Abstract
As the population ages, the incidence and prevalence of neurodegenerative disorders will continue to increase. Persons with neurodegenerative disease frequently experience sleep disorders, which not only affect quality of life, but potentially accelerate progression of the disease. Unfortunately, pharmacological interventions are often futile or have adverse effects. Therefore, investigation of non-pharmacological interventions has the potential to expand the treatment landscape for these disorders. The last decade has observed increasing recognition of the beneficial role of exercise in brain diseases, and neurodegenerative disorders in particular. In this review, we will focus on the therapeutic role of exercise for sleep dysfunction in four neurodegenerative diseases, namely Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis. Available data suggest that exercise may have the potential to improve sleep disorders and attenuate neurodegeneration, particularly in Alzheimer’s disease and Parkinson’s disease. However, additional research is required in order to understand the most effective exercise therapy for these indications; the best way to monitor the response to interventions; the influence of exercise on sleep dysfunction in Huntington’s disease and amyotrophic lateral sclerosis; and the mechanisms underlying exercise-induced sleep modifications.
Keywords: Sleep, Exercise, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis
INTRODUCTION:
Neurodegenerative disorders (NDD), such as Alzheimer’s disease (AD), Parkinson’s disease (PD) Huntington’s disease (HD) and amyotrophic lateral sclerosis (ALS), are characteristically defined by protein accumulation, synaptic loss or dysfunction of a specific group of neurons, and anatomic susceptibility leading to neuronal dysfunction and death1,2. Although the existing diagnostic gold standard is a neuropathological assessment at autopsy, NDD are clinically diagnosed based on their main phenotypic features. For example, AD includes problems with recent memory, word-finding, and language difficulties, with slow progression to global cognitive impairment and functional impairment3. In PD, diagnosis is made based on motor symptoms, which include bradykinesia, rest tremor, rigidity, and postural instability4. Persons with PD also experience non-motor symptoms, including cognitive decline, autonomic dysfunction, neuropsychiatric symptoms, and sleep disorders. The seminal features of HD are a triad of progressive cognitive decline, motor dysfunction (chorea, akathisia, bradykinesia, spasticity) and psychiatric symptoms and are confirmed by a genetic testing revealing a trinucleotide repeat expansion5,6. ALS involves degeneration of alpha motor neurons, resulting in both upper motor neuron signs (increased muscle tone, spastic paresis, and pseudobulbar palsy) and lower motor neuron signs (fasciculations, atrophy, and weakness)7. Sleep disorders occur in all these NDD and are correlated with a higher occurrence of both cognitive and neuropsychiatric problems affecting the quality of life of patients and their caregivers8.
Many persons with NDD suffer from sleep disorders8. The exact pathogenesis underlying sleep dysfunction in NDD is unknown (Figure 1), but it can be categorized into primary and secondary mechanisms. Primary mechanisms include loss or morphological modifications of the neurons in the hypothalamus or caudal brainstem involved in sleep-wake generation, which changes the equilibrium between sleep and wakefulness and results in circadian rhythm disorders, insomnia, sleep fragmentation, hypersomnia, or parasomnia9. Secondary or indirect mechanisms include poor sleep hygiene, environmental factors, psychiatric co-morbidity, medication side effects, sleep-related breathing disorders, physical immobility, or increased frequency of nocturia, which results in sleep-maintenance insomnia, fatigue, irritability, and daytime somnolence10. Treatment of sleep disorders is complex and also important, as there is increasing evidence that inadequate sleep leads to accelerated progression of NDD and possibly plays a role in their pathogenesis11. Unfortunately, medications used to treat these symptoms are often not sufficiently effective and can cause intolerable adverse effects12. Non-pharmacologic interventions such as light therapy, cognitive behavioral therapy, behavioral modifications, and exercise have the potential to change the treatment landscape in NDD. While additional research is needed, many of these interventions have shown promise for potential to improve sleep in NDD13–15.
Fig. 1.
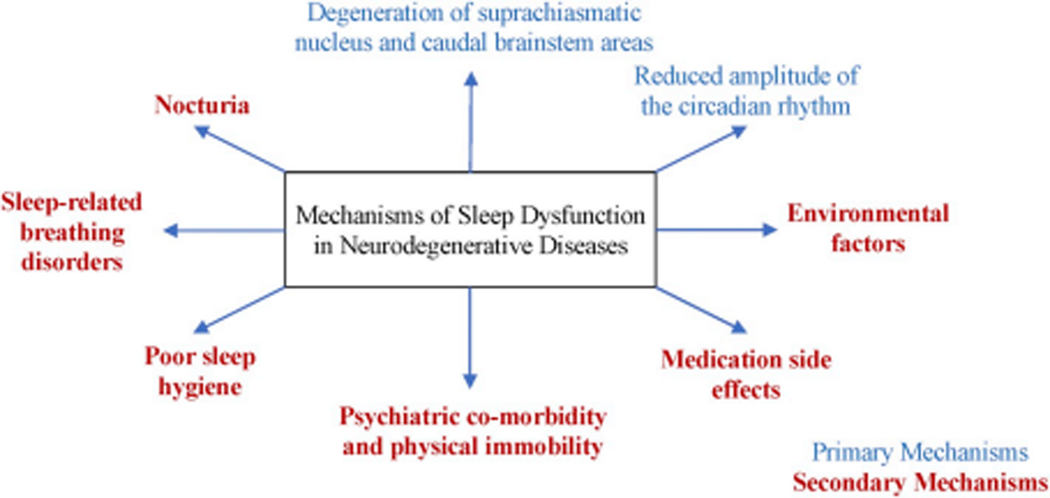
Potential mechanisms of sleep dysfunction in neurodegenerative disease
The last decade has witnessed a growing appreciation of the beneficial role of exercise in NDD as a non-pharmacological therapeutic intervention. Besides preventing brain tissue loss, exercise has shown moderate improvements in subjective sleep quality in older adults with sleep problems16. In this review, we discuss the available evidence supporting the use of exercise for the treatment of sleep disorders in four neurodegenerative disorders.
EFFECTS OF EXERCISE ON SLEEP IN HEALTHY ADULTS
The basis for investigation of potential exercise-induced sleep benefit in NDD stems from prior work evaluating the influence of exercise on sleep in healthy populations. Different exercise modalities, intensities, and durations have been explored in healthy adults, and the type of exercise may shape outcomes (i.e. endurance training impacts aerobic fitness and resistance training improves strength). While cardiorespiratory (aerobic) and resistance (anaerobic) exercise are most often discussed, other modalities such as flexibility exercises or neuromotor exercises emphasizing balance, proprioception, coordination, and agility have also been explored in the research setting17. Incorporating a cognitive component into exercise (mind-body exercise) with exercises such as Tai chi or Qigong may have additional benefit18. Other considerations that influence research outcomes include duration of the intervention, ranging from acute (single bout) to regular (chronic training)19. Many forms of exercise, including both acute and regular exercise as well as both aerobic and resistance training improve subjective sleep quality16,20. Studies exploring objective sleep outcomes due to exercise are more limited than survey based analyses; however, it is known that acute exercise enhances both total sleep time and slow wave sleep, reduces REM sleep, and delays REM latency21. Additionally, chronic exercise training improves sleep efficiency, decreases latency to sleep onset, and increases total sleep time and slow wave sleep19,22.
Several potential mechanisms for these beneficial sleep changes have been proposed, including exercise-induced reduction of inflammation, alterations of core body temperature, changes in neurotransmitters important for sleep regulation, increases in growth hormone and brain derived neurotrophic factor (BDNF), and changes in heart rate variability and autonomic function19,22–25. Additionally, exercise can promote entrainment of the circadian rhythm for improved sleep/wake regulation26. In mammalian models, timing of scheduled daily exercise can change the time of peak heart rate and body temperature as well as adrenal activity27–29. Human studies suggest that both acute bouts of exercise and chronic timed exercise interventions can modulate circadian function including shifting the onset of melatonin excretion, changing temperature regulation, and promoting phase shift of sleep-wake activity cycle30–32. Although there is the potential that some aspects of aging or neurodegeneration could blunt or alter the exercise-induced sleep benefit in these populations, evidence discussed in this review suggest that these benefits are maintained.
