Abstract
Objective:
A decrease in intracochlear electrocochleographic (ECochG) amplitude during cochlear implantation has been associated with poorer postoperative hearing preservation in several short-term studies. Here, we relate the stability of ECochG during surgery to hearing preservation at 3- and 12-months.
Methods:
Patients with hearing ≤80-dB HL at 500 Hz were implanted with a slim-straight electrode array. ECochG responses to short, high-intensity, 500-Hz pure tones of alternating polarity were recorded continuously from the apical-most electrode during implantation. No feedback was provided to the surgeon. ECochG amplitude was derived from the difference response, and implantations classified by the presence (“ECochG drop”) or absence (“no drop”) of a ≥30% reduction in ECochG amplitude during insertion. Residual hearing (relative and absolute) was reported against the ECochG class.
Results:
ECochG was recorded from 109 patients. Of these, interpretable ECochG signals were recorded from 95. Sixty-six of 95 patients had an ECochG drop during implantation. Patients with an ECochG drop had poorer preoperative hearing above 1000 Hz. Hearing preservation (in decibels, relative to preoperative levels and functionally) was significantly poorer at 250-, 500-, and 1000-Hz at 3 months in patients exhibiting an ECochG drop. Twelve-month outcomes were available from 85 patients, with significantly poorer functional hearing, and greater relative and absolute hearing loss from 250 to 1000 Hz, when an ECochG drop had been encountered.
Conclusion:
Patients exhibiting ECochG drops during implantation had significantly poorer hearing preservation 12 months later. These observational outcomes support the future development of surgical interventions responsive to real-time intracochlear ECochG. Early intervention to an ECochG drop could potentially lead to prolonged improvements in hearing preservation.
Keywords: Cochlea, Cochlear implant, Cochlear microphonic, Electrocochleography, Hearing loss
Electrocochleography (ECochG) has opened up new possibilities for monitoring cochlear function during cochlear implantation. ECochG can be recorded directly from the intracochlear electrodes, in real time during cochlear implantation (1,2). It is now possible to observe when an electrode array interferes with cochlear function, as evidenced by a reduction in the elicited ECochG response. Several types of interaction between the cochlea and the electrode that could affect hearing have been proposed. The first is a damping of basilar membrane movement by direct contact of the electrode (1,3–6), and the second is intracochlear trauma arising from contact between the electrode and the cochlear walls. Trauma can involve abrasion and/or bleeding of the endosteum or epithelium, fracture of the osseous spiral lamina, up-lifting of the basilar membrane, or translocation of the electrode (7). The long-term goal of intraoperative ECochG monitoring is to detect electrode–cochlear interactions affecting hearing as they occur, and to restrict or if possible reverse the detrimental effect upon hearing. For example, damping of the basilar membrane could potentially be released by electrode manipulation, and cochlear trauma minimized by not advancing the electrode further. But before the potential of ECochG for minimizing hearing loss can be explored, it is first necessary to establish whether changes in ECochG signal observed during surgery and hearing loss are related. In “observational” studies, where ECochG has been recorded without providing any feedback to the surgeon, we have previously reported that reductions in the amplitude of the ECochG signal (whether transient or persistent) are associated with a hearing loss 1 month after surgery (2). These preliminary results supported the notion that ECochG signal reduction is associated with a loss of residual hearing, but longer-term follow-up of a larger patient cohort has been required. Here, we present 3- and 12-month audiometric data from cochlear implant recipients who have undergone observational monitoring of ECochG during their surgeries. From these data it will be possible to determine whether ECochG monitoring informs hearing preservation over clinically-meaningful timeframes.
METHODS
This study was conducted at the Royal Victorian Eye and Ear Hospital, Melbourne, and St Vincent's Private Hospital, East Melbourne, under the auspices of Human Research Ethics Committee of the Royal Victorian Eye and Ear Hospital (#14/1171H). Eight surgeons performed the implantations. One hundred nine patients were recruited into this study.
Electrocochleography was recorded directly from the cochlear implant array, during insertion of Cochlear Limited's Nucleus CI 422 or 522 implants. These devices have a flexible, thin 25-mm-long straight (lateral wall) electrode. All patients had sufficient residual hearing to record ECochG before implantation, with all patients having a threshold of 80 dB or better at 500 Hz. Cochlear implantation was undertaken through the round window membrane incision, approached through a posterior tympanotomy. Electrodes were inserted to a depth between 20 and 25 mm, at the surgeon's discretion. The duration of the electrode insertion was not prescribed, and ranged from 60 to 120 seconds. The round window was sealed with thin fascia or periosteum. These procedures were video recorded from the operating microscope, for later review of the surgical technique.
