Abstract
Clear cell renal cell carcinoma (ccRCC) represents the most common subtype of renal cell carcinoma (RCC). In spite of recent advances in the treatment armamentarium and outcomes with the combined use of immune checkpoint and angiogenesis inhibitors, prediction of responses and selection of patients remain a challenge. This is a case of ccRCC with recurrence to the liver 1 year following right radical nephrectomy, who rapidly progressed on frontline therapy with axitinib/pembrolizumab. The clinical course and targeted tumor sequencing findings are discussed. In addition to established clinical prognostication in RCC, several surrogate markers of efficacy or/and resistance have been proposed for immunotherapy or/and anti-angiogenic therapy. Since the majority of patients will still progress after these combinations, it is becoming increasingly important to develop robust predictive biomarkers to guide patient selection and sequencing of targeted therapies.
Keywords: angiogenesis, molecular biomarker, mutation, immunotherapy, renal cell carcinoma
Introduction
Recent advances in the frontline therapy of advanced renal cell carcinoma (RCC) have led to novel combinations of agents targeting programmed death-1/programmed death-1 ligand (PD-1/PD-L1) and the angiogenesis/vascular endothelial growth factor receptor (VEGFR) pathway. The anti PD-1 monoclonal antibody (Mab) pembrolizumab (anti-PD-1) and the anti-PD-L1 avelumab were both FDA approved, each in combination with the VEGFR tyrosine kinase inhibitor (TKI) axitinib for previously untreated patients with advanced RCC, after improving the objective response rate (ORR), progression-free survival (PFS), or/and overall survival (OS), compared to the prior standard, sunitinib (1–3). This benefit was observed across all International Metastatic Renal Cell Carcinoma Database Consortium risk groups (favorable, intermediate, and poor risk) (1–4). In addition, the combination of the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor ipilimumab and the PD-1 inhibitor nivolumab was approved for intermediate- and poor-risk, patients with advanced RCC (5). Nevertheless, metastatic RCC remains a lethal disease overall, with a 5-year survival of approximately 10–20% (6), and a proportion of patients will still progress in spite of optimal therapy. At present, the majority of clinicians are not using any predictive biomarkers for treatment decision-making (7). A small proportion (10.9–12.4%) of advanced RCC patients treated with frontline PD-1/VEGFR-targeted combination therapy have demonstrated progressive disease as best response in the two major phase III studies of axitinib/pembrolizumab and axitinib/avelumab, respectively (1–3). The underlying biology of this intrinsic resistance is poorly understood. Herein, the clinical and genomic evaluation of a case of advanced refractory RCC with lack of response to first-line axitinib/pembrolizumab is presented. The aim of molecular analysis of a liver metastasis from this patient was to assess for somatic alterations that could potentially be indicators of primary resistance to the combination or/and sensitivity to other agents.
Case Report
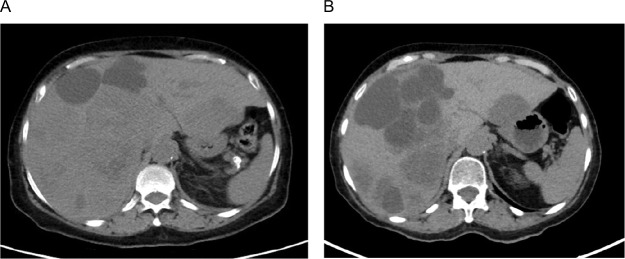
A 70-year-old woman presented with worsening right upper quadrant pain of 2 weeks duration, associated with anorexia and 10 lb weight loss. She had a history of right nephrectomy 13 months earlier for a grade 3, stage III pT3aNxM0 clear cell renal cell carcinoma (ccRCC), with extension to the renal vein and uninvolved margins; however, she did not follow up after surgery. No family history of cancer was reported. A computed tomography (CT) scan of her abdomen and pelvis disclosed hepatomegaly and several hypodense lesions throughout the liver parenchyma consistent with liver metastases (Figure 1A). Labs were remarkable for a hemoglobin level of 11.2 g/dL and an elevated serum lactate dehydrogenase level of 612 U/L.
