Abstract
Background
Malnutrition on the background of HIV (Human Immunodeficiency Virus) infection is a complex medical condition that carries significant morbidity and mortality for affected children, with greater mortality from SAM (Severe Acute Malnutrition) among HIV-positive children than their HIV-negative peers. HIV-induced immune impairment heightened risk of opportunistic infection and can worsen nutritional status of children. HIV infection often leads to nutritional deficiencies through decreased food intake, mal-absorption and increased utilization and excretion of nutrients, which in turn can hasten death.
Objective
The aim of this systematic review and meta-analysis was to assess the magnitude of underweight, wasting and stunting among HIV positive children in East Africa.
Methods
The authors systematically reviewed and meta-analyzed studies that assessed the prevalence of underweight, wasting and stunting among HIV positive children in East Africa from PubMed, Cochrane Library, Google Scholar, and Gray Literatures using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guideline. The last search date was December 30/2019. The data was extracted in excel sheet considering country, study design, year of publication, prevalence reported. Then the authors transformed the data to STATA 14 for analysis. Heterogeneity across the studies was assessed by the Q and the I2 test. A weighted inverse variance random-effects model was used to estimate the magnitude of underweight, wasting and stunting. The subgroup analysis was done by country, year of publication, and study design. To examine publication bias, a funnel plot and Egger’s regression test were used.
Results
For the analysis a total of 22 studies with 22074 patients were used. The pooled prevalence of under-weight, wasting, and stunting among HIV positive children in East Africa was found to be 41.63% (95%CI; 35.69–47.57; I2 = 98.7%; p<0.001), 24.65% (95%CI; 18.34–30.95; I2 = 99.2%; p<0.001), and 49.68% (95%CI; 42.59–56.77; I2 = 99.0%; p<0.001) respectively. The prevalence of under-weight among HIV positive children was found to be 49.67% in Ethiopia followed by 42.00 in Rwanda. It was high among cohort studies (44.87%). Based on the year of publication, the prevalence of under-weight among HIV positive children was found to be 40.88% from studies conducted from January 2008-December 2014, while it was 43.68% from studies conducted from 2015–2019. The prevalence of wasting among HIV positive children was found to be 29.7% in Tanzania followed by 24.94% in Ethiopia. Based on the study design, the prevalence of wasting among HIV positive children was found to be high in cohort studies (31.15%). The prevalence of stunting among HIV positive children was found to be 51.63% in Ethiopia, followed by 48.21% in Uganda.
Conclusions
The results presented above provide evidence of a higher prevalence of under nutrition among HIV positive children in East Africa. Despite the country level variations of child under nutrition in East Africa, still it is high in all aspects compared to the studies from other parts of Africa. It is recommended that further systematic review and meta-analysis need to be conducted on magnitude of malnutrition among HIV positive children in Sub-Saharan Africa as a whole.
Introduction
According to WHO malnutrition, in all its forms, includes under-nutrition (wasting, stunting, underweight), inadequate vitamins or minerals, overweight, obesity, and resulting diet-related non communicable diseases [1, 2]. The term under-nutrition is defined as the outcome of insufficient food intake (hunger) and repeated infectious disease; which includes being underweight for once age, too short for once age(stunted), dangerously thin(wasted) and deficient in vitamins and minerals (micronutrient malnutrition) [3]. Malnutrition on the background of HIV infection is a complex medical condition that carries significant morbidity and mortality for affected children, with greater mortality from SAM among HIV-positive children than their HIV-negative peers [4].
Nutritional and micronutrient deficiencies are common in HIV-infected persons and play a major and synergistic role in disease progression and in the retardation of growth and development of children. HIV/AIDS is most prevalent in sub-Saharan Africa, where it combines with other common conditions such as malnutrition and opportunistic infections to cause devastation effect among families, communities, and nations [5, 6].
Human Immune Virus and under nutrition interact in a vicious cycle; HIV-induced immune impairment heightened risk of opportunistic infection and can worsen nutritional status of children. HIV infection often leads to nutritional deficiencies through decreased food intake, mal-absorption and increased utilization and excretion of nutrients, which in turn can hasten death. Likewise, nutritional status modulates the immunological response to HIV infection, affecting the overall clinical outcome, since malnutrition is the primary cause of immunodeficiency [7–10].
Malnutrition is responsible for 11% of the total global disease burden and 35% of child deaths worldwide. In developing countries an estimated 19 million children are severely wasted [4, 11]. In some regions, notably sub-Saharan Africa, human immunodeficiency virus (HIV) infection poses an added challenge to the care of malnourished children. While the clinical context and interventions for many common causes of childhood mortality worldwide have been addressed over the last decade [12, 13], the management of malnutrition in children particularly in those infected with HIV remains poorly addressed [14].
In sub-Saharan Africa, the epidemiology of severe malnutrition has been increased in children requiring hospitalization composed of those who are HIV infected or HIV exposed with case-fatality rates reaches as high as 20–50% [15, 16]. Large percentages of HIV-positive children have an episode of severe malnutrition as their first AIDS-defining illness. Under nutrition is an important factor which might predict disease progression of HIV-infected individuals. It also results in higher risk of morbidity and mortality in both HIV-infected adults and children. Wasting and weight loss are common features of HIV infection, especially in resource-limited settings. It is known that children with HIV and severe malnutrition invariably have lower nutritional recovery and higher mortality rates than their HIV-negative counterparts [17, 18]. ART initiation in children can also cause metabolic disorders, and adverse effects on the nutritional status, especially in the first months of treatment, by causing complications, such as nausea and vomiting, or reduced bone mineral density [5, 19].
