidance (Figure 1). Biopsy of the lesion was attempted through the coaxial needle. A post-procedure CT scan was performed immediately afterward, followed by at least one chest x-ray to assess for complications. Specimens were sent to the pathology lab for processing and analysis and on-site cytology was not used during the 12-year study period.
Figure 1.
Coaxial needle placement into a peripheral lesion of the left lung.
Statistical Analysis Plan
A power analysis was conducted in IBM SPSS SamplePower 3.0 using logistic regression to test the difference in nondiagnostic yield between PGY 2 and PGY 5. Yield rates were established from a prior study evaluating fine needle aspiration biopsies of thyroid nodules;11 event rates were 14.6% for PGY 2s and 9.5% for PGY 5s. A sample size of 1,297 would be sufficient to detect a difference of 5.1% between groups with 80% power and a 5% significance level.
Data were de-identified and descriptive statistics conducted by outcome(s). Continuous variables were assessed for normality with the Kolmogorov-Smirnov test and were summarized with medians, means, and standard deviations. Bivariable associations by outcome(s) were evaluated with nonparametric Mann-Whitney U tests, Chi-square tests of independence, and, when data were sparse, by Fisher’s exact tests. Missing values were evaluated; however, due to non-randomness, no imputations were conducted.
RESULTS
Data were collected from 1,318 cases where lung biopsies were conducted (Figure 2). One case was excluded where the patient was less than 18 years of age. Forty-eight cases were excluded where a resident did not participate. Seventy-four cases were excluded where patients were participants in a clinical trial to prevent pneumothorax, and four cases were excluded in which the patient was both involved in the clinical trial and did not have a resident participating in the procedure. The analysis included 1,191 cases. Of these, 53% (626 of 1,191) patients were male and 47% (565/1,191) were female; the average age was 67 years, and median lesion size was 3.2 cm. A total of 51 residents conducted biopsies under supervision. Case distribution was 41%, 26%, 18%, and 15% for PGY 2 – 5, respectively. Percentage of nondiagnostic yield was 11.7% (139/1,191), while major complication was 12.4% (148/1,191).
Figure 2.
Participant flow.
Bivariate associations with diagnostic yield are shown in Table 1. Incidence by PGY 2 – 5 was 13.0%, 13.3%, 12.9%, and 9.2%, respectively (Figure 3). No significant results were observed by postgraduate year, p = 0.430. Significant differences were observed for patient age, lesion size, needle size, and number of biopsy cores. Incidence of benign findings on initial biopsy was 18.3% (218/1,191), and malignant was 70.0% (834/1,191). Updated classifications revealed that a total of 74.2% [(834+50)/1,191] were malignant after taking into account repeated biopsies.
Table 1.
Characteristics of patients and procedure by diagnostic yield.
| Diagnostic yield | ||||||
|---|---|---|---|---|---|---|
| Diagnostic biopsy | Nondiagnostic biopsy | |||||