Figure 1:
(A) Computed tomography (CT) abdomen and pelvis (pre-treatment) showing hepatomegaly and several hypodense lesions throughout the liver parenchyma consistent with liver metastases. (B) CT abdomen and pelvis (post-treatment) showing enlargement of existing lesions and new hepatic metastases.
The patient was started on pembrolizumab 200 mg intravenously every 3 weeks and axitinib 5 mg orally twice daily as first-line therapy for her recurrent/metastatic disease after completing full restaging, which was otherwise negative. She required dose reduction of her axitinib to 3 mg twice daily due to fatigue, and experienced elevated thyroid stimulating hormone (TSH) of 16.5 mcIU/mL (normal free T4), which improved on levothyroxine. Unfortunately, after completing 12 weeks on combination axitinib/pembrolizumab, repeat CT scans for assessment of response disclosed progression of liver metastases (Figure 1B).
A left hepatic lobe lesion was biopsied under CT guidance. Four core specimens were obtained. Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) were extracted from macrodissected, paraffin-embedded tumor of the patient using the Maxwell 16 instrument (Promega, Madison, WI) and RecoverAll Total Nucleid Acid Isolation Kit (Life Technologies, Walltham, MA), respectively. The extracted DNA and synthesized complementary DNA (cDNA) from the extracted RNA were amplified by the Oncomine Comprehensive Panel (OCP) and subjected to Next Generation Sequencing (NGS) using the Ion Torrent S5TM (Life Technologies), as previously described (8). OCP was developed, and its performance characteristics were determined by the Clinical Genomics Laboratory, Englander Institute for Precision Medicine, Department of Pathology and Laboratory Medicine at Weill Cornell Medicine, New York-Presbyterian Hospital. OCP is approved by the New York State Department of Health (NYS-DOH). Targeted tumor sequencing of patient’s CT-guided liver biopsy showed loss of function (pathogenic) mutations in von Hippel-Lindau (VHL) and BRCA1-associated protein (BAP1) genes, as well as copy-number loss of cyclin-dependent kinase inhibitor 2A (CDKN2A) (Table 1). Tumor purity (neoplastic content) of the tissue analyzed was 80%.
Table 1:
Genomic alterations (pathogenic) of liver metastasis from clear cell renal cell carcinoma.
| Gene | Alteration | Type | VAF |
|---|---|---|---|
| VHL | c.162_163insCG, p.Glu55Argfs*13 | Mutation – frameshift insertion | 32.8% |
| BAP1 | c.509T>G, p.Phe170Cys | Mutation – missense | 42.6% |
| CDKN2A | loss | Copy number alteration – loss | N/A |
VAF: variant allele frequency, N/A: not applicable, VHL, von Hippel-Lindau, BAP1, BRCA1-associated protein, CDKN2A, cyclin-dependent kinase inhibitor 2A.
Discussion
Resistance to systemic therapies in advanced RCC, either intrinsic due to presence of resistant clones or acquired after initial tumor regression can directly impact the clinical course and additional treatment approach of these patients in contemporary practice.
While the underlying mechanisms are a field of ongoing investigation, numerous studies have identified molecular alterations in primary and metastatic RCC tumors that may be contributing to the development of resistance (9).
The VHL gene is the most frequently mutated gene in the majority (80–90%) of sporadic RCCs (9–11). Mechanistically, the loss of VHL protein function leads to the accumulation of hypoxia-inducible factor (HIF) that promotes angiogenesis and tumor growth (9, 11). It has been found that the effect of VHL mutation on responses to VEGFR TKIs in patients with metastatic ccRCC is minimal, if any (12). Furthermore, although VHL is an inducer of PD-L1 through upregulation of HIF-2α (13), PD-L1 immunohistochemical expression status had no effect on ORR and PFS in neither of the two large phase 3 trials testing the PD-1/PD-L1 plus VEGFR pathway inhibitors combination (1–3). Other studies have demonstrated that overexpression and activation of the receptor tyrosine kinases MET and AXL due to VHL inactivation is implicated in resistance to VEGFR-targeted therapies (14). Specifically, combination of axitinib with the c-met inhibitor crizotinib in RCC patient-derived xenograft models resulted in decreased tumor microvessel density, growth inhibition, and improved survival (15).