In East Africa, variety of previous studies have reported that the magnitude of under nutrition; accordingly the prevalence of under-weight, wasting and stunting was ranged from 19.4% to 77.1%, 7% to 77.1% and 13% to 71.8% respectively. This showed pronounced discrepancies among reports of under nutrition across different geographical settings and different time periods. Moreover, there is no regionally represented pooled data of under nutrition in East Africa. Therefore, this systematic review and meta-analysis was aimed to estimate the pooled prevalence of underweight, wasting and stunting in the East African context.
Methods
Review question
The review questions of this systematic review and meta-analysis were:
What is the pooled prevalence of under-weight, wasting and stunting among HIV positive children in East Africa context?
Study selection and screening
To exclude duplicate studies the retrieved studies were exported to Endnote version 8 reference managers. Before retrieval of full-text papers two investigators (BB and TG) independently screened the selected studies using article's title and abstracts. We used pre-specified inclusion criteria to further screen the full-text articles. Disagreements were resolved with third reviewer (GT) for the final decision on the selection of studies to be included in the analysis [20].
Inclusion and exclusion criteria
Those studies had reported the prevalence of at least one under-weight, wasting and stunting and published in English language from January 2008 to December 2019. Studies conducted with cross-sectional, and cohort study design was included. Studies conducted on marginalized groups/populations like children with any medical diseases, chronic diseases, or street mothers were excluded. Studies conducted on HIV/AIDS children in East Africa. The prevalence of under-weight, wasting, and stunting was considered when weight/ age, weight /height and height per age Z score <-2sd respectively within a specific population and multiply by 100 to be prevalence report.
Searching strategy
This review identified studies that provide data on the prevalence of under-weight, wasting, and stunting with the context of Eastern Africa. In the searching engine, mainly PubMed, Cochrane library, Google Scholar, and Gray Literatures were retrieved. The last search date was December 30/2019. The Authors searched using keywords that are the amalgamations of population, condition/outcome, and context. A snowball searching of the references of relevant papers for linked articles were also performed. Those search terms or phrases including were: “children”, “child”, “infant”, “under-nutrition”, “underweight”, “wasting”, stunting, HIV, AIDS and Eastern Africa. Using those key terms, the following search map was applied: (prevalence OR magnitude) AND (children [MeSH Terms] OR child OR infant) AND (under-nutrition [MeSH Terms] OR underweight OR wasting OR stunting) AND (HIV OR AIDS) AND Eastern Africa on PubMed database (S1 Table). Thus, the PubMed search combines #1 AND #2 AND #3 (S1 Table). These search terms were further paired with names of each East African country. On both Cochran Library, and Google scholar, a build in text search were used on the advanced search section of the sources. The search date was December 30/2019.
Quality assessment
The quality of the studies were appraised by three authors independently using the Joanna Briggs Institute (JBI) checklist [21]. The disagreement was resolved by the interference of a third reviewer. Studies were considered as high risk or poor quality, when it scored 3 and below [20–22] (S2 Table).
Data extraction
The authors developed data extraction form on the excel sheet in considering country, year of publication, study design and prevalence of underweight, wasting and stunting reported. The data extraction sheet was piloted using selecting four papers randomly the data extraction sheet was piloted and adjustment were made after the template were piloted. Using the extraction form two authors (BB and TG) extracted the data in collaboration. The third author (GT) independently assessed the accuracy of the data and discussions with a third reviewer were done when disagreements between reviewers occurred [20, 22].
Synthesis of results
The authors transformed the data to STATA 14 for analysis after it was extracted in excel sheet. Using a random effect meta-analysis model we pooled the overall prevalence estimates of underweight, wasting and stunting. Using Q statistic and the I2 statistics we assessed the heterogeneity of effect size. The I2statistic value of zero, 25, 50, and 75% indicates true homogeneity, low heterogeneity, moderate heterogeneity and high heterogeneity, respectively. Using study design, study country, and year of publication subgroup analysis was done by. Sensitivity analysis was done to assess the impact of a single study on the pooled estimate. Using funnel plot subjectively and objectively by Egger’s regression test publication bias was assessed [20].
Results
A total of 3094 studies were identified; 2050 from PubMed, 12 from Cochrane Library, 1010 from Google Scholar and 22 from other sources. After duplication removed, a total of 970 articles remained (2127 removed by duplication). Finally, 230 studies were screened for full-text review, and 22 articles with (n = 22074 patients) were selected for the analysis (Fig 1).
Fig 1. PRISMA–Adapted flow diagram showed the results of the search and reasons for exclusion [23, 24].
Characteristics of included studies
Twenty two studies were included in this systematic review and meta-analysis [18, 25–45]. Of them 12 studies were done in Ethiopia [18, 25–34, 37], 1 in Kenya [41], while 2 were in Uganda [38, 39], 1 in Rwanda [35], and 6 in Tanzania [36, 40, 42–45]. Based on the study design used 14 studies were done by cross-sectional study design [18, 26, 27, 30, 31, 33–35, 37, 38, 40, 41, 43, 45] and while other 8 studies were conducted by cohort study design [25, 27–29, 32, 36, 39, 42, 44]. 14/22(63.6%) were published between 2008 and 2014 and the remaining 8/22 (33.4%) were published between 2015 and 2019. The total number of participants in the included studies were ranges from 96 [32] to 5951 [38] (Table 1).