| Description | n = 1,052 | 88.3% | n = 139 | 11.7% | p | |
| Sex | Male | 557 | 52.9 | 69 | 49.6 | 0.463 |
| Female | 495 | 47.1 | 70 | 50.4 | ||
| Median patient age; year; mean (SD) | 68.0; 67.0 (12.0) | 65.0; 64.0 (14.0) | 0.017* | |||
| Age group, years | < 60 | 301 | 28.6 | 53 | 38.1 | 0.021 |
| > 60 | 751 | 71.4 | 86 | 61.9 | ||
| Median chest wall thickness; mm; mean (SD) | 39.2; 41.4 (15.8) | 40.9; 42.6 (15.4) | 0.294 | |||
| Biopsy site | Lung | 1,003 | 95.3 | 132 | 95.0 | 0.843 |
| Mediastinum | 49 | 4.7 | 7 | 5.0 | ||
| Biopsy region | Right upper lobe | 285 | 28.4 | 42 | 31.8 | 0.566 |
| Left upper lobe | 241 | 24.0 | 35 | 26.5 | ||
| Right lower lobe | 217 | 21.6 | 27 | 20.5 | ||
| Left lower lobe | 193 | 19.2 | 18 | 13.6 | ||
| Right middle lobe | 67 | 6.7 | 10 | 7.6 | ||
| Median lesion size, cm; mean (SD) | 33.2; 40.5 (26.2) | 28.7; 35.9 (25.6) | 0.013* | |||
| Lesion size; cm | < 20 | 236 | 22.4 | 47 | 33.8 | 0.032 |
| 20 – 29.99 | 226 | 21.5 | 26 | 18.7 | ||
| 30 – 49.99 | 285 | 27.1 | 32 | 23.0 | ||
| ≥ 50 | 305 | 29.0 | 34 | 24.5 | ||
| Resident year** | PGY 2 | 429 | 41.1 | 55 | 39.6 | 0.430 |
| PGY 3 | 265 | 25.4 | 44 | 31.7 | ||
| PGY 4 | 195 | 18.7 | 22 | 15.8 | ||
| PGY 5 | 155 | 14.8 | 18 | 12.9 | ||
| Patient position | Prone | 470 | 44.7 | 59 | 42.4 | 0.880 |
| Supine | 432 | 41.1 | 59 | 42.4 | ||
| Other | 150 | 14.3 | 21 | 15.1 | ||
| Biopsy approach | Anterior | 332 | 31.7 | 44 | 31.7 | 0.806 |
| Posterior | 482 | 46.0 | 64 | 46.0 | ||
| Lateral | 129 | 12.3 | 20 | 14.4 | ||
| Other | 105 | 10.0 | 11 | 7.9 | ||
| Needle size (gauge) | ≤ 18 | 782 | 74.4 | 68 | 48.9 | < 0.001 |
| > 18 | 269 | 25.6 | 71 | 51.1 | ||
| Number of biopsy cores | ≤ 2 | 225 | 21.5 | 31 | 22.6 | 0.001 |
| 3 | 418 | 40.0 | 36 | 26.3 | ||
| 4 | 230 | 22.0 | 30 | 21.9 | ||
| > 4 | 173 | 16.5 | 40 | 29.2 | ||
| Lesion abutting the pleura | Yes | 611 | 58.2 | 84 | 60.4 | 0.623 |
| No | 438 | 41.8 | 55 | 39.6 | ||
| Median total tissue; mm; mean (SD) | 53.2; 56.3 (23.8) | 54.0; 57.4 (24.3) | 0.732* | |||
| Tissue traversed; mm | < 20 | 36 | 3.4 | 3 | 2.2 | 0.730 |
| 20 – 40 | 259 | 24.7 | 35 | 25.2 | ||
| > 40 | 754 | 71.9 | 101 | 72.7 | ||
Mann-Whitney U test.
Eight cases had missing resident experience level data.
Figure 3.
Diagnostic yield by resident level of experience.
Incidence of major complications included pneumothorax not requiring intervention was 32.9%, for pulmonary hemorrhage it was 20.4%, and for pneumothorax requiring intervention it was 12.0%, among others. Table 2 shows characteristics of patients, procedure, and findings by major complication. Compared to females, a greater proportion of males experienced major complications, 61.5% vs. 38.5%; p = 0.020. Major complications were observed for smaller median lesion, 25.0 cm vs. 34.1 cm, p < 0.001; and were more likely to occur when the patient position was supine, p = 0.039. Significant differences also were observed when lesions abutted the pleura, and when greater median total tissue was traversed.
Table 2.
Characteristics of patients, procedure, and findings by major complications.