Electrocochleography was recorded real time during implantation during cochlear implantation insertion with the University of Melbourne's ECochG system, using methods previously described (1,2,8). Briefly, cochlear potentials were recorded from the apical-most intracochlear electrode using the cochlear implant's in-built Neural Response Telemetry amplifier, in response to acoustic tones delivered via an EARTONE-3A earphone (Etymotic Research, IL). The potentials were communicated back to the laptop via the implant's telemetry system and a Universal Serial Bus Nucleus Implant Communicator with a Freedom Speech Processor. The system was controlled by in-house custom-written software, which controlled the CI using the Cochlear Device Interface libraries (4.15.02). Continuous monitoring of monopolar (MP+1) electrode impedance, interleaved with ECochG, provided a method of estimating the electrode array's insertion length. An electrode contact was deemed to have entered the cochlea when its impedance fell to measurable levels. These data were used together with the surgical videos to estimate the length of the electrode array within the cochlea at any point in time. The ECochG measurement added 5 minutes set-up time to the procedure. No extra time was required at the end of the operation because postinsertion ECochG measurements were made as the wound was being closed.
The acoustic stimulus was a 12 ms duration acoustic tone pip of 500 Hz (rise/fall times of 1 ms, presentation rate of 14 Hz) presented with alternating polarity at 100 or 110 dB Hearing Level (HL). A running average of the difference “Difference Response (DIF)” signal of the ECochG (derived by subtracting the responses to the rarefaction and condensation stimuli) was presented to the observer once a second. Common-ground electrode impedances were interleaved with the ECochG recordings, so that the point in time when more basal implant electrodes entered the perilymph could be determined. This was an observational protocol, in which the surgeons were not given any feedback on the ECochG during the electrode insertion.
Pure-tone audiometry was analyzed from before surgery, and at 3 and 12 months after implantation. In some patients a 3-month audiogram was not performed; in this situation an audiogram had been done between 6 and 10 weeks and these data were analyzed. If the hearing loss at 3 months exceeded 100 dB across frequency, the clinic protocol was not to repeat the audiogram at 12 months. For the 12-month analysis of these patients thresholds were reported as a total loss of hearing. Total hearing loss was defined on a frequency specific basis, after Skarzynski et al. (9), namely 105, 110, 120, 120, and 115 dB HL for 0.25, 0.5, 1, 2, and 4 kHz, respectively. Hearing at 3 and 12 months is presented using a variation on the “relative hearing loss” proposed by Skarzynski et al. (9). In that article, relative hearing loss was calculated on the pure-tone average. Here, we use the same approach to calculate the hearing loss at each frequency, using formula (1):
where for each frequency PostHL is the audiometric threshold at the postoperative time-point (3 or 12 mo), PreHL is the audiometric threshold preoperatively, and MaxHL is the frequency-specific total hearing loss defined above. We report both relative hearing loss and absolute loss in decibels. These data were collected between March 26, 2015 and the February 5, 2019.
Functional hearing was defined as a pure-tone average threshold across 250 and 500 Hz of 80 dB or better. This is an adaptation of the method recommended by Adunka et al. (10). We excluded 125 Hz thresholds from the average as these data were not available in our clinical records.
The intraoperative DIF ECochG traces were filtered around the fundamental frequency (0.9–1.1 times F0, with a 50th order digital bandpass filter, then a hamming window was applied over the first and last 1-ms). The response amplitude was derived by an fast Fourier transfer of the DIF response, zero-padded to 1,000 samples (for a 20-Hz bin width) at the fundamental frequency and plotted against time. Noise was measured by taking the standard deviation of 6 fast Fourier transfer bins, taken 3 bins above and below the bin containing the fundamental frequency. The DIF response was considered to contain a significant ECochG response if the amplitude was three times larger than the measured noise. The time at which each of the implant electrodes entered the perilymph was ascertained from when its impedance decreased from that indicating an open circuit to that of a closed circuit.
