Abstract
The zoonotic malaria parasite, Plasmodium knowlesi, is now a substantial public health problem in Malaysian Borneo. Current understanding of P. knowlesi vector bionomics and ecology in Sabah comes from a few studies near the epicentre of human cases in one district, Kudat. These have incriminated Anopheles balabacensis as the primary vector, and suggest that human exposure to vector biting is peri-domestic as well as in forest environments. To address the limited understanding of vector ecology and human exposure risk outside of Kudat, we performed wider scale surveillance across four districts in Sabah with confirmed transmission to investigate spatial heterogeneity in vector abundance, diversity and infection rate. Entomological surveillance was carried out six months after a cross-sectional survey of P. knowlesi prevalence in humans throughout the study area; providing an opportunity to investigate associations between entomological indicators and infection. Human-landing catches were performed in peri-domestic, farm and forest sites in 11 villages (3–4 per district) and paired with estimates of human P. knowlesi exposure based on sero-prevalence. Anopheles balabacensis was present in all districts but only 6/11 villages. The mean density of An. balabacensis was relatively low, but significantly higher in farm (0.094/night) and forest (0.082/night) than peri-domestic areas (0.007/night). Only one An. balabacensis (n = 32) was infected with P. knowlesi. Plasmodium knowlesi sero-positivity in people was not associated with An. balabacensis density at the village-level however post hoc analyses indicated the study had limited power to detect a statistical association due low vector density. Wider scale sampling revealed substantial heterogeneity in vector density and distribution between villages and districts. Vector-habitat associations predicted from this larger-scale surveillance differed from those inferred from smaller-scale studies in Kudat; highlighting the importance of local ecological context. Findings highlight potential trade-offs between maximizing temporal versus spatial breadth when designing entomological surveillance; and provide baseline entomological and epidemiological data to inform future studies of entomological risk factors for human P. knowlesi infection.
Author summary
The primate malaria parasite, Plasmodium knowlesi, is a common cause of human malaria in Malaysian Borneo. Most knowledge about the ecology and behaviour of mosquitoes transmitting P. knowlesi in Borneo comes from a limited number of sites near the major epicentre of human infection in Kudat District, Sabah. On this basis, human exposure to vectors was predicted to be higher in human settlement areas than in farming or forest habitats. Here we aimed to characterise the diversity and abundance of P. knowlesi vectors over a wider area of Sabah to test hypotheses about vector-habitat relationships and associated human exposure risk. Working in 11 villages across 4 districts in Sabah, we found low densities of the P. knowlesi vector, An. balabacensis. However, vector densities were higher in farm and forest habitats than in villages across this broader area, in contrast to findings from small scale study in Kudat. No association was observed between mean An. balabacensis abundance and P. knowlesi seropositivity in communities; however the ability to detect such an association, even if present, was limited by the relatively small number of mosquitoes collected.
Introduction
Human infection with the simian malaria parasite, Plasmodium knowlesi is now widespread across South East Asia with a large focus of transmission occurring in Malaysian Borneo. Anopheles mosquitoes in the Leucosphyrus complex are responsible for transmitting P. knowlesi [1], and the species An. balabacensis has been confirmed as the primary vector in the largest hotspot of human infection in the Kudat district of Sabah, Malaysian Borneo. Identification of vector species responsible for P. knowlesi transmission and habitats associated with human exposure is a vital first step for planning control measures. However, most of our current understanding of P. knowlesi ecology comes from intensive study within the Kudat epicentre [2–4]. Although human P. knowlesi cases have been reported throughout the state of Sabah [5], detailed study of vector ecology has been mostly restricted to a 2x3 km intensive study site in Kudat (Fig 1) and two sites on the neighbouring Banggi island [2]. One study compared An. balabacensis vector density, infection rates and survival in a village, plantation and secondary forest site [2], and concluded that An. balabacensis density was highest in the peri-domestic setting; challenging the previous paradigm of humans only being at risk if spending long periods of time in the forest [6]. However, this study also found that vector survival and infection rates were higher at forest and farm sites than those in the village [2]; indicating a higher “per mosquito bite” risk of infection in these habitats. Further investigations in villages in Kudat indicated that An. balabacensis were common in peri-domestic settings, and more abundant at households where human P. knowlesi cases were reported than homes of uninfected controls [3]. The higher abundance of vectors in peri-domestic settings and association between peri-domestic vector abundance and human cases suggests exposure may also be happening primarily around houses. However, a more recent study at one site in Kudat reported no difference in An. balabacensis density at peri-domestic, farm and forest edge habitats [7]. Most recently, Fornace et al [8] combined data on habitat-specific vector abundance and human movement data within Kudat District to show that most human exposure likely occurs in areas close to both secondary forest and houses. While this body of work is vital for understanding the current transmission hotspot centred in Kudat [2,3,8], it remains unclear how generalizable these findings are to other areas of Sabah, Malaysia or SE Asia in general where P. knowlesi is emerging.
Fig 1.
A) Location of Sabah in Malaysian Borneo (Image source: https://commons.wikimedia.org/wiki/Atlas_of_the_world) and B) Map of Northern Sabah indicating the eleven villages across 4 districts where entomological sampling was conducted in this study between March to June 2016.
Longitudinal sampling at three sentinel sites in Kudat demonstrated that An. balabacensis is the dominant vector species (95.1%) [2]. However, other members of the Leucosphyrus complex and An. donaldi have been implicated in P. knowlesi transmission in other parts of Malaysia [2,3,9–14]. Evidence suggests that there is substantial heterogeneity in vector diversity and density even between villages only two kilometres apart due to environmental factors such as land-cover, type of agriculture, availability of mosquito breeding sites, temperature, topography and elevation [15–17]. The landscape in Kudat is a fragmented mix of forest, farm and deforested areas, but is relatively similar in altitude, with no major urbanization. However, across the state of Sabah, there is substantial variation in elevation, the size and distribution of forest areas, and local agricultural activities thus it is likely that P. knowlesi vector ecology in Kudat district may not fully represent the state as a whole.
Vector density and sporozoite infection rates are key entomological indicators frequently investigated as proxies of human exposure risk [18]. Vector density has been associated with human Plasmodium prevalence and incidence in some contexts [19–23], but not others [24–27]. Entomological indicators may not be robust predictors of infection burden given the nonlinear relationship between entomological inoculation rates (product of vector biting and infection rates) and Plasmodium prevalence [28]. Reliable entomological predictors of zoonotic malaria risk for humans may be especially difficult to define due to the additional interaction between vectors and macaque reservoir populations. At present, no robust entomological predictors of P. knowlesi human infection risk have been defined.
Investigation of entomological indicators of Plasmodium infection requires high resolution, spatially and temporally concurrent data on vector bionomics and infection prevalence or incidence. A particular challenge in the study of P. knowlesi epidemiology is that human infection rates are generally very low, thus requiring often prohibitively large sample sizes to reliably estimate prevalence. Given these difficulties in measuring “active” infection, serology may provide a more tractable alternative for indirectly measuring previous infection. As part of an interdisciplinary study on P. knowlesi epidemiology [“MonkeyBar” project, [29]], a large-scale cross-sectional survey was conducted throughout Sabah State, Malaysia, to estimate human exposure based on sero-prevalence (September to December 2015) [30]. This provided a unique opportunity to carry out complimentary entomological surveillance to assess spatial heterogeneity in P. knowlesi vector abundance and its concordance with human infection risk as estimated from serology.