The BAP1 gene is mutated in different cancers, including ccRCC in up to 11% of cases (10). The resultant loss of function of this tumor suppressor was associated with more aggressive morphologic features (16) and decreased OS both in The Cancer Genome Atlas (TCGA) RCC cohort and within the ccRCC group (10). Besides its prognostic relevance, BAP1 loss in non-RCC tumors was correlated with upregulation of suppressive immune gene responses, for example, HLA-DR, CD38, and CD74 (uveal melanoma) (17), and the promotion of an inflammatory tumor microenvironment (peritoneal mesothelioma) (18). Interestingly, an integrated biomarker analysis of 412 RCC patients who were treated on the phase 3 COMPARZ trial comparing pazopanib versus sunitinib (19) demonstrated that those with tumors harboring mutated BAP1 had inferior OS compared to wild-type ones (20). Angiogenesis gene expression scores from a 43-gene signature were lower in patients with BAP1-mutated tumors, suggesting that BAP1 loss correlates with lower angiogenic signaling and worse outcomes after treatment with TKIs (20). Overall, both BAP1 and VHL deleterious/loss of function mutations were identified in hyperprogressive tumors post anti-PD-1 treatment, suggesting a potential involvement in the development of resistance (21).
CDKN2A is a ubiquitously expressed gene that encodes two other key genes, the INK4 family member p16 (p16INK4a) and p14ARF, both of which act as tumor suppressors regulating the cell cycle. CDKN2A gene aberrations, including mutations, hypermethylation, or deletions, occur in approximately 16% of ccRCCs (10), with loss of the chromosome 9p region encoding CDKN2A being the most frequent event (12%) in these cases (10). CDKN2A loss is associated with a higher tumor stage at diagnosis (22) and predicts shorter OS across all TCGA RCC subtypes (10). Patients with tumors that harbor chromosomal CDKN2A loss are frequently characterized by resistance to immunotherapy, which has been mechanistically explained, at least partially, by a concurrent deletion of the interferon-gamma (IFNγ) signaling pathway gene JAK2 (23). In addition, copy-number variations of CDKN2A or/and other genes in the CDK4 pathway (CDK4, CCND1) are associated with innate resistance to anti-PD-1 therapy in patients with advanced melanoma, which can be reversed with addition of CDK4/6 inhibitors to anti-PD-1 antibodies (24). Interestingly, CDK4/6 inhibitors have shown activity in RCC in vitro, and the combination of abemaciclib with sunitinib resulted in a dramatic reduction in tumor sizes in a RCC mouse model (25). A phase 1 trial of these two agents is currently ongoing in progressing patients with metastatic ccRCC (NCT03905889).
Collectively, treatment of RCC patients with disease progression after axitinib/pembrolizumab or axitinib/avelumab is complex, and the current expert consensus agreement is on the use of another VEGFR pathway inhibitor, cabozantinib, which also has activity against MET, AXL, and RET kinases (7). A potential predictive value of T-effector gene expression and angiogenesis signatures has been supported from exploratory biomarker analyses of PD-1/PD-L1 plus VEGFR TKI combination studies (26–28); however, this is largely hypothesis-generating and not ready for clinical use. It also remains ill-defined as to how potential genomic biomarkers could be combined with the established clinical prognostication tools (the IMDC, the Cleveland Clinic Foundation CCF model, the International Kidney Cancer Working Group IKCWG model, the French model, and the Memorial Sloan-Kettering Cancer Center model) (4) to improve our prediction of effective therapies. In phase 2 and 3 studies of the anti-PD-L1 Mab atezolizumab combined with the anti-VEGF-A Mab bevacizumab, expression of angiogenesis genes was enriched in patients with favorable risk, compared to those with intermediate and poor risk according to the MSKCC risk stratification model (28).
Conclusion
This case of advanced refractory RCC with clinical and genomic evaluation highlights an unmet need for better characterizing the underlying biology of treatment-resistant RCC in order to improve our ability to conduct biology- and biomarker-driven trials to help guide selection of the right drug, for the right target, in the right patient, at the right time.