ECochG signals recorded from the tip-electrode usually grow in amplitude during electrode insertion (1,2,8) (Fig. 1A). The ECochG response at three time points (a,b,c) is illustrated in the lower panels. Our previous data has suggested that when the amplitude drops, even temporarily, that the postoperative hearing outcomes are poorer 1 month later (2). Here, we extend these preliminary observations, by relating ECochG amplitude drops observed during intraoperative ECochG to the postoperative hearing at 3 and 12 months. The definition of an ECochG drop in this study was a 30% reduction in the instantaneous signal amplitude of the DIF signal, once it had exceeded the noise floor, as defined above. There was no limit placed upon the time over which the 30%-amplitude reduction occurred, provided that it was observed during electrode insertion or the subsequent securing of the electrode leads into the mastoid. Therefore, the time taken for the ECochG amplitude to drop 30% could range from seconds to minutes. Figure 1B illustrates one example of an ECochG drop. A drop was said to have occurred even if the amplitude subsequently recovered, or subsequently exceeded the amplitude when the drop occurred (Fig. 1, C and D). An implantation was classed as exhibiting an ECochG drop if the patient experienced one, or more such events during their electrode insertion. The choice of a 30%-reduction as the criterion for a drop was empirical, and based upon previous observations (2), and was chosen as a change in amplitude that could potentially be detected by an observer during surgery.
FIG. 1.
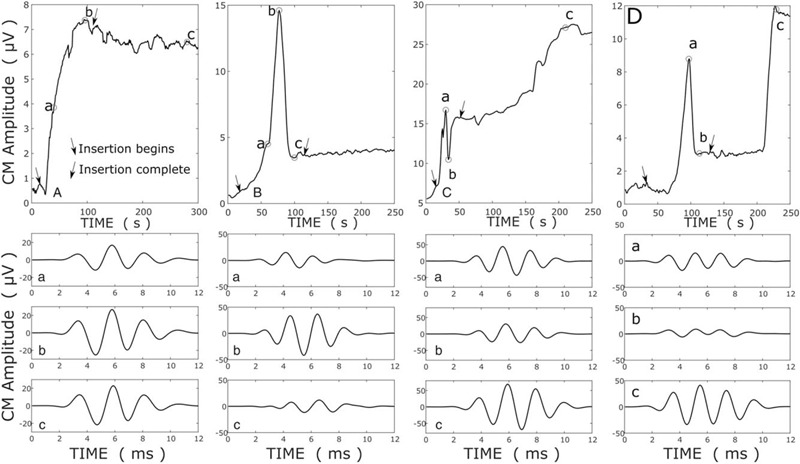
In the upper panels, ECochG amplitude growth is plotted over a routine cochlear implantation (A) and three examples of ECochG drops (B–D) are presented. The amplitude of “DIF” signal (the difference between responses to rarefaction and condensation 500 Hz tone pips) is a plotted against time from the beginning of the electrode insertion. The lower three panels show the ECochG traces at the times (a, b, c) indicated on the upper panel. ECochG indicates electrocochleographic.
RESULTS
Ninety-five of the 109 recruited patients had interpretable DIF responses during insertion, and for all data were available for analysis at the 3-month time point. Of these, the audiogram was acquired at 3 months for 75 patients and between 6 and 10 weeks for 20. Analysis of hearing outcomes was undertaken for 85 patients at 12 months. Of these, 21 patients had audiometric thresholds exceeding 100 dB across frequency at 3 months, so the audiogram was not repeated and hearing was recorded as a total loss for the 12-months analysis. Of the 10 patients in whom 12-month data were not available 4 were reviewed in regional centers via telehealth without access to audiometry, 4 patients’ care was dominated by other clinical issues such that audiometry was not performed, and 2 patients failed to attend their 12-month follow-up appointment.
A sudden drop in ECochG signal amplitude of ≥30% was observed in real-time intraoperative recordings in 69% (66 of 95) of cases. These drops occurred during advancement from 1 to 10 mm in 10 cases, 10 to 20 mm in 14 cases, and from 20 mm to full insertion in 29 cases. An additional nine cases had drops during fascia placement. In four cases we were unable to combine the video or impedance with the ECochG recordings to derive the timing of the drop. Patients exhibiting this response characteristic were classed as having experienced an ECochG “drop,” irrespective of whether the ECochG signal subsequently recovered or not (examples Fig. 1C and D). If the ECochG signal continued to increase in amplitude or remained constant throughout electrode insertion, the insertion was classed as “No drop” (Fig. 1A). Preoperative audiometric thresholds for each group are presented in Figure 2. Thresholds at 250 and 500 Hz did not differ when grouped by ECochG amplitude stability but were significantly poorer at ≥1 kHz in patients not exhibiting an ECochG drop. The scatter plot shows that this difference can be attributed to a greater number of patients having no recordable hearing in the “No drop” group. All but one patient (who experienced an ECochG drop) had functional hearing (<= 80 dB on a pure-tone average of 250 and 500 Hz) before surgery. The age at implantation did not differ with ECochG stability, with the mean age for those with ECochG drops being 68 years, and 65 years for those that did not (t test, p = 0.43).
FIG. 2.