The goal of this study was to investigate P. knowlesi vector species, density and infection rates across wider spatial scales in Sabah. Key aims were to identify associations with habitat type (forest, farm and village) to identify where human biting risk is highest. In addition, we investigated village-level associations between mean vector abundance and human P. knowlesi infection risk as estimated from the Monkeybar sero-prevalence study.
Methods
Village selection
A subset of 11 villages were selected from a larger group in Sabah province, Malaysia where a cross-sectional survey of P. knowlesi sero-positivity in people was conducted (September to December 2015 [29]). Three to four villages were selected from 4 districts to encompass a range of altitudes and habitat types: Kudat (altitude: 4–223m), Kota Marudu (8–745m), Pitas (7–218m) and Ranau (53–1275m) (Fig 1). Entomological sampling was carried out in all 11 villages approximately six months after the human sero-prevalence survey. All 11 villages were consecutively sampled over a 3-month period (21/03/16–16/06/16). One village was sampled per week, with mosquito collections being conducted over four consecutive nights. The research team attempted to visit a village from a different district on each week, so that district-level differences were not confounded by temporal autocorrelation. However this was not always logistically possible (see S1 Table for sampling dates).
Study sites within villages
Villages were accessible by tertiary or dirt track roads. All villages were rural, with small populations of < 750 residents. These were generally structured as a group of houses surrounded by a mosaic of crops (usually largely palm oil and rubber trees) and secondary forest patches. Thus there was a range of habitats available at each village. Within each village, mosquitoes were collected in three distinct habitat types: forest patch, farm and peri-domestic settings (e.g. Fig 2). This range of habitats replicated the sampling design used in a previous study in Kudat district [2]. The peri-domestic environment was defined as the outdoor garden area immediately surrounding a household (outside, < 5m from the main house). Farm sites were located in small plantations, and forest sites were in patches of secondary forest comprising non-agricultural trees. Due to the wide geographical range of our sampling, the farm habitat varied between villages depending on what crops were locally cultivated (S1 Table). Forest was distributed patchily throughout the area with patch sizes varying significantly between villages (0.075–10km2, S1 Table).
Fig 2.

Photos showing examples of typical peri-domestic (A, B), farm (C = rubber, D = palm, E = cabbage) and forest (F) habitats where mosquito collections were conducted in this study.
Mosquito sampling sites were selected by walking in and around each village at the start of each visit to identify all accessible locations within each of the 3 habitat types. One location per habitat type was selected based on the following criteria: peri-domestic- consent from household residents, farm- a point at least 25m from the nearest house to differentiate from peri-domestic sites, forest- a minimum patch size of 10x10m, with sampling occurring at least 20m from forest edge (if not possible, then centre of forest patch). On each night of sampling, one team of two people performed Human Landing Catches (HLC, details below) in each of the 3 habitat types, then the teams rotated between habitats on subsequent nights. Across all four sampling nights, a different sampling point was selected within each of the 3 focal habitat types each night. Each sampling point was at least 25 m from the location used the previous night. Only three nights of collections were performed for Sungai Pupu and Patiu villages due to heavy rainfall and fogging (for dengue control) taking place on the fourth day.
Mosquito sampling
Mosquitoes were collected using the Human Landing Catch (HLC) technique [2]. Previous studies in Sabah have evaluated a range of different trapping methods for P. knowlesi vectors (e.g CDC light traps, e-nets, monkey-baited traps, resting traps [31,32]) and found HLC to be the most effective. Briefly, volunteers were positioned in teams of two with their lower legs exposed, and trapped mosquitoes which landed on them to feed using 30ml plastic screw-top vials. One mosquito was trapped per vial and the hour and habitat of collection were recorded on each. Nightly collections per site represented the total number of mosquitoes caught in HLC carried out by two people. Catches were performed between 18:00–00:00 to include the peak biting time of Sabah’s primary P. knowlesi vector, An. balabacensis [2,33]. Previous studies in this area have shown the majority of An. balabacensis biting activity occurs before midnight [3,8]. All HLCs were conducted outdoors because P. knowlesi vectors exhibit exophilic host seeking behaviour [12].
Mosquito processing
At the end of each 6-hour sampling period, mosquitoes trapped inside vials were transported to the central field station and put in a -20˚C freezer. Mosquitoes were killed by storing at -20˚C overnight and identified to genera and species level the following day using the keys of Rattanarithikul et al (2005/6) [34–37]. Species belonging to the Leucosphyrus group were identified using [38]. Specimens were stored in 95% ethanol until further processing.
Plasmodium detection in Anopheles
DNA was prepared from all Leucosphyrus group Anopheles, and An. donaldi and An. maculatus (known malaria vectors, [14,33,39]). First the ethanol preservative was removed from these sample tubes and then DNA extracted from the whole body using the QIAGEN DNeasy Blood and Tissue Kit following the manufacturer’s instructions with the following minor modifications. Specimens were initially ground in 180 μl buffer ATL using a pestle and hand-held homogenisor, and lastly eluted in a volume of 25 μl TE buffer. Nested PCRs were conducted to screen samples for Plasmodium DNA using the method of Snounou and Singh [40], which identifies DNA of any species within the Plasmodium genus. Samples positive for Plasmodium DNA were subjected to a further PCR to identify the species present. Nine separate reactions were set up following the method of Ta et al [41] (to detect P. falciparum, P. vivax, P. malariae and P. ovale), Lee et al [42] (P. coatneyi, P. inui and P. cynomolgi) and Imwong et al [43] (P. knowlesi) (S2 Table).
Plasmodium knowlesi sero-prevalence in humans
Sero-prevalence data on Plasmodium exposure in humans in the study villages was obtained from a cross-sectional survey as described in [30]. In summary, no active Plasmodium infections were observed by either microscopy or PCR [29] in this survey, thus serological measures of previous P. knowlesi exposure were used to examine associations with the density of Leucosphyrus group Anopheles [44]. Measures of village level sero-positivity (the proportion of individuals from the total screened per village that were IgG positive for P. knowlesi) were estimated for the 11 villages in which entomological surveillance was conducted [29]. Serological screening can detect individuals infected with P. knowlesi at least within the previous 28 days [44] but it is unknown how long these antibodies can persist for.
Data analysis
Anopheles diversity across habitat types
Data were analysed using the R statistical programming software, version 3.4.2. The “vegan” package was used to measure four species diversity indices: species richness, rarefied species richness, Simpson’s index and the Shannon index. These measures were used to estimate and compare Anopheles diversity across habitat types (peri-domestic area, farm and forest). Species richness is the total number of different Anopheles species collected in each village. The rarefied species richness is the species richness if collections had the same Anopheles density (ie. set to the group with the lowest total density). Rarefaction is a method used to standardise unequal sampling sizes [45,46]. The Simpson’s index,
[λ = (n/n-1) x ∑ ps (1-ps)] [47], where
n = total Anopheles density
ps = each species count/n,
measures the probability that two individuals randomly sampled from the dataset will be of the same species [48]. The Simpson’s Index is noted to be sensitive to abundant species [49], thus the Shannon Index was also calculated as a comparison. The Shannon index,
H = -∑ (n/N) log (n/N) (48), where
N = total Anopheles density
ni = each species count,
measures the uncertainty in predicting the species of an individual randomly sampled from the dataset [49]. Confidence intervals for Simpson’s Diversity Index were calculated following Zhang [47].
