Supplemental Digital Content is available in the text.
Keywords: cardiac surgery, cardiopulmonary bypass, extracorporeal membrane oxygenation, failure to wean, mortality
Abstract
Objectives:
Extracorporeal membrane oxygenation has been used to support children who fail to wean from cardiopulmonary bypass after pediatric cardiac surgery, but little is known about outcomes. We aimed to describe epidemiology and extracorporeal membrane oxygenation factors associated with inhospital mortality in these patients.
Design:
Retrospective multicenter registry-based cohort study.
Setting:
International pediatric extracorporeal membrane oxygenation centers.
Patients:
Children less than 18 years old supported with extracorporeal membrane oxygenation for failure to wean from cardiopulmonary bypass after cardiac surgery during 2000–2016 and reported to Extracorporeal Life Support Organization’s registry.
Intervention:
None.
Measurements and Main Results:
The primary outcome measure was inhospital mortality. Cardiac surgical procedural complexity was assigned using risk adjustment in congenital heart surgery-1. Multivariable logistic regression was used to identify factors independently associated with the primary outcome. We included 2,322 patients, with a median age of 26 days (interquartile range, 7–159); 47% underwent complex surgical procedures (risk adjustment in congenital heart surgery 4–6 categories). Inhospital mortality was 55%. The multivariable model evaluating associations with inhospital mortality showed noncardiac congenital anomalies (odds ratio, 1.78; CI, 1.36–2.32), comorbidities (odds ratio, 1.59; CI, 1.30–1.94), preoperative cardiac arrest (odds ratio, 1.67; CI, 1.20–2.34), preoperative mechanical ventilation greater than 24 hours (odds ratio, 1.49; CI, 1.21–1.84), preoperative bicarbonate administration (odds ratio, 1.42; CI, 1.08–1.86), longer cardiopulmonary bypass time (> 251 min; odds ratio, 1.50; CI, 1.13–1.99), complex surgical procedures (odds ratio, 1.43; CI, 1.13–1.81), longer extracorporeal membrane oxygenation duration (> 104 hr, odds ratio, 1.54; CI, 1.17–2.02), and extracorporeal membrane oxygenation complications increased the odds of inhospital mortality. Age greater than 26 days (odds ratio, 0.56; CI, 0.42–0.75) reduced the odds of mortality.
Conclusions:
Children supported with extracorporeal membrane oxygenation for failure to wean from cardiopulmonary bypass after cardiac surgery are at high risk of mortality (55%). Younger patients, those with congenital abnormalities and comorbidities, undergoing complex procedures, requiring longer cardiopulmonary bypass, and experiencing extracorporeal membrane oxygenation complications and longer extracorporeal membrane oxygenation duration have higher mortality risk. These data can help assessing prognosis in this high-risk population.
Children with congenital heart disease (CHD) or acquired heart disease undergoing open-heart surgery and failing to wean from cardiopulmonary bypass (CPB) after surgery face imminent mortality (1). Failure to wean from CPB may result from severe post-CPB cardiac and/or pulmonary dysfunction or hemodynamically significant residual lesions. In these patients, transition to extracorporeal membrane oxygenation (ECMO) can provide longer duration of cardiopulmonary support while awaiting cardiac and/or pulmonary recovery, bridge to a surgical or catheter-based intervention aimed at correcting a residual lesion, or bridge to transplantation (1, 2). Previous reports of children supported with ECMO for failing to wean from CPB document variable inhospital mortality (23–60%) (3–6). These single-institution reports are limited by small sample size and generalizability (3–15).
We sought to estimate mortality for a large cohort of pediatric patients supported with ECMO after failing to wean from CPB following cardiac surgery, using multicenter data from the Extracorporeal Life Support Organization (ELSO) registry. Additionally, we explored demographics, presurgical support and surgical details, ECMO support, and complications independently associated with inhospital mortality.
MATERIALS AND METHODS
Data Source
The ELSO registry collects data on ECMO use and outcomes for a wide range of ages and indications. Over 300 United States and international centers contribute ECMO data to the registry, and data from greater than 100,000 patients are available for research (16). Member centers report data on voluntary basis, after approval by the local Institutional Review Boards. The data use agreement between the ELSO and member centers allows release of limited deidentified datasets to the member centers for research purposes, waiving the need for regulatory approval. The present study is qualified for human subject research exemption by the Boston Children’s Hospital Review Board.
Study Population
We extracted data from children (less than 18 yr old) who underwent a cardiac surgical procedure and required ECMO for failure to wean from CPB, during 2000–2016. “Failure to wean from CPB” is an extractable ECMO indication reported through the cardiac addendum. We excluded patients already on ECMO at the time of surgery, patients with no documented cardiac surgical procedure, those in whom the time of surgical procedure was not reported, and ECMO indication as respiratory failure or support of cardiopulmonary resuscitation. Finally, for patients with more than one ECMO run, only the first run was included in the analyses.
Data Collection
Data extracted included demographics, cardiac surgical procedure details, preoperative evaluation, pre-ECMO support variable, and ECMO support information and complications. Our primary outcome measure was inhospital mortality. Secondary outcome measures were successful wean-off ECMO and ECMO duration. We assumed pre-ECMO variables documented patient illness and support prior to cardiac surgery and ECMO. Patient selection, presurgical support, surgical decision-making including weaning from CPB, and management of ECMO patients were not standardized, and therefore subject to wide practice variability.
Data Categorization
Primary and secondary diagnoses, reported using the International Classification of Diseases, 9th Revision, Clinical Modification for cases recorded up to 2015 and International Classification of Diseases, 10th Revision, Clinical Modification for cases recorded from 2016, were used to classify noncardiac anomalies and comorbid conditions. Surgical procedures were reported using the common procedural terminology (CPT) codes (Supplemental Information, http://links.lww.com/DCR/B376). Cardiac surgical procedures were categorized based on complexity, using the Risk Adjustment in Congenital Heart Surgery-1 (RACHS-1) method (17). ECMO complications were grouped using the ELSO-registry complication codes using a previously described system (18).