ALZHEIMER’S DISEASE
Sleep disorders in Alzheimer’s disease
Sleep disorders are frequent in AD, affecting up to 45% of patients33,34. Sleep disturbance can be present in the early stages of AD, and its severity parallels the severity of dementia35,36. Sleep disorders in AD include trouble with falling or staying asleep, wandering, sleep fragmentation, and excessive daytime sleepiness33,37. AD patients also have alterations in sleep architecture, including prolonged REM sleep latency and decreased percentage of slow wave and REM sleep38. As the disease progresses, other findings include poorly formed sleep spindles and K-complexes, which makes it difficult to distinguish between stages N1 and N2 on electroencephalography39. Although these variations in sleep patterns can be components of the normal aging process, AD causes more degeneration of these patterns than in age matched control subjects40. Sleep dysfunction in AD enhances the probability of physical and psychological morbidity in both patients and their caregivers and is correlated with increased risk of early institutionalization41–49. Disrupted sleep not only negatively influences the quality of life of patients, but also adversely impacts caregivers46,50.
The cause of sleep disorders in AD is considered to be multifactorial. Proposed mechanisms (Figure 1) involve the degeneration of neural pathways controlling sleep-wake patterns, including the suprachiasmatic nucleus that regulates circadian function, as well as reduced expression of neuropeptides such as Arginine Vasopressin (AVP) and Vasoactive intestinal peptide (VIP), and changes in melatonin receptor (MT1) expression51. Additional mechanisms include underlying psychiatric and medical comorbidities, medication-related side effects, and altered light/dark patterns during the day or night34,52. Sleep interruption has also been proposed to hasten the advancement of AD through malfunctioning of the glymphatic system53 because sleep performs a crucial function in clearing noxious protein accumulation, such as beta-amyloid, from the central nervous system54–56.
In the forthcoming years, the aging population is expected to cause a dramatic worldwide increase in the number of people living with AD57. Thus, cost-effective interventions to improve disrupted sleep in individuals with AD are essential for the benefit of global economics as well as patient health. Pharmacological therapies for the treatment of sleep disorders in AD have limited efficacy and can be associated with severe adverse effects such as hypotension, dizziness, and falls58–61. Therefore, development of safe non-pharmacological approaches for treating sleep disorders in AD is a critical unmet need. Exercise and physical therapy are attractive treatment options for sleep dysfunction as these have already shown potential to improve cognition in AD62.
Impact of Exercise on Sleep in Alzheimer’s disease
Similar to findings in healthy adults, exercise has shown potential for improving sleep dysfunction in AD as well. Most published research on this topic describes observational studies evaluating the relationship between physical activity and sleep in AD. In 59 patients with dementia evaluated cross-sectionally with the Mini-Sleep Questionnaire, those who participated in regular physical activity had fewer sleep complaints compared to those who had lower levels of physical activity63. Another observational, cross-sectional study in 184 participants with dementia examined the influence of exercise (defined as number of hours per week of walking) on sleep quality and sundowning64. The findings showed that walking with relatives as compared to non-relatives and walking more hours per week was associated with less sundowning and with improved sleep quality in AD64. One limitation of this study was its reliance on caregiver recall64. Exercise has also been shown to delay or prevent the onset of behavioral problems in demented frail older people living in nursing homes. For example, one pilot longitudinal study showed that the combination of aerobic/endurance activities, strength training, balance, and flexibility training statistically reduced behavioral problems, including sleep disorders in dementia patients65.
Although additional study is needed, there have been a few randomized, controlled trials (RCT) investigating effects of exercise on sleep outcomes in AD. One RCT examined the benefits of physical activity (N=32), light exposure (N=34), and combination treatment (walking, light exposure, and guided sleep education) (N=33) for improving sleep dysfunction in AD compared to a contact control group (N=33). The primary outcome included actigraphically-measured total wake time at night. The exercise intervention included 30 minutes of continuous walking every day. Compared to the contact control group, participants in each active treatment arm had shorter actigraphic total wake time post-intervention. Patients in the exercise group were awake 33.1 fewer minutes per night compared to the control, suggesting that exercise improved objectively measured sleep66. Another RCT, the nighttime insomnia treatment and education for Alzheimer’s disease (NITE-AD) trial, included thirty-six community-dwelling patients with AD and their caregivers. Caregivers in both the active treatment arm (N=17) and the control arm (N=19) received specific suggestions regarding establishing and employing sleep hygiene programs. The caregivers in the active treatment arm were also encouraged to have patients walk 30 minutes daily and increase daytime light exposure. Patients in the NITE-AD active arm showed a significant reduction in the number of nighttime awakenings, total time awake at night, and depression. These benefits were continued up to six months of follow-up67. A pilot study revised the NITE-AD program and analyzed the intervention in a group rather than individual format with caregivers. The time frame in the modified NITE-AD program was six weeks instead of two months in the original NITE-AD program. While this program was pilot and only included seven participants, all showed reduced frequency, severity, and distress on the Sleep Disorder Inventory68. Another controlled study evaluated the effects of multimodal exercise conducted regularly over six months on instrumental activities of daily living (IADL) and subjective sleep disturbances, measured with the Mini-Sleep Questionnaire, in persons with AD69. Participants in the active exercise arm (N=19) had one-hour exercise sessions three times per week for six months compared to no exercise in the control group (N=16). Those in the exercise group showed improvement in IADLs and subjective sleep quality compared to the control group69.
In summary, several studies suggest that exercise has the potential to improve sleep in persons with AD. However, to our knowledge, there are no RCTs or observational studies investigating the relationship between physical activity and polysomnographically-measured sleep outcomes in this patient population. More study is needed to fill this gap and to determine the potential mechanisms underlying exercise-induced changes in sleep (Figure 2) as well as the optimal exercise modality, duration, and intensity to better optimize sleep in persons with AD.
Fig. 2.
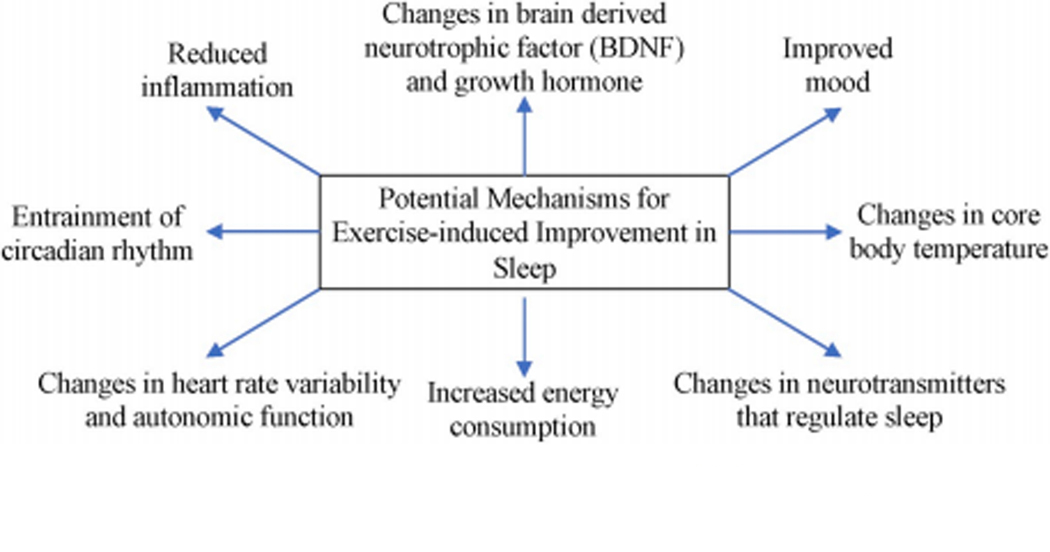
Potential machanisms for exercise-induced improvement In sleep.