| Major complications | ||||||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| Description | n = 148 | 12.4% | n = 1,043 | 87.6% | p | |
| Sex | Male | 91 | 61.5 | 535 | 51.3 | 0.020 |
| Female | 57 | 38.5 | 508 | 48.7 | ||
| Median patient age; year; mean (SD) | 67.0; 66.0 (12.0) | 68.0; 67.0 (13.0) | 0.265* | |||
| Age group, years | < 60 | 43 | 29.1 | 311 | 29.8 | 0.849 |
| > 60 | 105 | 70.9 | 732 | 70.2 | ||
| Median chest wall thickness; mm; mean (SD) | 38.2; 40.0 (13.8) | 39.8; 41.8 (16.0) | 0.264 | |||
| Biopsy site | Lung | 143 | 96.6 | 992 | 95.1 | 0.416 |
| Mediastinum | 5 | 3.4 | 51 | 4.9 | ||
| Biopsy region | Right upper lobe | 52 | 36.4 | 275 | 27.7 | 0.111 |
| Left upper lobe | 34 | 23.8 | 242 | 24.4 | ||
| Right lower lobe | 29 | 20.3 | 215 | 21.7 | ||
| Left lower lobe | 17 | 11.9 | 194 | 19.6 | ||
| Right middle lobe | 11 | 7.7 | 66 | 6.7 | ||
| Median lesion size, cm; mean (SD) | 25.0; 30.3 (17.6) | 34.1; 41.3 (26.9) | < 0.001* | |||
| Lesion size group; cm | < 20 | 49 | 33.1 | 234 | 22.4 | < 0.001 |
| 20 – 29.99 | 39 | 26.4 | 213 | 20.4 | ||
| 30 – 49.99 | 39 | 26.4 | 278 | 26.7 | ||
| ≥ 50 | 21 | 14.2 | 318 | 30.5 | ||
| Resident year** | PGY 2 | 63 | 42.6 | 421 | 40.7 | 0.575 |
| PGY 3 | 41 | 27.7 | 268 | 25.9 | ||
| PGY 4 | 28 | 18.9 | 189 | 18.3 | ||
| PGY 5 | 16 | 10.8 | 157 | 15.2 | ||
| Patient position | Prone | 57 | 38.5 | 472 | 45.3 | 0.039 |
| Supine | 75 | 50.7 | 416 | 39.9 | ||
| Other | 16 | 10.8 | 155 | 14.9 | ||
| Biopsy approach | Anterior | 56 | 38.4 | 320 | 30.7 | 0.062 |
| Posterior | 58 | 39.7 | 488 | 46.9 | ||
| Lateral | 13 | 8.9 | 136 | 13.1 | ||
| Other | 19 | 13 | 97 | 9.3 | ||
| Needle size (gauge) | ≤ 18 | 94 | 63.5 | 756 | 72.6 | 0.023 |
| > 18 | 54 | 36.5 | 286 | 27.4 | ||
| Number of biopsy cores | ≤ 2 | 41 | 27.7 | 215 | 20.8 | 0.209 |
| 3 | 53 | 35.8 | 401 | 38.7 | ||
| 4 | 33 | 22.3 | 227 | 21.9 | ||
| > 4 | 21 | 14.2 | 192 | 18.6 | ||
| Lesion abutting the pleura | Yes | 121 | 82.9 | 574 | 55.1 | < 0.001 |
| No | 25 | 17.1 | 468 | 44.9 | ||
| Median total tissue traversed; mm; mean (SD) | 65.6; 67.9 (25.1) | 52.0; 54.9 (23.3) | < 0.001* | |||
Mann-Whitney U test.
Eight cases had missing resident experience level data.
Incidence of major complication(s) by PGY 2–5 was 11.4%, 14.2%, 10.1%, and 10.4%, respectively (Figure 4). No significant results were observed by postgraduate year, p = 0.575.
Figure 4.
Major complication(s) by resident level of experience.
There was no indication of bias by resident level of experience. Bivariate results of the bias assessment for resident year showed that, except for needle size, p = 0.008, no other variables were significant, demonstrating that residents conducted biopsies on similar patients and obtained similar outcomes (results not shown).
A total of 87 patients were re-biopsied after their initial pathology reading. Of the 10 cases originally classified as malignant, all 10 were confirmed as malignant. Conversely, repeated biopsies showed that 50 patients had malignant lesions originally classified as either benign (16 cases) or nondiagnostic (36 cases), representing approximately 46% and 86% missed cancer diagnosis rate when benign or nondiagnostic biopsies were repeated, respectively. Figure 5 shows the proportion of malignancies confirmed on repeat biopsy by resident experience level. While PGY 5 had the highest proportion, they also had the fewest repeated biopsies. Last, repeated biopsies for the remaining 27 patients (originally classified as benign or nondiagnostic) were not malignant. Important, however, was the finding that no repeated biopsy was observed during the study period for 97 of the nondiagnostic cases or for 183 of the benign cases.
Figure 5.
Cancer found on repeated biopsy of originally nondiagnostic or benign tissue samples by resident level of experience.
DISCUSSION
Over the last 40 years, hundreds of articles have been published about CT-guided core needle lung biopsies. We believe that we have the first study to evaluate the role that resident seniority plays in the outcomes of such biopsies. Our results showed that an increasing level of resident experience was not associated significantly with lower nondiagnostic rates or complication rates. Similarly, the incidence of repeated biopsy and the incidence of missed malignancy discovered on repeated biopsy were not significantly different between postgraduate years. These results suggested that outcomes are not affected significantly by level of training and that programs may benefit from affording opportunities for newer PGY classes to participate in procedures.