Statistical Analysis
Data are reported as frequencies and percentages for categorical, and median and interquartile range (IQR, 25–75th percentile) for continuous variables. Demographic, clinical, preoperative and ECMO support details, and ECMO complications were compared between the survivors and nonsurvivors. The Pearson chi-square test was used to compare categorical data; the Fisher exact test was used when expected count in greater than 20% of cells was less than 5. The Mann-Whitney U test was used to compare continuous data.
Three multivariable logistic regression models were developed to evaluate independently the association of presurgical factors, ECMO-related factors, and both presurgical and ECMO factors with inhospital mortality. Candidate variables for inclusion in the first two models were selected from the univariate analysis comparing survivors and nonsurvivors. All variables with a univariate p value of less than 0.1 were selected for inclusion in the multivariable model. Variables with greater than 10% of missing data were excluded. A backward conditional strategy was used for entry and retention of variables in the model. A candidate variable was retained in the model if the p value was less than 0.05. All the variables significantly associated with mortality in the first two models were included in the third comprehensive model.
Variables containing continuous data that were retained in the multivariable model were tested for linear association and those not meeting the linearity assumption were categorized for inclusion in the model. Age and weight were tested for correlation using the Spearman Rho test; only age was used for modeling, as age and weight were collinear. Because comorbid conditions were only present in small number of subjects, a comprehensive “comorbid condition” variable was created for inclusion into the model, combining the following pre-ECMO variables: prematurity, heart failure, cardiogenic shock, respiratory disease, neurologic disease, renal disease, coagulation defects, hemorrhage, and hematologic or immunologic defect. Genetic syndrome and noncardiac congenital anomalies were included as a combined variable called “noncardiac congenital anomalies.” RACHS-1 categories were combined into a three-category variable (RACHS 1–3, RACHS 4–6, and not assigned).
All statistical analyses were performed using the IBM SPSS statistical software (Version 25.0; IBM Corp, Armonk, NY). Statistical significance was set at a two-sided p value of less than 0.05.
RESULTS
Study Population
Two-thousand nine-hundred fifteen patients underwent 2,950 runs for failure to wean from CPB during the study period. After application of the exclusion criteria, 2,322 patients (80%) were selected as our study cohort (Supplemental Fig. 1, http://links.lww.com/DCR/B376). Demographic and clinical characteristics of patients are described in Table 1. The median age at ECMO initiation was 26 days (IQR, 7–159). A genetic syndrome or congenital anomalies were present in 17%, single-ventricle CHD in 34% (n = 793), and 10% had preoperative cardiac arrest. Cardiac procedures in RACHS-1 1–3 categories included 1,108 patients (48%) and RACHS 4–6 1,103 patients (47%). The median CPB time was 251 minutes (IQR: 174–351). A compilation of survival to hospital discharge estimates based on surgical procedures is shown in Figure 1, and a detailed list is provided in Supplemental Table 1 (http://links.lww.com/DCR/B376).
Figure 1.
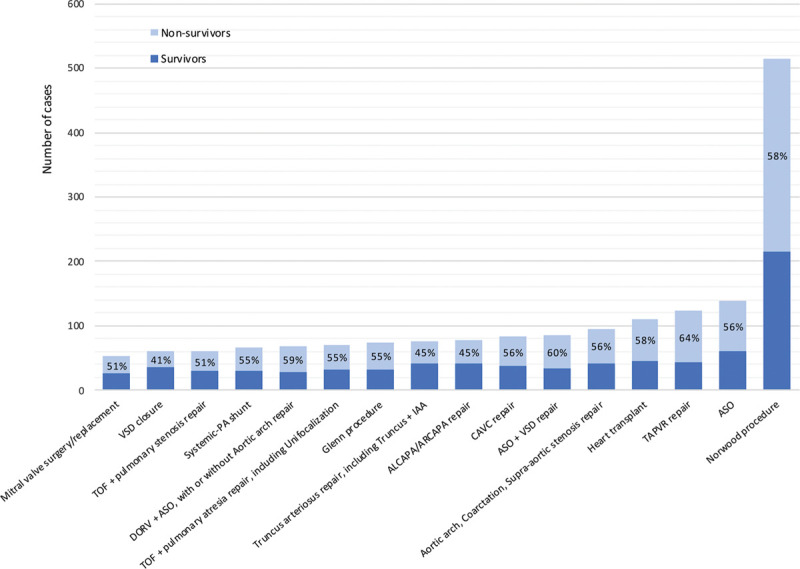
Number of survivors and nonsurvivors at hospital discharge according to most frequent cardiac procedures. Percentages are mortality at discharge. ALCAPA = anomalous left coronary artery from the pulmonary artery, ARCAPA = anomalous right coronary artery from the pulmonary artery, ASO = arterial switch operation, CAVC = complete atrioventricular canal, DORV = double outlet right ventricle, IAA = interrupted aortic arch, PA = pulmonary artery, TAPVR = total anomalous pulmonary venous return, TOF = tetralogy of Fallot, VSD = ventricular septal defect.
Pre-ECMO support and ECMO details are shown in Table 2. Preoperative mechanical ventilation (MV) was used in 1,198 patients (51%). The most common vascular access sites for ECMO cannulation included the aorta (n = 2,044, 88%) and the right atrium (n = 1,982, 85%); 13% (n = 312) had left atrial cannulation. The median duration of ECMO support was 104 hours (IQR, 65–169). ECMO complications in our study cohort are shown in Table 3.
ECMO was successfully weaned in 67% of patients (n =1,568). Three percent of patients (n = 70) were converted to a ventricular assist device (VAD) and 2% underwent transplant on-ECMO (n = 41, including 15 previously converted). Inhospital mortality was 55%, and survival did not change significantly over the study period (linear-by-linear association p = 0.13; Supplemental Fig. 2, http://links.lww.com/DCR/B376).