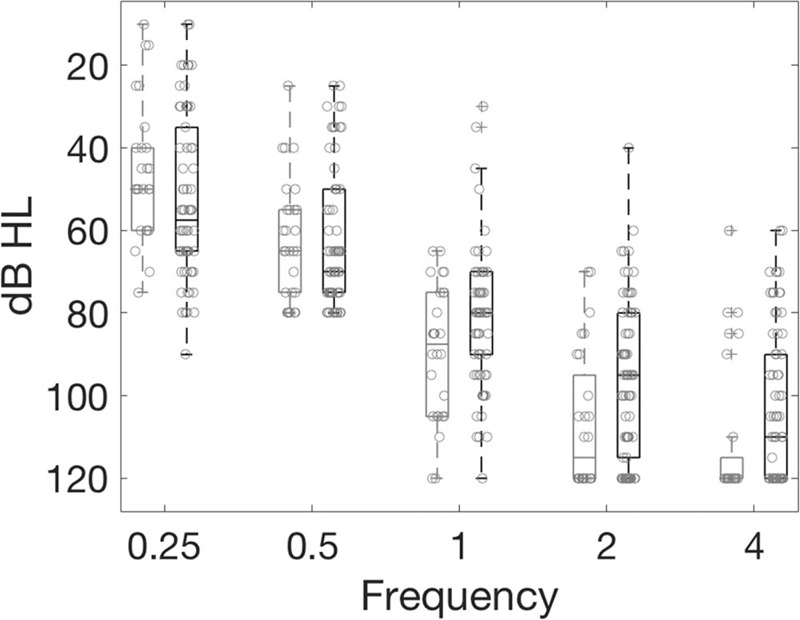
Preoperative audiometric thresholds. The light gray (left-hand) of each box plot denotes thresholds from patients in whom an ECochG drop was not observed. The black (right-hand) boxes denote data from patients with ECochG drops.
By measuring real-time ECochG, transient drops were captured in a manner that “interval” ECochG recording (i.e., recording extracochlear ECochG once preinsertion and once postinsertion) would not capture, for example Figure 1C and D. A proxy for interval ECochG recording was measured, using the maximum ECochG signal recorded divided by the final ECochG amplitude, which captured a significantly reduced number of ECochG drop patients (47 for interval, 66 with real-time, Fisher's exact test p < 0.01).
Audiometric thresholds at 3 months after surgery are plotted against preoperative hearing levels for the 95 patients with clear DIF responses during insertion and available audiometric measurements in Figure 3. It is apparent that patients exhibiting an ECochG drop were more prone to a lose all hearing, across frequency. Threshold shifts are presented as relative hearing loss, as defined in formula (1). The relative hearing lost was significantly greater in those patients experiencing ECochG drops at 250 Hz, where the median relative loss was 16.7% for no-drops and 75% when there was an ECochG drop (Kruskal Wallis, χ 2 = 15.7, p = 0.0001), at 500 Hz (drop: 29%, no-drop: 89%, χ 2 = 16.9, p < 0.0001), and 1 kHz (drop: 28%, no-drop: 80%, χ 2 = 16.9, p = 0.0015), but not 2 and 4 kHz. Similarly, the median decibel loss was greater when there was an ECochG drop at 250 Hz (drop: 25 dB, no-drop: 15 dB, χ 2 = 10.1, p = 0.0015), 500 Hz (drop: 35 dB, no-drop: 15 dB, χ 2 = 14.2, p = 0.0002), and 1 kHz (drop: 25 dB, no-drop: 10 dB, χ 2 = 13.3, p = 0.0003). Functional hearing was preserved more often in patients not experiencing an ECochG drop (χ 2 = 15.5, p < 0.0001).
FIG. 3.