PARKINSON’S DISEASE
Sleep Disorders in Parkinson’s Disease
Sleep disorders are nearly universal among patients with PD and include sleep fragmentation, REM sleep behavior disorder, insomnia, excessive daytime sleepiness (EDS), periodic limb movements of sleep, and circadian rhythm dysregulation70,71. In contrast to studies in AD, persons with PD have reduced amplitude of the circadian rhythm without any significant shift in circadian phases72. Compared to healthy older adults, persons with PD also have changes in sleep architecture, with reduced sleep efficiency and total sleep time and decreased amounts of REM and slow wave sleep71,73,74. Motor symptoms occurring during the night, as well as nocturia, constipation, mood disorders, and other non-motor symptoms of PD also contribute to insomnia and sleep fragmentation75. However, treatment of motor symptoms with dopaminergic therapy can also contribute to sleep dysfunction and daytime somnolence. For example, dopaminergic medications can alter circadian physiology. In one analysis of medication naïve patients with PD, introduction of levodopa caused an increase in melatonin production while concurrently delaying sleep onset76. Unfortunately, sleep dysfunction contributes to impaired quality of life and is associated with worse motor, cognitive, autonomic, and neuropsychiatric dysfunction in PD71,74,77–79.
Although poor sleep is a common source of morbidity in PD, interventions for treatment are limited. Available medications such as sedatives/hypnotics, antipsychotics, and antidepressants can have an intolerable side effect profiles in this population of patients who are at risk for balance and cognitive dysfunction. The sleep benefits of non-pharmacologic interventions are therefore of proportionally greater interest. Cognitive-behavioral therapy is recommended for treatment of insomnia by the American Academy of Sleep Medicine and may be beneficial in patients with PD13,80. Further, light therapy for circadian reconditioning demonstrates benefits on both sleep and mood in PD13.
Exercise is emerging as a beneficial nonpharmacologic aspect of PD care81–83. The effects of exercise on PD are wide ranging and include improvements in quality of life, motor symptoms, balance, strength, and endurance as well as skeletal muscle adaptions83,84. Non-motor symptoms also improve, with benefits on cognition, depression, constipation, and anxiety82,85. Similarly, the effects of exercise on sleep symptoms are beginning to be recognized.
Impact of Exercise on Sleep in Parkinson’s Disease
Several studies have evaluated the impact of exercise on subjective sleep quality in PD. One study retrospectively analyzed subjective sleep quality, as measured by the Parkinson’s Disease Sleep Scale (PDSS), in patients with PD who underwent multidisciplinary intensive rehabilitation treatment (MIRT) in an inpatient setting over 4 weeks compared to patients who did not undergo rehabilitation. The MIRT group had 3 one-hour sessions per day, five days per week, over four weeks. These sessions included a wide range of exercises including aerobic exercises, relaxation techniques, stretching, stabilometric platform exercises focusing on balance and gait, occupational therapy, and other exercises tailored to the individual patient such as speech therapy, hydrotherapy, and robotic-assisted walking training. The persons with PD in the MIRT group (N=89) demonstrated significant improvement in PDSS scores in all items except for two, which were in normal ranges prior to the intervention (distressing hallucinations during the night and daytime sleepiness) compared to patients who were kept on pharmacologic therapy only without rehabilitation (N=49)86.
Similar exercise-induced sleep quality improvement was found in a trial in which PD participants were assigned either to a no-exercise control group (N=19) or an intervention group (N=23) with three times weekly, 1-hour multimodal exercise sessions administered by physical education professionals over a 6-month intervention period69. The exercises were of moderate intensity aerobic exercise achieving 60 – 80% of maximal heart rate and utilizing multimodal exercises designed to benefit balance, resistance, and flexibility. The intervention arm demonstrated marked improvement in the Brazilian version of the Mini-Sleep Questionnaire while the control group did not change69.
Other less intensive interventions with more emphasis on cognitive outcomes have also been reported. One such therapy, Qigong, is a form of mild intensity exercise which incorporates mindfulness as well as balancing. In a pilot study, 7 patients with PD were compared before and after an 8-week program incorporating twice daily sessions of 15 – 20 minutes of Qigong exercise as well as weekly one-hour class-based interventions with an instructor. Participants showed a small but non-significant improvement in the PDSS87. A more robust analysis of this intervention was performed with an RCT comparing daily walking for 30 minutes (N=44) to an intervention with 4 instructor-led sessions of 45 minutes duration followed by four times weekly home-based Qigong sessions lasting approximately 20 minutes (N=45). After 6 months, the Qigong intervention group reported significant improvement in their PDSS-2 while controls did not experience a change88. A small pilot study89 investigated the effects of Qigong exercise on pro-inflammatory cytokines including IL-6, IL-1β, and TNF-α, which have been found to be increased in PD and have also been implicated in sleep-wake regulation90–93. Participants with mild to moderate PD were enrolled into either the control group (n=5) or the intervention group (n=5), which performed Qigong exercises twice daily at home for 15–20 min and also attended a group exercise session weekly for 45 – 60 min for a duration of 6 weeks. The control group maintained the same schedule with sham-Qigong which consisted of the same physical activities without the deep breathing or meditative components. Sleep quality was assessed subjectively with PDSS-2 which found statistically significant improvement in PD symptoms at night as well as improved overall PDSS-2 score in the intervention group compared to the control group. Additionally, TNF-α levels were found to be statistically lower in the intervention group89.
Another study investigated the effects of Tai Chi, a low intensity form of exercise focusing on slow smooth movements with concurrent breathing and meditative tasks, on subjective sleep quality in persons with PD. Participants were randomized to one of two interventions over 12 weeks: 1) three weekly sessions of Tai Chi supplemented by 2 weekly sessions of multimodal exercises including both aerobic and resistance training (N=19) or 2) five weekly sessions of multimodal exercise alone (N=22). Both groups demonstrated improvement in PDSS and PD motor symptoms, though there was no difference in outcomes between the intervention groups94.
Other forms of exercise have also been studied. A small RCT studied the effects of resistance training three times weekly for 12 weeks on subjective sleep quality. The intervention group (N=11) demonstrated improved sleep quality as measured by the PSQI compared to controls (N=11). In fact, at the end of this intervention, they reported better sleep quality than a group of age matched healthy controls95.
Many exercise trials are confounded by the social aspect of the study design. There is a potential bias if patients are actually experiencing benefit because of the cognitive stimulation that a group setting provides. In another study utilizing Tai Chi, the relative value of group setting compared to individual tutelage was evaluated in a small randomized controlled trial. Patients with PD received the exercise either individually (N=17) or in a group setting (N=19) three days a week for 13 weeks. There was significant though similar improvement in their questionnaire-based sleep evaluation in both groups compared to baseline. Though there was a trend toward more improvement in the group-based intervention, improvement was not statistically different between groups. The group-based intervention did have significantly better compliance96.
Most published studies of sleep outcomes incorporate only questionnaire-based assessment tools. However, a subanalysis of a trial of persons with PD incorporated activity monitoring with a triaxial accelerometer97. In this intervention lasting 6 months, an exercise group received twice weekly, hour-long exercise sessions (30 minutes resistance training plus 30 minutes of endurance training) (N=29) compared to a control group assigned to a handwriting task designed to mimic the cognitive tasks involved in exercise (N=36). The quality of sleep was evaluated both by analysis of sleep related questions from UPDRS parts I and II, as well as accelerometer data during periods of rest and activity. There was no improvement subjectively on questionnaires in either group, but both groups demonstrated decreased accelerometry-measured motor activity during sedentary times, thought to represent improved sleep quality with decreased restlessness or awakenings97.
We recently reported the first RCT investigating the impact of exercise on objective sleep outcomes measured with polysomnography in PD98. Persons with PD were randomized to either high-intensity progressive resistance training rehabilitation (N=27) or a no-exercise, sleep hygiene control group (N=28). Participants in the exercise group had supervised exercise training 3 times per week for 16 weeks, with a combination of resistance training (RT) and bodyweight functional mobility exercises with limited rest intervals designed to challenge strength, power, balance, and endurance. The sleep hygiene group received suggestions for improving sleep hygiene through discussion with a board-certified sleep medicine physician and were provided written materials. The exercise group had significant improvement in sleep efficiency (primary outcome), as well as total sleep time, wake after sleep onset, and time spent in slow wave sleep, compared to the sleep hygiene group98.