Analysis of environmental variables
Percentage forest cover in a 100m buffer (circle of radius 100m) around each sampling location for HLC was calculated using the Hansen global forest cover 2014 map, with forest defined as 50% canopy cover [50]. GLMMs were constructed in R using the lme4 package to extract the mean elevations and proportion of forest cover at all mosquito collection sites. A negative binomial model was used to predict mean elevation and a model with a binomial distribution was used for percentage forest cover. Elevation and percentage forest cover were the response variables and habitat was the explanatory variable, with date and village set as random effects.
Mosquito presence and density analyses
Statistical analysis was performed on two sets of mosquito data: 1) An. balabacensis only, and 2) All Leucosphyrus group Anopheles. The second group was inclusive of An. balabacensis (n = 32), An. latens (n = 7) and suspected An. balabacensis/An. latens (n = 2); defined as being either of these two species with identification to species level not being possible due to the loss of fragile scales on the wings necessary for morphological identification. Both An. balabacensis or An. latens are implicated in the transmission of P. knowlesi in Malaysian Borneo [2,9] thus were analysed as a whole. The packages lme4 and multcomp were used to analyse mosquito presence and density. Generalised Linear Mixed Models (GLMMs) were constructed to test for associations between the two response variables of mosquito presence (binary outcome, 0 = absent, 1 = present) and density (mean number caught per site per night), and the following explanatory variables: elevation, habitat type and forest cover. To relieve issues with scaling, elevation was converted from a continuous to a categorical variable by splitting into three elevation ranges: low (0 – 375m), medium (376 – 750m) and high (751 – 1125m). Models were fit with a negative binomial distribution for mosquito density and a binomial distribution for mosquito presence. In all models, random effects were included for village and date. The significance of explanatory variables in each of the models was tested by backward elimination using likelihood ratio tests. A Tukeys’ post hoc test was performed to assess differences between each of the 3 habitat types.
Biting time in Plasmodium vector species
The lme4 package was used to construct GLMMs in R to extract hourly biting rates of different Plasmodium vector species caught. Only An. balabacensis, An. donaldi and An. maculatus were examined because the overall density of An. latens (n = 7) was too low to analyse in this way. The number of mosquitoes of each species caught per hour throughout the night was examined, with the first hour as 18:00–19:00 and the last as 23:00–00:00. Hourly mosquito abundance was treated as the response variable with the main fixed effect being biting hour. A negative binomial distribution was used with date and village set as random effects. A Tukey’s post-hoc test (package multcomp) was used to assess differences in biting rates between hours within each species.
Associations between vector density and human P. knowlesi exposure
General linear models (GLMs) were constructed to test for associations between mosquito presence and density for 1) An. balabacensis only and 2) Leucosphyrus group Anopheles (An. balabacensis/An. latens) and village-level P. knowlesi sero-positivity. A GLMM with a negative binomial distribution was used to predict mean mosquito density from each village where mosquito density per night was the response variable and habitat and date were fit as random effects. A binomial GLMM was used to predict the probability of detecting a mosquito in each village where mosquito presence (1) or absence (0) per night was the response variable and habitat and date were fit as random effects. These village-specific estimates of mean vector density and probability of occurrence were used to test for associations with the proportion of individuals sero-positive for P. knowlesi antigens in each village. A binomial GLM was used with village sero-positivity as the response variable and mosquito presence or density as the fixed effect. Entomological collections began ~ 6 months after the cross-sectional survey thus did not run in parallel with human sampling. However, an assumption of this analysis is that entomological measures were assumed to be reflective of general differences between villages at the time of the cross-sectional survey. A post hoc power analysis was performed using the pwr.f2.test function from the pwr package in R (effect size = R2/(1−R2), significance level = 0.05, power = 0.8) to determine the sample size required to detect an association between village level human P. knowlesi sero-positivity rates and P. knowlesi vector presence or abundance. All data collected in this study is available from Harvard dataverse “https://doi.org/10.7910/DVN/3QG1HP”.
Ethics statement
This project was approved by the Malaysian Ministry of Health (NMRR-12-786-13048) and by the research ethics committees of the London School of Hygiene and Tropical Medicine (Ref. 6302). Homeowners gave permission to use the area around their houses for mosquito collection. All volunteers who carried out mosquito collections were adults and signed informed consent forms prior to the study. Volunteers were provided with antimalarial prophylaxis during participation and one month after performing HLC, volunteers were screened for Plasmodium by giemsa stained thick and thin blood smears. Participants were asked to immediately report if they felt ill or feverish and would be taken to the nearest medical facility for check-up and malaria treatment if required. No participants reported malaria infections during the study.
Results
In 42 nights of sampling, a total of 5588 mosquitoes belonging to eight genera were collected (S3 Table). The majority of specimens were from the Culex and Armigeres genera, with only 4% Anopheles and 8% Aedes. Five genera were found in peri-domestic habitats, six in farm and seven in forests (S1 Fig). Species known to transmit Plasmodium in Sabah (Anopheles balabacensis, An. latens and An. donaldi) comprised 1.1% of the total mosquito catch. Six species of Anopheles were collected (Table 1) with An. maculatus and An. barbumbrosus being the most abundant. Dengue vector species (Ae. albopictus and Ae. aegypti) comprised 6.9% of mosquitoes collected. The majority of Aedes specimens were Ae. albopictus (~90%) with only a few Ae. aegypti (~1%). The remaining Aedes specimens could not be identified to species level.
Table 1. Anopheles species caught in eleven villages within the four districts: Kudat, Kota Marudu, Pitas and Ranau in Sabah, sampled from March to June 2016.
Village names: SUV–Suvil, SUN–Sungai Pupu, BAR–Barankason, SOR–Sorinsim, PAT–Patiu, KOT–Kotud, PER–Perpaduan, SIN–Sinangip, LIP–Lipasu Lama, SIB–Siba Bundu Tuhan and GON—Gondohon.
| District of sampling | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kudat (villages) | Kota Marudu (villages) | Pitas (villages) | Ranau (villages) | |||||||||
| Mosquito genera/ species | SUV | SUN | BAR | SOR | PAT | KOT | PER | SIN | LIP | SIB | GON | Total (%) |
| Leucosphyrus gp. | 0 | 1 | 0 | 8 | 1 | 1 | 0 | 10 | 19 | 0 | 0 | 41 (19.3) |
| An. balabacensis*# | 0 | 1 | 0 | 3 | 1 | 1 | 0 | 7 | 12 | 0 | 0 | 32 (15.1) |
| An. latens* | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 7 (3.3) |
| An. balabcensis or An. latens* | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 (0.9) |
| Barbirostris gp | 3 | 33 | 1 | 19 | 3 | 3 | 0 | 23 | 0 | 1 | 3 | 89 (42.0) |
| An. barbumbrosus | 0 | 16 | 1 | 14 | 3 | 3 | 0 | 22 | 0 | 0 | 2 | 61 (28.8) |
| An. donaldi# | 3 | 16 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 23 (10.9) |
| An. maculatus# | 0 | 0 | 0 | 8 | 3 | 29 | 0 | 10 | 0 | 29 | 1 | 80 (37.7) |
| An. tesselatus | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 (0.9) |
| Total Anopheles sp. | 3 | 34 | 1 | 34 | 8 | 33 | 2 | 43 | 19 | 31 | 4 | 212 |
* vector of P. knowlesi
# vector of P. falciparum/ P. vivax
Anopheline species diversity was lower in peri-domestic and farm sites than at forest sites (Table 2). Both the rarefied species richness, Shannon and Simpson Indices estimated similar trends with forest sites having higher Anopheles species diversity, followed by farm sites and then peri-domestic sites (Table 2).