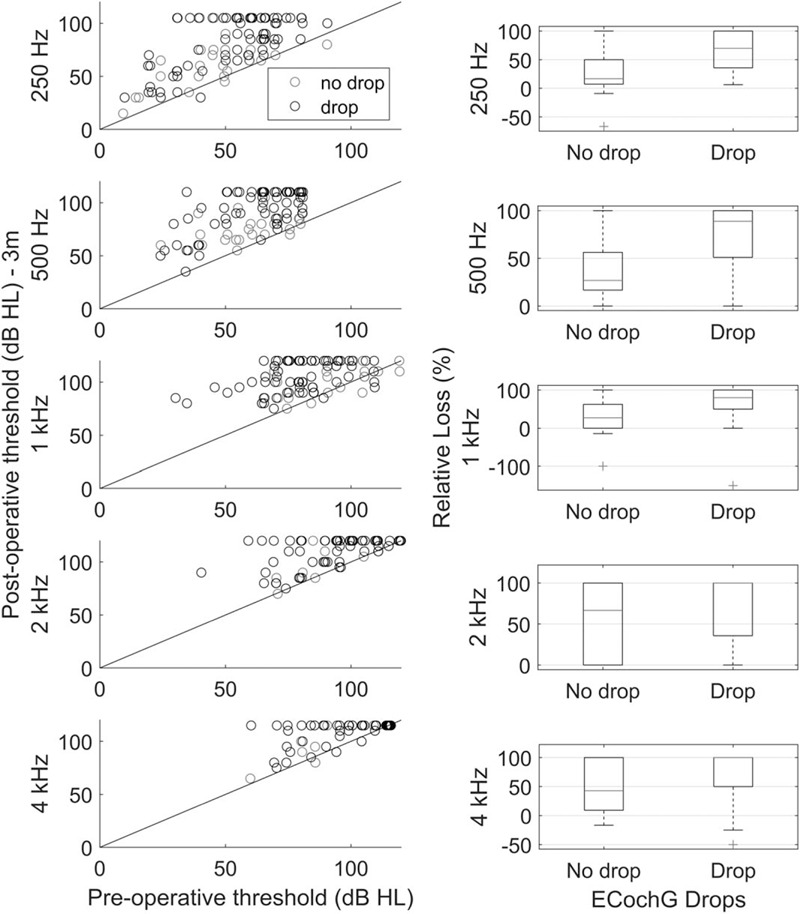
A to E, Preoperative plotted against 3-month postoperative audiometric thresholds. Data at each frequency is plotted on different rows. Red symbols are from patients who did not experience an ECochG drop, while black symbols are from patients who do exhibit a drop. The right column presents the relative hearing loss (%) at each frequency. These boxplots present median (red line), the interquartile range, with whiskers reflecting the range. Outliers are presented as red crosses. Improved hearing will have a negative value.
At 12 months (Fig. 4) there was significantly better hearing in patients who did not experience a drop in CM amplitude in surgery, compared with those who did. This was seen for relative hearing loss at 250 Hz (drop: 100%, no drop: 17%, χ 2 = 18.32, p = 0.00002), 500 Hz (drop: 100%, no drop: 33%, χ 2 = 18.65, p = 0.00005), and 1000 Hz (drop: 100%, no drop: 42%, χ 2 = 12.76, p = 0.0004), as well as for absolute hearing loss at 250 Hz (drop: 20 dB, no drop: 10 dB, χ 2 = 8.85, p = 0.0029), 500 Hz (drop: 25 dB, no drop: 15 dB, χ 2 = 9.8, p = 0.0017), and 1000 Hz (drop: 25 dB, no drop: 10 dB, χ 2 = 9.5, p = 0.0021). Similarly, functional hearing was preserved more often in patients without an ECochG drop (χ 2 = 11.76, p = 0.0006).
FIG. 4.
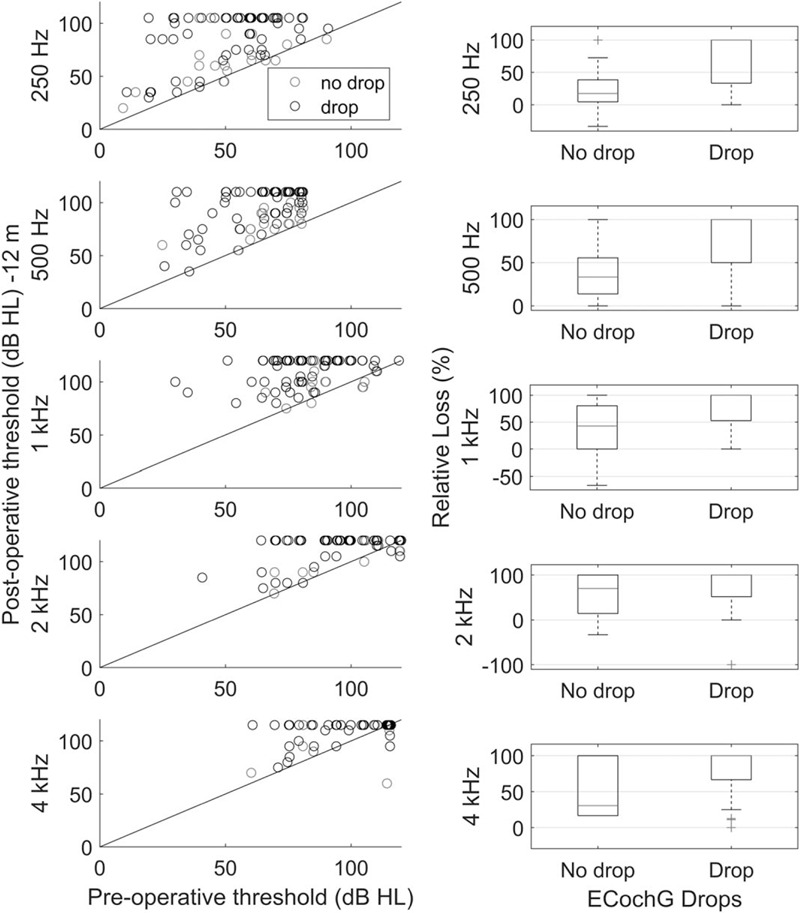
Preoperative plotted against 12-month audiometric thresholds, in the same format as Figure 3.