In summary, multiple different exercise modalities have potential to improve sleep quality in PD. However, more study is needed with dedicated RCTs addressing sleep as a primary outcome. The exercise modality, frequency, duration, and intensity needed for optimization of sleep is not known. Additionally, more investigations of objective sleep outcomes measured with polysomnography as well as additional study on influence of exercise on circadian rhythms in PD are needed.
HUNTINGTON’S DISEASE
Sleep Disorders in Huntington’s disease
HD is an autosomal dominant trinucleotide repeat neurodegenerative disorder that causes motor dysfunction (chorea, bradykinesia, rigidity, spasticity, tics, akathisia), progressive cognitive decline, and psychiatric symptoms. Persons with HD also have significant sleep and circadian dysfunction99. Mechanisms underlying circadian abnormalities in HD are largely unknown, and there is conflicting evidence for how melatonin secretion changes in HD. In one study, there was no difference in mean daytime melatonin level between HD patients and controls, but persons with HD had delayed onset of the diurnal melatonin rise100. Another study found reduced and flattening of the circadian rhythm of the 24-hour melatonin concentration101. Some of these circadian changes may be related to neurodegeneration within the hypothalamus and suprachiasmatic nucleus102. In addition to circadian changes, sleep disturbances are present in up to 90% of persons with HD and include insomnia, frequent awakenings, and daytime sleepiness99. In a meta-analysis of 7 studies that have measured laboratory-based polysomnography (PSG) in HD and control participants, a pooled sample of 152 HD and 144 controls were evaluated103. This analysis showed that, compared to controls, persons with HD had reduced sleep efficiency, lower percentage of slow wave sleep and REM sleep, increased latency to sleep onset, and increased light sleep and wake after sleep onset103. Some of these abnormalities were influenced by advanced age, longer CAG repeat length, and lower BMI103. In some cases, sleep disturbances measured by polysomnography in HD are often not concordant with subjective sleep complaints104. These changes in sleep architecture have been noted even in pre-manifest HD (mutation carriers prior to onset of subjective symptoms) and early HD105. Findings of REM sleep behavior disorders have also been reported in HD105. Sleep complaints in HD have been associated with worse depression, anxiety and disinhibition compared to those without sleep complaints and waking later in the morning is associated with worse cognitive impairment106,107. Treatments for sleep dysfunction in HD are limited.
Impact of Exercise on Sleep in Huntington’s disease
Although exercise has been investigated as an intervention for HD motor symptoms and quality of life, few studies have evaluated how exercise influences sleep in HD108,109. One controlled study explored the impact of a 9-month multimodal intervention (exercise, cognitive and dual task training, and social events) on sleep, daytime sleepiness, hypothalamic volume, melatonin, cortisol, and BDNF levels in patients with premanifest and prodromal HD110. Eighteen HD participants received the intervention and were compared to 11 HD participants in the control group. The exercise component of the intervention included twice weekly exercise sessions with 30 minutes of aerobic training and 30 minutes of resistance training. Sleep was measured subjectively with questionnaires including PSQI, Epworth Sleepiness Scale (ESS), and Consensus Sleep Diary. The results showed the hypothalamic volume loss was less in the intervention group compared to the control group and serum BDNF decreased in the control group but not in the intervention group110. There were no differences between groups in terms of changes in melatonin or cortisol levels. There was no difference between groups in change in PSQI-assessed subjective sleep quality or ESS-assessed daytime sleepiness. Participants in the control group reported increases in diary-recorded total sleep time and time spent in bed, while there were no changes in sleep habits in the intervention participants110. Given the lack of concordance between objective and subjective sleep in HD, this study would have been strengthened by inclusion of polysomnography or actigraphy104. Another cross-sectional study by the same group showed that, although persons with premanifest HD had lower physical activity levels compared to controls, there was no relationship between reported physical activity levels and subjective sleep quality111. In an uncontrolled study of influence of aquatherapy (one-on-one, individually-tailored sessions offered twice weekly for 6 weeks) on quality of life in mid- to late-stage HD, some of the 6 participants reported improvement in sleep quality in a 30-minute, structured post-intervention interview with open ended questions112.
In summary, available information about the impact of exercise on sleep dysfunction in HD is sparse. Additional studies, particularly RCTs with objective outcome measures, are needed to determine the impact of exercise and rehabilitation interventions on sleep and circadian function in HD.
AMYOTROPHIC LATERAL SCLEROSIS
Sleep Disorders in amyotrophic lateral sclerosis
ALS is a progressive neurodegenerative illness, which involves a wide-ranging clinical continuum, affecting both upper and lower motor neurons7,113. In a subcategory of patients, cognitive and behavioral defects or overt frontotemporal dementia is associated with the motor system deterioration114. Persons with ALS also have significant sleep disorders, which are often under-diagnosed or under-reported. Recent recognition of their significant role in affecting the quality of life in ALS patients has prompted increased attention to sleep dysfunction in these patients115,116. Sleep disorders are present in up to 59% of persons with ALS117 and include increased time spent awake during the sleep period, excessive daytime sleepiness, sleep-disordered breathing, and a substantial reduction in the quantity of deep sleep and REM sleep118. Insomnia (65%) and sleep-disordered breathing (52.5%) were the two most common sleep disorders reported in one observational study117. These disturbances are due to an extended range of considerations including nocturnal hypoventilation, restless legs syndrome, obstructive sleep apnea, difficulty positioning in bed due to lack of mobility, cramps, depressed mood, psychological stress, excessive secretions, and choking115,119,120. Nocturnal hypoventilation results from weakness of respiratory, bulbar, or diaphragmatic musculature, a challenge which is likely intensified by supine posture and failure of accessory respiratory muscle function during REM sleep. Unfortunately, ALS is demoralizing and fatal121, with limited treatment options. A recent review showed that endurance and/or resistance exercise have potential beneficial effects to improve quality of life in ALS, but without any known extension of life expectancy122. However, to our knowledge, no research to date has explored the impact of conventional exercise on sleep disorders in ALS. Hence, further investigations are necessary to determine any role of exercise in treating sleep dysfunction in ALS patients.
DISCUSSION
Persons with NDD frequently report sleep dysfunction, which is challenging to manage and decreases quality of life for both patients and caregivers. Physical activity, even at low intensities, has been reported to improve sleep quality, reduce time to fall asleep, and increase the duration of sleep in the elderly123,124. The precise mechanisms through which exercise benefits sleep-related disorders are still being explored, but evidence indicates that exercise increases total sleep time and slow-wave sleep, reduces REM, and delays REM sleep onset125. It is thought that these beneficial effects on sleep architecture are achieved in part by enhancement of numerous neurotransmitter systems, including norepinephrine and serotonin afferents to the hippocampus, and also by exercise-induced BDNF upregulation (Figure 2)126,127.
There is growing evidence that interrupted sleep accelerates progression of neurodegenerative disease. In this review, we discuss the evidence for potential exercise-induced improvements in sleep dysfunction, particularly in AD and PD. Unfortunately, there are considerations specific to persons with these NDD that may limit participation in exercise. For example, specific symptoms such as motor symptoms of PD and the risk of falls, cognitive impairment, excessive daytime sleepiness, depression, apathy, cardiac sympathetic denervation, and fatigue can decrease involvement in physical activity and lead to a more sedentary lifestyle128–132. Devising approaches to increase commitment to routine exercise poses a challenge for both researchers and caregivers. A few methods to enhance physical activity involvement include establishing community-based programs; treating depression; designing interventions to accommodate motor symptoms of PD (i.e., stationary bicycle); and reviewing obstacles to physical activity with both the patient and caregivers. Future studies could evaluate the influence of environmental, interpersonal, or physical attributes of patients and caregivers as well the amount of instructor contact that is required to develop sustainable physical activity programs in order to optimize sleep, motor, and cognitive outcomes in persons with NDD.