Table 1. Distribution of included studies on the prevalence underweight, wasting and stunting in East Africa, from January 2008-December 2019.
| Author name | Publication Year | Country | Region | Study design | Sample size | Under weight | Wasting | Stunting | Cut of point | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Z-scor | ||||||||||
| 1. Kedir et al | 2014 | Ethiopia | Adama/Et | Cohort | 560 | 51.6 | <-2sd | [25] | ||
| 2. Mekonen et al | 2014 | Ethiopia | AA/ET | Cross sectional | 255 | 47.5 | 71.3 | <-2sd | [26] | |
| 3. Jeylan et al | 2018 | Ethiopia | Adama/ET | Cross sectional | 412 | 21.8 | 13.4 | <-2sd | [27] | |
| 4. Tekleab et al | 2016 | Ethiopia | AA/ET | Cohort | 202 | 39.5 | 16.3 | 71.3 | <-2sd | [28] |
| 5. Yassin et al | 2017 | Ethiopia | Fiche/ET | Cohort | 269 | <3rd centile | [29] | |||
| 6. Abdulkadir et al | 2014 | Ethiopia | Gonder/ET | Cross sectional | 142 | 31.7 | 46.5 | NR | [30] | |
| 7. Haileselassie et al | 2019 | Ethiopia | Harar/Et | Cross sectional | 390 | 28.2 | 24.7 | <-2sd | [31] | |
| 8. Teklemariam et al | 2015 | Ethiopia | Harar/ET | Cross sectional | 108 | 51.6 | 31.5 | 49.1 | <-2sd | [18] |
| 9. Workneh et al | 2009 | Ethiopia | Jimma/ET | Cohort | 96 | 77.1 | 47.5 | 63.5 | <5th centile | [32] |
| 10. Wondimu et al | 2014 | Ethiopia | Hawassa/Et | Cross sectional | 455 | 41.2 | 21.4 | 60.5 | <-2sd | [33] |
| 11. Megabiaw et al | 2012 | Ethiopia | Gondar/ET | Cross sectional | 301 | 41.7 | 5.8 | 65 | <-2sd | [34] |
| 12. Arpadi et al | 2019 | Rwanda | Rwanda | Cross sectional | 374 | 42 | <-2sd | [35] | ||
| 13. Kamenju et al | 2017 | Tanzania | Tanzania | Cohort | 2092 | 25.4 | 21.6 | 27.1 | <-2sd | [36] |
| 14. Sewale et al | 2018 | Ethiopia | Gojjam/Et | Cross sectional | 372 | <-2 sd | [37] | |||
| 15. Nalwoga et al | 2010 | Uganda | Uganda | Cross sectional | 5951 | 30 | 10 | 42 | <-2 sd | [38] |
| 16. Arinaitwe et al | 2012 | Uganda | Uganda | Cohort | 358 | 39.7 | 71.8 | <-2 sd m-s | [39] | |
| 17. Sunguya et al | 2014 | Tanzania | Tanzania | Cross sectional | 748 | 40.6 | 30.2 | 60.8 | ≥-2sd | [40] |
| 18. Herman et al | 2102 | Kenya | Kenya | Cross sectional | 2275 | 19.4 | 7 | 28.6 | <-2 sd | [41] |
| 19. Mwiru et al | 2015 | Tanzania | Tanzania | Cohort | 3144 | 53 | 33 | 56 | <-2 sd | [42] |
| 20. Sunguya et al | 2011 | Tanzania | Tanzania | Cross sectional | 213 | 22.1 | 23.6 | 36.6 | <-2sd | [43] |
| 21. Mwiru et al | 2014 | Tanzania | Tanzania | Cohort | 3144 | 30 | 40 | 52 | <-2sd | [44] |
| 22. Dundigalla et al | 2015 | Tanzania | Tanzania | Cross sectional | 213 | 61 | 29.1 | 56.8 | <-2sd | [45] |
Meta-analysis
Underweight
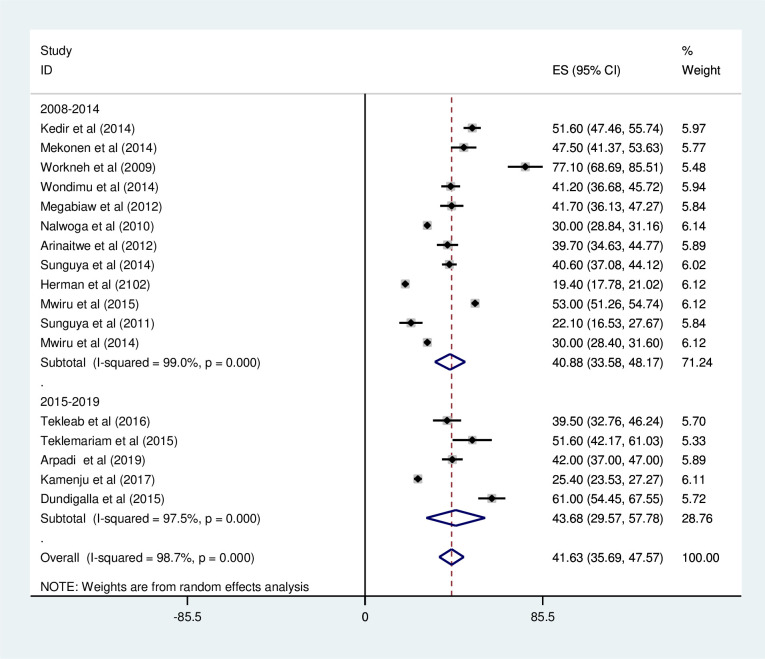
Prevalence of under-weight among HIV positive children. Most of the studies (n = 17) have reported the prevalence of under-weight among HIV positive children. The prevalence of under-weight was ranged from 19.4% [41] to 77.1% [32]. The pooled prevalence of under-weight among HIV positive children in East Africa using random-effects model analysis was found to be 41.63% (95%CI; 35.69–47.57; I2 = 98.7%; p<0.001) (Fig 2).
Fig 2. Forest plot showing the pooled prevalence of under-weight among HIV positive children in East Africa, from January 2008-December 2019.