Features of Survivors and Nonsurvivors
Demographic, clinical, and surgical characteristics of survivors and nonsurvivors are shown in Table 1. Age and body weight were significantly lower among nonsurvivors than survivors. The frequency of genetic syndromes, noncardiac abnormalities, comorbid conditions, and cardiac arrest prior to surgery was higher in nonsurvivors than survivors. Nonsurvivors had more complex surgery (higher RACHS-1 category) and longer duration of CPB.
TABLE 1.
Demographic and Clinical Features of Patients Using Extracorporeal Membrane Oxygenation After Failure to Wean From Cardiopulmonary Bypass
| Variable | Total (n = 2,322) | Survivors (n = 1,039) | Nonsurvivors (n = 1,283) | p |
|---|---|---|---|---|
| Age (d), median (IQR) | 26 (7–159) | 59 (9–234) | 15 (9–114) | < 0.001 |
| Age category, n (%) | ||||
| Neonates | 1,207 (52) | 446 (43) | 761 (59) | |
| Infants | 752 (32) | 379 (36) | 373 (29) | < 0.001 |
| Children | 363 (16) | 214 (21) | 149 (12) | |
| Weight (kg), median (IQR)a | 3.6 (3.0–5.7) | 4.0 (3.2–7.0) | 3.4 (2.9–4.7) | < 0.001 |
| Gender (male), n (%)b | 1,273 (55) | 565 (54) | 708 (55) | 0.184 |
| Race (White), n (%)c | 1,311 (58) | 619 (61) | 692 (55) | 0.005 |
| Comorbid conditions, n (%) | ||||
| Genetic syndrome | 215 (9) | 77 (7) | 138 (11) | 0.006 |
| Noncardiac congenital anomalies | 221 (9) | 81 (8) | 140 (11) | 0.011 |
| Prematuritye | 208 (9) | 48 (4) | 166 (13) | < 0.001 |
| Cardiac-associated disease | ||||
| Arrhythmia | 348 (15) | 148 (14) | 200 (16) | 0.367 |
| Heart failure | 516 (22) | 207 (20) | 309 (24) | 0.016 |
| Shock, cardiogenic | 142 (6) | 52 (5) | 90 (7) | 0.044 |
| Pulmonary hypertension | 103 (4) | 47 (4) | 56 (4) | 0.853 |
| Cardiomyopathy | 69 (3) | 32 (3) | 37 (3) | 0.782 |
| Other | 116 (5) | 49 (5) | 67 (5) | 0.578 |
| Respiratory disease | 449 (20) | 173 (17) | 276 (21) | 0.003 |
| Neurologic disease | 233 (10) | 78 (7) | 155 (12) | < 0.001 |
| Renal disease | 284 (12) | 59 (6) | 225 (17) | < 0.001 |
| Gastrointestinal disease | 139 (6) | 62 (6) | 77 (6) | 0.972 |
| Infectious disease | 175 (8) | 67 (6) | 108 (8) | 0.074 |
| Metabolic, endocrine, and electrolyte abnormalities | 111 (5) | 45 (4) | 66 (5) | 0.361 |
| Coagulation defects | 42 (2) | 8 (1) | 34 (3) | 0.001 |
| Hemorrhage | 112 (5) | 32 (3) | 80 (6) | < 0.001 |
| Immunologic or hematologic disease | 87 (4) | 29 (3) | 58 (4) | 0.029 |
| Other comorbidities | 221 (10) | 107 (10) | 114 (9) | 0.249 |
| Preoperative cardiac arrest, n (%)f | 238 (10) | 89 (8) | 149 (12) | 0.016 |
| Preoperative echocardiography, n (%) | 1,253 (54) | 592 (57) | 661 (51) | 0.009 |
| Preoperative cardiac catheterization, n (%) | 381 (17) | 174 (17) | 207 (16) | 0.692 |
| Main cardiac surgery procedure Risk Adjustment in Congenital Heart Surgery-1 score, n (%) | ||||
| 1 | 16 (1) | 7 (1) | 9 (1) | |
| 2 | 255 (11) | 141 (14) | 114 (9) | |
| 3 | 837 (36) | 433 (44) | 404 (33) | |
| 4 | 508 (22) | 193 (20) | 315 (25) | < 0.001 |
| 5 | 30 (1) | 8 (1) | 22 (2) | |
| 6 | 565 (24) | 196 (20) | 369 (30) | |
| Not assigned | 111 (5) | 61 (6) | 50 (4) | |
| Extracorporeal membrane oxygenation, yr, n (%) | ||||
| 2000–2005 | 559 (24) | 241 (23) | 318 (25) | |
| 2006–2011 | 896 (39) | 400 (38) | 496 (39) | 0.583 |
| 2012–2016 | 867 (37) | 398 (38) | 469 (37) | |
| Surgery details | ||||
| Cardiopulmonary bypass time (min), median (IQR)d | 251 (174–351) | 238 (169–336) | 266 (180–365) | < 0.001 |
| Aortic cross clamp, n (%) | 1,979 (85) | 911 (88) | 1,068 (83) | 0.003 |
| Deep hypothermic cardiac arrest, n (%) | 974 (42) | 412 (40) | 562 (44) | 0.044 |
IQR = interquartile range.
Missing data, n (survivors, nonsurvivors):
a19 (11, 8); b20 (13, 7); c65 (30, 35); and
d230 (89, 141).
ePrematurity is defined as gestational age less than or equal to 36 wk.
fWithin 24 h prior to extracorporeal membrane oxygenation.
Pre-ECMO support and ECMO details in survivors and nonsurvivors are shown in Table 2. Nonsurvivors had longer duration of pre-ECMO ventilatory support, lower Pao2 and lower standardized bicarbonate levels on blood gas measurements, and received bicarbonate more frequently. ECMO pump flows were significantly higher in nonsurvivors at both 4 and 24 hours after ECMO initiation. At 24 hours following ECMO deployment, use of high-flow oscillatory ventilation was more frequent, and Fio2 was lower among nonsurvivors than survivors. Additionally, nonsurvivors underwent an invasive procedure on ECMO more frequently and had longer duration of ECMO than survivors. ECMO complications were more common in nonsurvivors (Table 3). Patients transplanted during ECMO support had significantly lower mortality than patients not transplanted (22% vs 56%; p < 0.001). Mortality among patients converted to VAD after failure to wean from CBP did not significantly differ compared with the mortality of those not converted (61% vs 55%; p = 0.29).