Although exercise has proven to be a low risk and beneficial intervention to improve overall health and sleep disorders in AD and PD; several questions remain unanswered. For example, the modality of exercise, as well as the frequency and session duration needed for the maximum benefit are still unknown. Additionally, while there is evidence supporting the benefits of exercise on subjective sleep quality in AD and PD, the impact of exercise on objective sleep outcomes in these disorders is underexplored. Finally, factors that may predict responsiveness to specific exercise interventions are undetermined. Future studies investigating potential biomarkers of exercise efficacy and predictors of exercise response could lead to a more personalized approach to treatment for these conditions.
For other NDD, such as ALS and HD, available evidence about the effects of exercise on sleep dysfunction is scant. In persons with ALS, although some early data indicated a possible connection between a vigorously active lifestyle and a heightened risk of ALS133–135, the latest research suggests that physical activity is not a risk factor for ALS136–138. In fact, there is substantial data indicating that several exercise modalities, including resistance, stretching, endurance or a combination, can have positive impact on quality of life and physical functionality139–145, muscle strength143,146, and cardiorespiratory function140,143,144 in ALS. Exercise has also been shown to slow disease progression and reduce caregiver burden in ALS147. While there is a lack of data about the effects of physical activity on sleep disorders in both HD and ALS patients, this is an exciting potential avenue for future studies.
The beneficial effects of exercise and physical activity on overall health and well-being are well documented. Due to the increasing prevalence of NDD in our aging population and the potential for adverse effects of pharmacological treatments for sleep disorders in these patients, there is a critical need to expand our understanding of the influence of nonpharmacological interventions such as exercise on sleep dysfunction in NDD.
Footnotes
Declarations of Interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature. 2006;443(7113):796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorman AM. Neuronal cell death in neurodegenerative diseases: recurring themes around protein handling. J Cell Mol Med. 2008;12(6A):2263–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez OL, Dekosky ST. Clinical symptoms in Alzheimer’s disease. Handb Clin Neurol. 2008;89:207–216. [DOI] [PubMed] [Google Scholar]
- 4.Marsili L, Rizzo G, Colosimo C. Diagnostic Criteria for Parkinson’s Disease: From James Parkinson to the Concept of Prodromal Disease. Front Neurol. 2018;9:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72(6):971–983. [DOI] [PubMed] [Google Scholar]
- 6.Huntington G. On chorea. George Huntington, M.D. J Neuropsychiatry Clin Neurosci. 2003;15(1):109–112. [DOI] [PubMed] [Google Scholar]
- 7.Grad LI, Rouleau GA, Ravits J, Cashman NR. Clinical Spectrum of Amyotrophic Lateral Sclerosis (ALS). Cold Spring Harb Perspect Med. 2017;7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chokroverty S. Sleep and neurodegenerative diseases. Semin Neurol. 2009;29(4):446–467. [DOI] [PubMed] [Google Scholar]
- 9.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92(3):1087–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calkins AW, Hearon BA, Capozzoli MC, Otto MW. Psychosocial predictors of sleep dysfunction: the role of anxiety sensitivity, dysfunctional beliefs, and neuroticism. Behav Sleep Med. 2013;11(2):133–143. [DOI] [PubMed] [Google Scholar]
- 11.Malhotra RK. Neurodegenerative Disorders and Sleep. Sleep Med Clin. 2018;13(1):63–70. [DOI] [PubMed] [Google Scholar]
- 12.Iranzo A Sleep in Neurodegenerative Diseases. Sleep Med Clin. 2016;11(1):1–18. [DOI] [PubMed] [Google Scholar]
- 13.Amara AW, Chahine LM, Videnovic A. Treatment of Sleep Dysfunction in Parkinson’s Disease. Curr Treat Options Neurol. 2017;19(7):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferini-Strambi L, Galbiati A, Casoni F, Salsone M. Therapy for Insomnia and Circadian Rhythm Disorder in Alzheimer Disease. Curr Treat Options Neurol. 2020;22(2):4. [DOI] [PubMed] [Google Scholar]
- 15.Shub D, Darvishi R, Kunik ME. Non-pharmacologic treatment of insomnia in persons with dementia. Geriatrics. 2009;64(2):22–26. [PubMed] [Google Scholar]
- 16.Yang PY, Ho KH, Chen HC, Chien MY. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. J Physiother. 2012;58(3):157–163. [DOI] [PubMed] [Google Scholar]
- 17.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. [DOI] [PubMed] [Google Scholar]
- 18.Wu WW, Kwong E, Lan XY, Jiang XY. The Effect of a Meditative Movement Intervention on Quality of Sleep in the Elderly: A Systematic Review and Meta-Analysis. J Altern Complement Med. 2015;21(9):509–519. [DOI] [PubMed] [Google Scholar]
- 19.Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, Otto MW. The effects of physical activity on sleep: a meta-analytic review. J Behav Med. 2015;38(3):427–449. [DOI] [PubMed] [Google Scholar]
- 20.Rubio-Arias JA, Marin-Cascales E, Ramos-Campo DJ, Hernandez AV, Perez-Lopez FR. Effect of exercise on sleep quality and insomnia in middle-aged women: A systematic review and meta-analysis of randomized controlled trials. Maturitas. 2017;100:49–56. [DOI] [PubMed] [Google Scholar]
- 21.Youngstedt SD. Effects of exercise on sleep. Clin Sports Med. 2005;24(2):355–365, xi. [DOI] [PubMed] [Google Scholar]
- 22.Uchida S, Shioda K, Morita Y, Kubota C, Ganeko M, Takeda N. Exercise effects on sleep physiology. Front Neurol. 2012;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. Journal of psychiatric research. 2015;60:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGinty D, Szymusiak R. Keeping cool: a hypothesis about the mechanisms and functions of slow-wave sleep. Trends Neurosci. 1990;13(12):480–487. [DOI] [PubMed] [Google Scholar]
- 25.Sellami M, Bragazzi NL, Slimani M, et al. The Effect of Exercise on Glucoregulatory Hormones: A Countermeasure to Human Aging: Insights from a Comprehensive Review of the Literature. Int J Environ Res Public Health. 2019;16(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aoyama S, Shibata S. The Role of Circadian Rhythms in Muscular and Osseous Physiology and Their Regulation by Nutrition and Exercise. Front Neurosci. 2017;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrington ME. Exercise strengthens circadian clocks. J Physiol. 2012;590(23):5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schroeder AM, Truong D, Loh DH, Jordan MC, Roos KP, Colwell CS. Voluntary scheduled exercise alters diurnal rhythms of behaviour, physiology and gene expression in wild-type and vasoactive intestinal peptide-deficient mice. J Physiol. 2012;590(23):6213–6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tahara Y, Aoyama S, Shibata S. The mammalian circadian clock and its entrainment by stress and exercise. J Physiol Sci. 2017;67(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkinson G, Edwards B, Reilly T, Waterhouse J. Exercise as a synchroniser of human circadian rhythms: an update and discussion of the methodological problems. Eur J Appl Physiol. 2007;99(4):331–341. [DOI] [PubMed] [Google Scholar]
- 31.Schroeder AM, Colwell CS. How to fix a broken clock. Trends Pharmacol Sci. 2013;34(11):605–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Youngstedt SD, Elliott JA, Kripke DF. Human circadian phase-response curves for exercise. J Physiol. 2019;597(8):2253–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moran M, Lynch CA, Walsh C, Coen R, Coakley D, Lawlor BA. Sleep disturbance in mild to moderate Alzheimer’s disease. Sleep Med. 2005;6(4):347–352. [DOI] [PubMed] [Google Scholar]