Footnotes
How to cite: Panagiotis J. Vlachostergios. Resistance to pembrolizumab and axitinib in renal cell carcinoma: clinical and genomic evaluation. J Kidney Cancer VHL. 2020;7(1): 7–11.
Conflict of interest
The author declares no potential conflicts of interest with respect to research, authorship, and/or publication of this article.
References
- 1.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019. March 21;380(12):1116–27. 10.1056/NEJMoa1816714 [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019. March 21;380(12):1103–15. 10.1056/NEJMoa1816047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choueiri TK, Motzer RJ, Rini BI, Haanen J, Campbell MT, Venugopal B, et al. Updated efficacy results from the JAVELIN renal 101 trial: First-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol. 2020. April 24 pii: 10.1016/j.annonc.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol. 2013. February;14(2):141–8. 10.1016/S1470-2045(12)70559-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018. April 5;378(14):1277–90. 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NIH, National Cancer Institute, Surveillance Epidemiology and End Results Program. Cancer Stat Facts: Kidney and Renal Pelvis Cancer [Internet]. [Accessed 2020 May 1]. Available from: https://seer.cancer.gov/statfacts/html/kidrp.html
- 7.Rini BI, Battle D, Figlin RA, George DJ, Hammers H, Hutson T, et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of advanced renal cell carcinoma (RCC). J Immunother Cancer. 2019. December 20;7(1):354 10.1186/s40425-019-0813-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park K, Tran H, Eng KW, Ramazanoglu S, Marrero Rolon RM, Scognamiglio T, et al. Performance characteristics of a targeted sequencing platform for simultaneous detection of single nucleotide variants, insertions/deletions, copy number alterations, and gene fusions in cancer genome. Arch Pathol Lab Med. 2020. February 11 10.5858/arpa.2019-0162-OA [DOI] [PubMed] [Google Scholar]
- 9.Makhov P, Joshi S, Ghatalia P, Kutikov A, Uzzo RG, Kolenko VM. Resistance to systemic therapies in clear cell renal cell carcinoma: Mechanisms and management strategies. Mol Cancer Ther. 2018. July;17(7):1355–64. 10.1158/1535-7163.MCT-17-1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricketts CJ, De Cubas AA, Fan H, Smith CC, Lang M, Reznik E, et al. The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep. 2018. April 3;23(1):313–26.e5. 10.1016/j.celrep.2018.03.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh JJ, Le VH, Oyama T, Ricketts CJ, Ho TH, Cheng EH. Chromosome 3p loss-orchestrated VHL, HIF, and epigenetic deregulation in clear cell renal cell carcinoma. J Clin Oncol. 2018. October 29:JCO2018792549 10.1200/JCO.2018.79.2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choueiri TK, Vaziri SA, Jaeger E, Elson P, Wood L, Bhalla IP, et al. von Hippel-Lindau gene status and response to vascular endothelial growth factor targeted therapy for metastatic clear cell renal cell carcinoma. J Urol. 2008. September;180(3):860–5; discussion 865–6. [DOI] [PubMed] [Google Scholar]
- 13.Messai Y, Gad S, Noman MZ, Le Teuff G, Couve S, Janji B, et al. Renal cell carcinoma programmed death-ligand 1, a new direct target of hypoxia-inducible factor-2 alpha, is regulated by von Hippel-Lindau gene mutation status. Eur Urol. 2016. October;70(4):623–32. 10.1016/j.eururo.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 14.Rankin EB, Fuh KC, Castellini L, Viswanathan K, Finger EC, Diep AN, et al. Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc Natl Acad Sci U S A. 2014;111:13373–8. 