Many previous studies have analyzed the impact of resident experience level on non-procedural competency. One study which analyzed image reading discrepancies between PGY 2 – 5 residents and the attending physician found a small but significant decrease in discrepancy rate between 1st/2nd year residents and the 3rd/4th year residents.12 As expected, the highest discrepancy rates were among the 1st and 2nd year residents. Another study looking at diagnostic discordances for diffusion-weighted magnetic resonance imaging in the emergency department found similar results with the rate of discordance between residents and attendings to be highest for the PGY 2 resident and lowest for the PGY 5 resident.13
There is scant literature describing the impact that resident experience level has on outcomes of procedures. A recent 2014 study utilized eight years of data on resident-performed fine needle aspirations of the thyroid to assess nondiagnostic rate.11 Results showed a significant inverse relationship with nondiagnostic rate and postgraduate year for residents without prior surgical training or subsequent training in interventional radiology, potentially serving as an indicator of procedural interest. Another study looked at the difference in fluoroscopy time for isolated temporary jugular central venous catheterization procedures performed by radiology residents versus attending radiologists. 14 Mean fluoroscopy time of resident catheterizations was twice as long as that of attendings with increasing years of training for residents not significantly reducing fluoroscopy time. A handful of other studies have examined the associations of resident physician experience with procedural success in procedures such as chest port placement, uterine artery embolization, electromyography needle electrode placement, and prostate biopsies.15–18 Consistent with our study, these studies failed to show any significant difference between outcomes and operator experience level.
The body of literature on the attainment of procedural skills during residency has illuminated other variables that are associated with surgical skills beyond just post-graduate year. One study identified that a resident predilection toward an observation-style of learning as opposed to an action-based style was associated with transfer to a non-surgical residency or non-physician field.19 In addition to learning style, past hobby video game play has been suggested to predict laparoscopic surgical skills.20 Another study found that interns designated for a general surgery residency program performed better in a laparoscopic skills test than their peers while older trainees were slower to develop technical skills.21
Our study found several significant bivariate associations with both major complications and nondiagnostic outcomes. Successful diagnostic biopsies were more common in older patients, with larger lesions, using needle size ≤ 18 gauge, and obtaining less than four biopsy cores. Complications were avoided more often in female patients, with larger lesions, lesions not abutting the pleural surface, using prone positioning, and traversing less tissue. These findings highlighted the importance of the role patient selection plays in the successful outcome of procedures. The pattern associated with nondiagnostic biopsies may have revealed a bias to take younger patients with non-cancerous, smaller lesions to biopsy that otherwise may have been observed if they were of older age.
Prior studies have attempted to demonstrate factors associated with diagnostic and complication rates with core needle lung biopsies. A recent meta-analysis of 32 of these studies reported small lesion size, mean patient age, use of a coaxial needle, use of biopsy device, and use of CT-fluoroscopy to all be risk factors for complications, though none reached significance.6 A previous study analyzing diagnostic accuracy similarly did not find significant risk factors for a nondiagnostic sample when using a core needle, though the depth of the lesion from the pleural surface approached significance.22 Another showed that nondiagnostic biopsies were most likely to occur when there was a moderate or high pretest probability of infection, as opposed to malignancy, though this study was performed with fine needle aspirations as opposed to core needle biopsies.23 Only a handful of studies analyzing risk factors for nondiagnostic and complication rates of core needle lung biopsies have recorded operator experience as a variable, making our study unique. One 2004 study reported radiologist experience as the third major risk factor for pneumothorax, though this study only analyzed four radiologists. 24 The studies that looked at operator experience as a variable typically reported it as an overall mean value, rather than by individual operator.6
Of the 139 biopsies classified as nondiagnostic on initial biopsy in our study, less than 1/3 were re-sampled. Over 80% of those that got re-sampled contained cancer on re-biopsy. Biopsies classified as nondiagnostic on the pathology report should be viewed with caution and integrated with other clinical information before foregoing further diagnostic testing.
Limitations
There was considerable variability at our institution in operational autonomy granted by attending physicians. Some attendings give substantial free reign to the resident, whereas others are hesitant to let even senior residents become too involved in the case. Residents in the study were on their CT rotation, thus attending-resident pairing was by chance and changed daily. We were unable to quantify how much of a role the resident played in each procedure, however, it is reasonable to presume that more senior residents likely had more procedural involvement, regardless of the attending.