The change in hearing between 3 and 12 months is presented in Figure 5. This was only calculated when audiometric thresholds were recorded at both time points. The median deterioration was within 5 dB for most frequencies, but the distribution was skewed toward greater hearing loss at all frequencies.
FIG. 5.
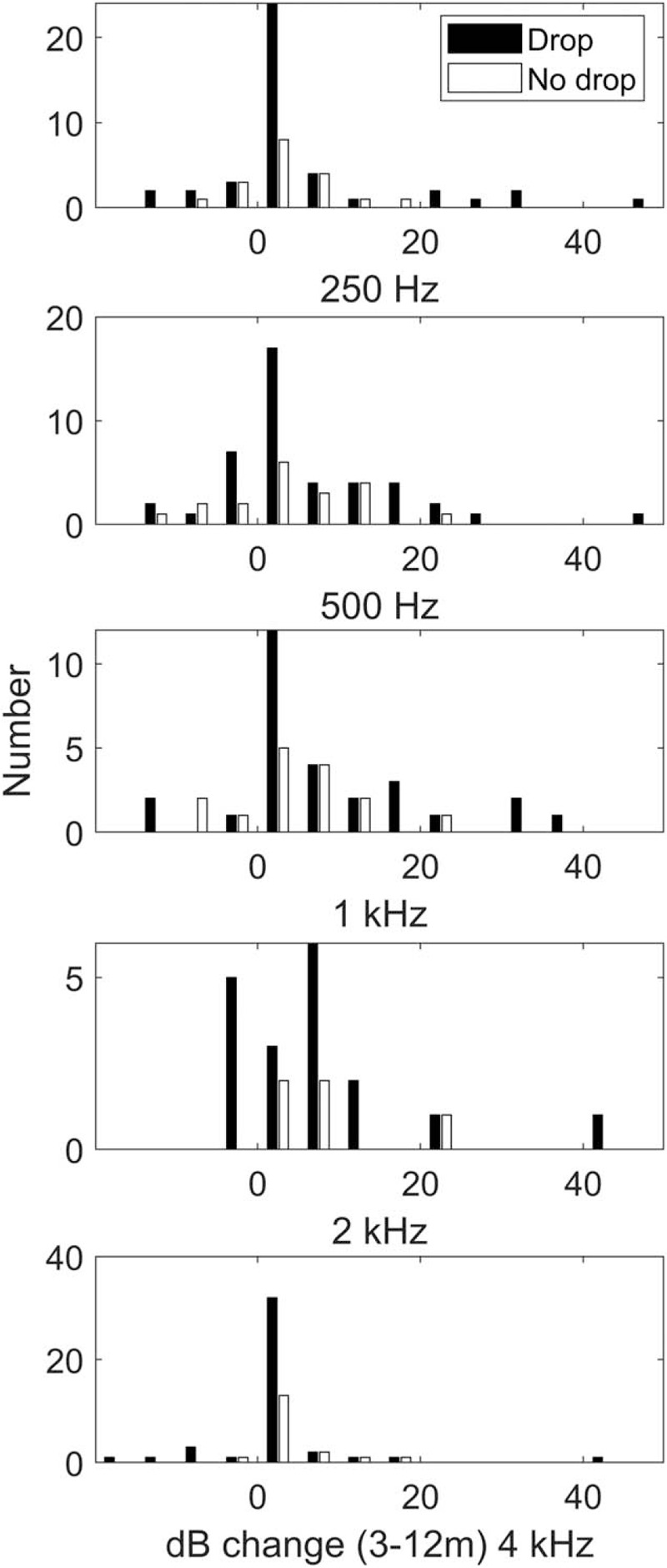
Hearing loss between 3 and 12 months after surgery, for those patients in whom audiometric thresholds could still be measured at 3 months.