10.1073/pnas.1404848111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciamporcero E, Miles KM, Adelaiye R, Ramakrishnan S, Shen L, Ku S, et al. Combination strategy targeting VEGF and HGF/c-met in human renal cell carcinoma models. Mol Cancer Ther. 2015;14:101–10. 10.1158/1535-7163.MCT-14-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu YF, Cohn S, Christie A, McKenzie T, Wolff N, Do QN, et al. Modeling renal cell carcinoma in mice: Bap1 and Pbrm1 inactivation drive tumor grade. Cancer Discov. 2017. August;7(8):900–17. 10.1158/2159-8290.CD-17-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Figueiredo CR, Kalirai H, Sacco JJ, Azevedo RA, Duckworth A, Slupsky JR, et al. Loss of BAP1 expression is associated with an immunosuppressive microenvironment in uveal melanoma, with implications for immunotherapy development. J Pathol. 2020. April;250(4):420–39. 10.1002/path.5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shrestha R, Nabavi N, Lin YY, Mo F, Anderson S, Volik S, et al. BAP1 haploinsufficiency predicts a distinct immunogenic class of malignant peritoneal mesothelioma. Genome Med. 2019. February 18;11(1):8 10.1186/s13073-019-0620-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013. August 22;369(8):722–31. 10.1056/NEJMoa1303989 [DOI] [PubMed] [Google Scholar]
- 20.Voss MH, Kuo F, Chen D, Marker M, Patel P, Redzematovic A, et al. Integrated biomarker analysis for 412 renal cell cancer (RCC) patients (pts) treated on the phase 3 COMPARZ trial: Correlating common mutation events in PBRM1 and BAP1 with angiogenesis expression signatures and outcomes on tyrosine kinase inhibitor (TKI) therapy. J Clin Oncol. 2017. May 20;35(15_Suppl):4523–3. 10.1200/JCO.2017.35.15_suppl.4523 [DOI] [Google Scholar]
- 21.Xiong D, Wang Y, Singavi AK, Mackinnon AC, George B, You M. Immunogenomic landscape contributes to hyperprogressive disease after anti-PD-1 immunotherapy for cancer. iScience. 2018. November 30;9:258–77. 10.1016/j.isci.2018.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nouhaud FX, Blanchard F, Sesboue R, Flaman JM, Sabourin JC, Pfister C. Clinical relevance of gene copy number variation in metastatic clear cell renal cell carcinoma. Clin Genitourin Cancer. 2018. August;16(4):e795–805. 10.1016/j.clgc.2018.02.013 [DOI] [PubMed] [Google Scholar]
- 23.Horn S, Leonardelli S, Sucker A, Schadendorf D, Griewank KG, Paschen A. Tumor CDKN2A-associated JAK2 loss and susceptibility to immunotherapy resistance. J Natl Cancer Inst. 2018. June 1;110(6):677–81. 10.1093/jnci/djx271 [DOI] [PubMed] [Google Scholar]
- 24.Yu J, Yan J, Guo Q, Chi Z, Tang B, Zheng B, et al. Genetic aberrations in the CDK4 pathway are associated with innate resistance to PD-1 blockade in Chinese patients with non-cutaneous melanoma. Clin Cancer Res. 2019. November 1;25(21):6511–23. 10.1158/1078-0432.CCR-19-0475 [DOI] [PubMed] [Google Scholar]
- 25.Small J, Washburn E, Millington K, Zhu J, Holder SL. The addition of abemaciclib to sunitinib induces regression of renal cell carcinoma xenograft tumors. Oncotarget. 2017. July 27;8(56):95116–34. 10.18632/oncotarget.19618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24(6):749–57. 10.1038/s41591-018-0235-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choueiri TK, Albiges L, Haanen JBAG, Larkin JMG, Uemura M, Pal SK, et al. Biomarker analyses from JAVELIN renal 101: Avelumab + axitinib (A+Ax) versus sunitinib (S) in advanced renal cell carcinoma (aRCC). J Clin Oncol. 2019;May 20;37(15_Suppl):101–1. 10.1200/JCO.2019.37.15_suppl.101 [DOI] [Google Scholar]
- 28.Rini B, Huseni M, Atkins MB, McDermott DF, Powles TB, Escudier B, et al. Molecular correlates differentiate response to atezolizumab + bevacizumab vs sunitinib. Ann Oncol. 2018. October 20;29(Suppl_8):LBA31 10.1093/annonc/mdy424.037 [DOI] [Google Scholar]