Subgroup analysis for the prevalence of under-weight among HIV positive children in Eastern Africa. Using country, study design, and year of publication as criteria subgroup analysis was done. Based on this, the prevalence of under-weight among HIV positive children was found to be 49.67% in Ethiopia, 42.00 in Rwanda, 38.59% in Tanzania, 34.52% in Uganda, and 19.4% in Kenya (Fig 3 and Table 2). The prevalence of under-weight among HIV positive children was found to be 39.33% in cross-sectional studies and 44.87% in cohort studies (Fig 4 and Table 2). It was found to be 40.88% from studies conducted from January 2008-December 2014, while it was 43.68% from studies conducted from 2015–2019 (Fig 5 and Table 2).
Fig 3. Forest plot showing the subgroup analysis of the prevalence of under-weight among HIV positive children by country in East Africa, from January 2008-December 2019.
Table 2. Summery of subgroup analysis of the prevalence of under-weight among HIV positive children in Eastern Africa by country, design and year of publication, from January 2008-December 2019.
| Variables | Characteristics | Pooled prevalence, %(95% CI) | I2, (P-value) |
|---|---|---|---|
| By country | Ethiopia | 49.67(42.06–57.27) | 91.3%(<0.001) |
| Rwanda | 42.00(37.00–47.00) | - | |
| Tanzania | 38.59(27.33–49.83) | 99.2%(<0.001) | |
| Uganda | 34.52(25.04–44.01) | 92.5%(<0.001) | |
| Kenya | 19.40(17.78–21.02) | - | |
| By study design | Cross-sectional | 39.33(32.54–46.12) | 97.8% (<0.001) |
| Cohort | 44.87(33.97–55.77) | 99.1%(<0.001) | |
| By year of publication | 2008–2014 | 40.88(33.58–48.17) | 99.0% (<0.001) |
| 2015–2019 | 43.68(29.57–57.78) | 97.5%(<0.001) |
Fig 4. Forest plot showing the subgroup analysis of the prevalence of under-weight among HIV positive children by study design in East Africa, from January 2008-December 2019.
Fig 5. Forest plot showing the subgroup analysis of the prevalence of under-weight among HIV positive children by year of publication in East Africa, from January 2008-December 2019.
Sensitivity analysis for under-weight
To assess the influence of individual study on the pooled prevalence of under-weight in Eastern Africa we employed a sensitivity analysis. Accordingly the findings were not dependent on all included studies. The prevalence of under-weight varied between 39.55(33.67–45.43) [32] and 42.71(36.31–49.11) [36] after deletion of a single study (S1 Fig).
Publication bias
Subjectively the funnel plot indicated symmetrical distributions which indicate the absence of publication bias. The Egger’s regression test- also revealed the absence of publication bias; P-value was 0.064, (S2 Fig).
Wasting
Prevalence of wasting among HIV positive children in East Africa. Most of the studies (n = 16) have reported the prevalence of wasting among HIV positive children. The prevalence of wasting was ranged from 7% [41] to 77.1% [32]. The prevalence of wasting among HIV positive children in East Africa was found to be 24.65% (95%CI; 18.34–30.95; I2 = 99.2%; p<0.001) (Fig 6).
Fig 6. Forest plot shows the pooled prevalence of wasting among HIV positive children in East Africa, from January 2008-December 2019.
Subgroup analysis of the prevalence of wasting among HIV positive children in Eastern Africa. The subgroup analysis was by country, study design, and year of publication. Accordingly, the prevalence of wasting among HIV positive children was found to be 24.94% in Ethiopia, 29.7% in Tanzania, 10.0% in Uganda, and 7.0% in Kenya (Fig 7 and Table 3). The prevalence of wasting among HIV positive children was found to be 21.22% in cross-sectional studies and 31.15% in cohort studies (Fig 8 and Table 3). Based on the year of publication, the prevalence of wasting among HIV positive children was found to be 24.7% from studies conducted from January 2008-December 2014, while it was 24.02% from studies conducted from 2015–2019 (Fig 9 and Table 3).
Fig 7. Forest plot shows the pooled prevalence of wasting by country in East Africa, from January 2008-December 2019.
Table 3. Summery of subgroup analysis of the prevalence of wasting among HIV positive children in Eastern Africa by country, design and year of publication, from January 2008-December 2019.
| Variables | Characteristics | Pooled prevalence, %(95% CI) | I2, (P-value) |
|---|---|---|---|
| By country | Ethiopia | 24.94(16.83–33.05) | 95.7%(<0.001) |
| Tanzania | 29.7(23.00–36.39) | 97.8%(<0.001) | |
| Uganda | 10.0(9.24–10.76) | - | |
| Kenya | 7.0(5.95–8.95) | - | |
| By study design | Cross-sectional | 21.22(16.56–25.88) | 97.6% (<0.001) |
| Cohort | 31.15(22.57–39.74) | 98.5%(<0.001) | |
| By year of publication | 2008–2014 | 24.7(16.11–33.30) | 99.5% (<0.001) |
| 2015–2019 | 24.02(20.35–27.70) | 76.9%(<0.001) |
Fig 8. Forest plot shows the pooled prevalence of wasting by year of publication in East Africa, from January 2008-December 2019.
Fig 9. Forest plot shows the pooled prevalence of wasting by study design in East Africa, from January 2008-December 2019.
Sensitivity analysis for wasting
To assess the influence of individual study on the pooled prevalence of wasting in Eastern Africa we employed a sensitivity analysis. Accordingly the findings were not dependent on all included studies. The prevalence of wasting varied between 23.29(16.85–29.74) [32] and 25.95(19.34–32.52) [34] after deletion of a single study (S3 Fig).
Publication bias
Subjectively the funnel plot indicated symmetrical distributions which indicate the absence of publication bias. The Egger’s regression test- also revealed the absence of publication bias; P-value was 0.068 (S4 Fig).