DISCUSSION
A drop in ECochG amplitude during CI surgery was associated with a greater relative and absolute loss of relative residual hearing at 0.25, 0.5, and 1 kHz Hz during the first 12 months after implantation. These results confirm that real-time intraoperative ECochG monitoring informs the preservation of hearing for extended periods of time after implantation.
This report strengthens the association between a reduction in ECochG signal and loss of residual hearing loss, reported previously from our group (2). It shows that a relatively simple metric, namely a drop in ECochG amplitude that could be detected during surgery, predicts hearing loss. A recent report suggests that the sensitivity and specificity of hearing-loss detection with intracochlear ECochG may be increased further by detailed analysis of the interactions between the response to the first harmonic (mainly derived from the cochlear microphonic) and the second harmonic (primarily the auditory neurophonic) (11), although the implementation of these methods real-time during surgery could be challenging. Another approach taken has been to determine intraoperative ECochG thresholds, which have been found to relate to postoperative preservation of residual hearing (12). While informative, this approach could not be used to provide real-time intraoperative monitoring because threshold estimation is quite time consuming.
The patient cohort reported here were older adults, with a mean age exceeding 65 years. The cohort is skewed toward older adults because of the exclusion of children. Age did not differ between those exhibiting ECochG drops and those who did not, so it is unlikely to have impacted upon the results reported here; especially when it is considered that hearing preservation is reported to be more strongly correlated with preoperative hearing than age (13).
The preoperative hearing of patients exhibiting ECochG drops was poorer at ≥1 kHz than for patients in whom an ECochG drop was not observed. This is perhaps not surprising, given that intracochlear ECochG records cochlear potentials in the vicinity of the electrode. Therefore, when hearing is poorer, there is less chance that a detectable ECochG response will be recorded, especially when the hearing is at profound levels. This observation has important ramifications for ECochG monitoring; if the patient has a “ski-slope” audiogram, then it may not be possible to measure ECochG until toward the end of the insertion (when the electrode tip is nearing a region with residual hair cells), and clearly it will not be possible to detect a drop in ECochG until then. This drawback is alleviated appreciably by the majority of drops occurring past 10-mm in insertion (85%, or 52/62 with known ECochG drop timing), at which point the majority of our patients showed a significant ECochG signal. An alternative explanation for these observations is that low-frequency hearing is more robust in patients with ski-slope audiograms. That better preoperative low-frequency hearing predicts greater postoperative speech perception has been reported (13), but how this could prevent a transient ECochG drop is not clear.
This study provides a basis for considering the use of ECochG for surgical decision making; that is, to change the operative approach if an ECochG drop occurs. The results presented here show the implications for residual hearing if nothing is done. Assuming that an ECochG drop reflects either interference with basilar membrane motion by contact with the electrode (4), or a traumatic interaction between the electrode and intracochlear structures (7), it is conceivable that the effect on hearing could be minimized or even reversed if the surgeon was aware of the ECochG change. For slim-straight electrodes, interventions such as electrode withdrawal or stopping the insertion may achieve these ends. If surgical intervention in response to an ECochG drop could achieve similar hearing preservation (≅15 dB) and duration of effect (12 mo) to that we have observed in the no-drop situation, then clinically meaningful hearing might be preserved. Whether this can be achieved will need to be tested by clinical trials.
Hearing deteriorated between 3 and 12 months in approximately half of the patients, irrespective of whether the ECochG fluctuated during surgery or not. It is apparent from the available data that the disturbance in cochlear function observed by ECochG during surgery is not a biomarker for a delayed hearing loss that emerges after 3 months. While not in itself conclusive, these observations argue against delayed hearing loss being predetermined by electrode insertion trauma. We had thought that this may have been a possibility, given that trauma and subsequent postoperative inflammation and fibrosis are related (7), and the latter might have predisposes to factors thought to contribute delayed hearing loss such as chronic inflammation (7,14), endolymphatic hydrops (15–17), or synaptopathy (18).
It is pertinent to note that real-time monitoring is required to detect ECochG drops, as CM drops occur rapidly and may also recover quickly. Interval or pre/post ECochG monitoring does not capture these transient events, and in our data it is estimated that this would miss 26% of the transient drops detected. Similarly, the sometimes rapid ECochG fluctuations seen here are not often observed in extracochlear ECochG recordings (3), where very different patterns of ECochG amplitude loss and recovery are encountered. It is clearly argued in Weder et al. (19) that these transient drops have just as significant an effect on postoperative hearing preservation as permanent drops. It seems likely, therefore, that the observations made here may not be directly applicable to the extracochlear ECochG approaches. There are however some similarities; a loss of ECochG amplitude (i.e., an ECochG drop without recovery) observed during surgery predicts postoperative hearing loss during extracochlear ECochG (3).