TABLE 2.
Preextracorporeal Membrane Oxygenation Support and Extracorporeal Membrane Oxygenation Details of Patients Who Failed to Wean From Cardiopulmonary Bypass
| Variable | Total (n = 2,322) | Survivors (n = 1,039) | Nonsurvivors (n = 1,283) | p |
|---|---|---|---|---|
| Pre-ECMO support, n (%) | ||||
| Inotropic/vasopressor drugs | 1,435 (62) | 645 (62) | 790 (62) | 0.804 |
| Vasodilator drugs | 440 (19) | 195 (19) | 245 (19) | 0.841 |
| Cardiac pacemaker | 196 (8) | 91 (9) | 105 (8) | 0.621 |
| Inhaled nitric oxide | 374 (16) | 170 (16) | 204 (16) | 0.763 |
| Steroids | 81 (3) | 34 (3) | 47 (4) | 0.610 |
| Pre-ECMO analgesia and sedation, n (%) | ||||
| Inhaled anesthetic | 160 (7) | 68 (6) | 92 (7) | 0.554 |
| Opioids | 1,226 (53) | 551 (53) | 675 (53) | 0.840 |
| Pre-ECMO neuromuscular blockers, n (%) | 1,142 (49) | 508 (49) | 634 (49) | 0.802 |
| Mechanical ventilation, n (%) | ||||
| Pre-ECMO | ||||
| Conventional | 1,174 (50) | 522 (49) | 652 (50) | 0.442 |
| HFOV | 24 (1) | 9 (1) | 17 (1) | 0.540 |
| At 24 hr after ECMO initiationa | ||||
| Conventional | 2,038 (99) | 926 (100) | 1,112 (99) | 0.010h |
| HFOV | 14 (1) | 1 (0) | 13 (1) | |
| Fio2 (%), median (IQR)b | ||||
| Pre-ECMO | 100 (55–100) | 99 (56–100) | 100 (52–100) | 0.456 |
| At 24 hr after ECMO initiation | 37 (25–40) | 40 (30–40) | 30 (21–40) | < 0.001 |
| Surfactant, pre-ECMO, n (%) | 21 (1) | 6 (1) | 15 (1) | 0.134 |
| Duration of MV prior to ECMO (hr), median (IQR)c | 14 (8–87) | 12 (7–43) | 18 (9–125) | < 0.001 |
| MV > 24 hr prior to ECMO, n (%)c | 900 (40) | 326 (32) | 574 (46) | < 0.001 |
| Arterial blood gas, pre-ECMOd | ||||
| pH, median (IQR) | 7.31 (7.23–7.40) | 7.31 (7.24–7.40) | 7.31 (7.21–7.39) | 0.074 |
| Paco2 (mm Hg), median (IQR) | 44 (37–54) | 44 (37–54) | 44 (36–55) | 0.705 |
| Pao2 (mm Hg), median (IQR) | 77 (39–242) | 104 (41–269) | 64 (37–225) | < 0.001 |
| Hco3 (mEq), median (IQR) | 23 (20–26) | 23 (20–26) | 23 (19–25) | 0.018 |
| Preoperative bicarbonate infusion, n (%) | 382 (16) | 133 (13) | 249 (19) | < 0.001 |
| ECMO indication subcategories, n (%) | ||||
| Low cardiac output syndrome | 1,123 (48) | 520 (50) | 603 (47) | 0.144 |
| Pulmonary hypertension | 178 (8) | 90 (9) | 88 (7) | 0.104 |
| Cardiac and pulmonary failure | 190 (8) | 59 (6) | 131 (10) | < 0.001 |
| ECMO cannulation sites, n (%)e | ||||
| Arterial cannulatione | ||||
| Aorta | 2,044 (88) | 921 (89) | 1,123 (87) | |
| Common carotid artery | 158 (7) | 56 (5) | 102 (8) | 0.150h |
| Femoral artery | 13 (1) | 9 (1) | 4 (1) | |
| Other | 25 (1) | 8 (1) | 17 (1) | |
| Venous cannulation | ||||
| Right atrium | 1,982 (85) | 879 (85) | 1,103 (86) | |
| Internal jugular vein | 154 (7) | 62 (6) | 92 (7) | 0.438 |
| Femoral vein | 31 (1) | 20 (2) | 11 (1) | |
| Other | 72 (3) | 35 (3) | 37 (3) | |
| Left atrium | 312 (13) | 154 (15) | 158 (12) | 0.078 |
| ECMO pump flow rates (mL/kg/min), median (IQR)f | ||||
| At 4 hr after ECMO initiation | 115 (95–141) | 109 (92–135) | 119 (99–145) | < 0.001 |
| At 24 hr after ECMO initiation | 119 (98–146) | 111 (91–140) | 124 (100–150) | < 0.001 |
| ECMO support duration (hr), median (IQR)g | 104 (65–169) | 86 (51–122) | 136 (71–219) | < 0.001 |
| Cardiac surgery on-ECMO, n (%) | 253 (11) | 107 (10) | 146 (11) | 0.406 |
| Cardiac surgery post-ECMO, n (%) | 25 (1) | 15 (1) | 10 (1) | 0.123 |
| Multiple cardiac surgery, n (%) | 276 (12) | 121 (12) | 155 (12) | 0.747 |
| Invasive procedure on ECMO, others, n (%) | 440 (19) | 170 (16) | 270 (21) | 0.004 |
| Cardiac catheterization within 24 hr from ECMO, n (%) | 320 (14) | 132 (12) | 188 (15) | 0.286 |
| Multiple ECMO runs, n (%) | 60 (3) | 27 (3) | 33 (3) | 0.968 |
ECMO = extracorporeal membrane oxygenation, HFOV = high-frequency oscillatory ventilation, IQR = interquartile range, MV = mechanical ventilation.