- 34.McCurry SM, Reynolds CF, Ancoli-Israel S, Teri L, Vitiello MV. Treatment of sleep disturbance in Alzheimer’s disease. Sleep Med Rev. 2000;4(6):603–628. [DOI] [PubMed] [Google Scholar]
- 35.Pat-Horenczyk R, Klauber MR, Shochat T, Ancoli-Israel S. Hourly profiles of sleep and wakefulness in severely versus mild-moderately demented nursing home patients. Aging (Milano). 1998;10(4):308–315. [DOI] [PubMed] [Google Scholar]
- 36.Vitiello MV, Prinz PN. Alzheimer’s disease. Sleep and sleep/wake patterns. Clin Geriatr Med. 1989;5(2):289–299. [PubMed] [Google Scholar]
- 37.Dauvilliers Y Insomnia in patients with neurodegenerative conditions. Sleep Med. 2007;8 Suppl 4:S27–34. [DOI] [PubMed] [Google Scholar]
- 38.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16(1):40–81. [DOI] [PubMed] [Google Scholar]
- 39.Ktonas PY, Golemati S, Xanthopoulos P, et al. Potential dementia biomarkers based on the time-varying microstructure of sleep EEG spindles. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:2464–2467. [DOI] [PubMed] [Google Scholar]
- 40.Peter-Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer’s disease. Sleep Med Rev. 2015;19:29–38. [DOI] [PubMed] [Google Scholar]
- 41.Pollak CP, Perlick D, Linsner JP, Wenston J, Hsieh F. Sleep problems in the community elderly as predictors of death and nursing home placement. J Community Health. 1990;15(2):123–135. [DOI] [PubMed] [Google Scholar]
- 42.Singer C, Tractenberg RE, Kaye J, et al. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer’s disease. Sleep. 2003;26(7):893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaugler JE, Edwards AB, Femia EE, et al. Predictors of institutionalization of cognitively impaired elders: family help and the timing of placement. J Gerontol B Psychol Sci Soc Sci. 2000;55(4):P247–255. [DOI] [PubMed] [Google Scholar]
- 44.Hope T, Keene J, Gedling K, Fairburn CG, Jacoby R. Predictors of institutionalization for people with dementia living at home with a carer. Int J Geriatr Psychiatry. 1998;13(10):682–690. [DOI] [PubMed] [Google Scholar]
- 45.Knopman DS, Kitto J, Deinard S, Heiring J. Longitudinal study of death and institutionalization in patients with primary degenerative dementia. J Am Geriatr Soc. 1988;36(2):108–112. [DOI] [PubMed] [Google Scholar]
- 46.McCurry SM, Logsdon RG, Teri L, Vitiello MV. Sleep disturbances in caregivers of persons with dementia: contributing factors and treatment implications. Sleep Med Rev. 2007;11(2):143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pollak CP, Perlick D. Sleep problems and institutionalization of the elderly. J Geriatr Psychiatry Neurol. 1991;4(4):204–210. [DOI] [PubMed] [Google Scholar]
- 48.Rongve A, Boeve BF, Aarsland D. Frequency and correlates of caregiver-reported sleep disturbances in a sample of persons with early dementia. J Am Geriatr Soc. 2010;58(3):480–486. [DOI] [PubMed] [Google Scholar]
- 49.Spira AP, Friedman L, Beaudreau SA, et al. Sleep and physical functioning in family caregivers of older adults with memory impairment. Int Psychogeriatr. 2010;22(2):306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gehrman P, Gooneratne NS, Brewster GS, Richards KC, Karlawish J. Impact of Alzheimer disease patients’ sleep disturbances on their caregivers. Geriatr Nurs. 2018;39(1):60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hood S, Amir S. Neurodegeneration and the Circadian Clock. Front Aging Neurosci. 2017;9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meeks TW, Ropacki SA, Jeste DV. The neurobiology of neuropsychiatric syndromes in dementia. Curr Opin Psychiatry. 2006;19(6):581–586. [DOI] [PubMed] [Google Scholar]
- 53.Wu YH, Swaab DF. Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer’s disease. Sleep Med. 2007;8(6):623–636. [DOI] [PubMed] [Google Scholar]
- 54.Spira AP, Gamaldo AA, An Y, et al. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70(12):1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fultz NE, Bonmassar G, Setsompop K, et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science. 2019;366(6465):628–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prince M, Ali GC, Guerchet M, Prina AM, Albanese E, Wu YT. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther. 2016;8(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forlenza OV, Loureiro JC, Pais MV, Stella F. Recent advances in the management of neuropsychiatric symptoms in dementia. Curr Opin Psychiatry. 2017;30(2):151–158. [DOI] [PubMed] [Google Scholar]
- 59.Alexopoulos GS, Jeste DV, Chung H, Carpenter D, Ross R, Docherty JP. The expert consensus guideline series. Treatment of dementia and its behavioral disturbances. Introduction: methods, commentary, and summary. Postgrad Med. 2005;Spec No:6–22. [PubMed] [Google Scholar]
- 60.Bliwise DL. Sleep disorders in Alzheimer’s disease and other dementias. Clin Cornerstone. 2004;6 Suppl 1A:S16–28. [DOI] [PubMed] [Google Scholar]
- 61.Paniagua MA, Paniagua EW. The demented elder with insomnia. Clin Geriatr Med. 2008;24(1):69–81, vii. [DOI] [PubMed] [Google Scholar]
- 62.Jia RX, Liang JH, Xu Y, Wang YQ. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: a meta-analysis. BMC Geriatr. 2019;19(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christofoletti G, Oliani MM, Bucken-Gobbi LT, Gobbi S, Beinotti F, Stella F. Physical activity attenuates neuropsychiatric disturbances and caregiver burden in patients with dementia. Clinics (Sao Paulo). 2011;66(4):613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shih YH, Pai MC, Huang YC, Wang JJ. Sundown Syndrome, Sleep Quality, and Walking Among Community-Dwelling People With Alzheimer Disease. J Am Med Dir Assoc. 2017;18(5):396–401. [DOI] [PubMed] [Google Scholar]
- 65.Landi F, Russo A, Bernabei R. Physical activity and behavior in the elderly: a pilot study. Arch Gerontol Geriatr Suppl. 2004(9):235–241. [DOI] [PubMed] [Google Scholar]
- 66.McCurry SM, Pike KC, Vitiello MV, Logsdon RG, Larson EB, Teri L. Increasing walking and bright light exposure to improve sleep in community-dwelling persons with Alzheimer’s disease: results of a randomized, controlled trial. J Am Geriatr Soc. 2011;59(8):1393–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCurry SM, Gibbons LE, Logsdon RG, Vitiello MV, Teri L. Nighttime insomnia treatment and education for Alzheimer’s disease: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(5):793–802. [DOI] [PubMed] [Google Scholar]
- 68.Tewary S, Cook N, Pandya N, McCurry SM. Pilot test of a six-week group delivery caregiver training program to reduce sleep disturbances among older adults with dementia (Innovative practice). Dementia (London). 2018;17(2):234–243. [DOI] [PubMed] [Google Scholar]
- 69.Nascimento CM, Ayan C, Cancela JM, Gobbi LT, Gobbi S, Stella F. Effect of a multimodal exercise program on sleep disturbances and instrumental activities of daily living performance on Parkinson’s and Alzheimer’s disease patients. Geriatr Gerontol Int. 2014;14(2):259–266. [DOI] [PubMed] [Google Scholar]
- 70.Lees AJ, Blackburn NA, Campbell VL. The nighttime problems of Parkinson’s disease. Clin Neuropharmacol. 1988;11(6):512–519. [DOI] [PubMed] [Google Scholar]
- 71.Chahine LM, Amara AW, Videnovic A. A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Med Rev. 2017;35:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leng Y, Musiek ES, Hu K, Cappuccio FP, Yaffe K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019;18(3):307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rye DB, Bliwise DL Movement Disorders Specific to Sleep and the Nocturnal Manifestations of Waking Movement Disorders In: Watts RL, Koller WC, ed. Movement Disorders: Neurologic Principles and Practice. 2 ed: McGraw-Hill; 2004:855–890. [Google Scholar]