Table 2. Measures of diversity in Anopheles species across different habitat types sampled in eleven villages in Sabah from March to June 2016.
| Habitat | Anopheles abundance | Species richness | Rarefied species richness | Shannon index | Simpson’s index | Simpson’s index ± 95% confidence intervals |
|---|---|---|---|---|---|---|
| Peri-domestic | 22 | 4 | 2.380 | 0.969 | 0.5 | 0.52 ± 0.22 |
| Farm | 85 | 4 | 2.858 | 1.276 | 0.694 | 0.73 ± 0.05 |
| Forest | 98 | 5 | 3.139 | 1.477 | 0.750 | 0.79 ± 0.04 |
Averaging over all villages, there was no systematic difference in the mean altitude of the forest, farm and peri-domestic mosquito sampling sites (P > 0.05, S4 Table); thus habitat type was not confounded by altitudinal variation. As expected, the percentage of tree cover above sampling points in farms and forests was higher than in peri-domestic settings, however this result was not significant ((P > 0.05, S4 Table).
Vector density and distribution
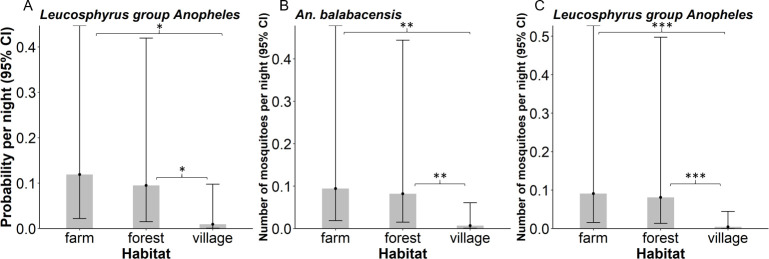
The major P. knowlesi vector An. balabacensis (n = 32) was found in approximately 14% of collections, with no significant difference in probability of detection between habitats (X2 = 5.33, df = 2, P = 0.07), or in association with percentage forest cover (X2 = 3.16, df = 1, P = 0.08) or elevation (X2 = 0.21, df = 2, P = 0.90). Pooling all Anopheles species in the Leucosphyrus group (n = 41), the probability of detection varied with habitat (X2 = 7.42, df = 2, P = 0.02) but not forest cover (X2 = 3.31, df = 1, P = 0.07) or elevation (X2 = 0.34, df = 2, P = 0.85). Leucosphyrus group mosquitoes were more likely to be caught in farm (P = 0.02) and forest (P = 0.02) sites than in peri-domestic environments (Fig 3A).
Fig 3.
Predicted A) Probability of catching Leucosphyrus group Anopheles B) Mean density of An. balabacensis and C) Mean density of Leucosphyrus group Anopheles in farm, forest and peri-domestic habitats. Error bars represent 95% confidence intervals.
The density of An. balabacensis varied with habitat (X2 = 9.82, df = 2, P < 0.01) but not with elevation (X2 = 0.13, df = 2, P = 0.93) or percentage forest cover (X2 = 3.16, df = 1, P = 0.08). Anopheles balabacensis was significantly more abundant in farm (P < 0.01) and forest (P < 0.01) habitats than in peri-domestic areas (Fig 3B, S2 Fig for raw data). Similarly, habitat was a significant predictor of the mean density of the Leucosphyrus group in general (X2 = 12.92, df = 2, P < 0.01); with their density being significantly lower in peri-domestic environments than in farm (P < 0.001) or forest (P < 0.001) habitats (Fig 3C, S2 Fig for raw data).The mean density of the Leucosphyrus group did not vary in relation to local forest cover (X2 = 4.12, df = 1, P = 0.04) or elevation of the collection site after accounting for habitat differences (X2 = 1.64, df = 1, P = 0.20).
Biting patterns of Plasmodium vector species
The biting patterns of the three known Plasmodium vector species (An. maculatus, An. donaldi and An. balabacensis) were assessed. Too few Anopheles latens individuals (a vector of P. knowlesi) were sampled for robust description (n = 7). These three species were found during all sampling hours (18:00–00:00), with some tendency for higher activity during the early evening hours (18:00–20:00). However due to the relatively small numbers collected and high variability in hourly catches, no clear peaks in biting time were evident (Tukey’s, P > 0.05, S3 Fig).
Plasmodium infection rates
Of the 144 female mosquitoes that were potential Plasmodium vector species (An. Leucosphyrus gp, An. donaldi and An. maculatus), only one tested positive for Plasmodium. This was an An. balabacensis collected in a forest patch in Sinangip village, Pitas, which was infected with P. knowlesi. This represents an infection rate of ~3% (n = 1/32) in An. balabacensis.
Association between Plasmodium vector density and human P. knowlesi exposure
Seroprevalence rates of P. knowlesi in people across the study area were provided by the Monkeybar large cross-sectional survey [8]. Within the subset of 11 villages where entomological surveillance was conducted, sero-positivity rates ranged from 0% (Sib and Sun) to 13.9% (in Sor). In these villages, the probability of trapping An. balabacensis per night in an HLC ranged from 0.11–0.42 (Fig 4A), and 0.11–0.50 for the Leucosphyrus group overall (Fig 4B). No significant relationship was detected between human P. knowlesi sero-positivity rates and the probability of detecting An. balabacensis or Leucosphyrus group Anopheles (P > 0.05, S5 Table). No significant relationship was detected between human P. knowlesi sero-positivity rates and the density of An. balabacensis as measured 6 months afterwards (Fig 4C) or Leucosphyrus group Anopheles (Fig 4D) (P > 0.05, S5 Table). However, post hoc power analysis indicated that the study had limited power to detect an association between seropositivity rates and vector abundance given the lower than anticipated densities of primary vector species. With the low vector densities observed here, and based on the GLM described above, 142 and 390 villages respectively would need to be sampled to detect a positive significant relationship (P = 0.05) between the presence of An. balabacensis and An. Leucosphyrus gp. and human P. knowlesi seropositivity rates with 80% power (S5 Table). The sample sizes required to detect a positive significant relationship (P = 0.05) between the density of An. balabacensis and An. Leucosphyrus gp. and human P. knowlesi seropositivity rates with 80% power would be derived from sampling 159 and 113 villages respectively (S5 Table).