Stunting
Prevalence of stunting among HIV positive children. Most of the studies (n = 18) have reported the prevalence of stunting among HIV positive children. The prevalence of stunting was ranged from 13% [27] to 71.8% [39]. The pooled prevalence of stunting among HIV positive children in East Africa using a random-effects model analysis was found to be 49.68% (95%CI; 42.59–56.77; I2 = 99.0%; p<0.001) (Fig 10).
Fig 10. Forest plot shows the pooled prevalence of stunting in East Africa, from January 2008-December 2019.
Subgroup analysis of the prevalence of stunting among HIV positive children in Eastern Africa. Using country, study design, and year of publication as criteria subgroup analysis was done. Accordingly, the prevalence of stunting among HIV positive children was found to be 51.63% in Ethiopia, 38.59% in Tanzania, 48.21% in Uganda, and 28.60% in Kenya (Fig 11 and Table 4). Based on the study design, the prevalence of under-weight among HIV positive children was found to be 46.16% in cross-sectional studies and 56.73% in cohort studies (Fig 12 and Table 4). The prevalence of stunting among HIV positive children was found to be 54.44% from studies conducted from January 2008-December 2014, while it was 40.11% from studies conducted from 2015–2019 (Fig 13 and Table 4).
Fig 11. Forest plot shows the pooled prevalence of stunting by country in East Africa, from January 2008-December 2019.
Table 4. Summery of subgroup analysis of the prevalence of stunting among HIV positive children in Eastern Africa by country, design and year of publication, from January 2008-December 2019.
| Variables | Characteristics | Pooled prevalence, %(95% CI) | I2, (P-value) |
|---|---|---|---|
| By country | Ethiopia | 51.63(34.68–68.59) | 98.9%(<0.001) |
| Tanzania | 48.21(36.52–59.91) | 99.2%(<0.001) | |
| Uganda | 56.81(27.61–86.02) | 99.3%(<0.001) | |
| Kenya | 28.60(26.74–30.46) | - | |
| By study design | Cross-sectional | 46.16(37.21–55.11) | 98.8% (<0.001) |
| Cohort | 56.73(43.68–69.77) | 99.3%(<0.001) | |
| By year of publication | 2008–2014 | 54.44(47.20–61.68) | 98.8% (<0.001) |
| 2015–2019 | 40.11(25.93–54.30) | 98.6%(<0.001) |
Fig 12. Forest plot shows the pooled prevalence of stunting by year of publication in East Africa, from January 2008-December 2019.
Fig 13. Forest plot shows the pooled prevalence of stunting by study design in East Africa, from January 2008-December 2019.
Sensitivity analysis
To assess the influence of individual study on the pooled prevalence of stunting in Eastern Africa we employed a sensitivity analysis. Accordingly the findings were not dependent on all included studies. The prevalence of stunting varied between 48.3(41.27–55.44) [39] and 51.8(45.07–58.62) [27] after deletion of a single study (S5 Fig).
Publication bias
Subjectively the funnel plot indicated symmetrical distributions which indicate the absence of publication bias. The Egger’s regression test- also revealed the absence of publication bias; P-value was 0.068 (S6 Fig).
Discussion
Based on this systematic review and meta-analysis, it was found that the pooled prevalence of underweight in eastern Africa is 41%. This result is higher than the study conducted among HIV positive children, in Nigeria (12.1%), Cameroon (20.5%, 37.8%) and, and Burkina Faso (31%) [46–49] respectively. But this result is lower compared to large scale study conducted in southern Africa(47.3%) [50]. The discrepancy might be due to the difference in number of study participants across the studies.
The sub group analysis based on country revealed that, the pooled prevalence of under-weight among HIV positive children was found to be 49.67% in Ethiopia, followed by 42.00% in Rwanda. The result is higher compared to large scale DHS study in sub Saharan Africa, which accounts 31.2% in Ethiopia and 18.5% in Rwanda respectively [51], and EDHS 2109 mini report which accounts overall 21% [52]. Based on the study design, the pooled prevalence of under-weight among HIV positive children was 39.33% in cross-sectional studies and 44.87% in cohort studies respectively. This may be due to cohort studies apply strict follow-up trend of the patients; through this they can record more reliable reports of the patients overall character. The pooled prevalence of underweight on the studies conducted from (2015–2019) found to be increased (43.68%) compared to the studies conducted from January 2008-2014(40.88%). This indicates that underweight is still an alarming issue among HIV positive children’s in East Africa.
The pooled prevalence of wasting among HIV positive children in East Africa found to be 24.65% (95%CI; 18.34–30.95). This result is higher compared to the study conducted in central and West African countries (16%) [9], the study conducted by Pendal et al in Cameroon(18.4%) [49], and large scale study conducted in southern Africa(21.3%) [50]. The discrepancy might be due to the emphasis given by the government as well as stakeholders of the area regarding the effects of HIV/AIDS on child growth and development.
Regarding sub group analysis by country the prevalence of wasting is higher in Tanzania (29%) followed by Ethiopia (24.94%). This result is higher compared to the data reported by Ethiopian Demographic Health Survey (EDHS), 2019 mini report which accounted 7% of overall children wasted [52]. This shows that these two countries needs great emphasis to decrease the burden of acute under-nutrition due to HIV infection; and it is better to invite governmental and non-governmental organizations regarding nutritional support to HIV-infected children at ART initiation. The prevalence of wasting among HIV positive children in studies conducted by follow up were found to be higher compared to cross-sectional once.