As opposed to what one may think, difficult cases generally are not handled by more senior residents, but were assigned randomly a week ahead of time. We also recognized the subjective nature of our definition of a diagnostic sample: “sufficient material to establish a pathologic diagnosis or guide appropriate patient management”.4 This could be interpreted differently depending on the physician.
The number of additional days of hospitalization required with pneumothoraxes that required intervention were not recorded. This limited our understanding of which pneumothoraxes were truly major complications and falsely elevated our major complication rate. In addition, the retrospective nature of the study limited our data, preventing consistent recording of patient body mass index, smoking status, comorbidities, and resident level of interest in procedures. We also did not collect information on whether residents had completed a surgical intern year or if they eventually underwent an interventional radiology fellowship, which may have served as markers of higher interest in mastering their procedural skills. Last, the study was performed at a single institution/residency program and our procedural technique may not match that of other programs, potentially limiting its generalizability to other residency programs.
CONCLUSION
Of 1,191 biopsies analyzed, nearly 12% were nondiagnostic and over 12% had major complications; neither being associated with resident level of experience. These results suggested outcomes were not affected significantly by level of training. Programs may benefit from affording opportunities for newer PGY classes to participate in procedures. These results should provide confidence to the patients undergoing lung biopsies at an academic institution. They can be reassured that regardless of the seniority of their resident operator, they will receive an equivalent quality of care when it comes to diagnostic sampling. Furthermore, nondiagnostic cases may benefit from repeat biopsy procedure and operators should consider having a low threshold for re-biopsy.
REFERENCES
- 1.Birchard KR. Transthoracic needle biopsy. Semin Intervent Radiol. 2011;28(1):87–97. doi: 10.1055/s-0031-1273943. PMID: 22379279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leyden I. Uber infectiose pneumonie. Deutsche medicinische Wochenschrift. 1883;9:52–54. [Google Scholar]
- 3.DiBardino DM, Yarmus LB, Semaan RW. Transthoracic needle biopsy of the lung. J Thorac Dis. 2015;7(suppl 4):S304–316. doi: 10.3978/j.issn.2072-1439.2015.12.16. PMID: 26807279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta S, Wallace MJ, Cardella JF, et al. Quality improvement guidelines for percutaneous needle biopsy. J Vasc Interv Radiol. 2010;21(7):969–975. doi: 10.1016/j.jvir.2010.01.011. PMID: 20304676. [DOI] [PubMed] [Google Scholar]
- 5.Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic prostate cancer in the United States (2004–2013) Prostate Cancer Prostatic Dis. 2016;19(4):395–397. doi: 10.1038/pcan.2016.30. PMID: 27431496. [DOI] [PubMed] [Google Scholar]
- 6.Heerink WJ, de Bock GH, de Jonge GJ, Groen HJ, Vliegenthart R, Oudkerk M. Complication rates of CT-guided transthoracic lung biopsy: Meta-analysis. Eur Radiol. 2017;27(1):138–148. doi: 10.1007/s00330-016-4357-8. PMID: 27108299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Council for Graduate Medical Education. The Diagnostic Radiology Milestone Project. Jul, 2015. [Accessed March 5, 2020]. https://www.acgme.org/Portals/0/PDFs/Milestones/DiagnosticRadiologyMilestones.pdf.
- 8.American Council for Graduate Medical Education. The Interventional Radiology Milestone Project. Feb, 2016. [Accessed March 5, 2020]. https://www.acgme.org/Portals/0/PDFs/Milestones/InterventionalRadiologyMilestones.pdf?ver=2016-02-03-091337-810.