Fig 4.
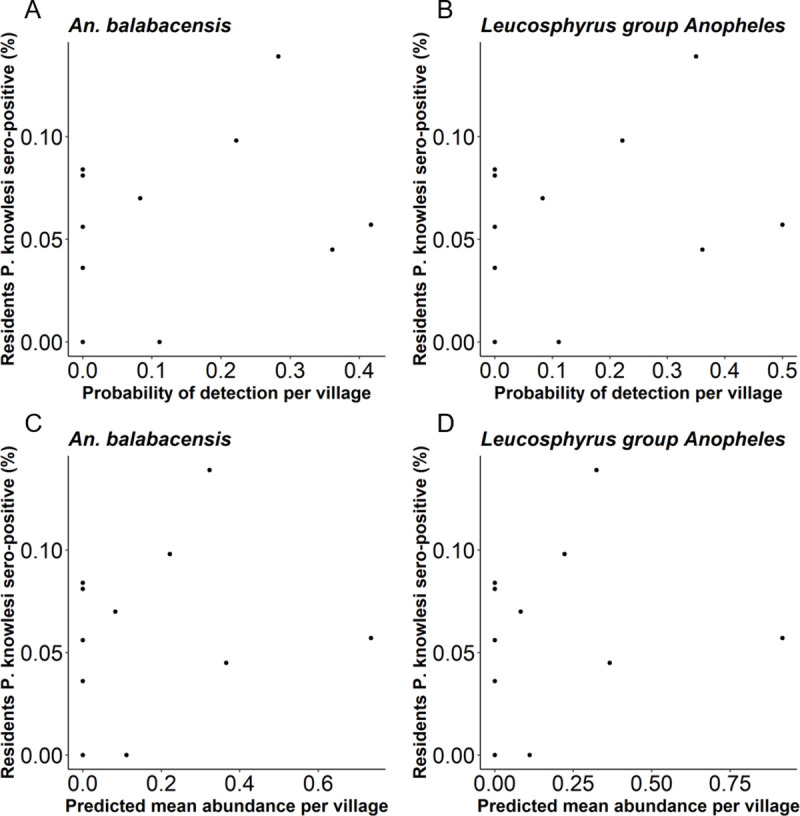
Association between the proportion of individuals in a village sero-positive for P. knowlesi antigens and the A) detection of An. balabacensis B) detection of Leucosphyrus group Anopheles C) density of An. balabacensis and D) density of Leucosphyrus group Anopheles caught in the village per night.
Discussion
Here we describe the density and diversity of P. knowlesi vectors across 4 districts in Malaysian Borneo where this parasite is a significant public health problem. This entomological surveillance covered a wider geographical region than has been investigated before. We found that An. balabacensis, the P. knowlesi vector, is widely distributed across 4 districts of Sabah, Malaysian Borneo; but at a lower relative abundance (within the Anopheline community) than has been previously reported near the epicentre of human cases in Kudat. There was substantial heterogeneity in the density and diversity of vector populations both within and between districts. Vector surveillance over this wider geographic area indicated a different pattern of vector-habitat relationships than hypothesized from single site studies in Kudat; with vector abundance being higher in forest and farm habitats than in peri-domestic environments. Using human P. knowlesi sero-positivity data gathered from a large cross-sectional survey, no significant correlation was detected between village-level human infection exposure and vector density. However, power to detect such an association was limited by low vector density throughout the study area and resultant small sample sizes. Larger-scale and longer-term studies thus may be required for robust investigation of entomological indicators of P. knowlesi infection.
Anopheles balabacensis, the primary vector incriminated in P. knowlesi transmission in Sabah, was found throughout the study area but at considerably lower density than previously estimated in focal studies around Kudat. Based on surveillance at a few sites in Kudat, this vector was previously reported to be the dominant Anopheline biting humans (e.g. 95.1% of Anophelines, [2,3]). However, An. balabacensis accounted for only 15.1% of the human-biting Anophelines in this study. We note that during the study there were droughts across Sabah due to the El Nino; which could have had impact on vector densities. However it is difficult to assess the potential effects on vectors from available data (collated in [8]) because there were no sites consistently sampled before, during and after the El Nino. Another factor that could account for the lower estimates of An. balabacensis density observed in this study compared to previous ones in Kudat is the duration and selection of sampling sites. In a previous study in Kudat, the farm, forest and village site were selected based on having high An. balabacensis density, as required to generate sufficient sample sizes for parasite screening [2]. Here mosquitoes were sampled for only 3–4 nights per site over a 3-month period (in 2016), whereas previous work sampled mosquitoes over 12 months (3 nights/month, 2013–2014). If vector population dynamics are highly seasonal, this shorter-term sampling could substantially over or underestimate average annual densities. However, previous studies indicate there is little seasonality in An. balabacensis abundance with vector numbers staying relatively constant across months in this area [2,30]. Given the potentially minor role of seasonality in these vector populations, the shorter period of sampling used here may be sufficient to reflect general differences in vector abundance between villages and sites. However, a more detailed understanding of temporal variation in these vector populations would be of great value for refining estimates of spatial variation.
Habitat type was a major predictor of Anopheles presence and density in this study. Both An. balabacensis and the Leucosphyrus group were more abundant in farm and forest than peri-domestic habitats. Similar vector-habitat associations have previously been reported in Kapit, Sarawak [9], and in Peninsular Malaysia [12], but a previous study conducted in a single farm, forest and peri-domestic site in Kudat found An. balabacensis to be most abundant in the village [2]. A further study conducted in Kudat found similar densities of An. balabacensis at all habitat types sampled (peri-domestic, farm and forest edge) [7]. Differences reported in Wong et al [2] and in Chua et al [7] may have been due to site specific factors rather than habitat, highlighting the need for replicated sampling over wide geographical areas for robust habitat prediction [51]. The sampling period applied here (11 villages, 3–4 nights per village) was shorter than the longer period used previously in Kudat (3 sites, 2–3 nights per month for 12 months) [2]. These differences in sampling design limit direct comparison of vector densities between these studies, however it does provide an opportunity to make qualitative comparisons of vector density between sites, even if more precise quantification is limited by the shorter collection period. Future studies investigating vector habitat associations over wider geographic regions in Sabah would require substantial depth (time and resources) to rigorously assess differences with previous studies conducted in the Kudat district.
Recent epidemiological studies have identified forest and agricultural-related work activities as risk factors for P. knowlesi infection in Sabah [29,52]. Forest cover and historical forest loss have also been significantly associated with the occurrence of human cases of P. knowlesi in this area [53] and that increasing distance from the forest reduces the chance of being bitten by an infected mosquito [8]. Additionally, investigations into human movement patterns in rural villages in Sabah indicate that during mosquito biting hours (18:00–06:00), people are less likely to use areas further away from the home indicating that people are at highest risk of exposure if their houses are in closer proximity to forested areas [8]. Therefore whilst our study found highest densities of P. knowlesi vectors in forest and farm sites, people are less likely to use these areas throughout the night, and so it is the proximity of the home to these habitats that is the key risk for human exposure.