The pooled prevalence of stunting among HIV positive children in East Africa found to be 49.68% (95%CI; 42.59–56.77). This result is higher compared to large scale study conducted on children with HIV positive in Central and West African (33%) HIV care programmes supported by the Growing up Programme in 2011 [9]. But this study is lower than the study conducted in southern Africa, which accounts (61.1%) of HIV positive children were chronically under nourished. This difference might be due to the action of non-governmental organizations on providing child nutrition compared to our study area.
The sub group analysis result based on country shows, greater than half (51.63%) of HIV positive children’s in Ethiopia found to be stunted followed by nearly half (48.21%) of HIV positive children’s in Uganda are under this scheme. This result is higher compared the data reported by mini EDHS, 2019 accounted (37%) [52], and large scale DHS study conducted in sub-Saharan countries, which accounted 26.2% in Ethiopia and 38.2% in Rwanda [51]. The inconsistency between results might be due to difference number and geographical area of study participants. This result calls the integration of nutritional support for HIV positive children and early initiation of ART to loosen the burden of chronic under nutrition in East African Countries.
The relationship between nutrition and HIV infection is very complex and is modified by factors such as nutritional status, including wasting or obesity, and micronutrient deficiencies along with HIV disease stage. Starting assessment, counseling, and education regarding nutrition shortly after HIV diagnosis is imperative. Good nutrition has been proven to increase resistance to infection and disease and improves energy. Severe malnutrition in HIV-infected persons is recognized as the “wasting syndrome,” defined by the Centers for Disease Control and Prevention (CDC) as a body weight loss equal to or greater than 10% with associated fatigue, fever, and diarrhea unexplained by another cause [53].
HIV positive children are the most vulnerable group for underweight, wasting and stunting among and need more medical and research attention [54].
Conclusion
The findings of this review results revealed a higher prevalence of under-nutrition among HIV positive children in East Africa. Despite the country level variations of child under-nutrition in East Africa, still it is high in all aspects compared to the studies from other parts of Africa. It is recommended that further systematic review and meta-analysis need to be conducted on magnitude of malnutrition among HIV positive children in Sub-Saharan Africa as a whole.
Strength and limitations
As strength the authors used a standardized JBI quality assessment checklist and the included studies were low risk of bias. Moreover, we employed subgroup analysis based on study country, study design, and year of publication and sensitivity analysis to identify the small study effect and the risk of heterogeneity. Nevertheless, there are a few limitations to consider in the present study. First, due to the cross-sectional design, the observed results cannot be interpreted as causal. Second, the self-reported measures of variables are subject to measurement, self-report, social desirability, and recall biases. Third, publication bias may occur because all grey literature may not be included and language bias; since all included studies are published in English only. Forth, since only HIV positive children were taken as a study subjects it is difficult to present the result comparing with HIV negative children.
Recommendations
Special attention and efforts to reduce the burden of under-nutrion in HIV positive children should be applied in East Africa. Health professionals working with HIV positive children should routinely screen and manage under nutrition (underweight, wasting and stunting). Policy makers should incorporate strategies regarding prevention, screening and management of under nutrition in management of HIV/AIDS. Parents of HIV positive children should improve their feeding practice so as to prevent under nutrition.
Hence, nutritional attention is needed for children living with HIV/AIDS and at the time of ART initiation. Future researchers should conduct comparative studies on nutritional status between HIV positive and negative children so that meta-analysis of those comparative studies is recommended by authors of this study.
Supporting information
(DOC)
(DOCX)
Using Joanna Briggs Institute (JBI) quality appraisal checklist [16].
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Abbreviations and acronyms
- DHS
Demographic Heath Survey
- WHO
World Health Organization
- CI
Confidence interval
- AOR
Adjusted odds ratio
- SAM
Severe Acute Malnutrition
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
The authors received no specific funding for this work
References
- 1.Musaiger AO, Hassan AS, Obeid O. The paradox of nutrition-related diseases in the Arab countries: the need for action. International journal of environmental research and public health. 2011;8(9):3637–71. 10.3390/ijerph8093637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, et al. Maternal and child undernutrition: consequences for adult health and human capital. The lancet. 2008;371(9609):340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNICEF. Progress for children: a report card on nutrition: Unicef; 2006. [Google Scholar]
- 4.Rose AM, Hall CS, Martinez-Alier N. Aetiology and management of malnutrition in HIV-positive children. Archives of disease in childhood. 2014;99(6):546–51. 10.1136/archdischild-2012-303348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anabwani G, Navario P. Nutrition and HIV/AIDS in sub-Saharan Africa: an overview. Nutrition. 2005;21(1):96–9. 10.1016/j.nut.2004.09.013 [DOI] [PubMed] [Google Scholar]
- 6.Menamo ED. Impact of Household Food Insecurity on Adherence to Antiretroviral Therapy (ART) among Urban PLHIV: The case of Hawassa City, SNNPR State, Ethiopia: Anchor Academic Publishing (aap_verlag); 2014. [Google Scholar]