Footnotes
S.O. is funded by the National Health and Medical Research Council (Australia). The corresponding author's University receives research grants from Cochlear Ltd. This research was conducted at the Royal Victorian Eye and Ear Hospital and funded by the National Health and Medical Research Council (Australia).
The authors disclose no conflicts of interest.
REFERENCES
- 1. Campbell L, Kaicer A, Briggs R, et al. Cochlear response telemetry: Intracochlear electrocochleography via cochlear implant neural response telemetry pilot study results. Otol Neurotol 2016; 36:399 405. [DOI] [PubMed] [Google Scholar]
- 2. Campbell L, Kaicer A, Sly D, et al. Intraoperative real-time cochlear response telemetry predicts hearing preservation in cochlear implantation. Otol Neurotol 2016; 37:332 338. [DOI] [PubMed] [Google Scholar]
- 3. Dalbert A, Pfiffner F, Röösli C, et al. Extra- and intracochlear electrocochleography in cochlear implant recipients. Audiol Neurotol 2015; 20:339 348. [DOI] [PubMed] [Google Scholar]
- 4. Verberne J, Risi F, Campbell L, et al. The effect of scala tympani morphology on basilar membrane contact with a straight electrode array: A human temporal bone study. Otol Neurotol 2017; 38:47 53. [DOI] [PubMed] [Google Scholar]
- 5. Choudhury B, Adunka OF, Demason CE, et al. Detection of intracochlear damage with cochlear implantation in a gerbil model of hearing loss. Otol Neurotol 2011; 32:1370 1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giardina CK, Khan TE, Pulver SH, et al. Response changes during insertion of a cochlear implant using extracochlear electrocochleography. Ear Hear 2018; 39:1146 1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O’Leary SJ, Monksfield P, Kel G, et al. Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hear Res 2013; 298:27 35. [DOI] [PubMed] [Google Scholar]
- 8. Bester CW, Campbell L, Dragovic A, et al. Characterizing electrocochleography in cochlear implant recipients with residual low-frequency hearing. Front Neurosci 2017; 11:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skarzynski H, Van De Heyning P, Agrawal S, et al. Towards a consensus on a hearing preservation classification system. Acta Otolaryngol 2013; 133:3 13. [DOI] [PubMed] [Google Scholar]
- 10. Adunka OF, Gantz BJ, Dunn C, et al. Minimum reporting standards for adult cochlear implantation. Otolaryngol Head Neck Surg 2018; 159:215 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giardina CK, Brown KD, Adunka OF, et al. Intracochlear electrocochleography: Response patterns during cochlear implantation and hearing preservation. Ear Hear 2019; 40:833 848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haumann S, Imsiecke M, Bauernfeind G, et al. Monitoring of the inner ear function during and after cochlear implant insertion using electrocochleography. Trends Hear 2019; 23:2331216519833567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moran M, Dowell RC, Iseli C, et al. Hearing preservation outcomes for 139 cochlear implant recipients using a thin straight electrode array. Otol Neurotol 2017; 38:678 684. [DOI] [PubMed] [Google Scholar]
- 14. Seyyedi M, Nadol JB., Jr Intracochlear inflammatory response to cochlear implant electrodes in humans. Otol Neurotol 2014; 35:1545 1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee J, Ismail H, Lee JH, et al. Effect of both local and systemically administered dexamethasone on long-term hearing and tissue response in a Guinea pig model of cochlear implantation. Audiol Neurootol 2013; 18:392 405. [DOI] [PubMed] [Google Scholar]
- 16. Smeds H, Eastwood HT, Hampson AJ, et al. Endolymphatic hydrops is prevalent in the first weeks following cochlear implantation. Hear Res 2015; 327:48 57. [DOI] [PubMed] [Google Scholar]
- 17. Handzel O, Burgess BJ, Nadol JB., Jr Histopathology of the peripheral vestibular system after cochlear implantation in the human. Otol Neurotol 2006; 27:57 64. [DOI] [PubMed] [Google Scholar]
- 18. Reiss LA, Stark G, Nguyen-Huynh AT, et al. Morphological correlates of hearing loss after cochlear implantation and electro-acoustic stimulation in a hearing-impaired Guinea pig model. Hear Res 2015; 327:163 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weder S, Bester C, Collins A, Shaul C, Briggs, O’Leary SJ. Towards a Better Understanding of Electrocochleography: Analysis of Real-time Recordings. Ear and Hearing 2020; In press. [DOI] [PubMed] [Google Scholar]