Only one Plasmodium infected mosquito was found across the study area, an An. balabacensis infected with P. knowlesi caught in a forest patch, corresponding to a total infection rate of ~3% (1 out of 32 tested). Whilst this is in line with the expectation that P. knowlesi infection rates are highest in An. balabacensis found in forests [2], the sample size of infected mosquitoes was too low to draw any significant conclusions about habitat-dependent mosquito infection rates. In previous studies in Kudat, the P. knowlesi infection rate of An. balabacensis ranged from 0–0.88% [2–4,7], with overall infection rates (all Plasmodium species) ranging from 1.45–3% [2–4,7]. No P. knowlesi infections were detected in An. balabacensis caught in Kudat here, which may be due to the low number of samples tested. However, we note that the absence of P. knowlesi in the An. balabacensis tested from Kudat here coincides with a recent reduction in the number of human P. knowlesi cases in this district [54]. The sample size of An. balabacensis obtained in this study was too low to draw conclusions on infection rates across different habitats or districts. Given the low vector densities and sporozoite rates in the study area, quantification of spatial variation in mosquito infection rates would likely require years of continual sampling in a large number of sites. Such high-resolution intensive sampling was not possible within the scope of this project. This highlights the trade-offs between spatial breadth and temporal depth that must be considered in designing surveillance programmes for zoonotic vectors. Given the difficulty of achieving both depth and breadth, the best solution may be a mixed approach combining short-term sampling at a wide range of sites coupled with intensive long-term sampling at a smaller number of fixed sentinel sites. A further factor influencing vector infection (and density) could be the presence and abundance of the macaque reservoir in the study area. Collecting this type of data is labour intensive and was outwith the scope of this study however is an important part of this malaria system that future studies should consider.
Altitude has long been recognized as a significant predictor of malaria transmission [55–60], however it was not a significant predictor of Anopheles presence and density across the wide gradient investigated here (13–1125). Across villages investigated here, there is substantial variation in climate and local tree species as well as elevation thus the significant additional environmental heterogeneity introduced by sampling over such a wide geographical range which may have swamped the more modest impact of elevation. All sites sampled may also have been within the altitudinal/temperature range suitable for An. balabacensis.
No significant association between the density of mosquito vectors (An. balabacensis and all An. Leucosphyrus group mosquitoes) and human sero-positivity for P. knowlesi at the village-level was detected. A notable limitation of our study design was that entomological sampling was performed six months after human data was collected. Thus, there was a temporal mismatch in the timing of human and entomological sampling which could have limited the strength of any association. The entomological data was only collected for a few days and may not have been an accurate representation of vector conditions at the time of human sampling. Alternatively, even if sampling was conducted concurrently there may be no link between vector density and human infection. Malaria vector densities do not always correlate with human risk [24–27]; with a lack of synchrony perhaps being more likely with zoonotic malaria due to the additional complexity introduced by the macaque reservoir. However, our ability to test these hypotheses was limited by a lack of statistical power; which post hoc analysis indicated that considerably larger sample sizes (approximately x 14) would have been required to robustly detect an association between An. balabacensis vector density and human sero-positivity with (80%) power. Thus although data collected here was not sufficient for robust analysis of entomological indicators, it can provide a useful guide for the design of future studies into the epidemiology of this complex malaria system.
Supporting information
Characteristics of the eleven villages in Sabah State, Malaysia, where mosquito vectors were sampled. “Crops” describes the dominant types of subsistence farming occurring in the village. “Approximate area of forest patch” refers to the size of the forest patch (estimated from map) in which mosquito collections were conducted within the forest habitat type. “Population size” refers to the estimated number of residents derived from household enumeration conducted as part of the Monkeybar cross-sectional survey in September to December 2015.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
GLMs were used to compute (R2 and effect sizes) for subsequent power analyses to determine sample sizes required to generate the same associations (P = 0.05) with 80% power.
(DOCX)
(DOCX)
(DOCX)
Predicted mean number of A) An. balabacensis, B) An. donaldi and C) An. maculatus biting per hour between 18:00–24:00 hrs, pooled across all sites and habitat types. Error bars are 95% confidence intervals.
(DOCX)
Acknowledgments
The authors would like to thank Albert M. Lim, Benny O. Manin and the MonkeyBar field team in Sabah for their support throughout the study, particularly field assistants Mohd Fazreen Abdullah and Nemran Bayan for their hard work. We thank the village leaders and all communities participating in entomological collections for their cooperation and interest during this research. We thank Universiti Malaysia Sabah for the use of their lab facilities.
Data Availability
The data underlying the results presented in the study are available from Harvard dataverse: https://doi.org/10.7910/DVN/3QG1HP.
Funding Statement
This work was funded by the Biotechnology and Biological Sciences Research Council Doctoral Training Programme (Grant No. 1517720; https://bbsrc.ukri.org/skills/investing-doctoral-training/dtp/ to HF and RB) and the Medical Research Council (Grant No. G1100796; https://mrc.ukri.org/funding/browse/esei-specification/environmental-and-social-ecology-of-human-infectious-diseases-esei-specification/ to TC, KF, CD, IV and HF). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Singh B, Daneshvar C. Human infections and detection of Plasmodium knowlesi. Clin Microbiol Rev [Internet]. 2013. April [cited 2014 Oct 17];26(2):165–84. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3623376&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong ML, Chua TH, Leong CS, Khaw LT, Fornace K. Seasonal and spatial dynamics of the primary vector of Plasmodium knowlesi within a major transmission focus in Sabah, Malaysia. PLoS Negl Trop Dis. 2015;9(10):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manin BO, Ferguson HM, Vythilingam I, Fornace K, William T, Torr SJ, et al. Investigating the contribution of peri-domestic transmission to risk of zoonotic malaria infection in humans. PLoS Negl Trop Dis [Internet]. 2016;10(10):1–14. Available from: http://dx.plos.org/10.1371/journal.pntd.0005064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chua TH, Manin BO, Daim S, Vythilingam I, Drakeley C. Phylogenetic analysis of simian Plasmodium spp. infecting Anopheles balabacensis Baisas in Sabah, Malaysia. PLoS Negl Trop Dis. 2017;11(10):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.William T, Jelip J, Menon J, Anderios F, Mohammad R, Awang Mohammad T a, et al. Changing epidemiology of malaria in Sabah, Malaysia: increasing incidence of Plasmodium knowlesi. Malar J [Internet]. 2014. January [cited 2014 Nov 21];13(1):1–11. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4195888&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warren M, Cheong WH, Fredericks HK, Coatney AR. Cycles of jungle malaria in west Malaysia. Am J Trop Med Hyg. 1970;19(3):383–93. [DOI] [PubMed] [Google Scholar]