- 7.Byron E, Gillespie S, Nangami M. Integrating nutrition security with treatment of people living with HIV: Lessons being learned in Kenya. IFPRI/RENEWAL. 2006. [DOI] [PubMed] [Google Scholar]
- 8.Katona P, Katona-Apte J. The interaction between nutrition and infection. Clinical Infectious Diseases. 2008;46(10):1582–8. 10.1086/587658 [DOI] [PubMed] [Google Scholar]
- 9.Jesson J, Masson D, Adonon A, Tran C, Habarugira C, Zio R, et al. Prevalence of malnutrition among HIV-infected children in Central and West-African HIV-care programmes supported by the Growing Up Programme in 2011: a cross-sectional study. BMC infectious diseases. 2015;15(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jesson J, Leroy V. Challenges of malnutrition care among HIV-infected children on antiretroviral treatment in Africa. Medecine et maladies infectieuses. 2015;45(5):149–56. 10.1016/j.medmal.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 11.Kerac M, McGrath M, Grijalva-Eternod C, Bizouerne C, Saxton J, Bailey H, et al. Management of Acute Malnutrition in Infants (MAMI) Project: summary report. Oxford: ENN Network; 2009. [Google Scholar]
- 12.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? The lancet. 2003;361(9376):2226–34. [DOI] [PubMed] [Google Scholar]
- 13.Trehan I, O'Hare BA, Phiri A, Heikens GT. Challenges in the management of HIV-infected malnourished children in sub-Saharan Africa. AIDS research and treatment. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hesseling AC, Westra A, Werschkull H, Donald P, Beyers N, Hussey G, et al. Outcome of HIV infected children with culture confirmed tuberculosis. Archives of disease in childhood. 2005;90(11):1171–4. 10.1136/adc.2004.070466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heikens GT, Bunn J, Amadi B, Manary M, Chhagan M, Berkley JA, et al. Case management of HIV-infected severely malnourished children: challenges in the area of highest prevalence. The Lancet. 2008;371(9620):1305–7. [DOI] [PubMed] [Google Scholar]
- 16.Byron E, Gillespie S, Nangami M. Integrating nutrition security with treatment of people living with HIV: lessons from Kenya. Food and Nutrition Bulletin. 2008;29(2):87–97. 10.1177/156482650802900202 [DOI] [PubMed] [Google Scholar]
- 17.Fergusson P, Tomkins A. HIV prevalence and mortality among children undergoing treatment for severe acute malnutrition in sub-Saharan Africa: a systematic review and meta-analysis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009;103(6):541–8. 10.1016/j.trstmh.2008.10.029 [DOI] [PubMed] [Google Scholar]
- 18.Teklemariam Z, Mitiku H, Mesfin F. Prevalence of anemia and nutritional status among HIV-positive children receiving antiretroviral therapy in Harar, eastern Ethiopa. HIV/AIDS (Auckland, NZ). 2015;7:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali MK, Magee MJ, Dave JA, Ofotokun I, Tungsiripat M, Jones TK, et al. HIV and metabolic, body, and bone disorders: what we know from low-and middle-income countries. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2014;67:S27–S39. 10.1097/QAI.0000000000000256 [DOI] [PubMed] [Google Scholar]
- 20.Beletew B, Mengesha A, Wudu M, Abate M. Prevalence of neonatal hypothermia and its associated factors in East Africa: a systematic review and meta-analysis. BMC pediatrics. 2020;20(1):1–14. 10.1186/s12887-019-1898-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markos Y, Dadi AF, Demisse AG, Ayanaw Habitu Y, Derseh BT, Debalkie G. Determinants of Under-Five Pneumonia at Gondar University Hospital, Northwest Ethiopia: An Unmatched Case-Control Study. Journal of environmental and public health. 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beletew B, Mengesha A, Wudu M, Abate M. Prevalence of neonatal hypothermia and its associated factors in East Africa: a systematic review and meta-analysis. BMC pediatrics. 2020;20:1–14. 10.1186/s12887-019-1898-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hailu F. Assessement of Traditional Medicine Utilization for Children and Associated Factors Among Parents in Tole Woreda, Southwest Shoa, Oromia, Ethiopia, 2017: Addis Ababa University; 2017. [Google Scholar]
- 25.Kedir A, Desta A, Fesseha G. Factors affecting survival of HIV positive children taking antiretroviral therapy at Adama Referral Hospital and Medical College, Ethiopia. J AIDS Clin Res. 2014;5(3):1–6. [Google Scholar]
- 26.Mekonnen A. Assessment of magnitude and factors affecting nutritional status of HIV infected under-five children at five public hospitals in Addis Ababa and its programmatic implication 2014. [Google Scholar]
- 27.Jeylan A, Mohammed E, Girma A. Magnitude of Stunting, Thinness and Associated Factors among HIV Positive Children Attending Chronic HIV Care and Support in Adama Hospital Medical College, Adama, Oromia Regional State, Ethiopia. [Google Scholar]
- 28.Tekleab AM, Tadesse BT, Giref AZ, Shimelis D, Gebre M. Anthropometric improvement among HIV infected pre-school children following initiation of first line anti-retroviral therapy: implications for follow up. PloS one. 2016;11(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yassin S, Gebretekle GB. Magnitude and predictors of antiretroviral treatment failure among HIV‐infected children in Fiche and Kuyu hospitals, Oromia region, Ethiopia: a retrospective cohort study. Pharmacology research & perspectives. 2017;5(1):e00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gondar E. PREVALENCE OF MALNUTRITION AND ASSOCIATED FACTORS AMONG HIV-INFECTED CHILDREN AGED 6–59 MONTHS AT GONDAR UNIVERSITY HOSPITAL, NORTHWEST ETHIOPIA: DEPARTMENT OF PEDIATRICS AND CHILDHEALTH, COLLEGE OF MEDICINE AND HEATLH …; 2014. [Google Scholar]
- 31.Haileselassie B, Roba KT, Weldegebreal F. Undernutrition and its Associated Factors among Pediatric Age Children Attending Antiretroviral Therapy in Eastern Ethiopia. East African Journal of Health and Biomedical Sciences. 2019;3(1):1–12. [Google Scholar]