Missing data, n (survivors, nonsurvivors):
a270 (112, 158);
bpre-ECMO 1,099 (493, 606) and on-ECMO: 214 (82, 132);
c71 (25, 46);
dpH and Pco2: 638 (290, 348), Po2: 646 (292, 354), and bicarbonate: 804 (371, 433);
earterial cannulation: 124 (58, 66) and venous cannulation: 147 (70, 77);
fat 4 hr: 121 (52, 69) and at 24 hr: 233 (99, 134);
g18 (8, 10);
hFisher exact test.
TABLE 3.
Extracorporeal Membrane Oxygenation-Related Complications in Patients Who Failed to Wean From Cardiopulmonary Bypass
| Variable | Total (n = 2,322) | Survivors (n = 1,039) | Nonsurvivors (n = 1,283) | p |
|---|---|---|---|---|
| ECMO circuit complications, n (%) | 906 (39) | 319 (31) | 587 (46) | < 0.001 |
| Mechanical problems | 245 (11) | 71 (7) | 174 (14) | < 0.001 |
| Clots in ECMO circuit | 696 (30) | 237 (23) | 459 (36) | < 0.001 |
| Air embolus | 90 (4) | 30 (3) | 60 (5) | 0.026 |
| Cannula problems | 119 (5) | 40 (4) | 79 (6) | 0.012 |
| CNS complications, n (%) | 409 (18) | 107 (10) | 302 (23) | < 0.001 |
| Seizures | 148 (6) | 43 (4) | 105 (8) | < 0.001 |
| Cerebral infarction or intracranial hemorrhage | 298 (13) | 81 (8) | 217 (17) | < 0.001 |
| Brain death | 30 (1) | 0 (0) | 30 (2) | < 0.001 |
| Cardiac complications, n (%) | 1,739 (75) | 722 (69) | 1,017 (79) | <0.001 |
| Cardiac arrhythmia requiring treatment | 403 (17) | 125 (12) | 278 (22) | < 0.001 |
| Cardiopulmonary resuscitation on ECMO | 74 (3) | 10 (1) | 64 (5) | < 0.001 |
| Cardiac tamponade | 243 (10) | 97 (9) | 146 (11) | 0.110 |
| Myocardial stun at echocardiography evaluation | 203 (9) | 63 (6) | 140 (11) | < 0.001 |
| Need for inotropic drugs | 1,503 (65) | 609 (59) | 894 (70) | < 0.001 |
| Hypertension requiring vasodilators | 369 (16) | 168 (16) | 201 (16) | 0.742 |
| Peripheral vascular complications, n (%) | 10 (0) | 0 (0) | 10 (1) | 0.003c |
| Pulmonary complications, n (%) | ||||
| Pneumothorax requiring treatment | 50 (2) | 17 (2) | 33 (3) | 0.122 |
| Pulmonary hemorrhage | 187 (8) | 39 (4) | 148 (11) | < 0.001 |
| Hemorrhagic complications (other than pulmonary), n (%) | ||||
| Cannulation site bleeding | 431 (19) | 166 (16) | 265 (21) | 0.004 |
| Surgical site bleeding | 1,099 (47) | 441 (42) | 658 (51) | < 0.001 |
| Gastrointestinal bleeding | 29 (1) | 7 (1) | 22 (2) | 0.025 |
| Hemolysisa, n (%) | 288 (12) | 103 (10) | 185 (14) | 0.001 |
| Disseminate intravascular coagulation, n (%) | 115 (5) | 26 (2) | 89 (7) | < 0.001 |
| Infectious complications, n (%) | ||||
| Culture proven infection | 215 (9) | 71 (7) | 144 (11) | < 0.001 |
| WBC count < 1,500/mL | 24 (1) | 5 (0) | 19 (1) | 0.018 |
| Renal complications, n (%) | ||||
| Renal failure | 299 (13) | 78 (7) | 221 (17) | < 0.001 |
| Serum creatinine 1.5–3.0 mg/dL | 169 (7) | 41 (4) | 128 (10) | < 0.001 |
| Serum creatinine > 3.0 mg/dL | 54 (2) | 20 (2) | 34 (3) | 0.249 |
| Dialysis required | 282 (12) | 61 (6) | 221 (17) | < 0.001 |
| Hemofiltration required | 650 (28) | 198 (19) | 452 (35) | < 0.001 |
| Metabolic complications, n (%) | ||||
| Arterial pH < 7.20 | 146 (6) | 39 (4) | 107 (8) | < 0.001 |
| Arterial pH > 7.60 | 111 (5) | 54 (5) | 57 (4) | 0.397 |
| Blood glucose < 40 mg/dL | 41 (2) | 14 (1) | 27 (2) | 0.168 |
| Blood glucose > 240 mg/dL | 368 (16) | 144 (14) | 224 (17) | 0.018 |
| Hyperbilirubinemiab | 132 (6) | 48 (5) | 84 (6) | 0.046 |
ECMO = extracorporeal membrane oxygenation.
aHemolysis is defined as plasma-free hemoglobin > 50 mg/dL.
bHyperbilirubinemia is defined as direct bilirubin > 2.0 mg/dL or total bilirubin > 15.0 mg/dL.
cFisher exact test.
Multivariable Models of Factors Associated With Mortality
Three multivariable models evaluating factors associated with mortality are presented in Supplemental Table 2 (http://links.lww.com/DCR/B376) and Table 4. The first model included demographic and pre-ECMO factors. Older age than neonatal one and White race were both associated with lower mortality. Presence of genetic syndrome and noncardiac anomalies, comorbidities, pre-ECMO cardiac arrest, pre-ECMO MV, and bicarbonate replacement all increased mortality odds. Finally, more complex operations and longer duration of CPB increased mortality.