- 7.Chua TH, Manin BO, Vythilingam I, Fornace K, Drakeley CJ. Effect of different habitat types on abundance and biting times of Anopheles balabacensis Baisas (Diptera: Culicidae) in Kudat district of Sabah, Malaysia. Parasit Vectors [Internet]. 2019;12(364):1–13. Available from: 10.1186/s13071-019-3627-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fornace KM, Alexander N, Abidin TR, Brock PM, Chua TH, Vythilingam I, et al. Local human movement patterns and land use impact exposure to zoonotic malaria in Malaysian Borneo. Elife. 2019;8(e47602):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan CH, Vythilingam I, Matusop A, Chan ST, Singh B. Bionomics of Anopheles latens in Kapit, Sarawak, Malaysian Borneo in relation to the transmission of zoonotic simian malaria parasite Plasmodium knowlesi. Malar J. 2008;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vythilingam I, Noorazian YM, Huat TC, Jiram AI, Yusri YM, Azahari AH, et al. Plasmodium knowlesi in humans, macaques and mosquitoes in peninsular Malaysia. Parasit Vectors [Internet]. 2008. January [cited 2014 Dec 8];1(1):1–10. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2531168&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vythilingam I, Lim Y, Venugopalan B, Ngui R, Leong CS, Wong ML, et al. Plasmodium knowlesi malaria an emerging public health problem in Hulu Selangor, Selangor, Malaysia (2009–2013): epidemiologic and entomologic analysis. Parasit Vectors [Internet]. 2014. January [cited 2014 Oct 14];7(436):1–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25223878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiram AI, Vythilingam I, NoorAzian YM, Yusof YM, Azahari AH, Fong M-Y. Entomologic investigation of Plasmodium knowlesi vectors in Kuala Lipis, Pahang, Malaysia. Malar J [Internet]. 2012. January [cited 2014 Nov 30];11(213):1–10. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3476358&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wharton RH, Eyles DE. Anopheles hackeri, a vector of Plasmodium knowlesi in Malaya. Science (80-). 1961;134(3474):279–80. [DOI] [PubMed] [Google Scholar]
- 14.Hawkes FM, Manin BO, Cooper A, Daim S, Homathevi R, Jelip J, et al. Vector compositions change across forested to deforested ecotones in emerging areas of zoonotic malaria transmission in Malaysia. Nat Sci Reports. 2019;9(13312):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Zhong D, Cui L, Lee M, Yang Z, Yan G, et al. Population dynamics and community structure of Anopheles mosquitoes along the China-Myanmar border. Parasit Vectors [Internet]. 2015;1–8. Available from: 10.1186/s13071-015-1057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obsomer V, Defourny P, Coosemans M. The Anopheles dirus complex: spatial distribution and environmental drivers. Malar J. 2007;6(26):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manh C Do, Beebe NW, Van VNT, Quang T Le, Lein CT, Nguyen D Van, et al. Vectors and malaria transmission in deforested, rural communities in north-central Vietnam. Malar J. 2010;9(259):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Malaria surveillance, monitoring & evaluation: a reference manual [Internet]. Geneva: CC BY-NC-SA 3.0 IGO; 2018. Available from: https://apps.who.int/iris/bitstream/handle/10665/272284/9789241565578-eng.pdf?ua=1
- 19.Onyido AE, Obinatu SC, Umeanaeto PU, Obiukwu MO, Egbuche MC. Malaria prevalence and mosquito vector abundance in Uli town, Ihiala local government area, Anambra State, Nigeria. African J Biomed Res. 2011;14:175–82. [Google Scholar]
- 20.Janko MM, Irish SR, Reich BJ, Peterson M, Doctor SM, Mwandagalirwa MK, et al. The links between agriculture, Anopheles mosquitoes, and malaria risk in children younger than 5 years in the Democratic Republic of the Congo: a population-based, cross-sectional, spatial study. Lancet Planet Heal [Internet]. 2018;2(2):74–82. Available from: 10.1016/S2542-5196(18)30009-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Killeen GF, Govella NJ, Mlacha YP, Chaki PP. Suppression of malaria vector densities and human infection prevalence associated with scale-up of mosquito-proofed housing in Dar es Salaam, Tanzania: re-analysis of an observational series of parasitological and entomological surveys. Lancet Planet Heal [Internet]. 2019;3:e132–43. Available from: 10.1016/S2542-5196(19)30035-X [DOI] [PubMed] [Google Scholar]
- 22.Pinto J, Sousa CA, Gil V, Ferreira C, Goncalves C, Lopes D, et al. Malaria in São Tomé and Príncipe: parasite prevalences and vector densities. Acta Trop. 2000;76(2):185–93. [DOI] [PubMed] [Google Scholar]
- 23.Charlwood JD, Smith T, Lyimo E, Kitua AY, Masanja H, Booth M, et al. Incidence of Plasmodium falciparum infection in infants in relation to exposure to sporozoite-infected anophelines. Am J Trop Med Hyg. 1998;59(2):243–51. [DOI] [PubMed] [Google Scholar]
- 24.Moreno JE, Rubio-Palis Y, Paez E, Perez E, Sanchez V. Abundance, biting behaviour and parous rate of anopheline mosquito species in relation to malaria incidence in gold‐mining areas of southern Venezuela. Med Vet Entomol. 2007;21(4):339–49. [DOI] [PubMed] [Google Scholar]
- 25.Mbogo CM, Mwangangi JM, Nzovu J, Gu W, Yan G, Gunter JT, et al. Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. Am J Trop Med Hyg. 2003;68(6):734–42. [PubMed] [Google Scholar]
- 26.Thomson MC, D’Alessandro U, Bennett S, Connor SJ, Langerock P, Jawara M, et al. Malaria prevalence is inversely related to vector density in The Gambia, West Africa. Trans R Soc Trop Med Hyg. 1994;88(6):638–43. [DOI] [PubMed] [Google Scholar]
- 27.Das MK, Prajapati BK, Tiendrebeogo RW, Ranjan K, Adu B, Srivastava A, et al. Malaria epidemiology in an area of stable transmission in tribal population of Jharkhand, India. Malar J. 2017;16:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith DL, Dushoff J, Snow RW, Hay SI. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature. 2005;438:492–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fornace KM, Herman LS, Abidin TR, Chua TH, Daim S, Lorenzo PJ, et al. Exposure and infection to Plasmodium knowlesi in case study communities in Northern Sabah, Malaysia and Palawan, The Philippines. PLoS Negl Trop Dis. 2018;12(6):e0006432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fornace KM, Brock PM, Abidin TR, Grignard L, Herman LS, Chua TH, et al. Environmental risk factors and exposure to the zoonotic malaria parasite Plasmodium knowlesi across northern Sabah, Malaysia: a population-based cross-sectional survey. Lancet Planet Heal [Internet]. 2019;3:179–86. Available from: https://apps.who.int/iris/bitstream/handle/10665/272284/9789241565578-eng.pdf?ua=1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawkes F, Manin BO, Ng SH, Torr SJ, Drakeley C, Chua TH, et al. Evaluation of electric nets as means to sample mosquito vectors host-seeking on humans and primates. Parasit Vectors [Internet]. 2017;10(1):338 Available from: http://parasitesandvectors.biomedcentral.com/articles/10.1186/s13071-017-2277-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown R, Hing CT, Fornace K, Ferguson HM. Evaluation of resting traps to examine the behaviour and ecology of mosquito vectors in an area of rapidly changing land use in Sabah, Malaysian Borneo. Parasites and Vectors. 2018;11(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vythilingam I, Chan ST, Shanmugratnam C, Tanrang H, Chooi KH. The impact of development and malaria control activities on its vectors in the Kinabatangan area of Sabah, East Malaysia. Acta Trop [Internet]. 2005. October [cited 2014 Nov 11];96(1):24–30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16076459 [DOI] [PubMed] [Google Scholar]