- 74.Yong MH, Fook-Chong S, Pavanni R, Lim LL, Tan EK. Case control polysomnographic studies of sleep disorders in Parkinson’s disease. PLoS One. 2011;6(7):e22511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suzuki K, Miyamoto M, Miyamoto T, Hirata K. Parkinson’s disease and sleep/wake disturbances. Curr Neurol Neurosci Rep. 2015;15(3):8. [DOI] [PubMed] [Google Scholar]
- 76.Bolitho SJ, Naismith SL, Rajaratnam SM, et al. Disturbances in melatonin secretion and circadian sleep-wake regulation in Parkinson disease. Sleep Med. 2014;15(3):342–347. [DOI] [PubMed] [Google Scholar]
- 77.Erro R, Santangelo G, Picillo M, et al. Link between non-motor symptoms and cognitive dysfunctions in de novo, drug-naive PD patients. J Neurol. 2012;259(9):1808–1813. [DOI] [PubMed] [Google Scholar]
- 78.Riedel O, Klotsche J, Spottke A, et al. Frequency of dementia, depression, and other neuropsychiatric symptoms in 1,449 outpatients with Parkinson’s disease. J Neurol. 2010;257(7):1073–1082. [DOI] [PubMed] [Google Scholar]
- 79.Kurtis MM, Rodriguez-Blazquez C, Martinez-Martin P, Group E. Relationship between sleep disorders and other non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2013;19(12):1152–1155. [DOI] [PubMed] [Google Scholar]
- 80.Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An american academy of sleep medicine report. Sleep. 2006;29(11):1415–1419. [PubMed] [Google Scholar]
- 81.David FJ, Rafferty MR, Robichaud JA, et al. Progressive resistance exercise and Parkinson’s disease: a review of potential mechanisms. Parkinsons Dis. 2012;2012:124527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.David FJ, Robichaud JA, Leurgans SE, et al. Exercise improves cognition in Parkinson’s disease: The PRET-PD randomized, clinical trial. Mov Disord. 2015;30(12):1657–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kelly NA, Ford MP, Standaert DG, et al. Novel, high-intensity exercise prescription improves muscle mass, mitochondrial function, and physical capacity in individuals with Parkinson’s disease. Journal of applied physiology. 2014;116(5):582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fox SH, Katzenschlager R, Lim SY, et al. International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2018;33(8):1248–1266. [DOI] [PubMed] [Google Scholar]
- 85.Cusso ME, Donald KJ, Khoo TK. The Impact of Physical Activity on Non-Motor Symptoms in Parkinson’s Disease: A Systematic Review. Front Med (Lausanne). 2016;3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frazzitta G, Maestri R, Ferrazzoli D, et al. Multidisciplinary intensive rehabilitation treatment improves sleep quality in Parkinson’s disease. J Clin Mov Disord. 2015;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wassom DJ, Lyons KE, Pahwa R, Liu W. Qigong exercise may improve sleep quality and gait performance in Parkinson’s disease: a pilot study. Int J Neurosci. 2015;125(8):578–584. [DOI] [PubMed] [Google Scholar]
- 88.Xiao CM, Zhuang YC. Effect of health Baduanjin Qigong for mild to moderate Parkinson’s disease. Geriatrics & gerontology international. 2016;16(8):911–919. [DOI] [PubMed] [Google Scholar]
- 89.Moon S, Schmidt M, Smirnova IV, Colgrove Y, Liu W. Qigong Exercise May Reduce Serum TNF-alpha Levels and Improve Sleep in People with Parkinson’s Disease: A Pilot Study. Medicines (Basel). 2017;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krueger JM, Fang J, Taishi P, Chen Z, Kushikata T, Gardi J. Sleep. A physiologic role for IL-1 beta and TNF-alpha. Ann N Y Acad Sci. 1998;856:148–159. [DOI] [PubMed] [Google Scholar]
- 91.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82(5):1313–1316. [DOI] [PubMed] [Google Scholar]
- 92.Nagatsu T, Mogi M, Ichinose H, Togari A. Cytokines in Parkinson’s disease. J Neural Transm Suppl. 2000(58):143–151. [PubMed] [Google Scholar]
- 93.Rockstrom MD, Chen L, Taishi P, et al. Tumor necrosis factor alpha in sleep regulation. Sleep Med Rev. 2018;40:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu M, Zhang Y, Pan J, Fu C, Wang Y. Effect of simplified Tai Chi exercise on relieving symptoms of patients with mild to moderate Parkinson’s disease. J Sports Med Phys Fitness. 2019. [DOI] [PubMed] [Google Scholar]
- 95.Silva-Batista C, de Brito LC, Corcos DM, et al. Resistance Training Improves Sleep Quality in Subjects With Moderate Parkinson’s Disease. J Strength Cond Res. 2017;31(8):2270–2277. [DOI] [PubMed] [Google Scholar]
- 96.Yang JH, Wang YQ, Ye SQ, Cheng YG, Chen Y, Feng XZ. The Effects of Group-Based versus Individual-Based Tai Chi Training on Nonmotor Symptoms in Patients with Mild to Moderate Parkinson’s Disease: A Randomized Controlled Pilot Trial. Parkinsons Dis. 2017;2017:8562867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Coe S, Franssen M, Collett J, et al. Physical Activity, Fatigue, and Sleep in People with Parkinson’s Disease: A Secondary per Protocol Analysis from an Intervention Trial. Parkinsons Dis. 2018;2018:1517807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amara AW, Wood KH, Joop A, et al. Randomized, Controlled Trial of Exercise on Objective and Subjective Sleep in Parkinson’s Disease. Mov Disord. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Herzog-Krzywoszanska R, Krzywoszanski L. Sleep Disorders in Huntington’s Disease. Front Psychiatry. 2019;10:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aziz NA, Pijl H, Frolich M, et al. Delayed onset of the diurnal melatonin rise in patients with Huntington’s disease. J Neurol. 2009;256(12):1961–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kalliolia E, Silajdzic E, Nambron R, et al. Plasma melatonin is reduced in Huntington’s disease. Mov Disord. 2014;29(12):1511–1515. [DOI] [PubMed] [Google Scholar]
- 102.Cheong RY, Gabery S, Petersen A. The Role of Hypothalamic Pathology for Non-Motor Features of Huntington’s Disease. J Huntingtons Dis. 2019;8(4):375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Y, Ren R, Yang L, et al. Sleep in Huntington’s disease: a systematic review and meta-analysis of polysomongraphic findings. Sleep. 2019;42(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Piano C, Della Marca G, Losurdo A, et al. Subjective Assessment of Sleep in Huntington Disease: Reliability of Sleep Questionnaires Compared to Polysomnography. Neurodegener Dis. 2017;17(6):330–337. [DOI] [PubMed] [Google Scholar]
- 105.Arnulf I, Nielsen J, Lohmann E, et al. Rapid eye movement sleep disturbances in Huntington disease. Arch Neurol. 2008;65(4):482–488. [DOI] [PubMed] [Google Scholar]
- 106.Baker CR, Dominguez DJ, Stout JC, et al. Subjective sleep problems in Huntington’s disease: A pilot investigation of the relationship to brain structure, neurocognitive, and neuropsychiatric function. J Neurol Sci. 2016;364:148–153. [DOI] [PubMed] [Google Scholar]
- 107.Aziz NA, Anguelova GV, Marinus J, Lammers GJ, Roos RA. Sleep and circadian rhythm alterations correlate with depression and cognitive impairment in Huntington’s disease. Parkinsonism Relat Disord. 2010;16(5):345–350. [DOI] [PubMed] [Google Scholar]
- 108.Fritz NE, Rao AK, Kegelmeyer D, et al. Physical Therapy and Exercise Interventions in Huntington’s Disease: A Mixed Methods Systematic Review. J Huntingtons Dis. 2017;6(3):217–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mueller SM, Petersen JA, Jung HH. Exercise in Huntington’s Disease: Current State and Clinical Significance. Tremor Other Hyperkinet Mov (N Y). 2019;9:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bartlett DM, Dominguez DJ, Lazar AS, et al. Multidisciplinary rehabilitation reduces hypothalamic grey matter volume loss in individuals with preclinical Huntington’s disease: A nine-month pilot study. J Neurol Sci. 2019;408:116522. [DOI] [PubMed] [Google Scholar]