The second model explored associations of ECMO support variables and complications with mortality (Supplemental Table 2, http://links.lww.com/DCR/B376). Use of left atrial cannulation and lower Fio2 at 24-hour post-ECMO reduced and need for higher pump flow at 4-hour post-ECMO, longer ECMO duration, and ECMO complications all increased mortality.
In the comprehensive multivariable model including both pre-ECMO and ECMO factors (Table 4), only older age (> 26 d) lowered mortality. Genetic syndrome or congenital anomalies, comorbidities, pre-ECMO cardiac arrest, pre-ECMO MV for greater than 24 hours, pre-ECMO bicarbonate infusion, longer duration of CPB, procedures of higher surgical complexity, longer ECMO duration, and ECMO complications were all associated with increased mortality.
TABLE 4.
Comprehensive Multivariable Model of Factors Associated With Mortality in Patients Using Extracorporeal Membrane Oxygenation After Failure to Wean From Cardiopulmonary Bypass
| Variables | OR (95% CI) | p |
|---|---|---|
| Demographic and baseline clinical characteristics | ||
| Age (d) | < 0.001 | |
| ≤ 7 | 1.0 (reference group) | — |
| > 7 and ≤ 26 | 0.802 (0.601–1.069) | 0.132 |
| > 26 and ≤ 159 | 0.558 (0.416–0.750) | < 0.001 |
| > 159 | 0.478 (0.346–0.661) | < 0.001 |
| Genetic syndrome or congenital anomalies | 1.776 (1.358–2.324) | < 0.001 |
| Comorbidities | 1.586 (1.297–1.941) | < 0.001 |
| Pre-ECMO cardiac arrest | 1.675 (1.200–2.338) | 0.002 |
| Surgery details | ||
| Cardiopulmonary bypass time (min) | < 0.001 | |
| ≤ 174 | 1 (reference group) | — |
| > 174 and ≤ 251 | 0.967 (0.730–1.281) | 0.816 |
| > 251 and ≤ 351 | 1.501 (1.132–1.990) | 0.005 |
| > 351 | 1.609 (1.209–2.142) | 0.001 |
| RACHS-1 category | 0.004 | |
| RACHS 1–3 | 1 (reference group) | — |
| RACHS 4–6 | 1.435 (1.133–1.816) | 0.003 |
| RACHS not assigned | 0.745 (0.451–1.231) | 0.251 |
| Pre-ECMO details | ||
| Pre-ECMO mechanical ventilation duration > 24 hr | 1.494 (1.211–1.843) | < 0.001 |
| Pre-ECMO bicarbonate infusion | 1.419 (1.084–1.857) | 0.011 |
| ECMO factors | ||
| ECMO support duration (hr) | < 0.001 | |
| ≤ 65 | 1 (reference group) | — |
| > 65 and ≤ 104 | 0.765 (0.581–1.009) | 0.057 |
| > 104 and ≤ 169 | 1.539 (1.173–2.018) | 0.002 |
| > 169 | 3.472 (2.557–4.715) | < 0.001 |
| Complications | ||
| ECMO circuit complications | 1.259 (1.018–1.558) | 0.034 |
| CNS complications | 1.793 (1.358–2.366) | < 0.001 |
| Pulmonary hemorrhage | 2.527 (1.672–3.819) | < 0.001 |
| Renal failure | 1.698 (1.232–2.341) | 0.001 |
| Hemofiltration required | 1.540 (1.224–1.939) | < 0.001 |
ECMO = extracorporeal membrane oxygenation, OR = odds ratio, RACHS = Risk Adjustment in Congenital Heart Surgery.
Model: Candidate variables: age, race (White), genetic syndrome or congenital anomalies, comorbidities, pre-ECMO cardiac arrest, cardiopulmonary bypass time, RACHS-1 category, pre-ECMO mechanical ventilation duration > 24 hr, pre-ECMO bicarbonate infusion, left atrium cannulation, ECMO pump flow at 4 hr, ECMO support duration, Fio2 at 24 hr after ECMO initiation, ECMO circuit complications, CNS complications, pulmonary hemorrhage, renal failure, and hemofiltration required.
n = 2,024; p = 0.538 for Hosmer-Lemeshow test; area under the curve = 0.769.
− refers that no p value is calculable for that group because it is a reference group. It is a common sign for the reference group if there are multiple categories.
DISCUSSION
ECMO is used in many centers performing pediatric cardiac surgery to rescue children with refractory cardiopulmonary failure or cardiac arrest after cardiac surgery (2, 16). ECMO has also been used to bridge successfully children failing to wean from CPB following cardiac surgery to survival. We demonstrate that, although mortality when ECMO is used to support children failing to wean from CPB is high (55%), it is similar to mortality reported for cardiac ECMO support of all indications (ELSO 2019 International Summary survival to discharge neonatal cardiac ECMO: 43%; pediatric cardiac ECMO: 52%) (16). Among our high-risk ECMO cohort, neonates, those with comorbid conditions, those undergoing complex congenital cardiac surgery, those requiring long duration of CPB, and those with ECMO complications, not surprisingly, had reduced survival. The use of ECMO to support children who fail to wean off CPB has increased significantly; however, survival has remained unchanged.
In a two-center report of postoperative ECMO use in children with biventricular CHD undergoing cardiac surgery, Chaturvedi et al (19) reported improved survival in patients in whom ECMO was initiated in the operating room, some of whom failed to wean CPB, compared with ECMO initiated in the ICU (64% vs 29%). They suggested that avoiding prolonged exposure to inadequate cardiac output and cardiac arrest in postoperative period improved outcomes for these children. Our analysis showed that ECMO used in the context of failure to wean from CPB in children undergoing cardiac surgery is associated with high mortality (55%). Poor outcomes in our study may be related to the inclusion of a broader variety of CHD diagnosis, including many with complex single-ventricle CHD. The prior study included children who weaned CPB but required ECMO for unstable status in the operating room. Reasons for failure to wean CPB or if a period of separation from CPB occurred in our study cohort were not available to inform our analyses. Although our findings suggest that ECMO for failure to wean from CPB after pediatric cardiac surgery is associated with poor survival, these patients face imminent mortality without ECMO support. Decisions regarding use of ECMO in children failing to wean from bypass have to be made rapidly, and often without optimal clinical and/or imaging information. ECMO deployment in these circumstances may provide an opportunity for a careful evaluation of reversible conditions that may be amenable for correction.