- 34.Rattanarithikul R, Harbach RE, Harrison BA, Panthusiri P, Jones JW, Coleman RE. Illustrated keys to the mosquitoes of Thailand II. Genera Culex and Lutzia. Southeast Asian J Trop Med Public Health. 2005;36:1–97. [PubMed] [Google Scholar]
- 35.Rattanarithikul R, Harrison B a., Panthusiri P, Coleman RE. Illustrated keys to the mosquitoes of Thailand. I. Background; geographic distribution; lists of genera, subgenera, and species; and a key to the genera. Southeast Asian J Trop Med Public Health. 2005;36:1–80. [PubMed] [Google Scholar]
- 36.Rattanarithikul R, Harrison B a., Panthusiri P, Peyton EL, Coleman RE. Illustrated keys to the mosquitoes of Thailand: III. Genera Aedeomyia, Ficalbia, Mimomyia, Hodgesia, Coquillettidia, Mansonia, and Uranotaenia. Southeast Asian J Trop Med Public Health. 2006;37:1–10. [PubMed] [Google Scholar]
- 37.Rattanarithikul R, Harrison BA, Harbach RE, Panthusiri P. Illustrated keys to the mosquitoes of Thailand IV. Anopheles. 2006;37:1–128. [PubMed] [Google Scholar]
- 38.Sallum M, Peyton EL, Harrison BA, Wilkerson RC, Pesquisa N De, Médica E, et al. Revision of the Leucosphyrus group of Anopheles (Cellia). Rev Bras Entomol. 2005;49:1–152. [Google Scholar]
- 39.Rahman WA, Hassan AA, Adanan CR, Rashid MR. The prevalence of Plasmodium falciparum and P. vivax in relation to Anopheles maculatus densities in a Malaysian village. Acta Trop. 1993;55(4):231–5. [DOI] [PubMed] [Google Scholar]
- 40.Snounou G, Singh B. Nested PCR analysis of Plasmodium parasites. Methods Mol Med [Internet]. 2002. January;72:189–203. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12125116 [DOI] [PubMed] [Google Scholar]
- 41.Ta TH, Hisam S, Lanza M, Jiram AI, Ismail N, Rubio JM. First case of a naturally acquired human infection with Plasmodium cynomolgi. Malar J [Internet]. 2014;13:1–7. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3937822&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee KS, Divis PCS, Zakaria SK, Matusop A, Julin RA, Conway DJ, et al. Plasmodium knowlesi: reservoir hosts and tracking the emergence in humans and macaques. PLoS Pathog. 2011;7(4):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imwong M, Tanomsing N, Pukrittayakamee S, Day NPJ, White NJ, Snounou G. Spurious amplification of a Plasmodium vivax small-subunit RNA gene by use of primers currently used to detect P. knowlesi. J Clin Microbiol [Internet]. 2009. December [cited 2014 Nov 3];47(12):4173–5. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2786678&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herman LS, Fornace K, Phelan J, Grigg MJ, Anstey NM, William T, et al. Identification and validation of a novel panel of Plasmodium knowlesi biomarkers of serological exposure. PLoS Negl Trop Dis. 2018;12(6):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hurlbert SH. The nonconcept of species diversity: A critique and alternative parameters. Ecology. 2012;52(4):577–86. [DOI] [PubMed] [Google Scholar]
- 46.Sanders HL. Marine benthic diversity: A comparative study. Am Nat. 1968;102(925):243–82. [Google Scholar]
- 47.Zhang Z. Confidence Intervals for Simpson’s Diversity Index. 2012;(704):1–6. Available from: http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Confidence+Intervals+for+Simpson+’+s+Diversity+Index+*#7 [Google Scholar]
- 48.Peet RK. The measurement of species diversity. Annu Rev Ecol Syst [Internet]. 1974;5(1):285–307. Available from: http://www.annualreviews.org/doi/10.1146/annurev.es.05.110174.001441 [Google Scholar]
- 49.Morris EK, Caruso T, Buscot F, Fischer M, Hancock C, Maier TS, et al. Choosing and using diversity indices: insights for ecological applications from the German Biodiversity Exploratories. Ecol Evol. 2014;4(18):3514–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansen MC. High-resolution global maps of 21st-century forest cover change. Science (80-). 2013;342:850–4. [DOI] [PubMed] [Google Scholar]
- 51.Troyo A, Fuller DO, Calderon-Arguedas O, Beier JC. A geographical sampling method for surveys of mosquito larvae in an urban area using high-resolution satellite imagery. J Vector Ecol. 2014;33(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grigg MJ, Cox J, William T, Jelip J, Fornace KM, Brock PM, et al. Individual-level factors associated with the risk of acquiring human Plasmodium knowlesi malaria in Malaysia: a case-control study. Lancet Planet Heal [Internet]. 2017;1(3):97–104. Available from: http://linkinghub.elsevier.com/retrieve/pii/S2542519617300311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fornace KM, Abidin TR, Alexander N, Brock P, Grigg MJ, Murphy A, et al. Association between landscape factors and spatial patterns of emergent Plasmodium knowlesi infections in Sabah, Malaysia. Eid. 2016;22(2):201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooper DJ, Rajahram GS, William T, Jelip J, Mohammad R, Benedict J, et al. Plasmodium knowlesi malaria in Sabah, Malaysia, 2015–2017: Ongoing increase in incidence despite near- elimination of the human-only Plasmodium species. Clin Infect Dis. 2019;1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lindsay SW, Martens WJ. Malaria in the African highlands: past, present and future. Bull World Health Organ. 1998;76(12):33–45. [PMC free article] [PubMed] [Google Scholar]
- 56.Lardeux FJ, Tejerina RH, Quispe V, Chavez TK. A physiological time analysis of the duration of the gonotrophic cycle of Anopheles pseudopunctipennis and its implications for malaria transmission in Bolivia. Malar J [Internet]. 2008;7:1–17. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2518372&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beck-johnson LM, Nelson WA, Paaijmans KP, Read AF, Thomas MB, Bjørnstad ON. The effect of temperature on Anopheles mosquito population dynamics and the potential for malaria transmission. 2013;8(11):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patz J a Olson SH. Malaria risk and temperature: influences from global climate change and local land use practices. Proc Natl Acad Sci U S A. 2006;103(15):5635–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paaijmans KP, Blanford S, Chan BHK, Thomas MB. Warmer temperatures reduce the vectorial capacity of malaria mosquitoes. Biol Lett [Internet]. 2012;8(3):465–8. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-84861516095&partnerID=tZOtx3y1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Afrane YA, Little TJ, Lawson BW, Githeko AK, Yan G. Deforestation and vectorial capacity of Anopheles gambiae giles mosquitoes in malaria transmission, Kenya. Emerg Infect Dis. 2008;14(10):1533–8. [DOI] [PMC free article] [PubMed] [Google Scholar]