- 9.Richards TB, Doria-Rose VP, Soman A, et al. Lung Cancer screening inconsistent with U.S. Preventive Services Task Force recommendations. Am J Prev Med. 2019;56(1):66–73. doi: 10.1016/j.amepre.2018.07.030. PMID: 30467092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. PMID: 18929686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beland MD, Anderson TJ, Atalay MK, Grand DJ, Cronan JJ. Resident experience increases diagnostic rate of thyroid fine-needle aspiration biopsies. Acad Radiol. 2014;21(11):1490–1494. doi: 10.1016/j.acra.2014.06.006. PMID: 25088838. [DOI] [PubMed] [Google Scholar]
- 12.Tomich J, Retrouvey M, Shaves S. Emergency imaging discrepancy rates at a level 1 trauma center: Identifying the most common on-call resident “misses”. Emerg Radiol. 2013;20(6):499–505. doi: 10.1007/s10140-013-1146-4. PMID: 23887692. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Baek HJ, Jung HK, et al. Interpretations of diffusion-weighted MR imaging by radiology residents in the emergency department: Is diagnostic performance influenced by the level of residency training? Radiol Med. 2017;122(1):35–42. doi: 10.1007/s11547-016-0688-4. PMID: 27670660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu BJ, Duszak R, Jr, McGinnis RS, Stanfill JG, O’Rear J, An AQ. Increased fluoroscopy time for central venous catheter placement by radiology residents versus staff radiologists. J Am Coll Radiol. 2013;10(7):518–522. doi: 10.1016/j.jacr.2012.12.023. PMID: 23827004. [DOI] [PubMed] [Google Scholar]
- 15.Silas AM, Perrich KD, Hoffer EK, McNulty NJ. Complication rates and outcomes of 536 implanted subcutaneous chest ports: Do rates differ based on the primary operator’s level of training? Acad Radiol. 2010;17(4):464–467. doi: 10.1016/j.acra.2009.10.019. PMID: 20060749. [DOI] [PubMed] [Google Scholar]
- 16.Benham JR, Culp WC, Wright LB, McCowan TC. Complication rate of venous access procedures performed by a radiology practitioner assistant compared with interventional radiology physicians and supervised trainees. J Vasc Interv Radiol. 2007;18(8):1001–1004. doi: 10.1016/j.jvir.2007.05.014. PMID: 17675618. [DOI] [PubMed] [Google Scholar]
- 17.Benchikh El Fegoun A, El Atat R, Choudat L, et al. The learning curve of transrectal ultrasound-guided prostate biopsies: Implications for training programs. Urology. 2013;81(1):12–15. doi: 10.1016/j.urology.2012.06.084. PMID: 23273070. [DOI] [PubMed] [Google Scholar]
- 18.Das R, Lucatelli P, Wang H, Belli AM. Identifying the learning curve for uterine artery embolisation in an interventional radiological training unit. Cardiovasc Intervent Radiol. 2015;38(4):871–877. doi: 10.1007/s00270-014-1040-9. PMID: 25522982. [DOI] [PubMed] [Google Scholar]
- 19.Quillin RC, 3rd, Pritts TA, Hanseman DJ, Edwards MJ, Davis BR. How residents learn predicts success in surgical residency. J Surg Educ. 2013;70(6):725–730. doi: 10.1016/j.jsurg.2013.09.016. PMID: 24209648. [DOI] [PubMed] [Google Scholar]
- 20.Rosser JC, Jr, Lynch PJ, Cuddihy L, Gentile DA, Klonsky J, Merrell R. The impact of video games on training surgeons in the 21st century. Arch Surg. 2007;142(2):181–186. doi: 10.1001/archsurg.142.2.181. PMID: 17309970. [DOI] [PubMed] [Google Scholar]
- 21.Van Hove C, Perry KA, Spight DH, et al. Predictors of technical skill acquisition among resident trainees in a laparoscopic skills education program. World J Surg. 2008;32(9):1917–1921. doi: 10.1007/s00268-008-9643-4. PMID: 18553192. [DOI] [PubMed] [Google Scholar]
- 22.Anderson JM, Murchison J, Patel D. CT-guided lung biopsy: Factors influencing diagnostic yield and complication rate. Clin Radiol. 2003;58(10):791–797. doi: 10.1016/s0009-9260(03)00221-6. PMID: 14521889. [DOI] [PubMed] [Google Scholar]
- 23.Haas BM, Elicker BM, Nguyen J, et al. Nondiagnostic computed tomography-guided percutaneous lung biopsies are more likely when infection is suspected. J Thorac Imaging. 2016;31(3):151–155. doi: 10.1097/RTI.0000000000000207. PMID: 27043424. [DOI] [PubMed] [Google Scholar]
- 24.Yeow KM, Su IH, Pan KT, et al. Risk factors of pneumothorax and bleeding: Multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest. 2004;126(3):748–754. doi: 10.1378/chest.126.3.748. PMID: 15364752. [DOI] [PubMed] [Google Scholar]