We found many pre-ECMO and presurgical factors associated with mortality. The increased odds of death associated with neonatal age, race, and genetic and noncardiac comorbid conditions have been shown to be associated with poor outcomes when ECMO is used for other cardiac and noncardiac indications (4, 10, 14, 20). These factors offer little opportunity for improvement. Similarly, the association of presurgical factors, such as pre-ECMO cardiac arrest, MV, and need for correction of acidosis with increased mortality in our cohort, has been previously described both in cardiac ECMO and for children undergoing CHD surgery by the Society of Thoracic Surgeons, as factors associated with mortality (2).
Complex cardiac surgical procedures and longer CPB duration were both independently associated with mortality in our cohort. Prior reports of ECMO support following cardiac surgery for CHD have reported a high frequency of residual lesions in children requiring postoperative ECMO (15). These reports have also shown that prompt diagnosis and correction of residual lesions are essential to improve ECMO survival (15). One could argue that repair of these residual lesions at the time of the index operation may provide optimal hemodynamics for successful ECMO support. However, this would require long CPB duration or repeated exposures to CPB and may worsen outcomes for those who require postoperative ECMO support (21). Decisions regarding duration of CPB should be made by the primary surgeon and surgical team after weighing the consequences of continuing CPB to correct a residual lesion or deploying ECMO to provide a period of stability and attempting correction at a later time. We did not find an independent association between an additional cardiac surgery on-ECMO and mortality. We expected to find this, as correction of residual lesions after CHD surgery during ECMO has been shown to be associated with improved outcomes (15). However, data on residual lesions after CHD surgery are not mandatory enterable information in the ELSO registry, and thus subject to reporting bias.
Left atrial decompression with left atrial cannulation was identified as protective factor for mortality in the model that only includes ECMO factors. Because ECMO does not decompress the left ventricle, draining the left atrium can reduce left ventricular distension, allowing myocardial rest and recovery. Furthermore, it can also protect from lung injury due to cardiogenic pulmonary edema or hemorrhage from severe left atrial hypertension (22, 23). Even though statistical independence was not confirmed in the final model, we believe that in patients with left atrial hypertension could benefit from left-heart decompression. However, the association of left atrial decompression and improved survival should be interpreted cautiously, as there is wide variability in the use of left atrial decompression among ECMO centers and some high-risk procedures (e.g., Norwood operation) may not need left atrial decompression. Another interesting finding of our study is the improved survival for patients receiving heart transplantation on-ECMO. Our findings are similar to the improved survival described by Alsoufi et al (5). Thus, when there are no signs of recovery on ECMO support, early evaluation and listing for cardiac transplantation can be considered as an exit strategy.
ECMO complications in our study cohort were associated with increased mortality, as shown in many previous reports of ECMO (5, 6, 9, 11, 14). Surgical site bleeding is an anticipated complication in patients supported with ECMO after a surgical procedure. Surgical site bleeding was frequent in our cohort and was seen in 47% of patients and was associated with increased mortality (51%). The occurrence of surgical site bleeding in our cohort was higher than that reported by the ELSO registry for cardiac ECMO in neonates (26%) and children (25%) (24). The higher rate of surgical site bleeding in our study population may be related to increased risk of bleeding from fresh surgical incisions and residual anticoagulation from CPB. Reduction of surgical bleeding complications with aggressive modification of anticoagulation protocols, use of hemostatic agents, and surgical intervention for hemostasis are essential to improve outcomes for these patients.
We found that although the use of ECMO in patients failing to wean CPB increased overtime, survival did not improve. Although speculative, increasing use of ECMO in patients undergoing complex cardiac surgical procedures and in patients with noncardiac comorbid conditions may have resulted in no improvement in survival over time.
Several important limitations should be considered when interpreting our analyses. Data reported to the ELSO registry are not specific to studying outcomes for patients supported for failure to wean from CPB, thus, important confounders associated with survival may not have been collected. Both International Classification of Diseases and CPT codes do not adequately describe CHD and cardiac surgical procedures, and thus may have led to misclassification of complexity. Data reported to ELSO do not contain specific information on the exact reason for failure to wean from bypass, and presence and severity of residual surgical lesions after CHD surgery. Finally, the lack of short-term and long-term neurologic outcome data limits meaningful evaluation of survival. Despite these limitations, our study offers important information for assessing patients’ prognosis and for future investigation in this high-risk ECMO population.
CONCLUSIONS
In conclusion, we found that inhospital mortality was high for children undergoing ECMO for failure to wean from CPB. Younger children, those with genetic abnormalities and comorbid conditions, those with more severe preoperative illness, and those undergoing complex cardiac surgical procedures had higher mortality. ECMO factors including a longer support duration and on-ECMO complications are also independently associated with mortality. These data can guide prognostication in the high-risk population and offer object data for counseling families. Left atrial decompression may improve survival in some patients, and early consideration of heart transplantation represents an important ECMO exit strategy in patients showing no signs of cardiac recovery.