- 111.Bartlett DM, Dominguez DJ, Reyes A, et al. Investigating the relationships between hypothalamic volume and measures of circadian rhythm and habitual sleep in premanifest Huntington’s disease. Neurobiol Sleep Circadian Rhythms. 2019;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Plecash A, Coleman A, Leavitt BR. Feasibility and Safety of an Aquatherapy Program in Mid- to Late-Stage Huntington Disease. International Journal of Neurorehabilitation. 2015;2(4). [Google Scholar]
- 113.Hardiman O, Al-Chalabi A, Chio A, et al. Amyotrophic lateral sclerosis. Nat Rev Dis Primers. 2017;3:17085. [DOI] [PubMed] [Google Scholar]
- 114.Hortobagyi T, Cairns NJ. Amyotrophic lateral sclerosis and non-tau frontotemporal lobar degeneration. Handb Clin Neurol. 2017;145:369–381. [DOI] [PubMed] [Google Scholar]
- 115.Hetta J, Jansson I. Sleep in patients with amyotrophic lateral sclerosis. J Neurol. 1997;244(4 Suppl 1):S7–9. [DOI] [PubMed] [Google Scholar]
- 116.Lo Coco D, La Bella V. Fatigue, sleep, and nocturnal complaints in patients with amyotrophic lateral sclerosis. Eur J Neurol. 2012;19(5):760–763. [DOI] [PubMed] [Google Scholar]
- 117.Lo Coco D, Mattaliano P, Spataro R, Mattaliano A, La Bella V. Sleep-wake disturbances in patients with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2011;82(8):839–842. [DOI] [PubMed] [Google Scholar]
- 118.Boentert M Sleep disturbances in patients with amyotrophic lateral sclerosis: current perspectives. Nat Sci Sleep. 2019;11:97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aboussouan LS, Lewis RA. Sleep, respiration and ALS. J Neurol Sci. 1999;164(1):1–2. [DOI] [PubMed] [Google Scholar]
- 120.Ahmed RM, Newcombe RE, Piper AJ, et al. Sleep disorders and respiratory function in amyotrophic lateral sclerosis. Sleep Med Rev. 2016;26:33–42. [DOI] [PubMed] [Google Scholar]
- 121.Oliveira AS, Pereira RD. Amyotrophic lateral sclerosis (ALS): three letters that change the people’s life. For ever. Arq Neuropsiquiatr. 2009;67(3A):750–782. [DOI] [PubMed] [Google Scholar]
- 122.Tsitkanou S, Della Gatta P, Foletta V, Russell A. The Role of Exercise as a Nonpharmacological Therapeutic Approach for Amyotrophic Lateral Sclerosis: Beneficial or Detrimental? Front Neurol. 2019;10:783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.King AC, Oman RF, Brassington GS, Bliwise DL, Haskell WL. Moderate-intensity exercise and self-rated quality of sleep in older adults. A randomized controlled trial. JAMA. 1997;277(1):32–37. [PubMed] [Google Scholar]
- 124.Li F, Fisher KJ, Harmer P, Irbe D, Tearse RG, Weimer C. Tai chi and self-rated quality of sleep and daytime sleepiness in older adults: a randomized controlled trial. J Am Geriatr Soc. 2004;52(6):892–900. [DOI] [PubMed] [Google Scholar]
- 125.Driver HS, Taylor SR. Exercise and sleep. Sleep Med Rev. 2000;4(4):387–402. [DOI] [PubMed] [Google Scholar]
- 126.Rothman SM, Mattson MP. Sleep disturbances in Alzheimer’s and Parkinson’s diseases. Neuromolecular Med. 2012;14(3):194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ma Q Beneficial effects of moderate voluntary physical exercise and its biological mechanisms on brain health. Neurosci Bull. 2008;24(4):265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–2107. [DOI] [PubMed] [Google Scholar]
- 129.van Nimwegen M, Speelman AD, Hofman-van Rossum EJ, et al. Physical inactivity in Parkinson’s disease. J Neurol. 2011;258(12):2214–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dowling GA, Wiener CL. Roadblocks encountered in recruiting patients for a study of sleep disruption in Alzheimer’s disease. Image J Nurs Sch. 1997;29(1):59–64. [DOI] [PubMed] [Google Scholar]
- 131.McCurry SM, Pike KC, Logsdon RG, Vitiello MV, Larson EB, Teri L. Predictors of short- and long-term adherence to a daily walking program in persons with Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2010;25(6):505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nakamura T, Hirayama M, Hara T, Hama T, Watanabe H, Sobue G. Does cardiovascular autonomic dysfunction contribute to fatigue in Parkinson’s disease? Mov Disord. 2011;26(10):1869–1874. [DOI] [PubMed] [Google Scholar]
- 133.Beghi E, Logroscino G, Chio A, et al. Amyotrophic lateral sclerosis, physical exercise, trauma and sports: results of a population-based pilot case-control study. Amyotroph Lateral Scler. 2010;11(3):289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gotkine M, Friedlander Y, Hochner H. Triathletes are over-represented in a population of patients with ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(7–8):534–536. [DOI] [PubMed] [Google Scholar]
- 135.Strickland D, Smith SA, Dolliff G, Goldman L, Roelofs RI. Physical activity, trauma, and ALS: a case-control study. Acta Neurol Scand. 1996;94(1):45–50. [DOI] [PubMed] [Google Scholar]
- 136.Huisman MH, Seelen M, de Jong SW, et al. Lifetime physical activity and the risk of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2013;84(9):976–981. [DOI] [PubMed] [Google Scholar]
- 137.Luna J, Logroscino G, Couratier P, Marin B. Current issues in ALS epidemiology: Variation of ALS occurrence between populations and physical activity as a risk factor. Rev Neurol (Paris). 2017;173(5):244–253. [DOI] [PubMed] [Google Scholar]
- 138.Pupillo E, Messina P, Giussani G, et al. Physical activity and amyotrophic lateral sclerosis: a European population-based case-control study. Ann Neurol. 2014;75(5):708–716. [DOI] [PubMed] [Google Scholar]
- 139.Bello-Haas VD, Florence JM, Kloos AD, et al. A randomized controlled trial of resistance exercise in individuals with ALS. Neurology. 2007;68(23):2003–2007. [DOI] [PubMed] [Google Scholar]
- 140.Braga ACM, Pinto A, Pinto S, de Carvalho M. The Role of Moderate Aerobic Exercise as Determined by Cardiopulmonary Exercise Testing in ALS. Neurol Res Int. 2018;2018:8218697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Drory VE, Goltsman E, Reznik JG, Mosek A, Korczyn AD. The value of muscle exercise in patients with amyotrophic lateral sclerosis. J Neurol Sci. 2001;191(1–2):133–137. [DOI] [PubMed] [Google Scholar]
- 142.Lunetta C, Lizio A, Sansone VA, et al. Strictly monitored exercise programs reduce motor deterioration in ALS: preliminary results of a randomized controlled trial. J Neurol. 2016;263(1):52–60. [DOI] [PubMed] [Google Scholar]
- 143.Merico A, Cavinato M, Gregorio C, et al. Effects of combined endurance and resistance training in Amyotrophic Lateral Sclerosis: A pilot, randomized, controlled study. Eur J Transl Myol. 2018;28(1):7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pinto AC, Alves M, Nogueira A, et al. Can amyotrophic lateral sclerosis patients with respiratory insufficiency exercise? J Neurol Sci. 1999;169(1–2):69–75. [DOI] [PubMed] [Google Scholar]
- 145.Sanjak M, Bravver E, Bockenek WL, Norton HJ, Brooks BR. Supported treadmill ambulation for amyotrophic lateral sclerosis: a pilot study. Arch Phys Med Rehabil. 2010;91(12):1920–1929. [DOI] [PubMed] [Google Scholar]
- 146.Kato N, Hashida G, Konaka K. Effect of muscle strengthening exercise and time since onset in patients with amyotrophic lateral sclerosis: A 2-patient case series study. Medicine (Baltimore). 2018;97(25):e11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen A, Montes J, Mitsumoto H. The role of exercise in amyotrophic lateral sclerosis. Phys Med Rehabil Clin N Am. 2008;19(3):545–557, ix-x. [DOI] [PubMed] [Google Scholar]