- 32.Workneh N, Girma T, Woldie M. Immunologic and clinical outcomes of children on HAART: a Retrospective cohort analysis at Jimma University specialized hospital. Ethiopian Journal of Health Sciences. 2009;19(2). 10.4314/ejhs.v19i2.69419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wondimu WB. Nutritional status and associated factors in human immunodeficiency virus infected children in Hawassa University Referral Hospital Hawassa City. SNNPR, Ethiopia, EPHI; 2014. [Google Scholar]
- 34.Megabiaw B, Wassie B, Rogers NL. Malnutrition among HIV-Positive Children at two Referral Hospitals in Northwest Ethiopia. Ethiop J Health Biomed Sci. 2012;5:3–10. [Google Scholar]
- 35.Arpadi S, Lamb M, Nzeyimana IN, Vandebriel G, Anyalechi G, Wong M, et al. Better Outcomes Among HIV-Infected Rwandan Children 18–60 Months of Age After the Implementation of “Treat All”. Journal of acquired immune deficiency syndromes (1999). 2019;80(3):e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamenju P, Liu E, Hertzmark E, Spiegelman D, Kisenge R, Kupka R, et al. Nutritional status and complementary feeding among HIV‐exposed infants: a prospective cohort study. Maternal & child nutrition. 2017;13(3):e12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sewale Y, Hailu G, Sintayehu M, Moges NA, Alebel A. Magnitude of malnutrition and associated factors among HIV infected children attending HIV-care in three public hospitals in East and West Gojjam Zones, Amhara, Northwest, Ethiopia, 2017: a cross-sectional study. BMC research notes. 2018;11(1):1–6. 10.1186/s13104-017-3088-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nalwoga A, Maher D, Todd J, Karabarinde A, Biraro S, Grosskurth H. Nutritional status of children living in a community with high HIV prevalence in rural Uganda: a cross‐sectional population‐based survey. Tropical medicine & international health. 2010;15(4):414–22. [DOI] [PubMed] [Google Scholar]
- 39.Arinaitwe E, Gasasira A, Verret W, Homsy J, Wanzira H, Kakuru A, et al. The association between malnutrition and the incidence of malaria among young HIV-infected and-uninfected Ugandan children: a prospective study. Malaria journal. 2012;11(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sunguya BF, Poudel KC, Mlunde LB, Urassa DP, Yasuoka J, Jimba M. Poor nutrition status and associated feeding practices among HIV-positive children in a food secure region in Tanzania: a call for tailored nutrition training. PloS one. 2014;9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herman ND. HIV and child malnutrition: Examining the relationship of maternal HIV on nutritional outcomes for dependent children in Kenya: Georgetown University; 2012. [Google Scholar]
- 42.Mwiru RS, Spiegelman D, Duggan C, Seage III GR, Semu H, Chalamilla G, et al. Nutritional status and other baseline predictors of mortality among HIV-infected children initiating antiretroviral therapy in Tanzania. Journal of the International Association of Providers of AIDS Care (JIAPAC). 2015;14(2):172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sunguya BF, Poudel KC, Otsuka K, Yasuoka J, Mlunde LB, Urassa DP, et al. Undernutrition among HIV-positive children in Dar es Salaam, Tanzania: antiretroviral therapy alone is not enough. BMC public health. 2011;11(1):869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mwiru RS, Spiegelman D, Duggan C, Seage III GR, Semu H, Chalamilla G, et al. Growth among HIV-infected children receiving antiretroviral therapy in Dar es Salaam, Tanzania. Journal of tropical pediatrics. 2014;60(3):179–88. 10.1093/tropej/fmt104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dundigalla C, Chidugulla SK, Ashwani N, Divya BP, Dundigalla P. Study of Prevalence Of Malnutrition In HIV Positive Children and Its Correlation With Cd4 Count. IOSR J of Denl and Med Sci. 2015;14(12):50–7. [Google Scholar]
- 46.Anigilaje EA, Olutola A. Prevalence and risk factors of undernutrition among antiretroviral-therapy-naïve subjects aged under 5 years old in Makurdi, Nigeria: a retrospective study. International journal of general medicine. 2015;8:131 10.2147/IJGM.S73881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiabi A, Lebela J, Kobela M, Mbuagbaw L, Obama M, Ekoe T. The frequency and magnitude of growth failure in a group of HIV-infected children in Cameroon. Pan African Medical Journal. 2012;11(1). [PMC free article] [PubMed] [Google Scholar]
- 48.Poda GG, Hsu C-Y, Chao JC. Malnutrition is associated with HIV infection in children less than 5 years in Bobo-Dioulasso City, Burkina Faso: A case–control study. Medicine. 2017;96(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Penda CI, Moukoko ECE, Nolla NP, Evindi NOA, Ndombo PK. Malnutrition among HIV infected children under 5 years of age at the Laquintinie hospital Douala, Cameroon. The Pan African Medical Journal. 2018;30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davies M-A, Keiser O, Eley B, Rabie H, van Cutsem G, Giddy J, et al. Outcomes of the South African national antiretroviral treatment programme for children: the IeDEA Southern Africa collaboration. South African medical journal. 2009;99(10). [PMC free article] [PubMed] [Google Scholar]
- 51.Magadi MA. Household and community HIV/AIDS status and child malnutrition in sub-Saharan Africa: evidence from the demographic and health surveys. Social science & medicine. 2011;73(3):436–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ethiopian Public Health Institute (EPHI) [Ethiopia] and ICF. 2019. Ethiopia Mini Demographic and Health Survey 2019: Key Indicators. Rockville M, USA: EPHI and ICF. [Google Scholar]
- 53.De Pee S, Semba RD. Role of nutrition in HIV infection: review of evidence for more effective programming in resource-limited settings. Food and nutrition bulletin. 2010;31(4_suppl4):S313–S44. [PubMed] [Google Scholar]
- 54.Marotta C, Di Gennaro F, Pizzol D, Madeira G, Monno L, Saracino A, et al. The at risk child clinic (ARCC): 3 years of health activities in support of the most vulnerable children in Beira, Mozambique. International journal of environmental research and public health. 2018;15(7):1350. [DOI] [PMC free article] [PubMed] [Google Scholar]