Supplementary Material
Footnotes
Drs. Thiagarajan and Polito contributed equally.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
The study was supported, in part, by the Callaghan Family Chair in Cardiac ICU, Boston Children’s Hospital (to Dr. Thiagarajan).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Rihal CS, Naidu SS, Givertz MM, et al. ; Society for Cardiovascular Angiography and Interventions (SCAI), Heart Failure Society of America (HFSA), Society of Thoracic Surgeons (STS), American Heart Association (AHA), and American College of Cardiology (ACC) 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: Endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latino Americana de Cardiología Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J Am Coll Cardiol. 2015; 65:2140–2141 [DOI] [PubMed] [Google Scholar]
- 2.Mascio CE, Austin EH, 3rd, Jacobs JP, et al. Perioperative mechanical circulatory support in children: An analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. J Thorac Cardiovasc Surg. 2014; 147:658–664; discussion 664–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pourmoghadam KK, Olsen MC, Nguyen M, et al. Comparative review of outcomes in patients with congenital heart disease requiring cardiopulmonary support for failure to wean from cardiopulmonary bypass or for refractory sudden cardiac arrest. World J Pediatr Congenit Heart Surg. 2015; 6:387–392 [DOI] [PubMed] [Google Scholar]
- 4.Chrysostomou C, Morell VO, Kuch BA, et al. Short- and intermediate-term survival after extracorporeal membrane oxygenation in children with cardiac disease. J Thorac Cardiovasc Surg. 2013; 146:317–325 [DOI] [PubMed] [Google Scholar]
- 5.Alsoufi B, Al-Radi OO, Gruenwald C, et al. Extra-corporeal life support following cardiac surgery in children: Analysis of risk factors and survival in a single institution. Eur J Cardiothorac Surg. 2009; 35:1004–1011; discussion 1011 [DOI] [PubMed] [Google Scholar]
- 6.Balasubramanian SK, Tiruvoipati R, Amin M, et al. Factors influencing the outcome of paediatric cardiac surgical patients during extracorporeal circulatory support. J Cardiothorac Surg. 2007; 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ElMahrouk AF, Ismail MF, Hamouda T, et al. Extracorporeal membrane oxygenation in postcardiotomy pediatric patients-15 years of experience outside Europe and North America. Thorac Cardiovasc Surg. 2019; 67:28–36 [DOI] [PubMed] [Google Scholar]
- 8.McKenzie JM, Scodellaro T, d’Udekem Y, et al. Late-term gestation is associated with improved survival in neonates with congenital heart disease following postoperative extracorporeal life support. Pediatr Crit Care Med. 2017; 18:876–883 [DOI] [PubMed] [Google Scholar]
- 9.Bhat P, Hirsch JC, Gelehrter S, et al. Outcomes of infants weighing three kilograms or less requiring extracorporeal membrane oxygenation after cardiac surgery. Ann Thorac Surg. 2013; 95:656–661 [DOI] [PubMed] [Google Scholar]
- 10.Sasaki T, Asou T, Takeda Y, et al. Extracorporeal life support after cardiac surgery in children: Outcomes from a single institution. Artif Organs. 2014; 38:34–40 [DOI] [PubMed] [Google Scholar]
- 11.Kumar TK, Zurakowski D, Dalton H, et al. Extracorporeal membrane oxygenation in postcardiotomy patients: Factors influencing outcome. J Thorac Cardiovasc Surg. 2010; 140:330–336.e2 [DOI] [PubMed] [Google Scholar]
- 12.Peer SM, Emerson DA, Costello JP, et al. Intermediate-term results of extracorporeal membrane oxygenation support following congenital heart surgery. World J Pediatr Congenit Heart Surg. 2014; 5:236–240 [DOI] [PubMed] [Google Scholar]
- 13.Loforte A, Delmo Walter EM, Stiller B, et al. Extracorporeal membrane oxygenation for intraoperative cardiac support in children with congenital heart disease Interact Cardiovasc Thorac Surg. 2010; 10:753–758 [DOI] [PubMed] [Google Scholar]
- 14.Morris MC, Ittenbach RF, Godinez RI, et al. Risk factors for mortality in 137 pediatric cardiac intensive care unit patients managed with extracorporeal membrane oxygenation. Crit Care Med. 2004; 32:1061–1069 [DOI] [PubMed] [Google Scholar]
- 15.Agarwal HS, Hardison DC, Saville BR, et al. Residual lesions in postoperative pediatric cardiac surgery patients receiving extracorporeal membrane oxygenation support. J Thorac Cardiovasc Surg. 2014; 147:434–441 [DOI] [PubMed] [Google Scholar]
- 16.Nasr VG, Raman L, Barbaro RP, et al. ; ELSO Registry Scientific Oversight Committee Highlights from the Extracorporeal Life Support Organization registry: 2006-2017. ASAIO J. 2019; 65:537–544 [DOI] [PubMed] [Google Scholar]
- 17.Jenkins KJ, Gauvreau K, Newburger JW, et al. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002; 123:110–118 [DOI] [PubMed] [Google Scholar]
- 18.Thiagarajan RR, Laussen PC, Rycus PT, et al. Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation. 2007; 116:1693–1700 [DOI] [PubMed] [Google Scholar]
- 19.Chaturvedi RR, Macrae D, Brown KL, et al. Cardiac ECMO for biventricular hearts after paediatric open heart surgery. Heart. 2004; 90:545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thiagarajan RR, Barbaro RP, Rycus PT, et al. ; ELSO member centers Extracorporeal Life Support Organization registry international report 2016. ASAIO J. 2017; 63:60–67 [DOI] [PubMed] [Google Scholar]
- 21.Bronicki RA, Hall M. Cardiopulmonary bypass-induced inflammatory response: Pathophysiology and treatment. Pediatr Crit Care Med. 2016; 17:S272–S278 [DOI] [PubMed] [Google Scholar]
- 22.Kotani Y, Chetan D, Rodrigues W, et al. Left atrial decompression during venoarterial extracorporeal membrane oxygenation for left ventricular failure in children: Current strategy and clinical outcomes. Artif Organs. 2013; 37:29–36 [DOI] [PubMed] [Google Scholar]
- 23.Eastaugh LJ, Thiagarajan RR, Darst JR, et al. Percutaneous left atrial decompression in patients supported with extracorporeal membrane oxygenation for cardiac disease. Pediatr Crit Care Med. 2015; 16:59–65 [DOI] [PubMed] [Google Scholar]
- 24.Barbaro RP, Paden ML, Guner YS, et al. ; ELSO member centers Pediatric Extracorporeal Life Support Organization registry international report 2016. ASAIO J. 2017; 63:456–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


